Introduction
Endometriosis refers to the appearance of
endometrial tissue with growth function, including glands and
mesenchyme, in the endometrium-coated inner membrane and other
regions outside the muscle layers of the uterus body (1). As one of the common diseases in
gynecology and obstetrics, endometriosis is usually found in women
of childbearing age and seriously affects the quality of life of
patients (2). This disease is
predominantly found in women aged 25–45 years old with an incidence
of 10–15% in China, and incidence is significantly increasing with
a trend towards younger individuals (3). Although endometriosis is benign in
histopathology, it features hyperplasia, infiltration, metastasis,
recurrence and other malignant activity, with a malignant
transformation rate of 0.7–1.0% (4). It has various clinical manifestations
and the primary symptoms include dysmenorrhea, chronic pelvic pain,
sexual intercourse pain, infertility and menstrual changes
(5). At present, endometriosis is
treated medicinally and/or through surgery, however, there is no
ideal radical treatment, with the exception of radical surgery, for
pharmaceutical therapy or conservative surgery, for which the
recurrence rate remains high (6).
Endometriosis is one of the most common diseases in
women of childbearing age; however, its pathogenesis remains to be
fully elucidated (7). In previous
years, it has been shown that chemotaxis is important in the
development of epithelial-mesenchymal transition (EMT) (7). The overexpression of regulated on
activation, normal T cell expressed and secreted (RANTES) in
peritoneal fluid and ectopic foci is found in patients with
endometriosis (8). RANTES, as an
activating and regulatory factor, is formed on the expression and
secretion of normal T cells, has specific chemotactic properties on
memory T cells, monocytes and other immune cells, and is involved
in the development of EMT though regulation of the immune response
and interactions with other cellular factors, including ovarian
hormones (8,9).
Widely distributed all over China, whiteflower
lagopsis is a herb of Lagopsis, lamiales, also known as small
motherwort, floralwhite motherwort, foralwhite horebound and
lantern tree (10). It tastes
bitter and is neural in nature with marginal toxicity. With
functions, including the promotion of blood circulation, removal of
blood stasis and regulation of menstruation, it is a form of
gynecological medicine predominantly used to treat irregular
menstruation, hemiplegia, amenorrhea due to stagnation of blood,
anemia dizziness and other diseases (11). In previous years, investigations on
its chemical constituents have shown that the herb of whiteflower
lagopsis predominantly contains labdane-type diterpene, flavonoids,
phenethyl alcohol and other compounds (11). A previous study indicated that
marrubiin has several pharmacological activities, including
improving disturbances in blood and lymph microcirculation,
myocardial protection, anti-inflammation and anti-oxidation
(12). The aim of the present
study was to evaluate the protective effects of marrubiin on
endometriosis, via suppression of inflammation and downregulation
of the expression of RANTES.
Materials and methods
Animal endometriosis model
All experiments in the present study were approved
by the experimental animal committee of Nanhua Hospital Affiliated
to Nanhua University (Hengyang, China). Severe combined
immunodeficiency (SCID) female mice (20±2 g, 6–7 weeks) were
purchased from the Animal Laboratory of Nanhua University and
housed under a 12 h light-dark cycle at 22–24°C, with food and
water available ad libitum in pathogen-free conditions.
Endometriotic tissue was collected from the endometrium of the mice
under anesthesia and placed into sterile PBS, cut into coarse
fragments and suspended. The endometriotic cells (105
cell/ml) were intraperitoneally implanted into peritoneal cavity of
mice, and these mice were injected with estradiol benzoate (30
µg/kg) once a day for 14 days.
Groups and treatments
All SCID mice were randomly divided into the
following groups (n=12 per group): Sham group, endometriosis model
group, endometriosis model+12 mg/kg marrubiin treatment,
endometriosis model+25 mg/kg marrubiin treatment, endometriosis
model+50 mg/kg marrubiin treatment. After treatment with marrubiin,
mice were sacrificed using decollation under 30 mg/kg pentobarbital
anesthesia.
Extraction
Marrubiin was extracted from Marrubium
vulgare and Leonotis leonurus using a procedure
described previously with modifications (13). Acetone was added to the plant
material (10 ml/g) for organic extraction, and extraction was
performed for 1 h at 37°C. Subsequently, the mixture was filtered
through Whatman No. 1 filter paper. A rotary evaporator was used to
remove solvent, and distilled water was used to concentrate the
extract at 60°C.
Cell treatment
U937 cells were acquired from American Type Culture
Collection (Manassas, VA, USA) and cultured with RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA USA) and 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) supplemented 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a 5%
CO2 humidified atmosphere.
Hematoxylin and eosin (H&E)
staining
The implanted endometrium was fixed in 4%
paraformaldehyde for 1–2 days and embedded into paraffin.
Histological material was cut into 4 µm sections and stained with
H&E, which were visualized using an upright microscope (E600FN;
Nikon, Tokyo Japan).
Monocyte chemotaxis
Blood was obtained via cardiac puncture and
resuspended in 2 ml mouse monocyte isolation buffer. The layer
containing enriched monocytes was carefully removed following
centrifugation at 1,500 × g for 10 min at 4°C and washed with
Hank's solution. Monocyte purity was determined using flow
cytometric experiments as >95%.
Western blot analysis
The implanted endometrium was collected and
homogenized in lysis buffer with 1% protease inhibitor cocktail and
centrifuged for 15 min at 12,000 × g at 4°C. The protein
concentrations were determined using a BCA protein assay kit, and
50 mg total proteins were separated using 10–12% SDS-PAGE and
transferred onto nitrocellulose membranes. Following soaking in 5%
skim milk powder in TBS-Tween (0.1%), the membrane was incubated
with the following primary antibodies: Anti-RANTES (sc-20731;
1:3,00; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-TNF-α (sc-8301; 1:5,00; Santa Cruz Biotechnology, Inc.),
anti-prostaglandin E2 (PGE2; sc-514224; 1:2,00; Santa Cruz
Biotechnology, Inc.) and β-actin (sc-7210; 1:4,00; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membrane was developed
using the anti-rabbit or anti-mouse HRP-linked secondary antibody
(sc-2004 or sc-2005; 1:5,000, Santa Cruz Biotechnology, Inc.) at
37°C for 1 h and a chemiluminescent detection system.
ELISA
Expression levels of RANTES (E-EL-H0023c and
E-EL-M0009c; Elabscience Biotechnology Co., Ltd., Bethesda, MD,
USA), interleukin (IL)-6 and IL-1β (EM004-96 and EM001-96, both
from ExCell Bio Co., Ltd., Taicang, China) were determined by
ELISA, according to the manufacturer's protocol. Expression levels
were measured using a Tecan microplate reader (Safire2; Tecan,
Männedorf, Switzerland).
Calcium mobilization assay
Blood was resuspended in Tyrode's buffer containing
no calcium at a density of 3×108 platelets/ml, into
which Fura2-AM was added (4 M final concentration) and the mixture
was incubated at 37°C for 30 min. Calcium mobilization was measured
using a Tecan microplate reader (Safire2; Tecan).
Thromboxane B2 (TXB2) assay
TXB2 was assayed using a commercial enzyme
immunoassay kit according to the manufacturer's protocol
(E-EL-M1144c, Elabscience Biotechnology Co., Ltd.) and measured
using a Tecan microplate reader (Safire2; Tecan).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean, unless otherwise indicated. The results were evaluated
using the Mann-Whitney test. P<0.05 was considered to indicate a
statistically significant difference using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Protective effect of marrubiin
inhibits endometrial lesions
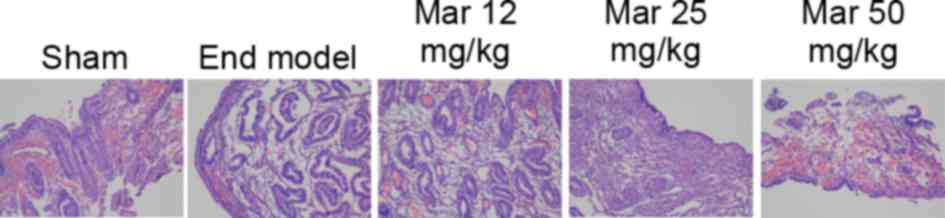
The endometrial lesions in each group were verified
using H&E staining, the results of which are shown in Fig. 1. The endometrial lesions in the
endometriosis model were more marked, compared with those in the
control group. Treatment with 25 or 50 mg/kg marrubiin
significantly reduced endometrial lesions in the mice with
endometriosis (Fig. 1).
Protective effect of marrubiin
inhibits monocyte chemotaxis
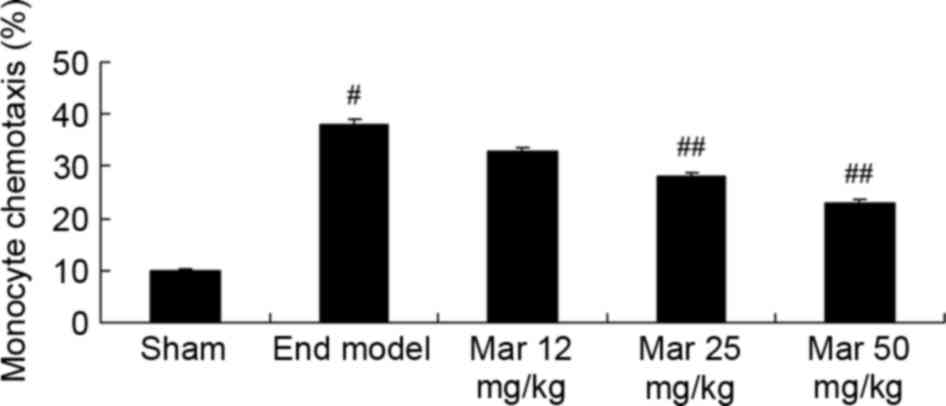
As shown in Fig. 2,
there was a marked increase in monocyte chemotaxis in the
endometriosis model, compared with the sham group. Treatment with
25 or 50 mg/kg marrubiin significantly inhibited monocyte
chemotaxis in the mice with endometriosis (Fig. 2).
Protective effect of marrubiin
inhibits U937 migration
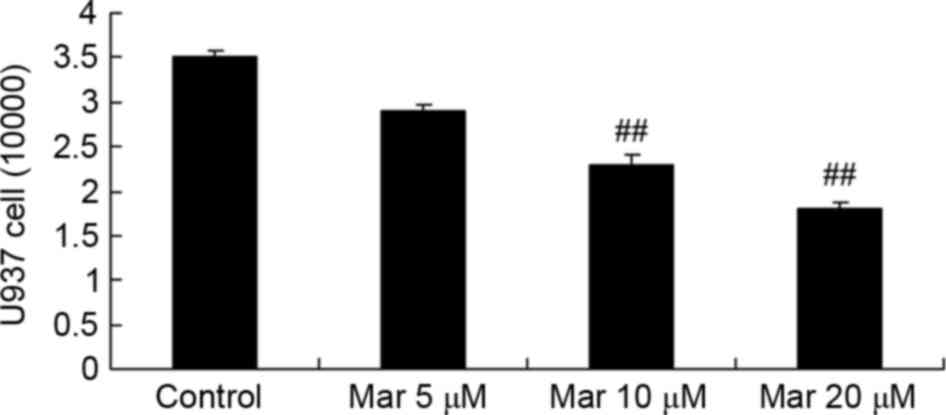
Compared with U937 migration in the control group,
marrubiin inhibited U937 migration in a dose-dependent manner. In
particular, treatment with 10 or 20 µM significantly inhibited U937
migration (Fig. 3).
Protective effects of marrubiin
inhibit the expression of RANTES
To examine the protective effects of marrubiin on
the mRNA expression of RANTES, RT-qPCR analysis was used to
determine the mRNA expression of RANTES in the SCID mice or U937
cells. As shown in Fig. 4A, the
expression of RANTES in the endometriosis model group was higher,
compared with that in the sham group. Marrubiin significantly
inhibited the expression of RANTES in the SCID mice and U937 cells
(Fig. 4A and B).
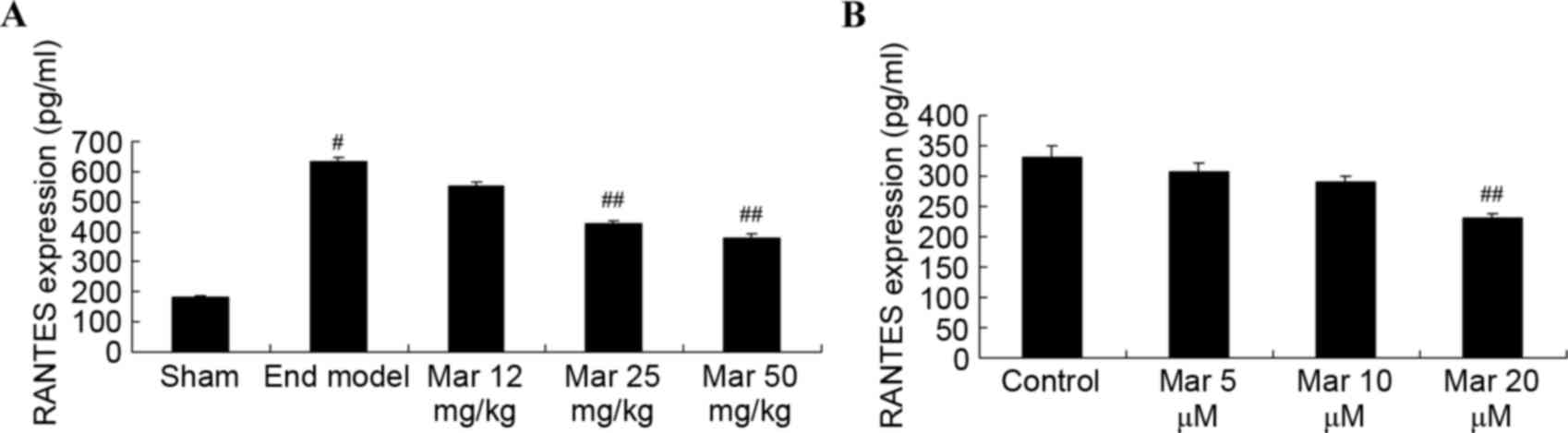 | Figure 4.Protective effect of Mar inhibits the
expression of RANTES levels. The effects of Mar on the expression
of RANTES levels were examined in (A) severe combined
immunodeficiency mice. Mar 12 mg/kg, endometriosis + 12 mg/kg Mar;
Mar 25 mg/kg, endometriosis + 25 mg/kg Mar; Mar 50 mg/kg,
endometriosis + 50 mg/kg Mar. #P<0.01, vs. sham;
##P<0.01, vs. End model. Effects of Mar on the mRNA
expression of RANTES were examined in (B) U937 cells.
##P<0.01, vs. control (0 µM Mar). RANTES, regulated
on activation, normal T cell expressed and secreted; Sham, sham
group; End model, endometriosis model; Mar, marrubiin. |
Protective effects of marrubiin
inhibit the protein expression of RANTES
To examine the protective effects of marrubiin on
the protein expression of RANTES, western blot analysis was used to
detect the protein expression of RANTES in the SCID mice and U937
cells. Compared with the protein expression levels of RANTES in the
sham and control groups, the protein expression of RANTES in the
endometriosis model group was higher (Fig. 5A). Treatment with marrubiin
significantly inhibited the protein expression of RANTES in the
mice with endometriosis and U937 cells (Fig. 5A and B).
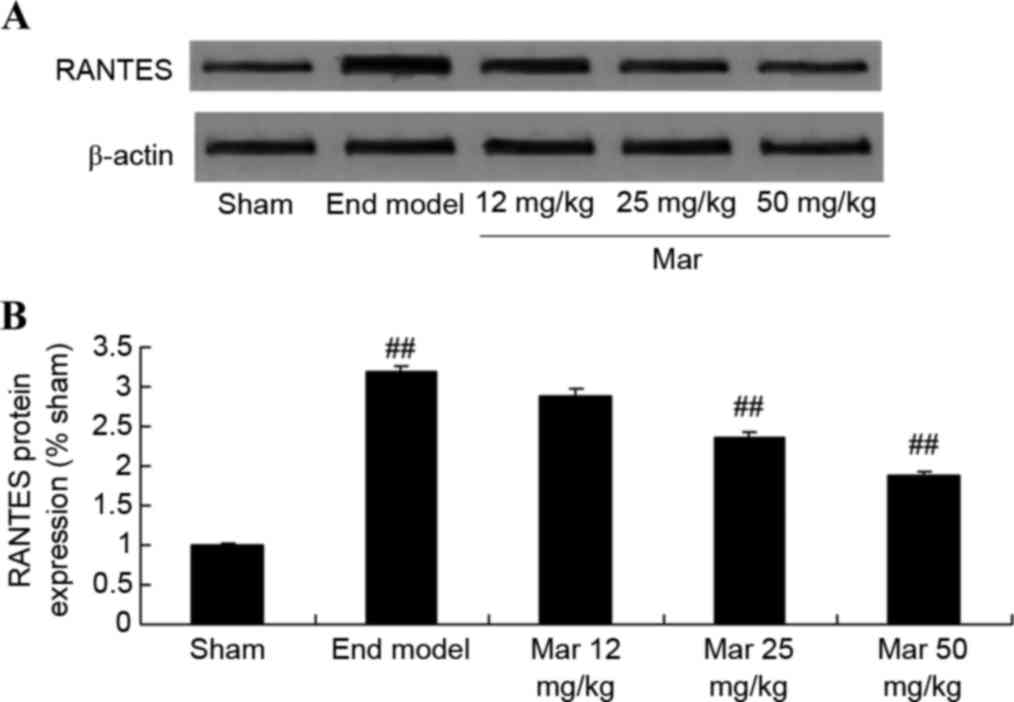 | Figure 5.Protective effect of Mar inhibits the
protein expression of RANTES. The effects of Mar on the protein
expression levels of RANTES were examined in (A and B) severe
combined immunodeficiency mice. Mar 12 mg/kg, endometriosis + 12
mg/kg Mar; Mar 25 mg/kg, endometriosis + 25 mg/kg Mar; Mar 50
mg/kg, endometriosis + 50 mg/kg Mar. #P<0.01, vs.
sham; ##P<0.01, vs. End model. Effects of Mar on the
protein expression levels of RANTES were examined in (C and D) U937
cells. ##P<0.01, vs. control (0 µM Mar). RANTES,
regulated on activation, normal T cell expressed and secreted;
Sham, sham group; End model, endometriosis model; Mar,
marrubiin. |
Protective effect of marrubiin
inhibits the protein expression of TNF-α
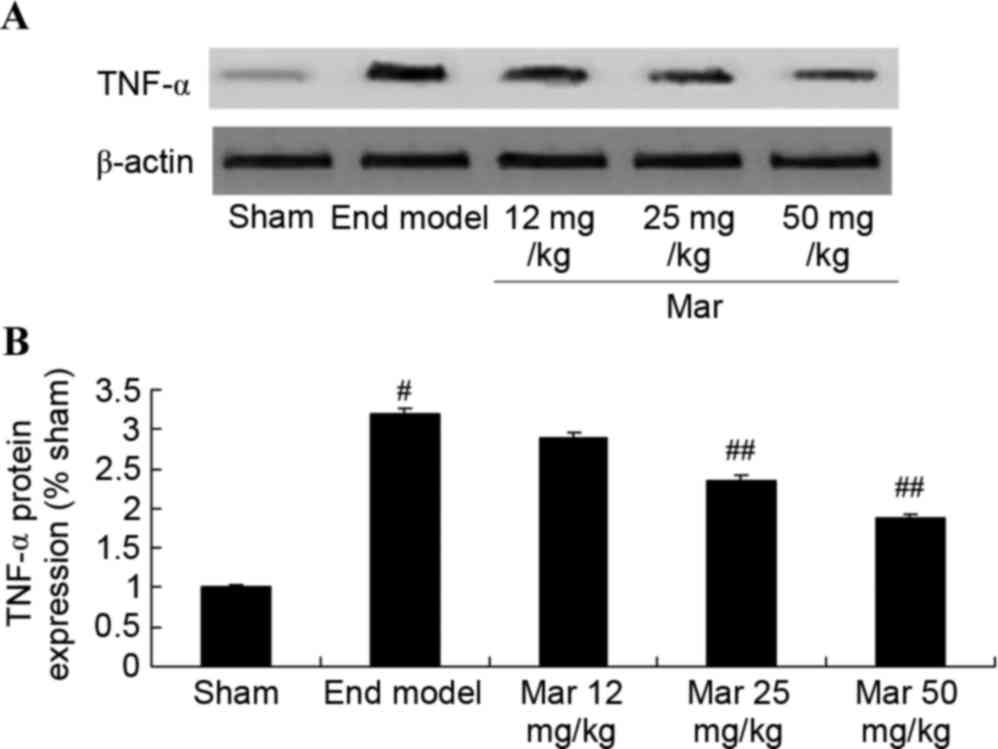
To probe the protective effects of marrubiin on the
protein expression of TNF-α, western blot analysis was used to
detect the protein expression of TNF-α. The results from the
western blot analysis showed that the protein expression of TNF-α
in the mouse model of endometriosis was higher, compared with that
in the sham group (Fig. 6).
Treatment with 25 or 50 mg/kg marrubiin significantly inhibited the
protein expression of TNF-α in the mice with endometriosis
(Fig. 6).
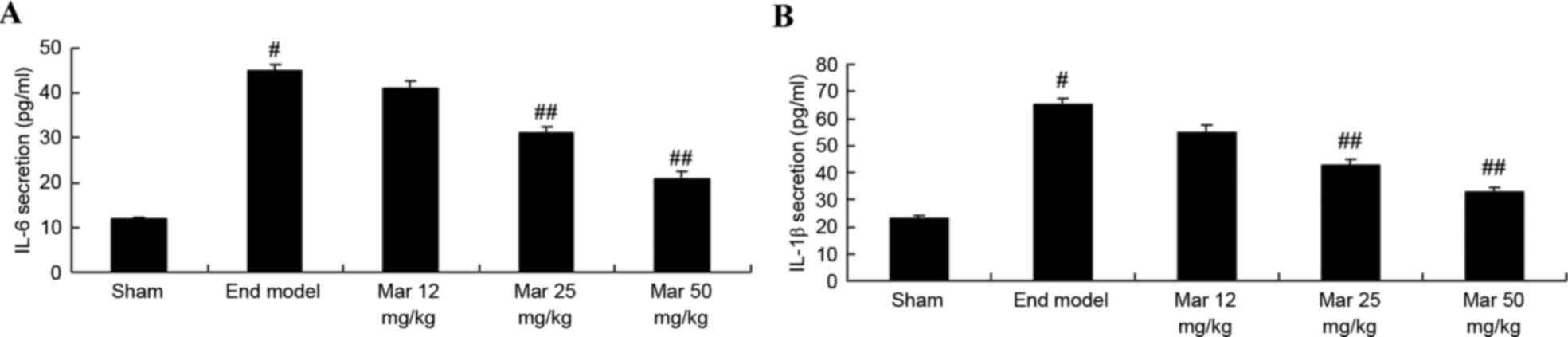
Protective effect of marrubiin
inhibits the expression levels of IL-6 and IL-1β
To examine the protective effects of marrubiin on
the expression levels of IL-6 and IL-1β, the activities of IL-6 and
IL-1β was measured using ELISA kits. As shown in Fig. 7, the activities of IL-6 and IL-1β
were higher, compared with those in the sham group. Treatment with
25 or 50 mg/kg marrubiin significantly inhibited the activities of
IL-6 and IL-1β in the mice with endometriosis (Fig. 7).
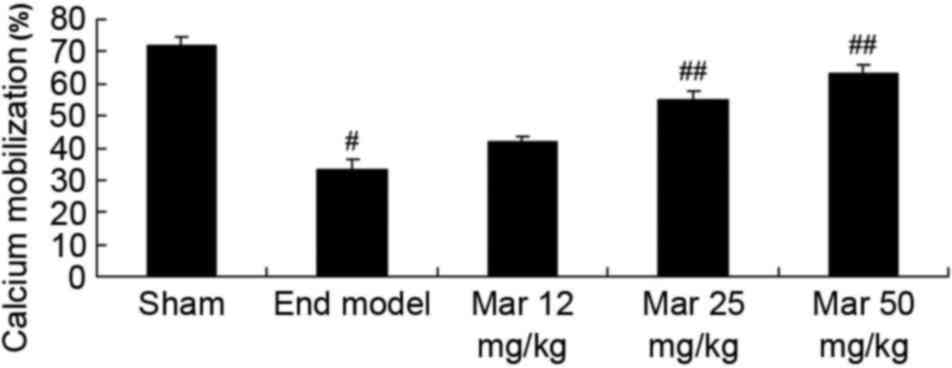
Protective effect of marrubiin induces
calcium levels
To examine the protective effects of marrubiin on
calcium levels, the levels of calcium were determined in the sham
and endometriosis groups of mice. Compared with level of calcium
migration in the sham group, the level in the endometriosis mice
was lower (Fig. 8). Treatment with
25 or 50 mg/kg marrubiin significantly increased calcium levels in
the mice with endometriosis (Fig.
8).
Protective effect of marrubiin
inhibits the protein expression of PGE2
To investigate the protective effects of marrubiin
on the protein expression of PGE2, western blot analysis was used
to measure the protein expression of PGE2. The results from the
western blot analysis showed that the protein expression of PGE2 in
the mouse model of endometriosis was signficantly higher, compared
with that in the sham group (Fig.
9). Treatment with 25 or 50 mg/kg marrubiin significantly
reduced the protein expression of PGE2 in the mice with
endometriosis (Fig. 9).
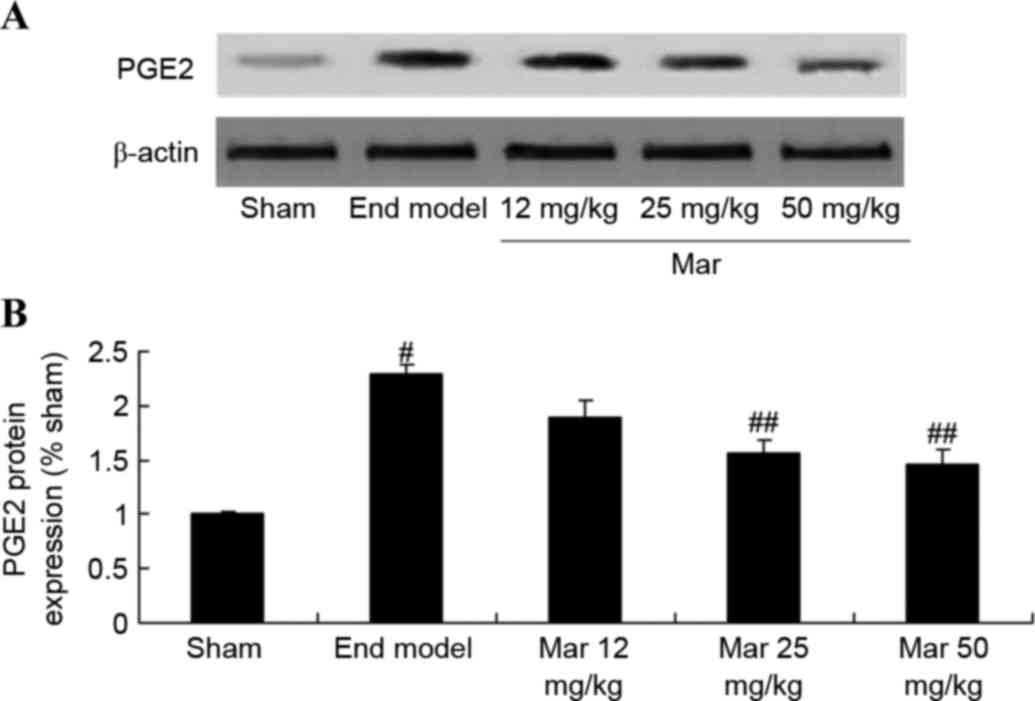 | Figure 9.Protective effect of Mar inhibits the
protein expression of PGE2. The effect of Mar on the protein
expression of PGE2 was examined using (A) western blot analysis.
(B) Statistical analysis of the protein expression of PGE2. Sham,
sham group; End model, endometriosis model; Mar 12 mg/kg,
endometriosis + 12 mg/kg Mar; Mar 25 mg/kg, endometriosis + 25
mg/kg Mar; Mar 50 mg/kg, endometriosis + 50 mg/kg Mar.
#P<0.01, vs. sham; ##P<0.01, vs. End
model. Sham, sham group; End model, endometriosis model; Mar,
marrubiin; PGE2, prostaglandin E2. |
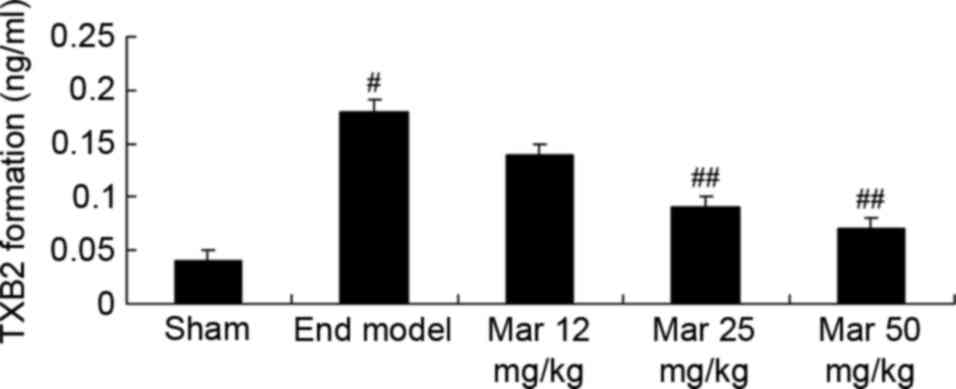
Protective effect of marrubiin
inhibits the formation of TXB2
The present study also investigated the protective
effects of marrubiin on TXB2 formation in mice with endometriosis.
The formation of TXB2 in the endometriosis model was higher,
compared with that in the sham group (Fig. 10). Treatment with 25 or 50 mg/kg
marrubiin significantly reduced the formation of TXB2 in the
endometriosis model (Fig.
10).
Discussion
Endometriosis is one of the common diseases
frequently occurring in women of childbearing age, and has a
significant impact on quality of life (4). The morbidity rate has increased
significantly, however, the etiology and pathogenesis remain to be
fully elucidated. The classic Sampson implantation theory has been
widely accepted, however, blood reflux is a common phenomenon,
whereas only a small proportion of individuals have disease
(14). The implantation theory
cannot explain this phenomenon, and investigations of endometriosis
have shown that the ectopic adhesion, invasion and angiogenesis of
endometrial tissue are closely associated with the successful
ectopic plantation of the endometrium (6). Therefore, a novel theory of etiology,
namely, the angiogenesis theory, has been suggested and has become
a focus among obstetricians and gynecologists. In the present
study, it was found that marrubiin significantly inhibited
endometrial lesions in a mouse model of endometriosis.
The current understanding of the function of ion
channel proteins has surpassed the traditional concept that the ion
channel is a channel only for the movement of ions into and out of
the cell (15). Studies have
indicated that potassium channels are widely distributed in various
tissues and have different functions, predominantly including
effects on cell growth, differentiation and apoptosis, effects on
cell volume and shape, the regulation of myocyte contraction and
relaxation, regulation of the release of neurotransmitters by
neurons, and regulation of hormone secretion by secretory cells
(15–17). Based on the physiological and
pathological effects of the Ca+-activated K+ pathway,
particularly its effects on the growth, differentiation and
apoptosis of cells, it is possible to develop
Ca+-activated K+ channels with a high level
of specificity (17). Channel
inhibitors and agonists may offer novel clinical therapeutic
options for several diseases (15). In the present study, it was found
that marrubiin significantly inhibited monocyte chemotaxis in mice
with endometriosis and inhibited U937 cell migration.
A previous study revealed that there is a
significant process of angiogenesis during endometriosis
plantation, and that the angiogenesis regulatory factors, TNF-α and
vascular endothelial growth factor, are important in the formation
of blood vessels, however, the mechanism of action in angiogenesis
in endometriosis remains to be elucidated (18). TNF-α is a type of cell factor with
vascular activity, which may be involved in the angiogenesis of
ectopic endometrium plantation (19). Investigations of TNF-α in
endometriosis have predominantly focussed on the blood and
peritoneal fluid, rather than the systematic investigation of
expression in normal and ectopic endometrial tissue (20). It is well known that marrubiin
significantly inhibits the protein expression of TNF-α and the
activities of IL-6 and IL-1β in mice with endometriosis. Mnonopi
et al (10) reported that
marrubiin inhibited the levels of IL-1β and IL-6 in a rat model of
obesity.
RANTES is an activation regulatory factor from the
expression and secretion of normal T cells (21). As an all-round type of chemokine,
RANTES belongs to the family of rapid growth chemokines, which are
primarily produced by memory T cells, epithelial cells and
mesothelial cells (9). RANTES can
exert chemotactic effects specifically on memory T cells, monocytes
and other immune cells, is involved in the regulation of the immune
response, and interacts with other cytokines and ovarian hormones
(22). Through its chemotaxic
effects, RANTES can amass inflammatory cells into a local lesion
for involvement in the inflammatory reaction, which can cause the
release of a variety of proinflammatory cytokines and angiogenic
factors, leading to a positive feedback effect and peritoneal
microenvironment change; this creates favorable conditions for the
incidence and development of endometriosis (23). It has been demonstrated that the
level of RANTES in the peritoneal fluid of patients with
endometriosis is significantly higher, compared with that in
patients without endometriosis, and this is positively correlated
with disease severity (24). The
present study showed that marrubiin significantly inhibited the
protein expression of RANTES in the endometriosis model and U937
cells, and induced calcium levels, protein expression levels of
PGE2 and the formation of TXB2 in the endometriosis model. Stulzer
et al (11) demonstrated
that marrubiin exerts pro-inflammatory agent-induced microvascular
extravasation of Evans blue in the mouse ear through PGE2.
In conclusion, the present study showed that
marrubiin significantly inhibited endometrial lesions and monocyte
chemotaxis in mice with endometriosis, and reduced the migration of
U937 cells. The mechanism underlying the protective effects of
marrubiin may involve the inhibition of inflammation and
downregulating the expression of RANTES in the mice with
endometriosis, followed by mediating the levels of calcium, PGE2
and TXB2. Further investigations of marrubiin may provide a basis
for the development of novel drugs for use in the treatment of
endometriosis.
References
|
1
|
Singh AK, Chattopadhyay R, Chakravarty B
and Chaudhury K: Markers of oxidative stress in follicular fluid of
women with endometriosis and tubal infertility undergoing IVF.
Reprod Toxicol. 42:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yun BH, Jeon YE, Chon SJ, Park JH, Seo SK,
Cho S, Choi YS, Lee JS and Lee BS: The Prognostic Value of
Individual Adhesion Scores from the Revised American Fertility
Society Classification System for Recurrent Endometriosis. Yonsei
Med J. 56:1079–1086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kondi-Pafiti A, Papakonstantinou E,
Iavazzo C, Grigoriadis C, Salakos N and Gregoriou O:
Clinicopathological characteristics of ovarian carcinomas
associated with endometriosis. Arch Gynecol Obstet. 285:479–483.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang KC, Chang WH, Lee WL, Huang N, Huang
HY, Yen MS, Guo CY and Wang PH: An increased risk of epithelial
ovarian cancer in Taiwanese women with a new surgico-pathological
diagnosis of endometriosis. BMC Cancer. 14:8312014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakr S, Naqvi H, Komm B and Taylor HS:
Endometriosis impairs bone marrow-derived stem cell recruitment to
the uterus whereas bazedoxifene treatment leads to endometriosis
regression and improved uterine stem cell engraftment.
Endocrinology. 155:1489–1497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston JL, Reid H and Hunter D:
Diagnosing endometriosis in primary care: Clinical update. Br J Gen
Pract. 65:101–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y,
Wang L and Li DJ: The high level of RANTES in the ectopic milieu
recruits macrophages and induces their tolerance in progression of
endometriosis. J Mol Endocrinol. 45:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Zhang X, Zhou C, Huang X, Lin J
and Xu H: Elevated immunoreactivity of RANTES and CCR1 correlate
with the severity of stages and dysmenorrhea in women with deep
infiltrating endometriosis. Acta Histochem. 115:434–439. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hornung D, Ryan IP, Chao VA, Vigne JL,
Schriock ED and Taylor RN: Immunolocalization and regulation of the
chemokine RANTES in human endometrial and endometriosis tissues and
cells. J Clin Endocrinol Metab. 82:1621–1628. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mnonopi N, Levendal RA, Mzilikazi N and
Frost CL: Marrubiin, a constituent of Leonotis leonurus, alleviates
diabetic symptoms. Phytomedicine. 19:488–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stulzer HK, Tagliari MP, Zampirolo JA,
Cechinel-Filho V and Schlemper V: Antioedematogenic effect of
marrubiin obtained from Marrubium vulgare. J Ethnopharmacol.
108:379–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Jesus RA, Cechinel-Filho V, Oliveira AE
and Schlemper V: Analysis of the antinociceptive properties of
marrubiin isolated from Marrubium vulgare. Phytomedicine.
7:111–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knöss W, Reuter B and Zapp J: Biosynthesis
of the labdane diterpene marrubiin in Marrubium vulgare via a
non-mevalonate pathway. Biochem J. 326:449–454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vercellini P, Consonni D, Barbara G,
Buggio L, Frattaruolo MP and Somigliana E: Adenomyosis and
reproductive performance after surgery for rectovaginal and
colorectal endometriosis: A systematic review and meta-analysis.
Reprod Biomed Online. 28:704–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palm F, Walter I, Budik S, Kolodziejek J,
Nowotny N and Aurich C: Influence of different semen extenders and
seminal plasma on PMN migration and on expression of IL-1beta,
IL-6, TNF-alpha and COX-2 mRNA in the equine endometrium.
Theriogenology. 70:843–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wessels JM, Linton NF, Croy BA and Tayade
C: A review of molecular contrasts between arresting and viable
porcine attachment sites. Am J Reprod Immunol. 58:470–480. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosh D and Sengupta J: Delineating the
prime mover action of progesterone for endometrial receptivity in
primates. Indian J Med Res. 140:(Suppl). S130–S136. 2014.PubMed/NCBI
|
|
18
|
Abutorabi R, Baradaran A, Mostafavi F
Sadat, Zarrin Y and Mardanian F: Evaluation of Tumor Necrosis
Factor Alpha Polymorphism Frequencies in Endometriosis. Int J
Fertil Steril. 9:329–337. 2015.PubMed/NCBI
|
|
19
|
Ozbilgin K, Turan A, Kahraman B, Atay C,
Vatansever S, Uluer ET and Ozçakir T: Distribution of furin, TNF-α
and TGF-β2 in the endometrium of missed abortion and voluntary
first trimester termination cases. Anal Quant Cytopathol
Histpathol. 37:123–133. 2015.PubMed/NCBI
|
|
20
|
Lu D, Song H and Shi G: Anti-TNF-α
treatment for pelvic pain associated with endometriosis. Cochrane
Database Syst Rev. 28:CD0080882013.
|
|
21
|
Agic A, Xu H, Rehbein M, Wolfler MM, Ebert
AD and Hornung D: Cognate chemokine receptor 1 messenger
ribonucleic acid expression in peripheral blood as a diagnostic
test for endometriosis. Fertil Steril. 87:982–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Schultze-Mosgau A, Agic A, Diedrich
K, Taylor RN and Hornung D: Regulated upon activation, normal T
cell expressed and secreted (RANTES) and monocyte chemotactic
protein 1 in follicular fluid accumulate differentially in patients
with and without endometriosis undergoing in vitro fertilization.
Fertil Steril. 86:1616–1620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebovic DI, Chao VA, Martini JF and Taylor
RN: IL-1beta induction of RANTES (regulated upon activation, normal
T cell expressed and secreted) chemokine gene expression in
endometriotic stromal cells depends on a nuclear factor-kappaB site
in the proximal promoter. J Clin Endocrinol Metab. 86:4759–4764.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hornung D, Bentzien F, Wallwiener D,
Kiesel L and Taylor RN: Chemokine bioactivity of RANTES in
endometriotic and normal endometrial stromal cells and peritoneal
fluid. Mol Hum Reprod. 7:163–168. 2001. View Article : Google Scholar : PubMed/NCBI
|