Introduction
As two of the most common inflammatory dermatoses,
atopic eczema (AE) and psoriasis threaten the quality of life and
health in humans. AE is an inflammation of the skin characterized
by pruritic, papulovesicular and occasional weeping dermatitis
(1), and its etiology is
hypothesized to be a combination of genetic and environmental
factors. It has been identified that AE may sometimes be an
allergic reaction to house dust mites (2). Psoriasis is clinically characterized
by dry, reddish and silvery-white scaly plaques. Psoriasis is
considered to be a primary T-lymphocyte-based immune-pathogenetic
disorder (3,4) and runs a chronic course with
recurrence caused by environmental factors, including trauma,
infection and stress (5,6). Elevated levels of the proinflammatory
cytokines interleukin (IL)-17 and IL-22 have been observed in
patients with psoriasis (7,8).
Psoriasis and AE are hypothesized to be associated with the
persistence of local proinflammatory activities (9,10).
However, the molecular mechanisms in the development of these
diseases remain to be further elucidated.
HMGB1, a 30-kDa nuclear protein referred to one of
the most important chromatin proteins, serves as a proinflammatory
cytokine mediator, following release by macrophages or monocytes,
or during various pathological necrotic conditions (11,12).
TLR4, as one of the HMGB1 receptors, is also involved in the
induction of inflammatory responses. Extracellular HMGB1 binds to
TLR4, which causes myeloid differentiation primary response gene 88
to activate nuclear factor κB (NF-κB) (13). Activated NF-κB modulates the immune
response through the transcriptional regulation of cytokines and
chemokines (14).
It has been recognized that HMGB1 serves significant
roles in autoimmunity diseases, epidermal tumors, toxic epidermal
necrolysis and Stevens-Johnson syndrome (15–18),
and TLR4 and NF-κB have been reported to take part in the
development of certain tumors and inflammatory diseases (14,16).
However, the effects of these mediators on human inflammatory
dermatoses have not been elucidated. In the present study, the
involvement of the HMGB1-TLR4-NF-κB signaling pathway in the
pathogenesis of AE and psoriasis was investigated.
Materials and methods
Tissue specimens
The present study was performed in accordance with
The Declaration of Helsinki 1964 and its later amendments, and was
approved by the Ethics Board of Tongji Medical College (Wuhan,
China). Diagnosis of AE and psoriasis was based on the clinically
apparent symptoms and histopathological criteria. The severity of
AE and psoriasis were assessed by two well-trained and experienced
dermatologists, according to the scoring AD (SCORAD) index and
Psoriasis Area and Severity Index (PASI), respectively (19,20).
Patients who had an illness duration of >2 years and exhibited a
moderate-severe condition (SCORAD index, 25–60; PASI score, 6–15)
were enrolled. Tissue specimens were obtained from active lesions
of patients through cutaneous biopsy following informed consent,
including 12 patients with psoriasis vulgaris (5 females and 7
males; age, 22–61 years) and 11 patients with chronic AE (6 females
and 5 males; age, 18–54 years). Normal skin specimens were obtained
from 10 cases (4 females and 6 males; age, 24–57 years) undergoing
reconstructive surgery. All specimens were collected between March
and December 2010. A therapeutic washout period of 4 weeks for oral
medication and 2 weeks for topical treatment was implemented prior
to specimen collection. Patients who had a history of other
autoimmune diseases, tumors or immunologic deficiency diseases were
excluded.
Antibodies and reagents
The antibodies and reagents used in the present
study include anti-HMGB1 (cat. no. 2600-1; Epitomics; Abcam,
Cambridge, UK), anti-TLR4 (cat. no. ab22048; Abcam), anti-NF-κB p65
(cat. no. SC-7151; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and REAL™ EnVision Detection kit (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Immunohistochemistry
The tissue specimen preparation and
immunohistochemical procedures were as previously described
(16). Tissue specimens were fixed
in formalin, embedded in paraffin and sectioned at 4-µm thickness.
Following deparaffinization, rehydration, antigen retrieval and
blocking of non-specific binding, the sections were incubated with
primary antibodies (anti-HMGB1, 1:800; anti-TLR4, 1:200; anti-NF-κB
p65, 1:200) at 4°C overnight. The specimens were subsequently
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:500; cat. nos. ab6789 and ab6721; Abcam) at room
temperature for 45 min. 3,3′-Diaminobenzidine substrate solution
was added, and dehydration, hyalinization and mounting were
routinely conducted, followed by observation under a Nikon Eclipse
Ti-SR microscope and the capturing of images with a Nikon DS-U3
digital camera (both Nikon Corporation, Tokyo, Japan). Negative
controls were obtained by omitting the primary antibodies. A total
of two pathologists counted and evaluated the staining
independently using a semi-quantitative scoring system as follows
(16): 0, No staining; 1, light
brown yellow staining; 2, brown staining; and 3, dark brown
staining. A total of 10 random fields of view (magnification, ×400)
were counted for each section and average positive expression was
scored as follows: 1, <25%; 2, between 25 and <50%; 3,
between 50 and <75%; and 4, ≥75%. The degree of staining and the
percentage of expression for each section provided a final score as
follows: Negative (−), 0–1 point; weakly positive (+), 2–3 points;
moderately positive (+ +), 4–6 points; and strongly positive (+ +
+), ≥7 points.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences between groups were analyzed using one-way
analysis of variance followed by the Bonferroni correction for
normally distributed datasets, or by Kruskal-Wallis one-way
analysis of variance followed by Nemenyi test for skewed datasets.
Spearman's rank correlation coefficient was used for correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using R
software (version 3.0, GNU Project, Boston, MA, USA).
Results
Expression of HMGB1 in lesional skin
of patients with psoriasis or AE
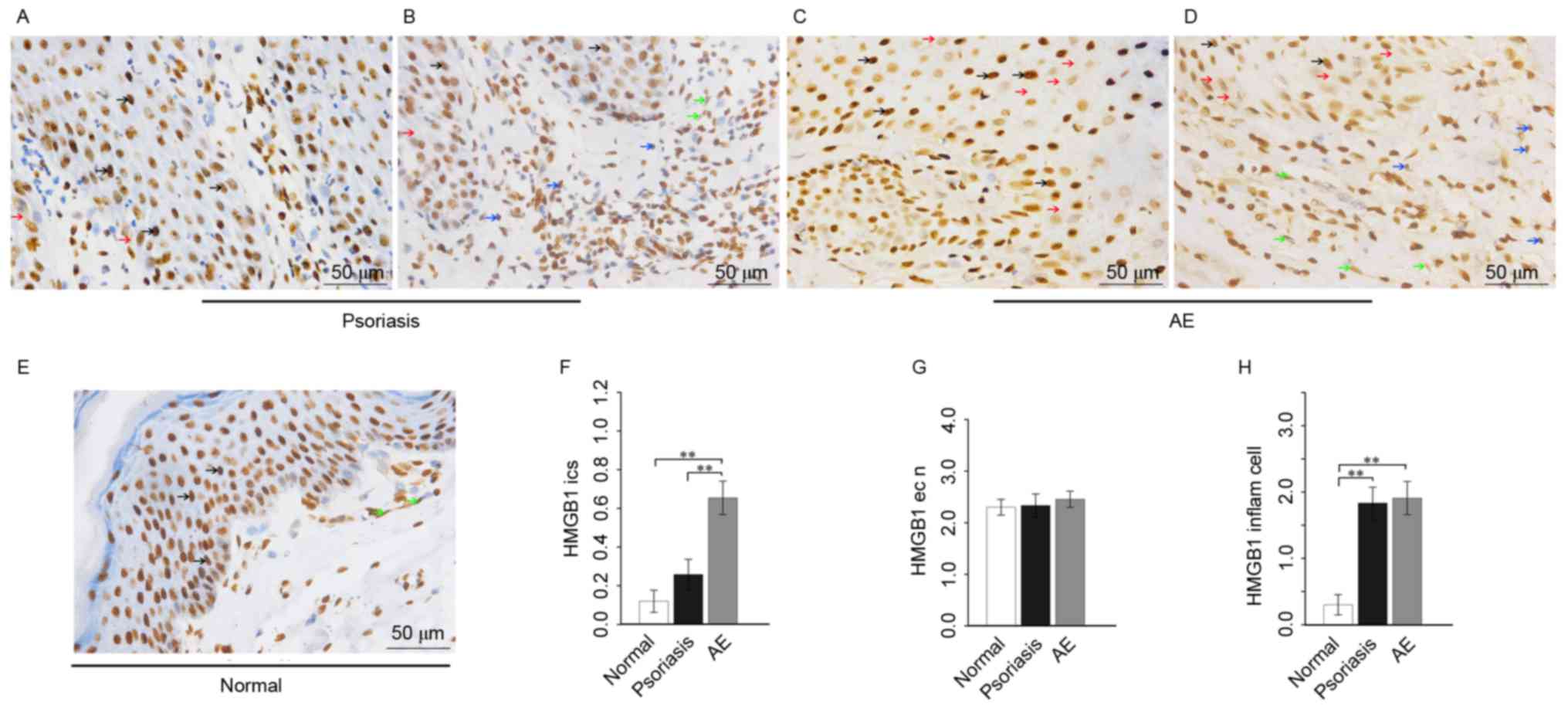
In psoriasis, moderate to strong positive HMGB1
diffuse expression was observed in the nuclei, with weak positive
focal expression in the cytoplasm of the squamous epithelium.
Extracellular HMGB1 was occasionally present in the intercellular
spaces of the epithelium. In the nuclei and cytoplasm of associated
inflammatory cells and vascular endothelial cells, moderate
positive HMGB1 diffuse expression was observed (Fig. 1A and B).
In AE, there was strong positive HMGB1 diffuse
expression in the nuclei, focal weak expression in the cytoplasm
and weak positive expression in the intercellular spaces of the
squamous epithelium. The nuclei and cytoplasm exhibited diffusive
moderate positive HMGB1 expression in the associated inflammatory
cells, while exhibiting moderate to strong expression in the
vascular endothelial cells (Fig. 1C
and D).
In healthy skin, moderate to strong positive HMGB1
expression was observed in the nuclei, with occasional focal
expression in the cytoplasm of the squamous epithelium. Little
HMGB1 expression was present in the epithelial intercellular
spaces. As for inflammatory cells, little HMGB1 expression was
observed in the nuclei and cytoplasm, and for vascular endothelial
cells, a moderate positive HMGB1 expression was observed (Fig. 1E).
Analysis of variance demonstrated that the HMGB1
expression in epithelial intercellular spaces in AE was
significantly increased compared with psoriasis (P=0.0023) and
normal skin (P=0.0001), and that the HMGB1 expression in epithelial
intercellular spaces in psoriasis was markedly increased compared
with normal skin (Fig. 1F). There
was no statistical difference among the HMGB1 expression in the
epithelial nuclei of psoriasis, AE and healthy skin (Fig. 1G). As for inflammatory cells, HMGB1
expression in psoriasis and AE was significantly increased compared
with healthy skin (P=0.0001 and P=0.0000, respectively), and the
HMGB1 expression in AE was markedly increased compared with
psoriasis (Fig. 1H).
Expression of TLR4 in lesional skin of
patients with psoriasis or AE
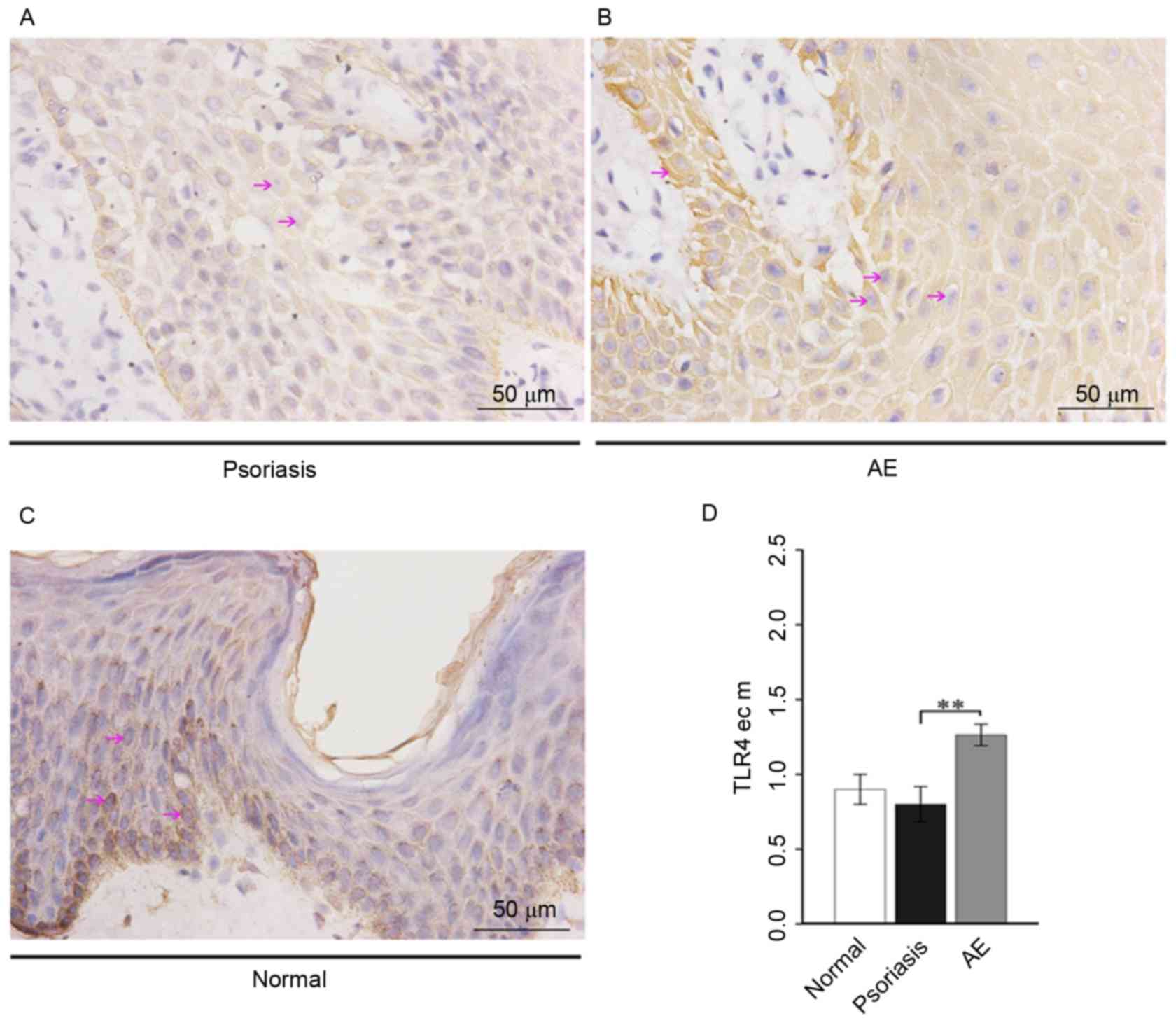
On the squamous epithelium membranes, weak positive
TLR4 expression was observed in psoriasis (Fig. 2A), weak to moderate positive
expression in AE (Fig. 2B), and
weak positive expression in healthy skin (Fig. 2C). Analysis of variance
demonstrated that epithelial cell membrane TLR4 expression in AE
was significantly increased compared with psoriasis (P=0.0066) and
was markedly increased compared with healthy skin, while the TLR4
expression in psoriasis was markedly decreased compared with
healthy skin (Fig. 2D).
Expression of NF-κB p65 in lesional
skin of patients with psoriasis or AE
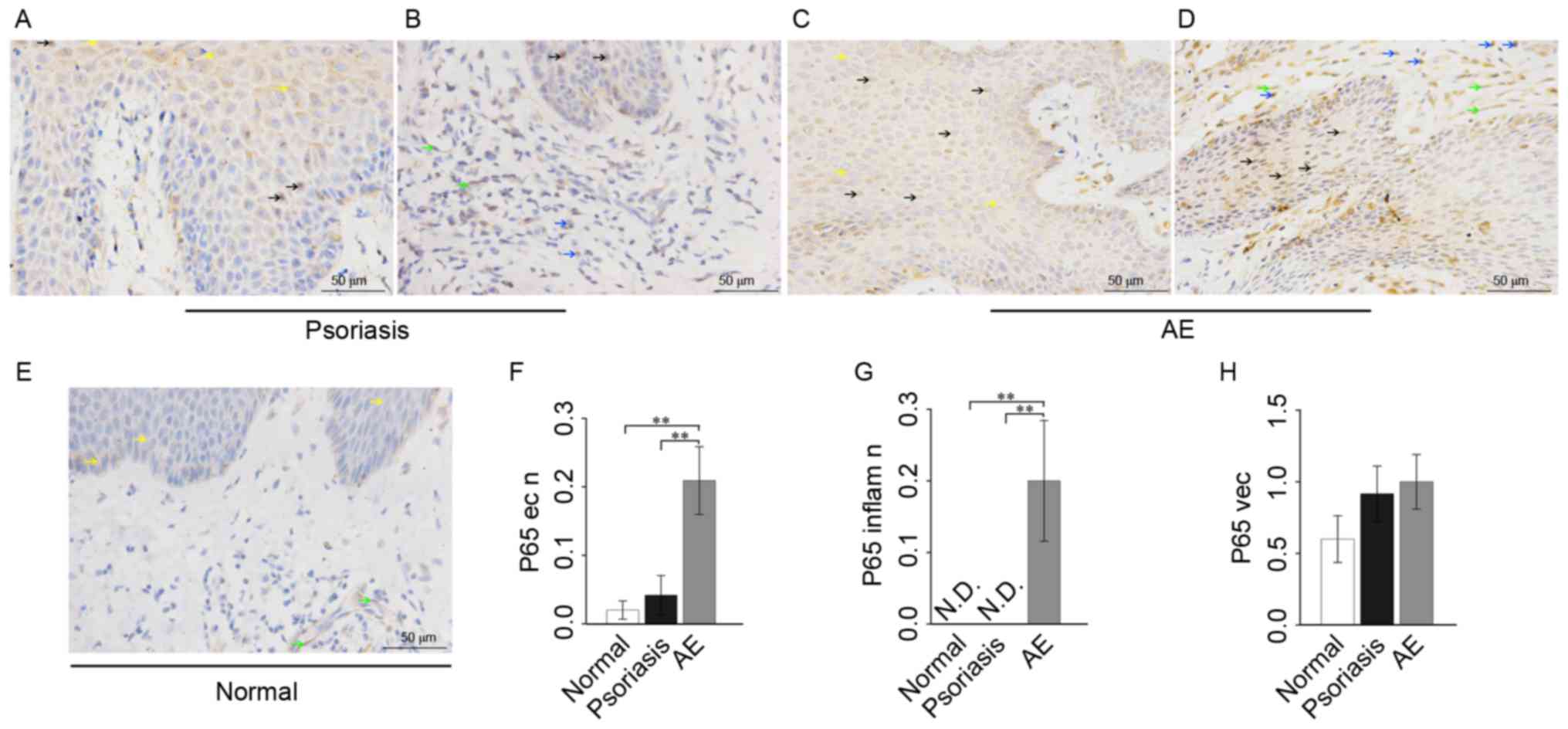
In psoriasis, sporadic weak positive p65 expression
was observed in the nuclei, with weak positive expression in the
cytoplasm of the epithelium. Extracellular p65 was occasionally
present in the epithelial intercellular spaces. In the associated
inflammatory cells, there was focal expression of p65 in the
cytoplasm and little expression of p65 in the nuclei. As for
vascular endothelial cells, there was a weak positive p65
expression in the cytoplasm; however, no p65 expression in the
nuclei (Fig. 3A and B).
In AE skin, there was a relatively high p65
expression in the nuclei and weak positive expression in the
cytoplasm of epithelium. There was also a relatively high p65
expression in the nuclei; however, weak positive focal expression
in the cytoplasm of associated inflammatory cells. However, in the
vascular endothelial cells, p65 was not observed in the nuclei and
exhibited weak positive staining in the cytoplasm (Fig. 3C and D).
In healthy skin, minimal p65 expression was observed
in the nuclei, with focal positive expression in the cytoplasm of
the epithelium. In the associated inflammatory cells there was
little p65 expression in the nuclei. As for vascular endothelial
cells, there was sporadic weak positive p65 expression in the
cytoplasm (Fig. 3E).
Analysis of variance showed that the p65 expression
of epithelial nuclei in AE was significantly increased compared
with healthy skin (P=0.0066) and psoriasis (P=0.0082), and p65
expression of epithelial nuclei in psoriasis was markedly increased
compared with healthy skin (Fig.
3F). The p65 expression of inflammatory cell nuclei in AE was
significantly increased compared with healthy skin (P=0.0029) and
psoriasis (P=0.0010) (Fig. 3G). As
for vascular endothelial cells, the p65 level in AE or psoriasis
was markedly increased compared with healthy skin (Fig. 3H).
Correlation analysis
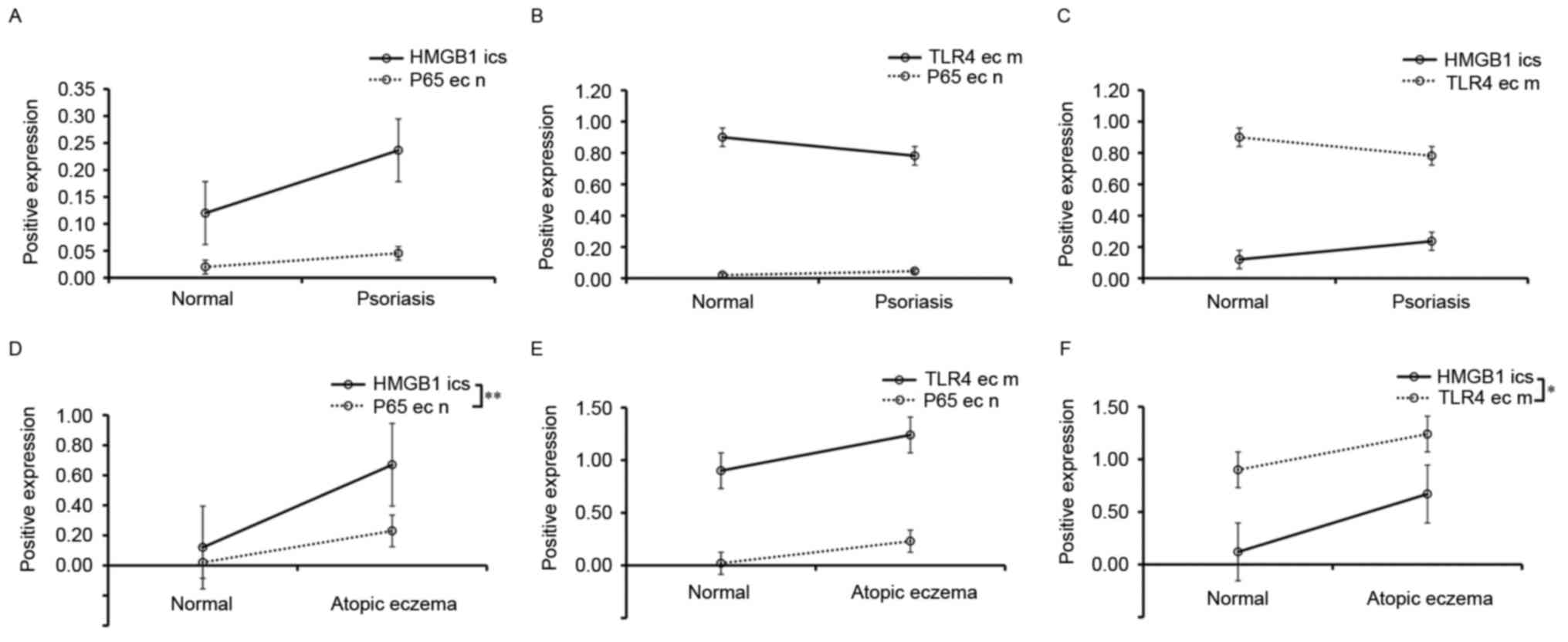
In healthy skin and psoriasis tissues, Spearman's
rank correlation coefficient analysis demonstrated that there was
no significant correlation between the p65 level in epithelial cell
nuclei and the HMGB1 level in epithelial intercellular spaces
(Fig. 4A) or the epithelial cell
membrane TLR4 level (Fig. 4B), or
between epithelial cell membrane TLR4 expression and epithelial
intercellular space HMGB1 expression (Fig. 4C). In AE tissues, Spearman's rank
correlation coefficient analysis demonstrated that the epithelial
nuclear p65 expression was positively correlated with the
epithelial intercellular space HMGB1 expression (r=0.5894;
P<0.01; Fig. 4D). The
epithelial nuclear p65 expression was not significantly correlated
with the epithelial cell membrane TLR4 expression, although there
was a trend (r=0.1381; P>0.05; Fig.
4E). However, the epithelial cell membrane TLR4 expression was
positively correlated with the epithelial intercellular space HMGB1
expression (r=0.3856; P<0.05; Fig.
4F).
Discussion
The pathogenesis of AE and psoriasis remains to be
completely elucidated. Despite the clinical distinctions, the two
diseases involve the activation of local proinflammatory mediators.
The purpose of the present study is to gain an improved
understanding of the interactions between HMGB1, TLR4 and NF-κB p65
in the two diseases. The results of the present study support
possible roles for these local proinflammatory mediators in the
pathogenesis of AE; however, not in psoriasis.
As important inflammatory cytokines and
transcription factors, the NF-κB family primarily consists of
transcription factor p65, NF-κB1, NF-κB2, proto-oncogene c-Rel and
transcription factor RelB (21,22),
which are commonly present in the cytoplasm in an inactive state.
The activity of NF-κB is primarily stimulated by lipopolysaccharide
signaling, the T-cell receptor or tumour necrosis factor (23). Activated by various inducers, NF-κB
protein translocates to the nucleus, regulates the expression of
inflammatory factors and becomes a hallmark of inflammatory
responses. Increased activation of NF-κB is frequently detected in
tumors (24,25), or the location of inflammation in
rheumatoid arthritis, inflammatory bowel diseases and asthma,
accompanied by the intensified production of proinflammatory
factors, including IL-1, IL-6 and TNF (23). The results of the present study
demonstrated that the p65 level of epithelial nuclei in AE was
increased compared with psoriasis and healthy skin, and the p65
level of epithelial nuclei in psoriasis was markedly increased
compared with healthy skin. These results indicated that the NF-κB
p65-regulated local inflammation was particularly enhanced in AE.
By contrast, obscure activation of NF-κB signaling was demonstrated
in psoriasis. The results were consistent with certain studies
demonstrating that enhanced NF-κB signaling was observed in atopic
dermatitis mouse models (26–28);
however, were not consistent with certain other studies
demonstrating that NF-kB may also act as a crucial mediator
involved in the pathogenesis of psoriasis (29–31).
Therefore, continued efforts will be required to identify the roles
of NF-κB signaling in the two inflammatory skin diseases.
To evaluate the effects of HMGB1 and TLR4 on NF-κB
p65-regulated inflammation, the significance of the
HMGB1-TLR4-NF-κB signaling pathway in the inflammatory dermatoses
was investigated. The data demonstrated that there were variant
levels of HMGB1 expression in the epithelial intercellular spaces
of the tissues, with major expression of extracellular HMGB1 in AE,
lesser expression of extracellular HMGB1 in psoriasis and little
expression of extracellular HMGB1 in normal skin. These results
implied that HMGB1 is released into the extracellular environment
in the inflammatory skin diseases, serving as a proinflammatory
mediator of the local inflammation. Analysis of variance
demonstrated that the expression of extracellular HMGB1 in AE was
increased compared with healthy skin, as well as in psoriasis, and
extracellular HMGB1 in psoriasis was markedly increased compared
with healthy skin, indicating that HMGB1 may be a significant
mediator in promoting local inflammation in AE; however, not in
psoriasis. These results are inconsistent with the study by Chen
et al (17), who observed
increased serum HMGB1 levels and altered HMGB1 distribution in the
lesional skin of patients with psoriasis vulgaris. It is possible
that, in the two studies, different localizations of HMGB1
expression were observed, or patients with different stages and
severity assessed by PSAI were enrolled. Therefore, further studies
are required to investigate this phenomenon.
TLRs are a type of pattern recognition receptor that
recognize structurally conserved molecules derived from microbes
and serve a key role in the innate immune system (32). Certain members of the TLR family
also recognize HMGB1 and trigger activation of inflammatory
responses (33). There is
currently sufficient evidence suggesting that HMGB1-TLR4
interactions are important for acute and chronic
inflammation-associated pathology (34–36).
Panzer et al (37) reported
that TLR4 expression was concentrated to the basal layers in
healthy skin, whereas it was pronounced in upper layers in atopic
dermatitis, contact dermatitis and psoriasis. The present results
also revealed that the expression of TLR4 on epithelial cell
membranes in AE was increased compared with psoriasis. However,
TLR4 on epithelial cell membranes in psoriasis was markedly
decreased compared with healthy skin. This indicated that TLR4
expression may be a considerable mediator in promoting inflammation
in AE; however, not in psoriasis. The results of the present study
suggest that extracellular HMGB1 interacts with TLR4 to activate
NF-κB p65 and results in p65-regulated inflammation in AE.
In addition, the present results demonstrated that
there was almost the same level of HMGB1 expression in the
epithelial nuclei of psoriasis, AE and healthy skin, indicating
that the nuclear HMGB1 contributes to the stabilization of DNA and
chromosomes, which is consistent with the authors' previous study
into the significance of nuclear HMGB1 in certain epidermal tumors
(16). The present results also
demonstrated that the HMGB1 level in the associated inflammatory
cells of AE and psoriasis was increased compared with healthy skin,
and the p65 level in inflammatory nuclei in AE was increased
compared with psoriasis, as well as in healthy skin, suggesting
that inflammatory skin diseases, particularly AE, may be mediated
by inflammatory cells. Furthermore, Spearman's rank correlation
coefficient analysis demonstrated that the HMGB1 level in
epithelial intercellular spaces was positively correlated with the
p65 level in epithelial nuclei, and was also positively correlated
with the TLR4 level on epithelial cell membranes in the lesional
skin of patients with AE, further indicating that extracellular
HMGB1 may bind TLR4 to activate NF-κB p65 and thus promote
p65-regulated local inflammation in AE.
In conclusion, the NF-κB p65-regulated inflammation
was intensified in AE; however, not in psoriasis in the present
study. Therefore, the HMGB1-TLR4-NF-κB signaling pathway may be
involved in the pathogenesis of AE and these molecules may be
promising targets for attenuating the inflammation in AE. However,
there are certain limitations to the present study, as the
inflammation intensity is correlated with the stage and severity of
skin diseases (17). Only a small
number of patients with a certain range of severity were enrolled
and only static expressions of three molecules in skin tissue were
examined. Therefore, only correlations, not mechanistic information
on function, were provided in the present study, and further
investigation and validation is required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460304) and the
Guangxi Natural Science Foundation (grant nos. 2015GXNSFAA139197
and 2015GXNSFDA139020).
References
|
1
|
Hanifin JM: Evolving concepts of
pathogenesis in atopic dermatitis and other eczemas. J Invest
Dermatol. 129:320–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werfel T, Breuer K, Ruéff F, Przybilla B,
Worm M, Grewe M, Ruzicka T, Brehler R, Wolf H, Schnitker J and Kapp
A: Usefulness of specific immunotherapy in patients with atopic
dermatitis and allergic sensitization to house dust mites: A
multi-centre, randomized, dose-response study. Allergy. 61:202–205.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lew W, Bowcock AM and Krueger JG:
Psoriasis vulgaris: Cutaneous lymphoid tissue supports T-cell
activation and ‘Type 1’ inflammatory gene expression. Trends
Immunol. 25:295–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyman O, Conrad C, Tonel G, Gilliet M and
Nestle FO: The pathogenic role of tissue-resident immune cells in
psoriasis. Trends Immunol. 28:51–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffiths CE and Barker JN: Pathogenesis
and clinical features of psoriasis. Lancet. 370:263–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boniface K, Guignouard E, Pedretti N,
Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G,
Yssel H, et al: A role for T cell-derived interleukin 22 in
psoriatic skin inflammation. Clin Exp Immunol. 150:407–415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi H, Tsuji H, Hashimoto Y,
Ishida-Yamamoto A and Iizuka H: Serum cytokines and growth factor
levels in Japanese patients with psoriasis. Clin Exp Dermatol.
35:645–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kasraie S and Werfel T: Role of
macrophages in the pathogenesis of atopic dermatitis. Mediators
Inflamm. 2013:9423752013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valledor AF, Comalada M, Santamaria-Babi
LF, Lloberas J and Celada A: Macrophage proinflammatory activation
and deactivation: A question of balance. Adv Immunol. 108:1–20.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai S, Sodhi C, Cetin S, Richardson W,
Branca M, Neal MD, Prindle T, Ma C, Shapiro RA, Li B, et al:
Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte
migration via activation of Toll-like receptor-4 and increased
cell-matrix adhesiveness. J Biol Chem. 285:4995–5002. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pisetsky D: Cell death in the pathogenesis
of immune-mediated diseases: The role of HMGB1 and DAMP-PAMP
complexes. Swiss Med Wkly. 141:w132562011.PubMed/NCBI
|
|
13
|
Tadie JM, Bae HB, Deshane JS, Bell CP,
Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E and Zmijewski
JW: Toll-like receptor 4 engagement inhibits adenosine
5′-monophosphate-activated protein kinase activation through a high
mobility group box 1 protein-dependent mechanism. Mol Med.
18:659–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh H and Ghosh S: NF-κB: Roles and
regulation in different CD4(+) T-cell subsets. Immunol Rev.
252:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harris HE, Andersson U and Pisetsky DS:
HMGB1: A multifunctional alarmin driving autoimmune and
inflammatory disease. Nat Rev Rheumatol. 8:195–202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng H, Deng Y, Xie Y, Liu H and Gong F:
Expression and significance of HMGB1, TLR4 and NF-κB p65 in human
epidermal tumors. Bmc Cancer. 13:3112013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen T, Guo ZP, Li L, Wang L, Jia RZ, Cao
N, Qin S and Li MM: Increased HMGB1 serum levels and altered HMGB1
expression in patients with psoriasis vulgaris. Arch Dermatol Res.
305:263–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima S, Watanabe H, Tohyama M, Sugita
K, Iijima M, Hashimoto K, Tokura Y, Nishimura Y, Doi H, Tanioka M,
et al: High-mobility group box 1 protein (HMGB1) as a novel
diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson
syndrome. Arch Dermatol. 147:1110–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oranje AP, Glazenburg EJ, Wolkerstorfer A
and de Waard-van der Spek FB: Practical issues on interpretation of
scoring atopic dermatitis: The SCORAD index, objective SCORAD and
the three-item severity score. Br J Dermatol. 157:645–648. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langley RG and Ellis CN: Evaluating
psoriasis with psoriasis area and severity index, psoriasis global
assessment, and lattice system physician's global assessment. J Am
Acad Dermatol. 51:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verma IM, Stevenson JK, Schwarz EM, Van
Antwerp D and Miyamoto S: Rel/NF-kappa B/I kappa B family: Intimate
tales of association and dissociation. Genes Dev. 9:2723–2735.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayo MW and Baldwin AS: The transcription
factor NF-kappaB: Control of oncogenesis and cancer therapy
resistance. Biochim Biophys Acta. 1470:M55–M62. 2000.PubMed/NCBI
|
|
25
|
Lin A and Karin M: NF-kappaB in cancer: A
marked target. Semin Cancer Biol. 13:107–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karuppagounder V, Arumugam S,
Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, Harima M,
Suzuki H, Nomoto M, Miyashita S, et al: Modulation of HMGB1
translocation and RAGE/NFκB cascade by quercetin treatment
mitigates atopic dermatitis in NC/Nga transgenic mice. Exp
Dermatol. 24:418–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HK, Kim HS, Kim YJ, Kim JS, Park YS,
Kang JS, Yuk DY, Hong JT, Kim Y and Han SB: Sophoricoside isolated
from Sophora japonica ameliorates contact dermatitis by
inhibiting NF-κB signaling in B cells. Int Immunopharmacol.
15:467–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JH, Kim MH, Yang G, Huh Y, Kim SH and
Yang WM: Effects of topical application of Astragalus membranaceus
on allergic dermatitis. Immunopharmacol Immunotoxicol. 35:151–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldminz AM, Au SC, Kim N, Gottlieb AB and
Lizzul PF: NF-κB: An essential transcription factor in psoriasis. J
Dermatol Sci. 69:89–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andrés RM, Montesinos MC, Navalón P, Payá
M and Terencio MC: NF-κB and STAT3 inhibition as a therapeutic
strategy in psoriasis: In vitro and in vivo effects of BTH. J
Invest Dermatol. 133:2362–2371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaisho T and Akira S: Toll-like receptor
function and signaling. J Allergy Clin Immunol. 117:979–987; quiz
988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JS, Gamboni-Robertson F, He Q,
Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama
I, Banerjee A, et al: High mobility group box 1 protein interacts
with multiple Toll-like receptors. Am J Physiol Cell Physiol.
290:C917–C924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nair AR, Ebenezer PJ, Saini Y and Francis
J: Angiotensin II-induced hypertensive renal inflammation is
mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial
cells. Exp Cell Res. 335:238–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang FC, Pei JX, Zhu J, Zhou NJ, Liu DS,
Xiong HF, Liu XQ, Lin DJ and Xie Y: Overexpression of HMGB1 A-box
reduced lipopolysaccharide-induced intestinal inflammation via
HMGB1/TLR4 signaling in vitro. World J Gastroenterol. 21:7764–7776.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z
and Wang X: TLR4 as receptor for HMGB1-mediated acute lung injury
after liver ischemia/reperfusion injury. Lab Invest. 93:792–800.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Panzer R, Blobel C, Fölster-Holst R and
Proksch E: TLR2 and TLR4 expression in atopic dermatitis, contact
dermatitis and psoriasis. Exp Dermatol. 23:364–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|