Introduction
The directional differentiation of stem cells
provides an important approach to cell therapy for the treatment of
neurological diseases (1,2). However, two primary issues remain to
be resolved: The determination of appropriate seed cells and the
identification of efficient inducers. The multi-directional
differentiation potential of mesenchymal stem cells (MSCs), which
has been well characterized by in vitro culture and
auto-transplantation approaches, has resulted in these cells being
widely used as seed cells for cell therapy. Meanwhile, the
potential usage of neurotrophins as inducers of stem cell
differentiation has received considerable attention (3,4).
Neuritin (Nrn1 or CPG15), a neurotrophic factor that
was identified in a study of plasticity-related genes, has been
demonstrated to promote neurite growth and the survival of cortical
neurons (5,6). While the effects of neuritin on cell
differentiation have yet to be reported, results obtained in a
previous study suggest that neuritin is involved in this process
(7).
In the present study, the effects of neuritin on the
directional differentiation of MSCs toward neuron-like (NL) cells
were evaluated by analyzing cell morphology, expression levels of
neuronal markers, and neuronal functions of rat bone marrow-derived
MSCs (rBM-MSCs) treated with recombinant neuritin protein purified
from Pichia pastoris (8).
Materials and methods
Ethics statement
Animal experiments were performed in accordance with
the National Institute of Health Guidelines for the Care and Use of
Laboratory Animals. Formal approval to conduct the experiments
described was obtained from the Animal Subjects Review board of The
First Affiliated Hospital of Shihezi University School of Medicine
(Shihezi, China; permit no.: 2011LL02). All efforts were made to
minimize suffering.
Isolation and culturing of rat
MSCs
In the present study, male and female Sprague-Dawley
rats (n=25; age, 4–6 weeks; weight, 80–100 g) (provided by the
Institute of Epidemiology, Xinjiang Uygur Autonomous Region,
Shihezi, China) were housed under standardized laboratory
conditions in an air-conditioned room at constant temperature
(23±2°C) and relative humidity of 45±5% on a 12 h light/dark cycle,
with free access to food and water. Rats were sacrificed by
cervical dislocation, and the tibias and femurs were isolated under
sterile conditions, as previously described (9,10).
Both ends of the bone were cut to expose the bone marrow cavity,
and bone marrow cells were collected by rinsing with 5 ml
L-Dulbecco's Modified Eagle's medium (DMEM; cat. no. SH30022.01;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) followed by
centrifugation at 156 × g for 10 min at room temperature. The
harvested cells were then seeded in culture dishes at a density of
1×106 cells/ml in L-DMEM/Nutrient Mixture F12 (cat. no.
12400024; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. 12478020;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin, and cultivated at 37°C in a 5% CO2
incubator for 24 h, following which non-adherent cells were
removed. The culture medium was replaced every 3 days until the
cells reached 90% confluency. Cells were then digested by treatment
with 1 ml 0.125% trypsin (cat. no. 25200072; Gibco; Thermo Fisher
Scientific, Inc.) for 5–8 min at 37°C, mixed with 1 ml L-DMEM, and
centrifuged at 156 × g for 10 min at room temperature. Collected
cells were then subcultured at a 1:2 ratio. The third passage of
MSCs were used for in vitro experiments.
Neuronal induction
rBM-MSCs (4×103 cells/ml) were initially
maintained in DMEM containing 10% FBS. The medium was replaced with
pre-induction medium consisting of DMEM supplemented with 10% FBS
24 h prior to induction, and 20 ng/ml basic fibroblast growth
factor (bFGF; cat. no. 13256029; Invitrogen; Thermo Fisher
Scientific, Inc.). To initiate neuronal differentiation, the
pre-induction medium was removed, and cells were washed with PBS
and incubated in neuronal induction medium, which consisted of DMEM
supplemented with 20 ng/ml bFGF, 2% B-27 supplement (cat. no.
0050129SA; Invitrogen; Thermo Fisher Scientific, Inc.), and 0.5–2.0
µg/ml neuritin (derived from Pichia pastoris) (8). Cells were incubated in media
containing His-tagged protein (cat. no. bs-0287P; Bioss, Beijing,
China) and 2% B-27 supplement wasused as a negative control.
Optimization of the induction
conditions for MSC differentiation
The number of cells exhibiting NL morphology was
counted in five randomly selected fields (minimum of 100
cells/field) at 0, 6, 24, 48 and 72 h post-induction. The
percentage of positive NL cells was calculated as follows: positive
NL cells (%) = (number of NL cells/total number of cells)
×100%.
To enumerate viable cells, trypan blue (0.4%) was
added to cell suspensions (4×103 cells/ml) at a ratio of
1:9 (v/v). Cells that absorbed trypan blue were considered
non-viable, and viable cells were counted using a hemocytometer
(cat. no. 02270113; QiuJing, Shanghai, China) at a magnification of
×400 under a light microscope within 3 min of dying. The total cell
numbers of 4 large fields of the hemocytometer were counted
according to the principle that cells above or to the left of the
boundary line were recorded. The percentage of viable cells was
calculated as follows: viable cells (%)=[1.00-(number of blue
cells/total cell numbers)] ×100%.
Analysis of MSC phenotypes by
immunofluorescence microscopy
Slides with MSCs (2.5×104 cells/ml) from
the third passage were rinsed three times with PBS and fixed with
PBS containing 4% paraformaldehyde for 30 min at room temperature.
The following operations were performed using a wet box: 5 min
washing with PBS, repeated 3 times, and slides containing MSCs were
incubated in blocking solution containing 10% goat serum (cat. no.
ZLI-9022; OriGene Technologies, Inc., Beijing, China) for 30 min at
room temperature. Cells were subsequently incubated overnight at
4°C with the following primary antibodies: CD29 (1:100; cat. no.
bs-20630R; Bioss), CD90 (1:100; cat. no. bs-0778R; Bioss), CD34
(1:100; cat. no. bs-0646R; Bioss), CD44 (1:100; cat. no. bs-0521R;
Bioss), or CD45 (1:100; cat. no. bs-0522R; Bioss). PBS was utilized
as a negative control. Subsequently, cells were incubated with
fluorescein isothiocyanate (FITC) -labeled goat anti-rabbit IgG
(1:100; cat. no. ab6717; Abcam, Cambridge, UK) for 2 h at 25°C in
the dark, washed with PBS, and observed in five randomly selected
fields (minimum 100 cells/field) by fluorescence microscopy (Carl
Zeiss AG, Oberkochen, Germany).
Detection of neural markers by
immunofluorescence microscopy
rBM-MSCs (2.5×104 cells/ml) were grown on
coverslips, as described, and treated with different concentrations
(0.5, 1, 1.5 and 2.0 µg/ml) of recombinant neuritin for 24 h.
Slides with MSCs were rinsed with 37°C PBS (pH=7.2) three times,
each time for 3 min, fixed by incubating in cold (−20°C) acetone
for 15 min, and then washed with PBS and blocked as described
above. Cells were incubated overnight at 4°C with the following
primary antibodies: Anti-neuron-specific enolase (NSE; 1:500; cat.
no. ab217778; Abcam), anti-microtubule associate protein 2 (MAP2;
1:500; cat. no. ab32454; Abcam), or anti-glial fibrillary acidic
protein (GFAP; 1:750; cat. no. ab7260; Abcam). PBS was utilized as
a negative control. Cells were subsequently treated with a
FITC-labeled goat anti-rabbit secondary antibody (1:100; cat. no.
ab6717; Abcam) for 1 h at 25°C in the dark, washed with PBS, and
observed in five randomly selected fields (minimum 100 cells/field)
under a fluorescence microscope (Carl Zeiss AG).
Western blot analysis
rBM-MSCs (8×106 cells/ml) were treated
with 0.5 µg/ml neuritin for 24 h. The cells were rinsed twice in
ice-cold PBS (pH 7.5) and lysed with radioimmunoprecipitation assay
buffer (cat. no. R0010; Solarbio, China) containing 1 mM PMSF (cat.
no. P0100; Beijing Solarbio Science and Technology Co., Ltd,
Beijing, China), and placed on ice for 30 min. The cell lysates
were centrifuged at 5,000 × g for 10 min at 4°C. The extractive
proteins were then separated by 10% SDS-PAGE and transferred to
nitrocellulose membranes. Membranes were blocked by incubating in
Tris-buffered saline containing 0.05% Tween-20 and 5% nonfat dry
milk for 1 h at room temperature, and then probed with MAP2
(1:1,000), NSE (1:1,000), and GFAP-specific (1:500) primary
antibodies diluted in blocking buffer overnight at 4°C.
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (goat anti-mouse;
1:50,000; cat. no. ZB-2301; OriGene Technologies, Inc.) for 1 h at
room temperature, and proteins were visualized by enhanced
chemiluminescence (cat. no. WBKLS0500; EMD Millipore, Billerica,
MA, USA). Detection of β-actin (mouse anti-β-actin; cat. no.
sc-47778; 1:1,500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was performed as an internal loading control.
Thin-layer chromatography (TLC)
The induced cell culture (4×103 cells/ml)
was blast chilled and ground into powder (Freeze Dryer Series; SIM
International Group Co., Ltd., Beijing, China). The powder was
dissolved with 1 ml carbinol and uninduced cell culture powder was
used as control. The dissolved powder samples, as well as serotonin
(used as a standard), were spotted on a thin-layer chromatogram
plate and treated with an n-butyl alcohol-acetic
acid-ddH2O solution (4:1:5). The chromatograph was then
sprayed with the color developing agent O-phthaldialdehyde (1 g in
100 mlethanol) and incubated at 110°C for 20 min.
Electrophysiological recordings
Conventional whole-cell recordings were performed as
previously described (11).
Briefly, cells exhibiting neuron-like morphologies were selected
for analysis, while uninduced MSCs were used as a control.
Recording pipettes were pulled from borosilicate glass capillaries
with a filament using a Sutter Instruments P-97 puller (Sutter
Instrument Company, Novato, CA, USA). The pipette had a resistance
of ~5 MΩ uponbeing filled with an internal solution comprised as
follows: 150 mM NaCl, 5.0 mM KCl, 2.5 mM CaCl2, 1.0 mM
MgCl2, 5.0 mM glucose and 10 mM
3-[4-(2-hydroxyethyl)-1-piperazinyl] propanesulfonic acid. The
passive membrane properties and ion channel of the cell samples
were determined by stimulating the cells with 20 mV hyperpolarizing
steps in a voltage clamp. The holding membrane potential was −60
mV. The membrane currents or voltage signals were low-pass filtered
at 10 kHz (−3 dB) using an Axon 700B amplifier (Axon Instruments;
Molecular Devices LLC, Sunnyvale, CA, USA), and were digitized
(Axon Digidata 1440; Axon Instruments; Molecular Devices LLC) and
analyzed using a pCLAMP (pClamp 10.2; Axon Instruments; Molecular
Devices LLC). All experiments were performed at room
temperature.
Statistical analysis
The results are presented as the mean ± standard
deviation. Repeated measures analysis of variance and the least
significant difference (LSD) post hoc test, and independent sample
Student's t-tests, were utilized to detect differences between
results. SPSS 20.0 software (IBM SPSS, Inc., Armonk, NY, USA) was
utilized for all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
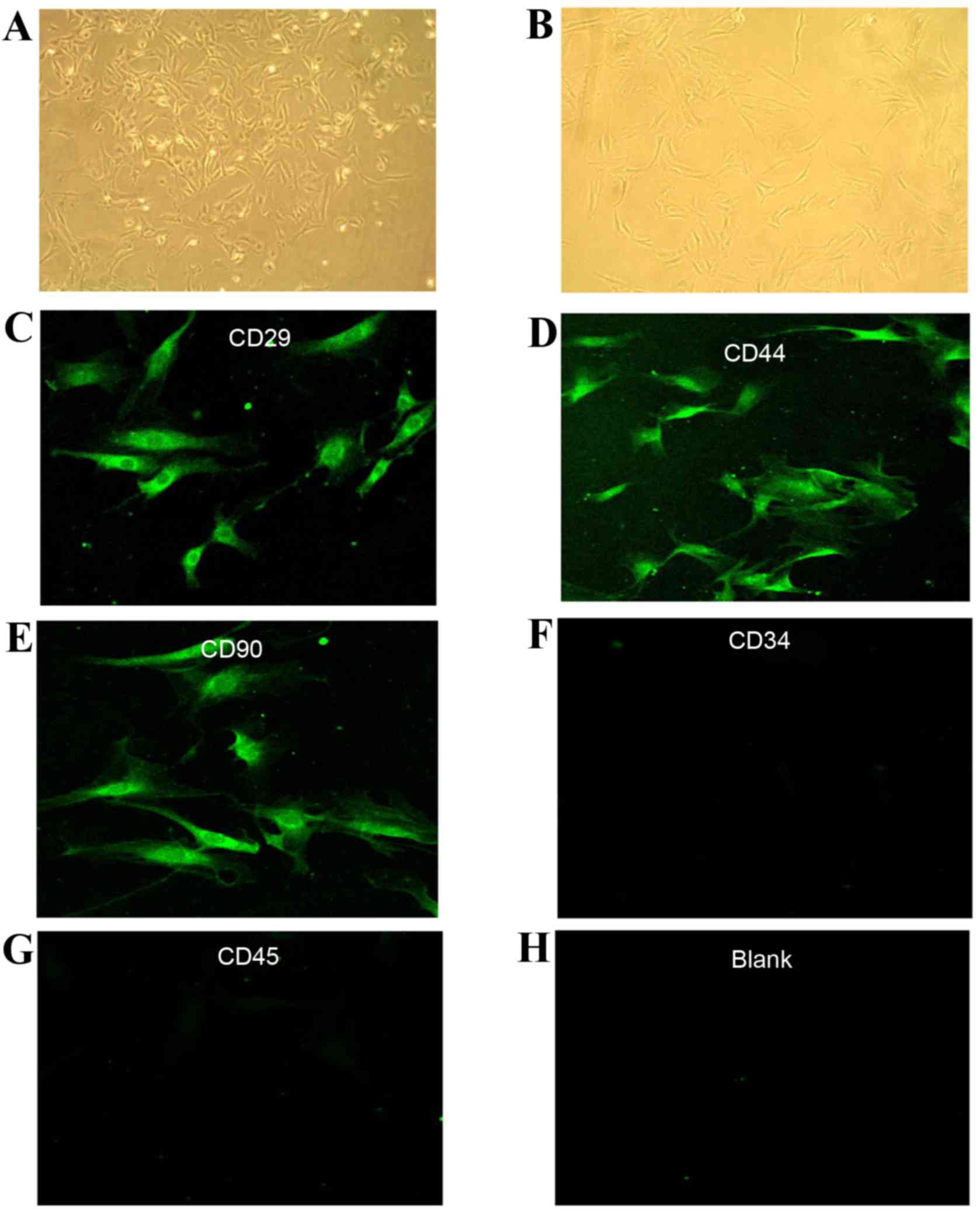
Characterization of cultured MSCs
Isolated rBM-MSCs were cultured and subjected to the
whole-cell adherence screening method as described previously
(12). Cell colonies were observed
96 h following primary culture, and the cells were spindled or
polygonal in shape (Fig. 1A).
Following three passages, the morphology of the cells was
fibroblast-like, and was consistent with that of MSCs (Fig. 1B). Indirect immunofluorescence
analysis detected the expression of the MSC-specific surface
antigens CD29, CD44, and CD90 in P3 cells (Fig. 1C-E, respectively), but not the
hematopoietic stem cell-specific surface antigens CD34 and CD45
(Fig. 1F and G, respectively),
which appeared the same as the blank control (Fig. 1H). These findings indicated that
the P3 cultures were comprised of MSCs.
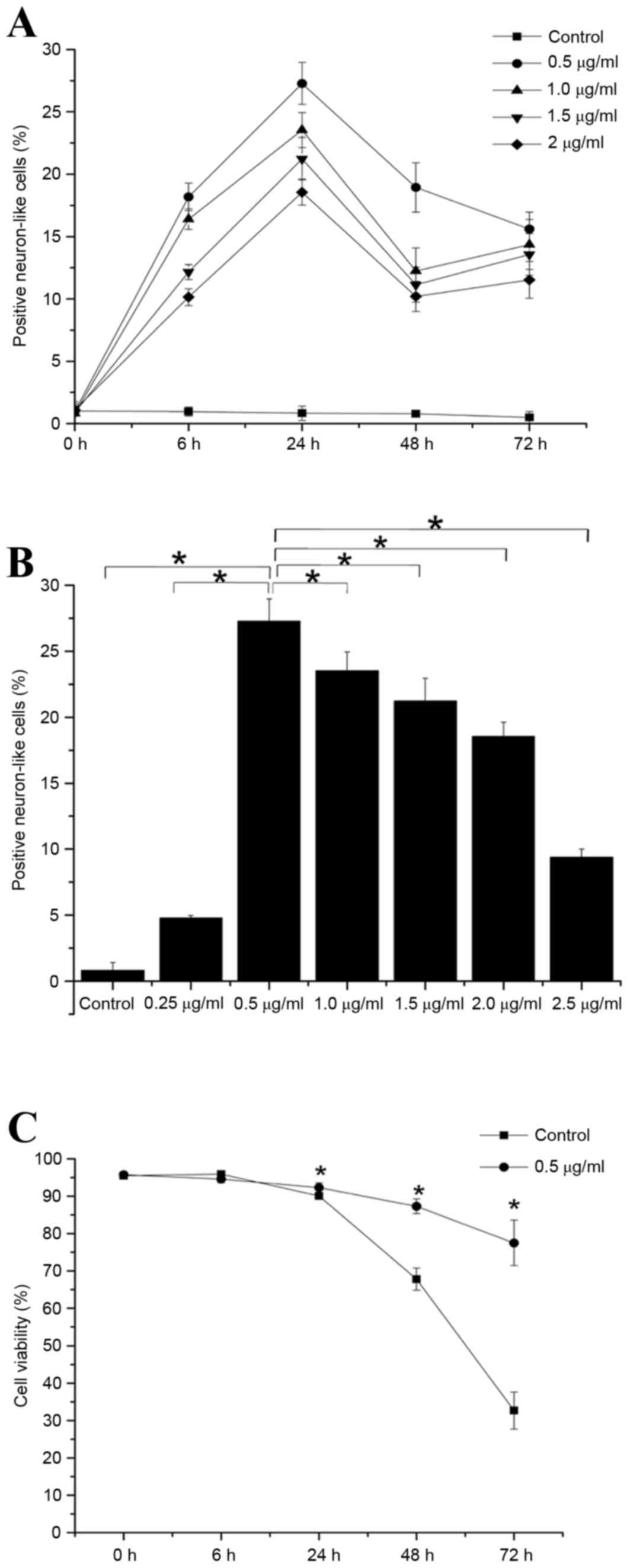
Optimization of cell differentiation
conditions
No significant change in the number of positive NL
cells in the control group was detected at any time point tested
(Fig. 2A). By contrast, the number
of positive NL cells in each of the neuritin groups increased
steadily with time, and were significantly increased at 6 h onwards
compared with present at the 0 h time point (P<0.05; Fig. 2A). Notably, the number of positive
cells in each neuritin treatment group was significantly higher at
24 h than at any other time point (P<0.05; Fig. 2A).
There was a dose-dependent increase in the number of
NL cells at neuritin concentrations between 0 and 0.5 µg/ml
(Fig. 2B). However, following 24 h
induction, the number of positive cells was significantly higher in
the 0.5 µg/ml neuritin group than in the other treatment groups
(P<0.05; Fig. 2B). Therefore,
0.5 µg/ml neuritin was utilized as the induction concentration for
further experiments.
To exclude the possibility that treatment with the
inducer resultedin cytotoxic effects on the rBM-MSCs, the viability
of treated cells was examined by trypan blue staining. The
viability of the cells induced with 0.5 µg/ml neuritin was
significantly higher than that of the control group at 24, 48 and
72 h (P<0.05; Fig. 2C), but not
at the 6 h time point (Fig.
2C).
Together, these results indicated that optimal
production of NL cells was achieved by treatment of rBM-MSCs with
0.5 µg/ml neuritin for 24 h.
Characterization of the morphology and
the expression patterns of neuron-specific proteins in MSCs treated
with neuritin
Cells in the control group exhibited a
fibroblast-like morphology (Fig.
3A). Following treatment with 0.5 µg/ml neuritin for 24 h,
however, 27% of the rBM-MSCs exhibited typical NL cell morphologies
(13) with long processes
(Fig. 3B). The cell body condensed
and became refractile, and the cells formed a network with bipolar
or multipolar neurites.
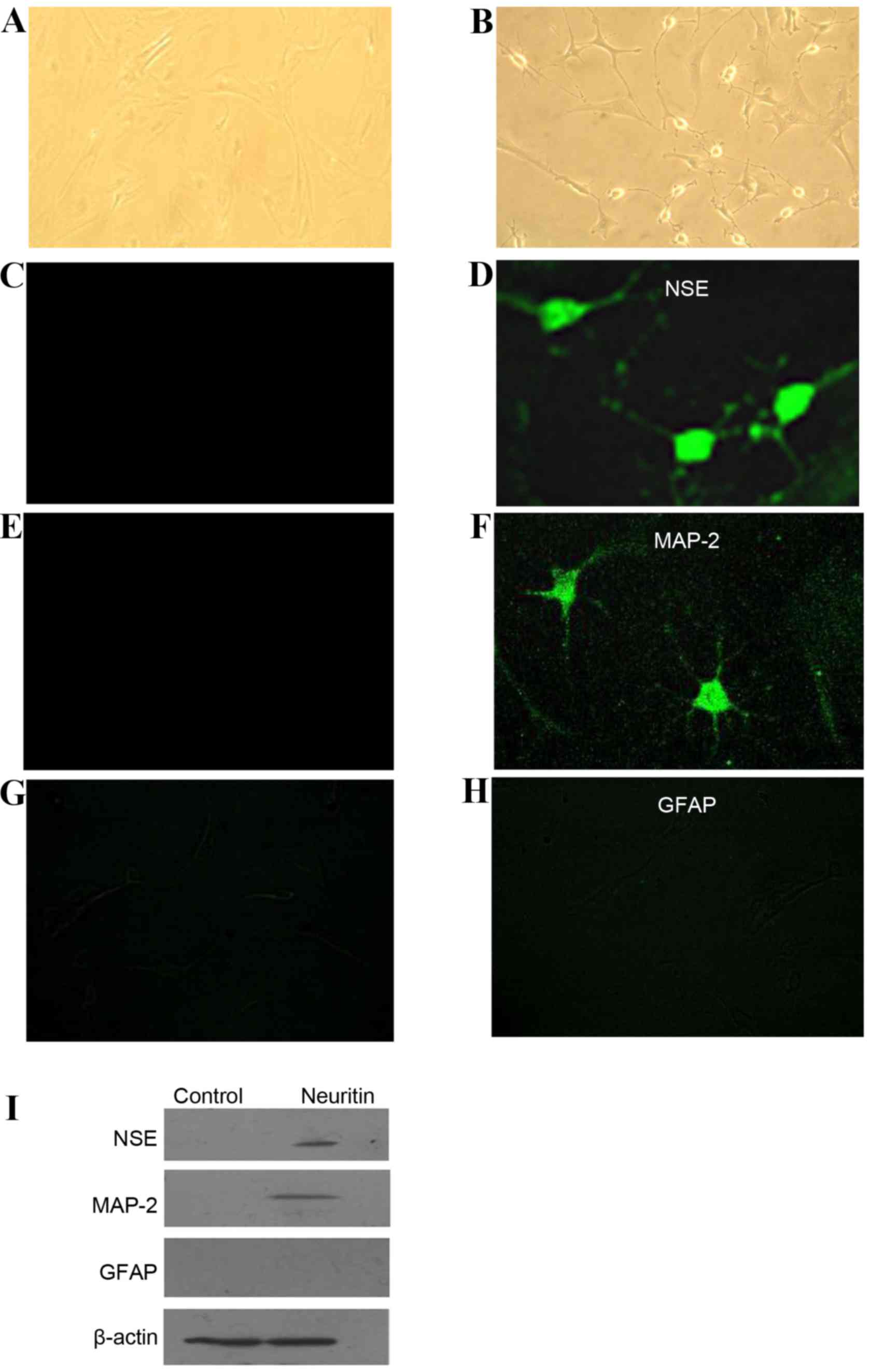 | Figure 3.Characterization of the morphology
and the expression patterns of neuron-specific proteins in rBM-MSCs
treated with neuritin. While cells in the (A) control group were
similar to fibroblasts, (B) differentiated rBM-MSCs exhibited
neuron-like cell morphologies. The cell body condensed and became
refractile, and the cells composed a network with bipolar or
multipolar long processes (magnification, ×200). (C-H) Indirect
immunofluorescence was performed to detect the expression (C and D)
NSE, (E and F) MAP2 and (G and H) GFAP following induction with
neuritin (magnification, ×400). (I) Similar results were obtained
by western blot analysis of NSE, MAP2, and GFAP expression in
neuritin-induced and control cells. rBM-MSCs, rat bone
marrow-derived mesenchymal stem cells; NSE, neuron-specific
enolase; MAP2, microtubule associated protein-2; GFAP, glial
fibrillary acidic protein. |
To confirm that the NL cells were of a neuronal
lineage, indirect immunofluorescence was utilized to examine the
expression of neuron-specific proteins. The mature neural marker
NSE (Fig. 3C and D) and the
dendritic marker MAP2 (Fig. 3E and
F) were expressed in the NL cells but not in the control cells.
Meanwhile, neither the neuritin-treated nor the control cells
exhibited positive expression of the astrocyte marker GFAP
(Fig. 3G and H). Similar findings
were obtained by western blot analysis (Fig. 3I). These results indicated that
rBM-MSCs acquired the cellular characteristics of cells in the
neuronal lineage, but not of astrocytes, following neuritin
treatment.
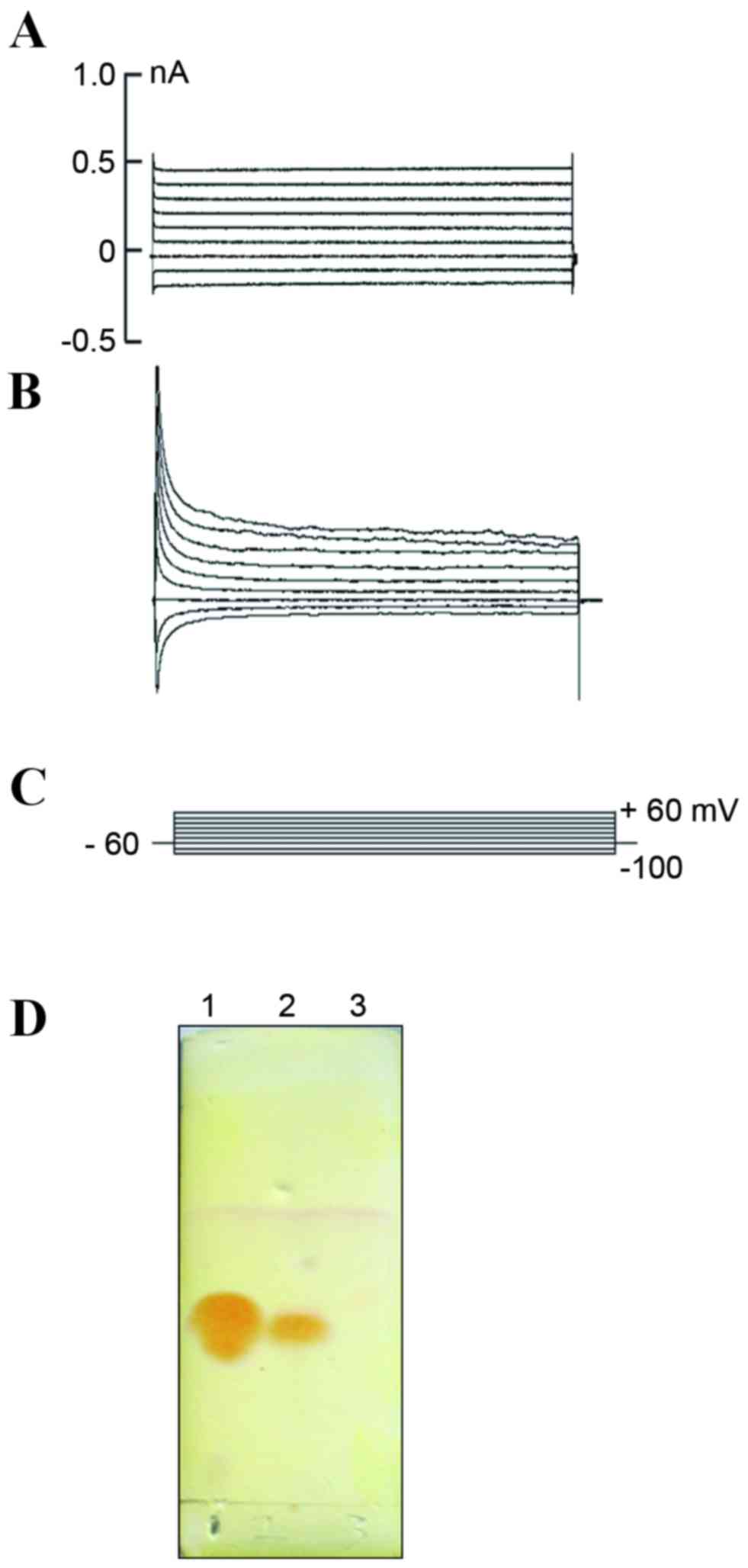
Characterization of the neuronal
functions of NL cells
To establish whether the NL cells produced by
neuritin treatment exhibited neuronal functions, the
electrophysiological properties of these cells were analyzed by
whole-cell patch clamp recording. The membrane capacitance (CM) and
resting membrane potential (RP) of the NL and control cells were
31.56±4.53 pF (n=8) and 15.55±2.40 pF (n=6), and −49.01±4.76 mV
(n=8) and −22.00±2.95 mV (n=6), respectively; and these differences
were statistically significant (P<0.01; Table I). The ionic current of the
neuritin-treated and control cells were then measured using the
voltage clamp method. No apparent channel opening was observed in
the control cells (Fig. 4A), but
an outward delayed rectifying current was detected in the treatment
group with increasing voltage (Fig. 4B
and C), which may indicate the presence of a voltage-dependent
K+ current. These results suggested that the
K+ channel maybe observed concomitantly with
morphological changes and the expression of certain neuron-specific
protein markers. Lastly, TLC was utilized to examine whether the
neurotransmitter 5-hydroxytryptamine (5-HT) was secreted from
neuritin-induced MSCs. A 5-HT-specific signal was detected in the
neuritin but not the control sample (Fig. 4D).
 | Table I.Comparison of membrane properties
between the control group and NL cells following induction. |
Table I.
Comparison of membrane properties
between the control group and NL cells following induction.
| Group | CM, pF | RP, mV |
|---|
| Control (n=6) | 15.55±2.40 | −22.00±2.95 |
| NL cells (n=8) |
31.56±4.53a |
−49.01±4.76a |
Discussion
The incidence of neurodegenerative diseases
hasincreased with aging in the population, but effective methods
are still lacking for treating these diseases. While nerve cell
therapy is currently considered one of the most promising solutions
to these diseases, there are several barriers including cell source
deficiency, immunological rejection, safety and ethical conflicts,
which continue to limit the application of this treatment. However,
previous studies have reported that stem cells exhibit the
potential to differentiate into nerve cells, which maybe
advantageous for nerve cell transplantation therapy (14,15).
MSCs are progenitor cells with multilineage
differentiation potential. These cells are capable of
differentiating into osteoblasts, chondrocytes and adipocytes from
the mesoderm (16) and into
neurons from the ectoderm (17).
Furthermore, due to several unique advantages including good
self-renewal capability, proliferating well in in vitro
culture, adequate resources, convenient access and the capacity to
be auto-transplanted, the rarity of immunological rejection and the
lack of ethical complications, MSCs are becoming an important
option for use as seed cells for cell replacement therapy.
While the bone marrow MSC content is limited
(18), the present studyovercame
this obstacle by adherent culturing of rBM-MSCs in vitro.
Following the third passage, the cells exhibited a typical MSC
morphology (19) and expressed the
MSC-specific surface antigens CD29, CD44 and CD90 (20), but not the hematopoietic stem
cell-specific surface antigens CD34 and CD45 (21). These results therefore excluded the
possibility that the cells were of hematopoietic origin.
Differentiation is a complex process involving the
activity of multiple factors. Combination induction is currently
the most extensively applied approach for inducing the
differentiation of MSCs into neurons (22). In accordance with the Woodbury
method (23), in the present study
MSCs were induced with neuritin following pre-induction with bFGF.
The optimal induction condition was established through detection
of the number of NL cells combined with the cell viability by which
cytotoxicity caused by the inducer was excluded (24,25),
and it was determined that exposure to 0.5 µg/ml neuritin for 24 h
comprised the optimal conditions for stimulating the
differentiation of MSCs into NL cells. Indeed, under these
conditions, MSCs effectively differentiated into typical NL cells
that contained neurites. The NL cells were then characterized by
examining the expression levels of specific neuronal markers and by
observing certain physiological functions. Immunofluorescence and
western blot analyses indicated that the NL cells expressed the
mature neural marker NSE (26) and
the dendritic cell marker MAP2 (3,27),
but not the astrocyte marker GFAP (28). These findings demonstrated that the
rBM-MSCs treated with neuritin differentiated into the neuronal
lineage and not into astrocytes. However, while the cell morphology
and the expression of neural-specific markers partially reflected
the level of cell differentiation, the functional characterization
of the NL cells was the key indicator for evaluating cell
differentiation (29). By patch
clamp analysis, differences in the CM and RP values were detected
in the NL cells compared with those of the control. An increased CM
indicates increased surface area of the cell membrane, and is
evidence supporting cellular vesicle secretion (30). Notably, the detection of 5-HT
secretion by NL cells in the present study is consistent with an
increased CM. Furthermore, secretion of this neurotransmitter is an
important indicator of neuron function. Meanwhile, the observed
decrease in RP denotes that the NL cells exhibited a membrane
potential similar to that of mature neurons, thereby confirming
that the cells were indeed differentiating into the neuronal
lineage (31). Finally, the
delayed rectifier K+-current recorded by the whole-cell
voltage clamp method indicated that the NL cells exhibited the
K+ properties of neurons. This finding was consistent
with the results obtained by physiological analysis of
differentiated embryo-derived neural stem cells (32,33).
In conclusion, the present study demonstrated that
neuritin-induced MSCs differentiated into NL cells. These cells
exhibited typical neuronal morphological features, expression of
neuronal markers and secretion of the neurotransmitter 5-HT.
Furthermore, these NL cells exhibited partial
neural-electrophysiological functions. The results of the present
study therefore demonstrated that neuritin is involved in the
directional differentiation potential of MSCs into NL cells.
Although still in a nascent stage, this novel methodology may
provide an alternative, potentially complementary tool for disease
modeling and the development of cell-based therapies.
Acknowledgements
The authors would like to thank Professor Ketao Ma
(Shihezi University, Shihezi, China) for the technical assistance
with the patch clamp. The present study was supported by the Key
Project of Xinjiang Province (grant. no. 2014AB048), the Major
Scientific and Technological Innovation Project of Hangzhou (grant.
no. 20152013A01) and the Key Project of Shihezi University (grant
no. ZRKX2010ZD03).
Glossary
Abbreviations
Abbreviations:
|
bFGF
|
basic fibroblast growth factor
|
|
CM
|
membrane capacitance
|
|
GFAP
|
glial fibrillary acidic protein
|
|
5-HT
|
5-hydroxytryptamine
|
|
MAP2
|
microtubule associated protein-2
|
|
NSE
|
neuron-specific enolase
|
|
RP
|
resting membrane potential
|
|
rBM-MSCs
|
rat bone marrow-mesenchymal stem
cells
|
|
TLC
|
thin layer chromatography
|
References
|
1
|
Bjorklund A and Kordower JH: Cell therapy
for Parkinson's disease: What next? Mov Disord. 28:110–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Freed CR: Will embryonic stem cells be a
useful source of dopamine neurons for transplant into patients with
Parkinson's disease? Proc Natl Acad Sci USA. 99:1755–1757. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanchez C, Díaz-Nido J and Avila J:
Phosphorylation of microtubule-associated protein 2 (MAP2) and its
relevance for the regulation of the neuronal cytoskeleton function.
Prog Neurobiol. 61:133–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim JY, Park SI, Oh JH, Kim SM, Jeong CH,
Jun JA, Lee KS, Oh W, Lee JK and Jeun SS: Brain-derived
neurotrophic factor stimulates the neural differentiation of human
umbilical cord blood-derived mesenchymal stem cells and survival of
differentiated cells through MAPK/ERK and PI3K/Akt-dependent
signaling pathways. J Neurosci Res. 86:2168–2178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naeve GS, Ramakrishnan M, Kramer R,
Hevroni D, Citri Y and Theill LE: Neuritin: A gene induced by
neural activity and neurotrophins that promotes neuritogenesis.
Proc Natl Acad Sci USA. 94:2648–2653. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Putz U, Harwell C and Nedivi E: Soluble
CPG15 expressed during early development rescues cortical
progenitors from apoptosis. Nat Neurosci. 8:322–331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Liu C, Xu F, Cui L, Tan S, Chen R,
Yang L and Huang J: Effects of neuritin on the migration,
senescence and proliferation of human bone marrow mesenchymal stem
cells. Cell Mol Biol Lett. 20:466–474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Zhang S, Xian L, Tang J, Zhu J,
Cui L, Li S, Yang L and Huang J: Expression and purification of
recombinant human neuritin from Pichia pastoris and a
partial analysis of its neurobiological activity in vitro. Appl
Microbiol Biotechnol. 99:8035–8043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zohar R, Sodek J and McCulloch CA:
Characterization of stromal progenitor cells enriched by flow
cytometry. Blood. 90:3471–3481. 1997.PubMed/NCBI
|
|
10
|
Sung JH, Yang HM, Park JB, Choi GS, Joh
JW, Kwon CH, Chun JM, Lee SK and Kim SJ: Isolation and
characterization of mouse mesenchymal stem cells. Transplant Proc.
40:2649–2654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma KT, Li XZ, Li L, Jiang XW, Chen XY, Liu
WD, Zhao L, Zhang ZS and Si JQ: Role of gap junctions in the
contractile response to agonists in the mesenteric artery of
spontaneously hypertensive rats. Hypertens Res. 37:110–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin ZH, Qu JM, Xu JF, Zhang J, Summah H,
Sai-Yin HX, Chen CM and Yu L: Intrapleural delivery of mesenchymal
stem cells: A novel potential treatment for pleural diseases. Acta
pharmacol Sin. 32:581–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang N, Ng YH, Pang ZP, Südhof TC and
Wernig M: Induced neuronal cells: How to make and define a neuron.
Cell Stem Cell. 9:517–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Potter ED, Ling ZD and Carvey PM:
Cytokine-induced conversion of mesencephalic-derived progenitor
cells into dopamine neurons. Cell Tissue Res. 296:235–246. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watmuff B, Pouton CW and Haynes JM: In
vitro maturation of dopaminergic neurons derived from mouse
embryonic stem cells: Implications for transplantation. PLoS One.
7:e319992012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K,
Zhou X, Park NH and Wang CY: Histone demethylases KDM4B and KDM6B
promotes osteogenic differentiation of human MSCs. Cell Stem Cell.
11:50–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Sun HM, Yan JH, Xue H, Wu B, Dong
F, Li WS, Ji FQ and Zhou DS: Conditioned medium from human amniotic
epithelial cells may induce the differentiation of human umbilical
cord blood mesenchymal stem cells into dopaminergic neuron-like
cells. J Neurosci Res. 91:978–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith RK, Werling NJ, Dakin SG, Alam R,
Goodship AE and Dudhia J: Beneficial effects of autologous bone
marrow-derived mesenchymal stem cells in naturally occurring
tendinopathy. PLoS One. 8:e756972013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erices A, Conget P and Minguell JJ:
Mesenchymal progenitor cells in human umbilical cord blood. Br J
Haematol. 109:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vogel W, Grünebach F, Messam CA, Kanz L,
Brugger W and Bühring HJ: Heterogeneity among human bone
marrow-derived mesenchymal stem cells and neural progenitor cells.
Haematologica. 88:126–133. 2003.PubMed/NCBI
|
|
21
|
Pei X: Who is hematopoietic stem cell:
CD34+ or CD34-? Int J Hematol. 70:213–215. 1999.PubMed/NCBI
|
|
22
|
Tomita M, Mori T, Maruyama K, Zahir T,
Ward M, Umezawa A and Young MJ: A comparison of neural
differentiation and retinal transplantation with bone
marrow-derived cells and retinal progenitor cells. Stem Cells.
24:2270–2278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neuhuber B, Gallo G, Howard L, Kostura L,
Mackay A and Fischer I: Reevaluation of in vitro differentiation
protocols for bone marrow stromal cells: Disruption of actin
cytoskeleton induces rapid morphological changes and mimics
neuronal phenotype. J Neurosci Res. 77:192–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnabé GF, Schwindt TT, Calcagnotto ME,
Motta FL, Martinez G Jr, de Oliveira AC, Keim LM, D'Almeida V,
Mendez-Otero R and Mello LE: Chemically-induced RAT mesenchymal
stem cells adopt molecular properties of neuronal-like cells but do
not have basic neuronal functional properties. PLoS One.
4:e52222009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin K, Mao XO, Batteur S, Sun Y and
Greenberg DA: Induction of neuronal markers in bone marrow cells:
Differential effects of growth factors and patterns of
intracellular expression. Exp Neurol. 184:78–89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ladrech S, Lenoir M, Ruel J and Puel JL:
Microtubule-associated protein 2 (MAP2) expression during synaptic
plasticity in the guinea pig cochlea. Hear Res. 186:85–90. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Messing A and Brenner M: GFAP: Functional
implications gleaned from studies of genetically engineered mice.
Glia. 43:87–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reh TA: Neural stem cells: Form and
function. Nat Neurosci. 5:392–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Angleson JK and Betz WJ: Monitoring
secretion in real time: Capacitance, amperometry and fluorescence
compared. Trends Neurosci. 20:281–287. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kohyama J, Abe H, Shimazaki T, Koizumi A,
Nakashima K, Gojo S, Taga T, Okano H, Hata J and Umezawa A: Brain
from bone: Efficient ‘meta-differentiation’ of marrow
stroma-derived mature osteoblasts to neurons with Noggin or a
demethylating agent. Differentiation. 68:235–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas
JD, Cheng J and Qu T: Generation of neural stem cell-like cells
from bone marrow-derived human mesenchymal stem cells. Neurol Res.
33:1083–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Risner-Janiczek JR, Ungless MA and Li M:
Electrophysiological properties of embryonic stem cell-derived
neurons. PLoS One. 6:e241692011. View Article : Google Scholar : PubMed/NCBI
|