Introduction
Colorectal carcinoma (CRC) is one of the most common
types of malignancy, comprising ~10% of all primary cancers
(1,2). In the last decade, advances have been
made in the use of neoadjuvant chemotherapy in combination with
surgery, increasing the long-term survival rate of patients with
CRC (3,4). Notably, 5-fluorouracil (5-FU) and
oxaliplatin are commonly used as first-line anticancer drugs
(5). However, patients who respond
poorly to these drugs may suffer metastasis and relapse.
Furthermore, drug-resistance often emerges following clinical
treatment; this is a primary obstacle limiting positive outcomes
(6,7). Thus, investigation into the molecular
mechanisms underlying chemotherapy resistance is required for the
development of novel clinical treatments for CRC.
Nucleus accumbens-associated protein 1 (NAC1) is a
member of the broad-complex, tramtrack and bric a brac/pox virus
and zinc finger protein family, which is involved in numerous
cellular processes including proliferation, apoptosis and
transcriptional regulation (8–10).
NAC1 contributes to cancer development and progression via
activating key proteins and interacting with signaling pathways.
Previous studies have demonstrated that NAC1 serves a key role in
carcinogenesis, including ovarian, breast and cervical cancers
(11). It was revealed that NAC1
expression levels were significantly increased in recurrent
post-treatment ovarian tumors, compared with pretreated tumors
(12,13). Furthermore, downregulated
expression levels of NAC1 maintained cell survival, prevented cell
senescence and activated autophagy via high mobility group box 1
protein (8,14). Additionally, NAC1 was demonstrated
to predominantly localize to the nucleus, directly bind to nanog
homeobox and interact with importin α3/4 to regulate the
pluripotency network (15).
Furthermore, NAC1 was identified to regulate forkhead box Q1
(FOXQ1) expression levels to mediate cell motility and invasion in
cancer cells (16). A previous
study indicated that NAC1 was additionally detected in the cytosol,
particularly during mitosis, suggesting that NAC1 serves various
functions in tumor initiation, development and progression
(17).
Homeobox (HOX) genes, comprising 39 genes organized
in four clusters, encode a class of transcription factors which
control self-renewal and differentiation of cells (18). Previous studies have identified
that HOX family genes are involved in cancer development and
therapy (19–21). NIH 3T3 fibroblast clones bearing
the activated HOX-2.4 gene produced fibrosarcomas in nude mice,
indicating its oncogenic potential (22). HOX protein HOXA9 is typically
expressed during the development of the reproductive tract, and is
overexpressed in ovarian cancer, acute leukemia and breast cancer
(23). Furthermore, HOXA9
expression levels in epithelial ovarian cancers cells have been
demonstrated to induce cancer-associated fibroblasts and promote a
microenvironment permissive for tumor growth (24). However, the potential roles of
HOXA9 in drug resistance remain to be fully elucidated.
In the present study, it was demonstrated that NAC1
was overexpressed in colorectal carcinoma tissues, and that
increased expression levels of NAC1 contributed to chemotherapy
resistance. These data suggested that overexpression of NAC1 in
HCT8 and SW480 cells conferred drug resistance and reduced
caspase-3/7 activity to inhibit cell apoptosis. Opposite effects
were observed when small interfering (si)RNA was used to knock down
NAC1 expression in HCT116 and SW620 cells. Further investigation
indicated that NAC1 promoted tumor progression viamediating HOXA9
expression. These findings identified a potential novel target for
the treatment of CRC.
Materials and methods
Patients and tissue samples
The present study was approved by the Research
Ethics Committee of Hangzhou First People's Hospital (Hangzhou,
China). A total of 94 CRC and paired non-tumorous tissues were
collected from patients following surgery at the Department of
Gastrointestinal Surgery, Hangzhou First People's Hospital. Written
informed consent was obtained from all patients. Tumor and paired
non-tumorous tissues were stored at −80°C.
Cell lines
HCT8, HCT116, SW480 and SW620 human CRC cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The HEK293T human embryogenic kidney cell line was
obtained from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin.
NAC1 overexpression and siRNA
transfection
For ectopic expression of NAC1, NAC1 cDNA was cloned
into the vector pLKO.1 (catalog no. 10878; Addgene, Inc.,
Cambridge, MA, USA). Lentivirals were constructed in HEK293T cells
and cell lines were infected to establish NAC1 stable
overexpression cells as described previously (25). The siRNAs targeting NAC1 (UGA UGU
ACA CGU UGG UGC CUG UCA CCA and GAG GAA GAA CUC GGU GCC CUU CUC
CAU) and HOXA9 (ACU ACU ACG UGG ACU CGU UC and AAU CAA CAA AGA CCG
AGC AAA) were purchased from Genepharm Biotech (Taipei, Taiwan). A
scrambled sequence was used as a negative control. Cell
transfection was performed using Lipofectamine® 3000
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from tissue samples and cells was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). First-strand cDNA was synthesized using
the PrimeScript cDNA Synthesis kit (Takara Biotechnology, Co.,
Ltd., Dalian, China), according to the manufacturer's protocol.
qPCR was performed using the SYBR® -Green Master mix kit
(Takara Biotechnology Co., Ltd., Dalian, China) and the Applied
Biosystems 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Cycling conditions were as follows: An
initial predenaturation step at 95°C for 30 sec, followed by 40
cycles of denaturation at 95°C for 30 sec and annealing at 60°C for
30 sec. All reactions were performed in triplicate. The primers
used for detecting NAC1 were as follows: Forward,
5′-AAGCTGAGGATCTGCTGGAA-3′ and reverse, 5′-CCAGACACTGCAGATGGAGA-3′.
The primers used for GAPDH were as follows: Forward,
5′-ACCACAGTCCATGCCATCA-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
The expression levels of NAC1 were normalized to that of GAPDH in
each sample using the 2−∆∆Cq method (26).
Western blot analysis
Total proteins from tissues and cells were extracted
using radioimmunoprecipitation assay buffer containing protease and
phosphatase inhibitors (Beyotime Institute of Biotechnology,
Haimen, China). For western blot analysis, 60 µg of total protein
lysates underwent 10% SDS-PAGE and were subsequently transferred
onto polyvinylidene difluoridemembranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) as described previously (27). The protein was detected using an
Enhanced Chemiluminescence substrate (Beijing Transgen Co., Ltd.,
Beijing, China). Rabbit anti-NAC1 (catalog no. ab29047) and rabbit
anti-HOXA9 (catalog no. ab83480) primary antibodies were purchased
from Abcam (Cambridge, UK). A mouse anti-β-actin (sc-47778) primary
antibody was obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA) and served as an internal control. Membranes were probed
with primary antibodies diluted 1:1,000 at 4°C overnight, followed
by a horse radish peroxidase-conjugated goat-anti-rabbit or
anti-mouse secondary antibody (catalog no. ab6789; Abcam,
Cambridge, UK) diluted 1:5,000 for 1 h at room temperature.
Immunohistochemical staining
CRC and paired non-tumorous tissues were fixed in
formalin, embedded in paraffin and sectioned into 4-µm thick
slices. Following deparaffinization and rehydration, antigen
retrieval was performed using boiling 0.01 M citrate buffer (Boster
Systems, Inc., Pleasanton, CA, USA) for 30 min and endogenous
peroxidases were blocked with 3% H2O2.
Sections were washed three times with PBS and goat serum (Boster
Systems, Inc.) was used to block nonspecific binding for 30 min.
Tissues were subsequently incubated with an anti-NAC1 antibody
(1:200 dilution; catalog no. ab29047; Abcam) at 4°C overnight
followed by incubation with a peroxidase-labeled goat anti-rabbit
secondary antibody (catalog no. BA1055; Boster Systems, Inc.) for 1
h at 37°C. 3′3-Diaminobenzidine (Boster Systems, Inc.) was used to
visualize tissue antigens. Following this, the sections were
counterstained with hematoxylin and dehydrated and observed under a
microscope.
Caspase-3/7 activity and cell
viability assay
The indicated cells were treated with 5-FU (50
ng/ml) oroxaliplatin (10 µM) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and cultured for 12 h at 37°C. To measure capase-3/7
activity of cells following treatment with a
Caspase-Glo® 3/7 assay kit (Promega Corporation,
Madison, WI, USA) was used according to the manufacturer's
protocol. Cell viability was evaluated using the Cell Counting
kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The
absorbance was measured at a wavelength of 450 nm using a
microplate reader. Experiments were repeated at least three
times.
Luciferase reporter assay
HCT116 and SW620 cells were transfected with NAC1 or
control siRNAs. The cells were subsequently transfected with a pGL3
control vector or the pGL3-HOXA9 promoter vector and
Renilla. A luciferase activity assay was performed using the
Dual-Luciferase® Reporter assay system (Promega
Corporation) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla activity.
Statistical analysis
SPSS software version 21.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. Data are presented as the
mean ± standard error of at least three experiments. Data were
analyzed by an unpaired Student's t-test for comparison between two
groups, or a one-way analysis of variance followed by
Student-Newman-Keuls post hoc test for comparison between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
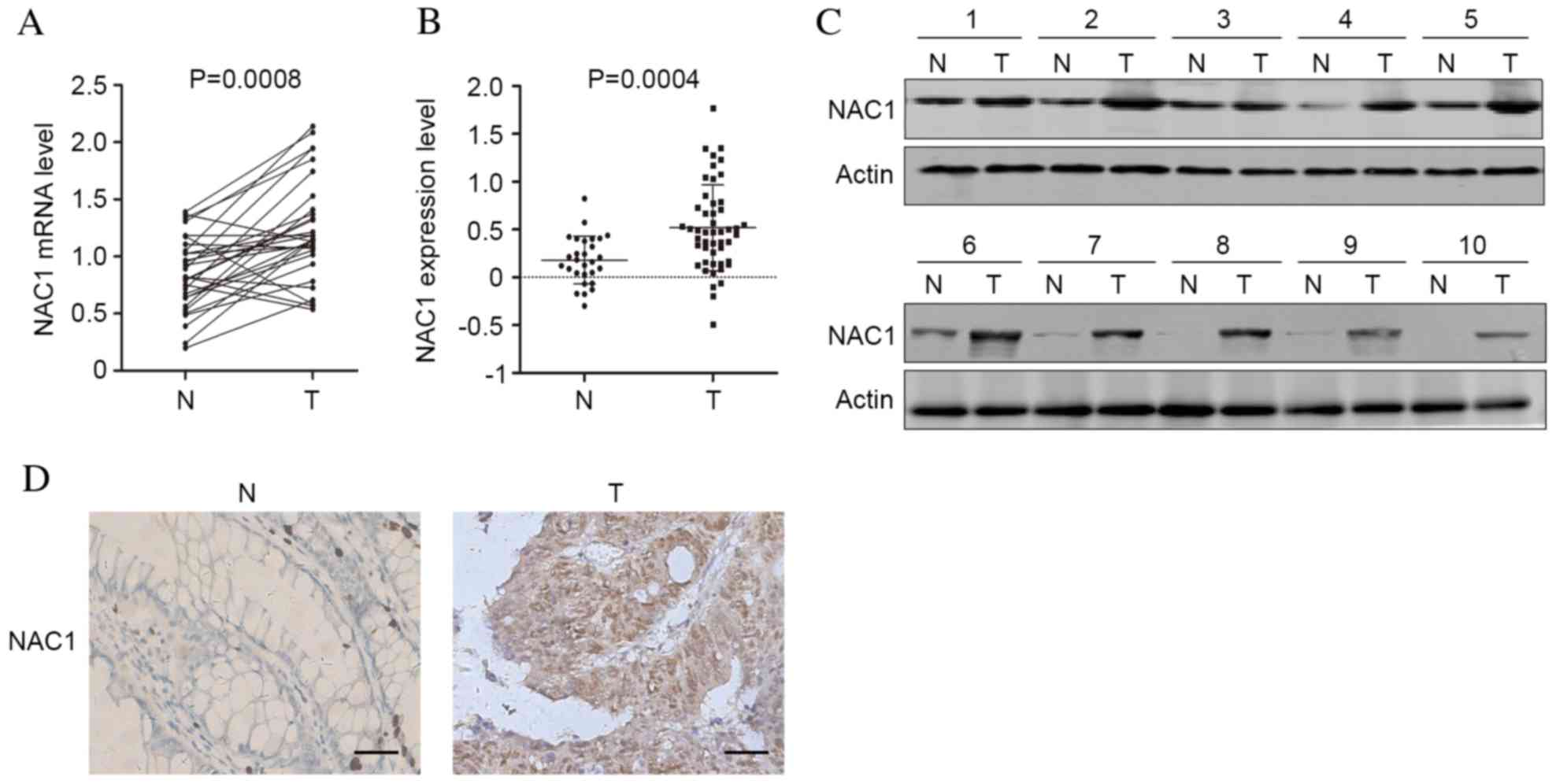
Expression levels of NAC1 are
significantly elevated in CRC tissue
To investigate the involvement of NAC1 in the
progression of CRC, the mRNA expression levels of NAC1 in 30 CRC
and adjacent non-tumorous tissues were analyzed by RT-qPCR. The
results indicated that compared with non-tumorous tissue, NAC1
expression levels were significantly upregulated in CRC tissue
(Fig. 1A; P=0.0008). Additionally,
a GSE6988 dataset generated from the Gene Expression Omnibus
database consisting of 28 healthy and 49 CRC tissues was
investigated, and the mRNA expression levels of NAC1 were
significantly increased in CRC tissues (Fig. 1B; P=0.004). Furthermore, western
blot analysis and immunohistochemistry revealed that the protein
expression levels of NAC1 were elevated in CRC tissue (Fig. 1C and D). The results indicated that
the expression levels of NAC1 were increased in tumor compared with
non-tumor tissues, implicating an oncogenic function for NAC1 in
CRC.
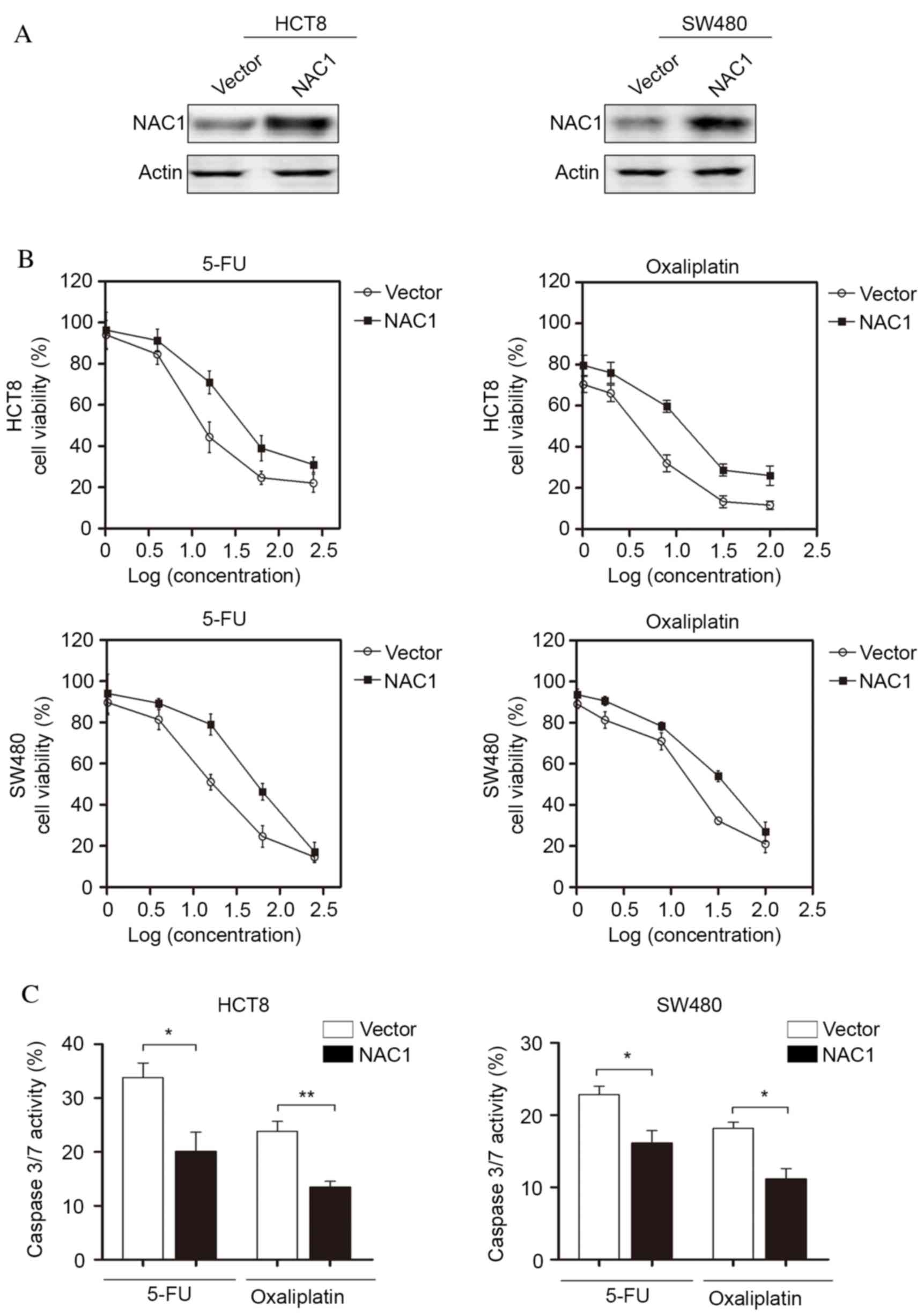
NAC1 confers resistance of CRC cells
to chemotherapy in vitro
Chemoresistance is a major challenge for CRC
treatment; therefore, the present study investigated the potential
function of NAC1 in CRC cells following chemotherapy. NAC1 was
stably expressed in HCT8 and SW480 cell lines and western blot
analysis was used to confirm the overexpression of NAC1 (Fig. 2A). The cells were subsequently
treated with 5-FU and oxaliplatin at a range of doses. The
concentrations of 5-FU were as follows: 1, 4, 16, 64 and 256 ng/ml,
and the concentrations of oxaliplatin were 1, 2, 8, 32 and 100 µM.
The results indicated that overexpression of NAC1 in HCT8 and SW480
cells significantly increased the resistance of cells to 5-FU and
oxaliplatin-induced cell death (Fig.
2B). In addition, caspase-3/7 activity was significantly
decreased following overexpression of NAC1. This suggested a low
level of apoptosis, and was consistent with the cell viability
assay (Fig. 2C). Taken together,
these data suggested that NAC1 increased the resistance of CRC
cells to cytotoxic drugs.
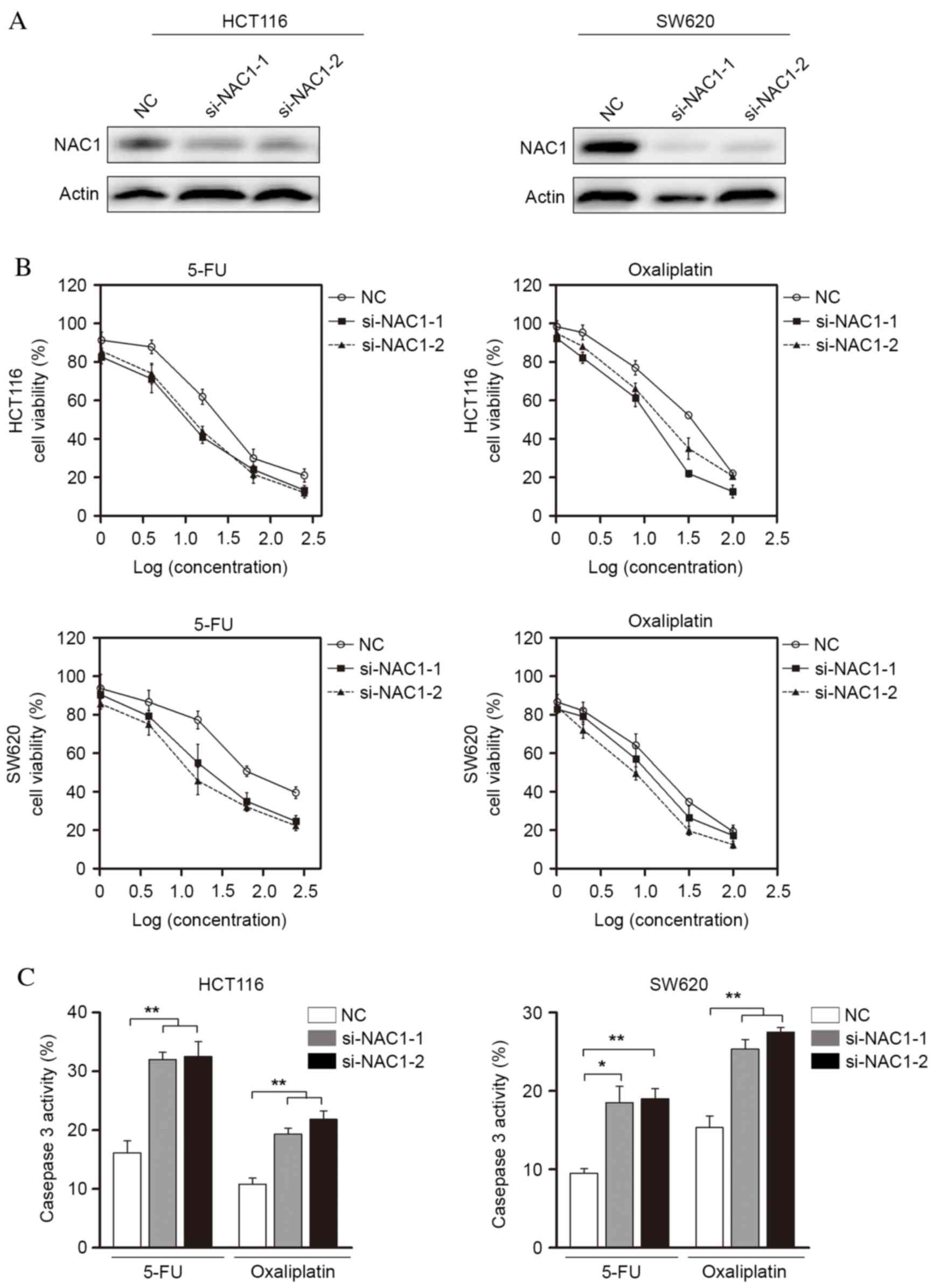
Knocking down the expression of NAC1
restores the chemosensitivity of CRC cells
To further characterize the role of NAC1 in the
regulation of CRC cell death, the present study transfected
target-specific siRNA against NAC1 into HCT116 and SW620 cells.
NAC1 siRNA led to a significant decrease of protein expression
levels in the two cell lines (Fig.
3A). These NAC1-knockdown cells became sensitive to apoptosis
induced by 5-FU and oxaliplatin (Fig.
3B), suggesting an anti-apoptotic role for NAC1 in CRC cells.
In addition, the cytotoxic agent-induced activation of caspase-3/7
significantly increased following NAC1 knockdown (Fig. 3C). Collectively, these data
indicated that knockdown of NAC1 expression resulted in restored
sensitivity to 5-FU and oxaliplatin in CRC cells.
NAC1 promotes chemoresistance of CRC
cells via increased HOXA9 expression
The present study investigated the potential
mechanism underlying NAC1-mediated chemoresistance in CRC cells.
NAC1 may transcriptionally regulate the gene expression of a
variety of pluripotency factors and modulate stem cell properties.
The HOX gene family was selected for investigation due to its
involvement in mediating cell stemness. It was revealed that HOXA9
promoter activity was significantly decreased following knockdown
of NAC1 (Fig. 4A). These findings
indicated that HOXA9 may be a downstream regulator of NAC1.
Following this, western blot analysis revealed that knockdown of
NAC1 suppressed HOXA9 expression (Fig.
4B). Furthermore, overexpression of NAC1 was demonstrated to
induce HOXA9 expression, which was subsequently silenced by siRNA
(Fig. 4C). The caspase-3/7
activity of CRC cells overexpressing NAC1 was determined, following
silencing of HOXA9 expression (Fig.
4D). The results revealed that the NAC1-induced reduction in
cell apoptosis was reversed by silencing HOXA9 expression,
suggesting that HOXA9 is a downstream regulator of NAC1-mediated
CRC cell resistance to anticancer drugs.
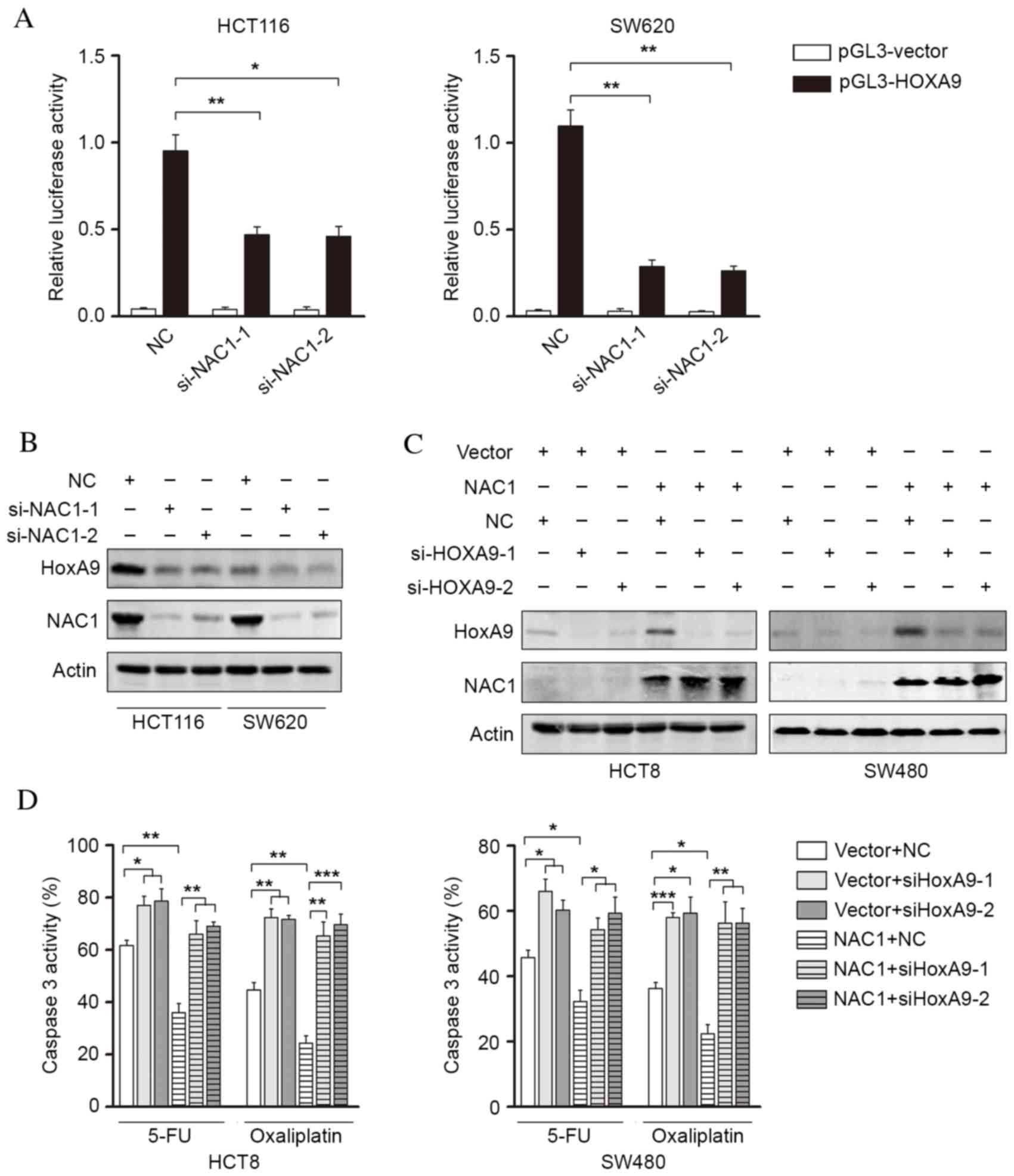 | Figure 4.HOXA9 is an NAC1 downstream gene
involved in NAC1-mediated chemoresistance. (A) Luciferase activity
of the HOXA9 promoter was measured in HCT116 and SW620 cells,
following transfection with control siRNA or siNAC1. Representative
western blot images and analysis of HCT116 and SW620 cells
demonstrating (B) an reduction in HOXA9 protein expression levels,
following NAC1 knockdown, and (C) an increase in HOXA9 protein
expression levels, following ectopic NAC1 expression. (D)
Caspase-3/7 activity in HCT8 and SW480 cells, following
overexpression of NAC1 and knockdown HOXA9 expression, was
assessed. Data are presented as the mean ± standard error (n=3).
*P<0.05; **P<0.01; ***P<0.001. HOXA9, homeobox A9; NAC1,
nucleus accumbens-associated protein 1; si, small interfering; NC,
negative control. |
Discussion
CRC is one of the most common types of malignancy
worldwide. Despite recent developments in therapeutic strategies,
finding an effective treatment approach remains challenging. For
example, 5-FU, the widely-used first-line anticancer drug, has an
efficacy of only ~20% when administered as a single drug treatment.
Numerous patients with CRC develop drug resistance and this may
lead to cancer progression, including metastasis and angiogenesis
(28). The present study
investigated whether NAC1-mediated upregulation of HOXA9
significantly contributes to drug resistance in CRC.
Emerging evidence has indicated that NAC1 has
multifunctional roles in tumorigenesis, including increasing cell
survival and proliferation, enhancing cell motility, and regulating
cellular senescence and autophagy. Although NAC1 overexpression has
frequently been observed in a wide variety of cancers, it has not
been studied in detail in CRC. The present study investigated the
clinical significance and biological function of NAC1 in CRC. It
was demonstrated that NAC1 was frequently upregulated in CRC
tissues, and that NAC1 may mediate CRC chemoresistance.
Furthermore, NAC1 siRNA was revealed to increase apoptosis in
response to anticancer drug-induced tumor cell apoptosis,
indicating that inhibition of NAC1 may be a novel strategy for CRC
treatment.
Cancer cell responses to chemotherapy have a variety
of underlying mechanisms, ranging from activation of survival
signaling pathways to inhibition of cell death, and from increasing
cell membrane transportation to initiating cell senescence
(29–31). However, the underlying molecular
mechanisms of NAC1-mediated chemoresistance remain unclear.
Recently, NAC1-mediated downregulation of various downstream genes
was investigated in the SKOV3 ovarian cancer cell line. The FOXQ1
gene was identified as a regulator of NAC1-mediated cell motility
(16). The present study examined
the potential involvement of the HOX gene family in tumor
chemoresistance, due to its roles in regulating cell transcription,
morphogenesis and differentiation (32). Luciferase activity experiments were
performed to assess HOX gene transcriptional regulation by knocking
down NAC1 expression levels. HOXA9 promoter activity was suppressed
when NAC1 expression was inhibited. Additionally, the present study
further revealed that the protein expression levels of HOXA9
decreased following NAC1 knockdown. Ectopic expression of NAC1 was
revealed to induce HOXA9 expression. Furthermore, inhibition of
NAC1 or HOXA9 increased the drug sensitivity of CRC cells. A
previous study demonstrated that the homeobox genes HOXA9 and A10
were upregulated in chemoresistant glioblastoma cells, and
increased levels of HOXA9 were associated with a reduced survival
rate (33). HOXA9 has additionally
been revealed to mediate resistance to temozolomide treatment in
gliomas, via upregulation of B-cell lymphoma 2 (34). Similarly, HOXA9 overexpression was
inversely correlated with drug resistance, relapse and overall
survival rate in acute leukemia (35). In addition, HOXA9 may induce
transforming growth factor-β2, consequently modulating tumor stroma
and promoting tumor growth and metastasis. Furthermore,
cancer-associated fibroblasts that may be induced by HOXA9 have
been revealed to mediate chemoresistance in cancer cells (36). Therefore, the present study
demonstrated that HOXA9 may be important to maintain NAC1-induced
chemoresistance.
In conclusion, the present study demonstrated the
upregulation of NAC1 in CRC samples, and that overexpression of
NAC1 in CRC cells induced resistance to anticancer drugs.
Furthermore, knockdown of NAC1 restored the chemosensitivity of CRC
cells, indicating an oncogenic role. The underlying molecular
mechanisms of NAC1 may be mediated via the upregulation of HOXA9.
This indicates that NAC1 may serve as a potential target for the
treatment of CRC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaukat A, Mongin SJ, Geisser MS, Lederle
FA, Bond JH, Mandel JS and Church TR: Long-term mortality after
screening for colorectal cancer. N Engl J Med. 369:1106–1114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schrag D, Weiser MR, Goodman KA, Gonen M,
Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem
JG, et al: Neoadjuvant chemotherapy without routine use of
radiation therapy for patients with locally advanced rectal cancer:
A pilot trial. J Clin Oncol. 32:513–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosset JF, Calais G, Mineur L, Maingon P,
Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC,
Bolla M, et al: Fluorouracil-based adjuvant chemotherapy after
preoperative chemoradiotherapy in rectal cancer: Long-term results
of the EORTC 22921 randomised study. Lancet Oncol. 15:184–190.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cecchin E, D'Andrea M, Lonardi S, Zanusso
C, Pella N, Errante D, De Mattia E, Polesel J, Innocenti F and
Toffoli G: A prospective validation pharmacogenomic study in the
adjuvant setting of colorectal cancer patients treated with the
5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen.
Pharmacogenomics J. 13:403–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong M, Zheng W, Lu X, Ao L, Li X, Guan Q,
Cai H, Li M, Yan H, Guo Y, et al: Identifying clinically relevant
drug resistance genes in drug-induced resistant cancer cell lines
and post-chemotherapy tissues. Oncotarget. 6:41216–41227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL,
Wu H, Patel R, Liu D, Qin ZH, Shih IM and Yang JM: NAC1 modulates
sensitivity of ovarian cancer cells to cisplatin by altering the
HMGB1-mediated autophagic response. Oncogene. 31:1055–1064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsunoda K, Oikawa H, Tada H, Tatemichi Y,
Muraoka S, Miura S, Shibazaki M, Maeda F, Takahashi K, Akasaka T,
et al: Nucleus accumbens-associated 1 contributes to cortactin
deacetylation and augments the migration of melanoma cells. J
Invest Dermatol. 131:1710–1719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Cheng Y, Ren X, Hori T,
Huber-Keener KJ, Zhang L, Yap KL, Liu D, Shantz L, Qin ZH, et al:
Dysfunction of nucleus accumbens-1 activates cellular senescence
and inhibits tumor cell proliferation and oncogenesis. Cancer Res.
72:4262–4275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jinawath N, Vasoontara C, Yap KL,
Thiaville MM, Nakayama K, Wang TL and Shih IM: NAC-1, a potential
stem cell pluripotency factor, contributes to paclitaxel resistance
in ovarian cancer through inactivating Gadd45 pathway. Oncogene.
28:1941–1948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakayama K, Nakayama N, Davidson B, Sheu
JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman
RJ, et al: A BTB/POZ protein, NAC-1, is related to tumor recurrence
and is essential for tumor growth and survival. Proc Natl Acad Sci
USA. 103:18739–18744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davidson B, Berner A, Trope' CG, Wang TL
and Shih IeM: Expression and clinical role of the bric-a-brac
tramtrack broad complex/poxvirus and zinc protein NAC-1 in ovarian
carcinoma effusions. Hum Pathol. 38:1030–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Yang JW, Ren X and Yang JM: NAC1
and HMGB1 enter a partnership for manipulating autophagy.
Autophagy. 7:1557–1558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okazaki K, Nakayama N, Nariai Y, Nakayama
K, Miyazaki K, Maruyama R, Kato H, Kosugi S, Urano T and Sakashita
G: Nuclear localization signal in a cancer-related transcriptional
regulator protein NAC1. Carcinogenesis. 33:1854–1862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao M, Wu RC, Herlinger AL, Yap K, Kim JW,
Wang TL and Shih IeM: Identification of the NAC1-regulated genes in
ovarian cancer. Am J Pathol. 184:133–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu PH, Hung SH, Ren T, Shih IeM and Tseng
Y: Cell cycle-dependent alteration in NAC1 nuclear body dynamics
and morphology. Phys Biol. 8:0150052011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Larochelle A, Choi U, Shou Y, Naumann N,
Loktionova NA, Clevenger JR, Krouse A, Metzger M, Donahue RE, Kang
E, et al: In vivo selection of hematopoietic progenitor cells and
temozolomide dose intensification in rhesus macaques through
lentiviral transduction with a drug resistance gene. J Clin Invest.
119:1952–1963. 2009.PubMed/NCBI
|
|
19
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashida T, Takahashi F, Chiba N,
Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco Md,
Wijendran V, Shioda T, et al: HOXB9, a gene overexpressed in breast
cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad
Sci USA. 107:1100–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Iwasaki M, Ficara F, Lin C,
Matheny C, Wong SH, Smith KS and Cleary ML: GSK-3 promotes
conditional association of CREB and its coactivators with MEIS1 to
facilitate HOX-mediated transcription and oncogenesis. Cancer Cell.
17:597–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aberdam D, Negreanu V, Sachs L and Blatt
C: The oncogenic potential of an activated Hox-2.4 homeobox gene in
mouse fibroblasts. Mol Cell Biol. 11:554–557. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng W, Liu J, Yoshida H, Rosen D and
Naora H: Lineage infidelity of epithelial ovarian cancers is
controlled by HOX genes that specify regional identity in the
reproductive tract. Nat Med. 11:531–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko SY, Barengo N, Ladanyi A, Lee JS,
Marini F, Lengyel E and Naora H: HOXA9 promotes ovarian cancer
growth by stimulating cancer-associated fibroblasts. J Clin Invest.
122:3603–3617. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferrarese R, Harsh GR VI, Yadav AK, Bug E,
Maticzka D, Reichardt W, Dombrowski SM, Miller TE, Masilamani AP,
Dai F, et al: Lineage-specific splicing of a brain-enriched
alternative exon promotes glioblastoma progression. J Clin Invest.
124:2861–2876. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Zhan S, Tan W, Cheng R, Gong H and
Zhu Q: P300 binds to and acetylates MTA2 to promote colorectal
cancer cells growth. Biochem Biophys Res Commun. 444:387–390. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Medina-Ramirez CM, Goswami S, Smirnova T,
Bamira D, Benson B, Ferrick N, Segall J, Pollard JW and Kitsis RN:
Apoptosis inhibitor ARC promotes breast tumorigenesis, metastasis,
and chemoresistance. Cancer Res. 71:7705–7715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei Y, Zou Z, Becker N, Anderson M,
Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et
al: EGFR-mediated Beclin 1 phosphorylation in autophagy
suppression, tumor progression, and tumor chemoresistance. Cell.
154:1269–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang WJ, Song MJ, Park EY, Lee JJ, Park
JH, Park K, Park JH and Kim HP: Transcription factors Sp1 and Sp3
regulate expression of human ABCG2 gene and chemoresistance
phenotype. Mol Cells. 36:368–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Breau MA, Wilkinson DG and Xu Q: A Hox
gene controls lateral line cell migration by regulating chemokine
receptor expression downstream of Wnt signaling. Proc Natl Acad Sci
USA. 110:16892–16897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaspar N, Marshall L, Perryman L, Bax DA,
Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis
RM, et al: MGMT-independent temozolomide resistance in pediatric
glioblastoma cells associated with a PI3-kinase-mediated HOX/stem
cell gene signature. Cancer Res. 70:9243–9252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brumatti G, Salmanidis M, Kok CH, Bilardi
RA, Sandow JJ, Silke N, Mason K, Visser J, Jabbour AM, Glaser SP,
et al: HoxA9 regulated Bcl-2 expression mediates survival of
myeloid progenitors and the severity of HoxA9-dependent leukemia.
Oncotarget. 4:1933–1947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alharbi RA, Pettengell R, Pandha HS and
Morgan R: The role of HOX genes in normal hematopoiesis and acute
leukemia. Leukemia. 27:1000–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johansson AC, Ansell A, Jerhammar F, Lindh
MB, Grénman R, Munck-Wikland E, Östman A and Roberg K:
Cancer-associated fibroblasts induce matrix
metalloproteinase-mediated cetuximab resistance in head and neck
squamous cell carcinoma cells. Mol Cancer Res. 10:1158–1168. 2012.
View Article : Google Scholar : PubMed/NCBI
|