Introduction
Ovarian cancer is the most common cause of mortality
among women in the world and its incidence has been rising
worldwide over the past few decades (1). Despite extensive efforts to improve
the diagnosis and treatment of ovarian cancer, limited progress has
been made (2–4). The majority of patients with ovarian
cancer are diagnosed at advanced stages due to the subtle symptoms
at early stages of ovarian carcinogenesis and the 5-year survival
rate of patients with ovarian cancer is <30% (5,6).
Therefore, there is a requirement to understand the underlying
mechanisms of ovarian cancer progression for the treatment of
ovarian cancer.
Protein Jumonji (JARID2) is a member of the family
of JmjC domain-containing proteins that remove methyl residues from
methylated lysine 4 on histone H3 lysine 4 (H3K4) (7). JARID2 is crucial for the maintenance
of pluripotency and differentiation in embryonic stem cells (ESCs)
(8). JARID2 depletion has been
reported to cause pronounced defects during ESC differentiation
(9,10). In addition, aberrant expression of
JARID2 has been reported in several types of cancers, and JARID2
may promote tumor growth and metastasis (11–13).
For example, Lei et al (14) reported that JARID2 expression was
increased in hepatocellular carcinoma (HCC) tissues compared with
in adjacent non-tumor liver tissues, and upregulation of JARID2
promoted HCC cell proliferation, migration and invasion in
vitro, and metastasis in vivo. However, the expression
pattern and role of JARID2 in ovarian cancer remains unclear.
Therefore, the present study investigated the role of JARID2 in
ovarian cancer and the associated underlying mechanisms. The
results demonstrated that knockdown of JARID2 inhibited the
proliferation, migration and invasion of ovarian cancer cells in
vitro. Therefore, JARID2 may represent a potential therapeutic
target for the treatment of ovarian cancer.
Materials and methods
Cell culture
A total of three human ovarian cancer cell lines
(SKOV3, 3AO and OVCAR3) and a human normal ovarian surface
epithelial cell line (HOSEpiC) were purchased from the American
Type Culture Collection (Manassas, VA, USA). All cells were
maintained in Dulbeccos Modified Eagles Medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA USA) supplemented with 10%
(v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from ovarian cancer cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and the cDNA was reverse transcribed using the EasyScript
First-Strand cDNA Synthesis SuperMix kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturers protocol. qPCR
reactions were performed on the Bio-Rad iQ5 real-time thermal
cyclers using SYBR Premix Ex Taq™ II kit (Takara Biotechnology Co.,
Ltd., Dalian, China). The following PCR primers were used: JARID2
forward, 5-GAC ACC AAA CCC AAT CAC CAC-3 and reverse, 5-GTT CAA CCT
GCC ACT GAC CTT-3; epithelial (E)-cadherin forward, 5-TAC ACT GCC
CAG GAG CCA GA-3′ and reverse, 5-TGG CAC CAG TGT CCG GAT TA-3;
neural (N)-cadherin forward, 5-TTT GAT GGA GGT CTC CTA ACA CC-3 and
reverse, 5-ACG TTT AAC ACG TTG GAA ATG TG-3; and β-actin forward,
5-TTA GTT GCG TTA CAC CCT TTC-3 and reverse, 5-ACC TTC ACC GTT CCA
GTT T-3. The PCR cycling program was as follows: 95°C for 5 min,
followed by 35 cycles of 95°C for 20 sec, 59°C for 20 sec and 72°C
for 20 sec, and a final extension at 72°C for 5 min. β-actin was
used as the internal reference gene. Then the PCR products were
separated by agarose gel electrophoresis to confirm successful
amplification. The relative expression levels were calculated using
the 2−ΔΔCq method (15).
Western blotting
Total protein was extracted from ovarian cancer
cells using radioimmunoprecipitation assay buffer containing
phosphatase and protease inhibitors (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The protein concentration was then determined
using the Bradford method. Protein lysates (30 µg) were separated
by 10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Then, the membrane
was blocked with 5% non-fat milk in TBS with Tween-20 [TBST; 10 mM
Tris-HCl (pH of 7.5), 150 mM NaCl and 0.05% Tween-20] for 1 h at
room temperature, followed by incubation with primary antibodies
anti-JARID2 (1:3,000; cat. no. SAB2105079; Sigma-Aldrich; Merck
KGaA), and E-cadherin (1:3,000; cat. no. sc-21791), N-cadherin
(1:2,000; cat. no. sc-53488), PI3K (1:2,000; cat. no. sc-365290),
phosphorylated-phosphoinositide 3-kinase (p-PI3K; 1:2,500; cat. no.
sc-293115), protein kinase B (Akt; 1:3,000; sc-5298), p-Akt
(1:2,500; cat. no. sc-52940) and GAPDH (1:2,000; cat. no.
sc-47724), from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA)
overnight at 4°C. Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibody (1:2,500; cat.
no. sc-2005; Santa Cruz Biotechnology, Inc.) at room temperature
for 1 h. Bound antibodies were detected using enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.).
Relative protein levels of p-PI3K and p-Akt were quantified using
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA).
RNA interference and cell
transfection
Small interfering RNA against JARID2 (si-JARID2,
5-AGG AAG AGG AGG AGG ACA A-3) and its negative control (si-NC,
5-GAG UGG GUC UGG GUC UUC CCG UAG A-3) were synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). SKOV3 cells were
seeded at a density of 1×105 cells/well in each cell of
a 24-well microplate, grown for 24 h to reach 40% confluence, and
then transfected with si-JARID2 or si-NC using 4 µl Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) for 24 h, according to the manufacturers protocol. The
efficacy of overexpression was confirmed by RT-qPCR and western
blot analysis.
Cell proliferation assay
Cell proliferation was measured using the MTT assay
(Sangon Biotech Co., Ltd., Shanghai, China). SKOV3 cells were
seeded at a density of 3×104 cells/well into 96-well
culture plates and cultured for 1–4 days following transfection. At
each time point, 20 µl PBS containing 5 mg/ml MTT/well was added
and incubated for 4 h at 37°C. The medium was removed, and dimethyl
sulfoxide was added to each well. The absorbance was measured at
490 nm with a microplate reader (Bio-Rad, Laboratories, Inc.,
Hercules, CA, USA).
Transwell migration and invasion
assays
Cell migration and invasion were detected using the
Transwell chamber assay. For the cell migration assay, transfected
ovarian cancer cells (1×105 cells/chamber) in 100 µl
serum-free media were plated into the upper chamber, while 500 µl
DMEM medium with 10% FBS was added into the lower compartment.
Following incubation for 24 h at 37°C, the non-migratory cells were
removed with cotton swabs from the upper surface of the membrane.
The cells on the lower surface of the membrane were fixed with 4%
paraformaldehyde, stained with 0.1% crystal violet at room
temperature for 10 min, and then counted under a light microscope
(magnification, ×100). For the invasion assay, the procedures were
the same as the migration assay except the chamber was pre-coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ USA).
Statistical analysis
All statistical analyses were conducted using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). Statistical
significance was analyzed with one-way factorial analysis of
variance followed by Tukeys post hoc test or Students two-tailed
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
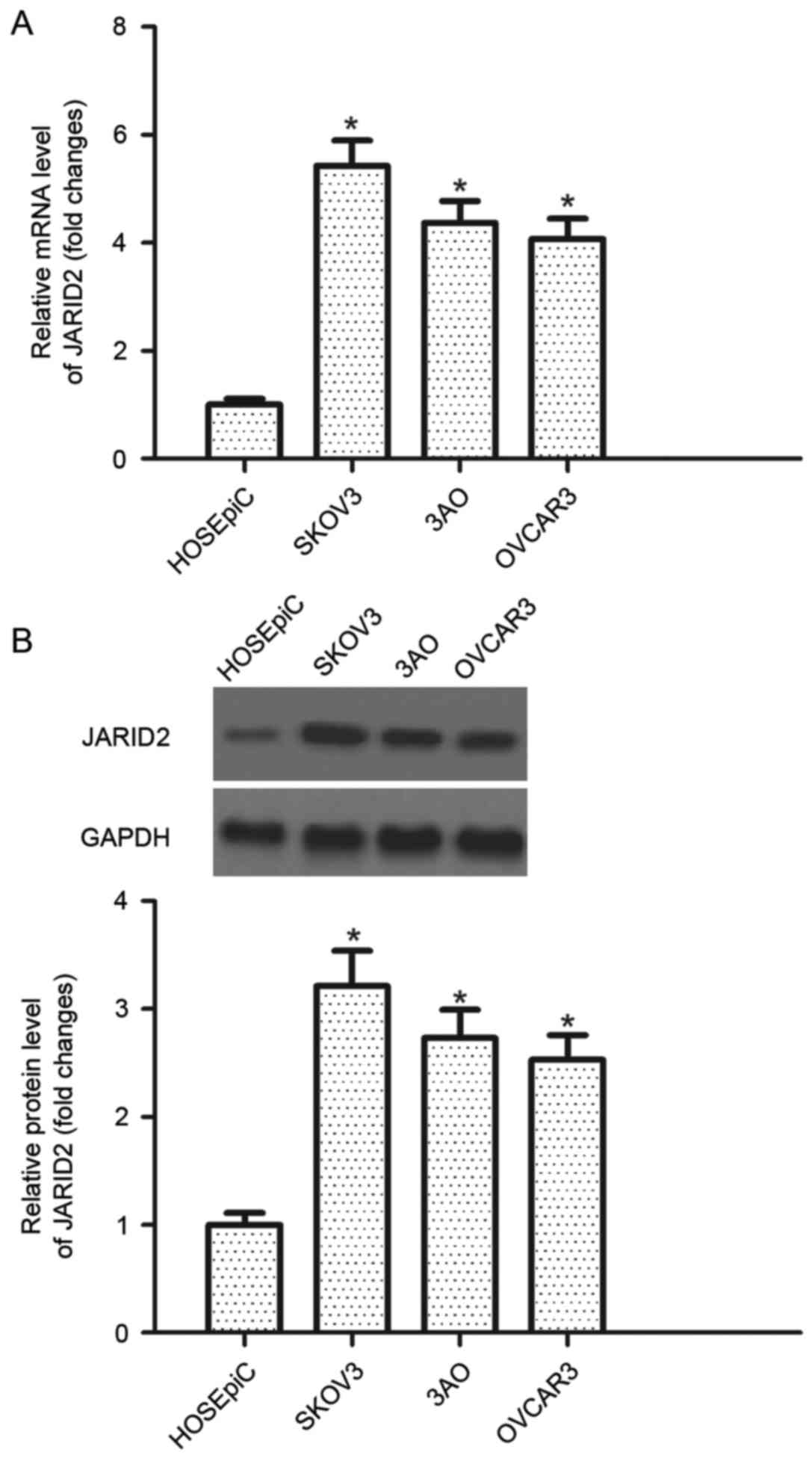
JARID2 is highly expressed in ovarian
cancer cell lines
RT-qPCR was performed to detect the level of JARID2
mRNA expression in human ovarian cancer cell lines. It was observed
that the mRNA expression levels of JARID2 were increased in human
ovarian cancer cell lines compared with the control group (Fig. 1A). Furthermore, the protein
expression of JARID2 in human ovarian cancer cell lines was
examined by western blotting. All cell lines investigated exhibited
increased JARID2 protein expression levels in human ovarian cancer
cell lines compared with the control HOSEpiC cell line (Fig. 1B).
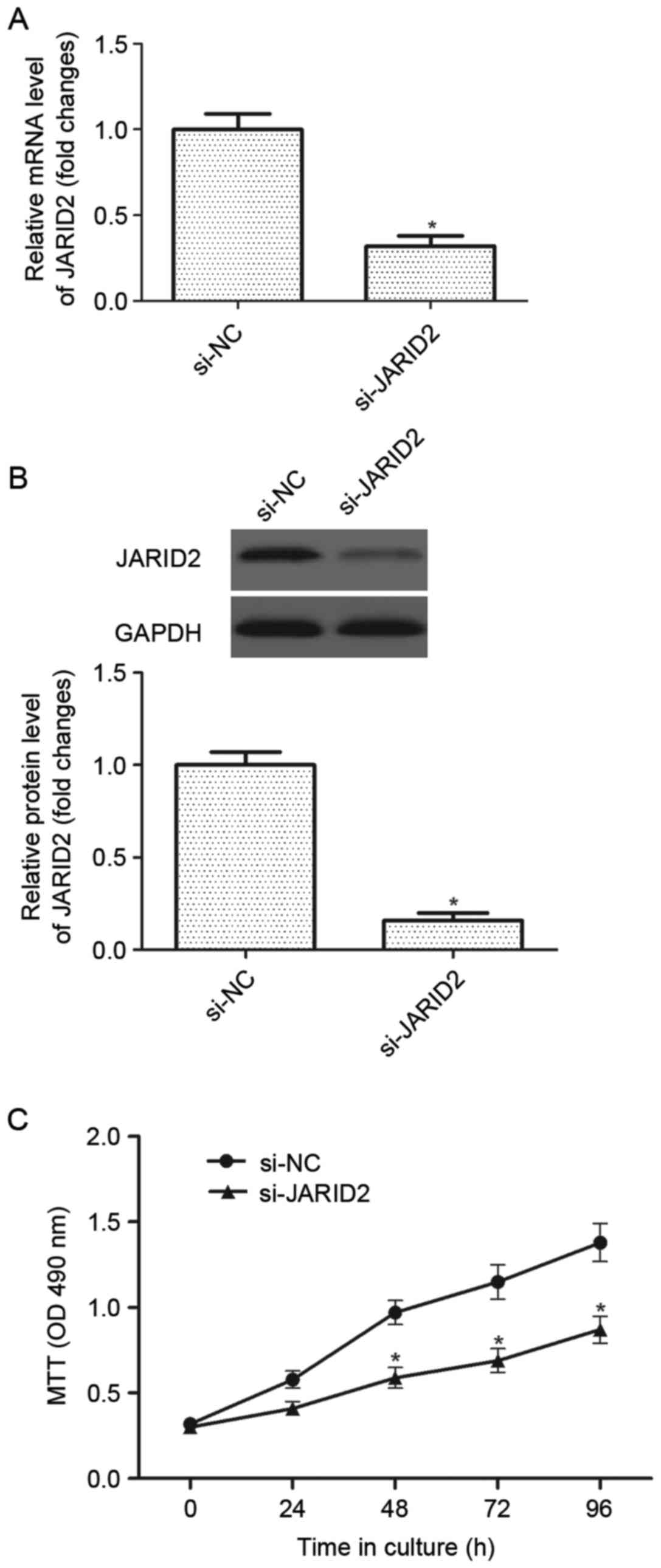
Downregulation of JARID2 inhibits the
proliferation of ovarian cancer cells
In order to investigate the role of JARID2 in
ovarian cancer carcinogenesis, a si-JARID2 was introduced into
ovarian cancer cells. The results of the RT-qPCR analysis
demonstrated that the mRNA expression of JARID2 was significantly
decreased in ovarian cancer cells infected with si-JARID2 compared
with the si-NC group (Fig. 2A).
Western blot analysis indicated that si-JARID2 also downregulated
the protein expression of JARID2 in ovarian cancer cells compared
with the si-NC group (Fig. 2B).
Then, the effect of JARID2 on ovarian cancer cell proliferation was
examined using the MTT assay. As presented in Fig. 2C, downregulation of JARID2
significantly suppressed the proliferation of ovarian cancer cells,
as compared with the si-NC group.
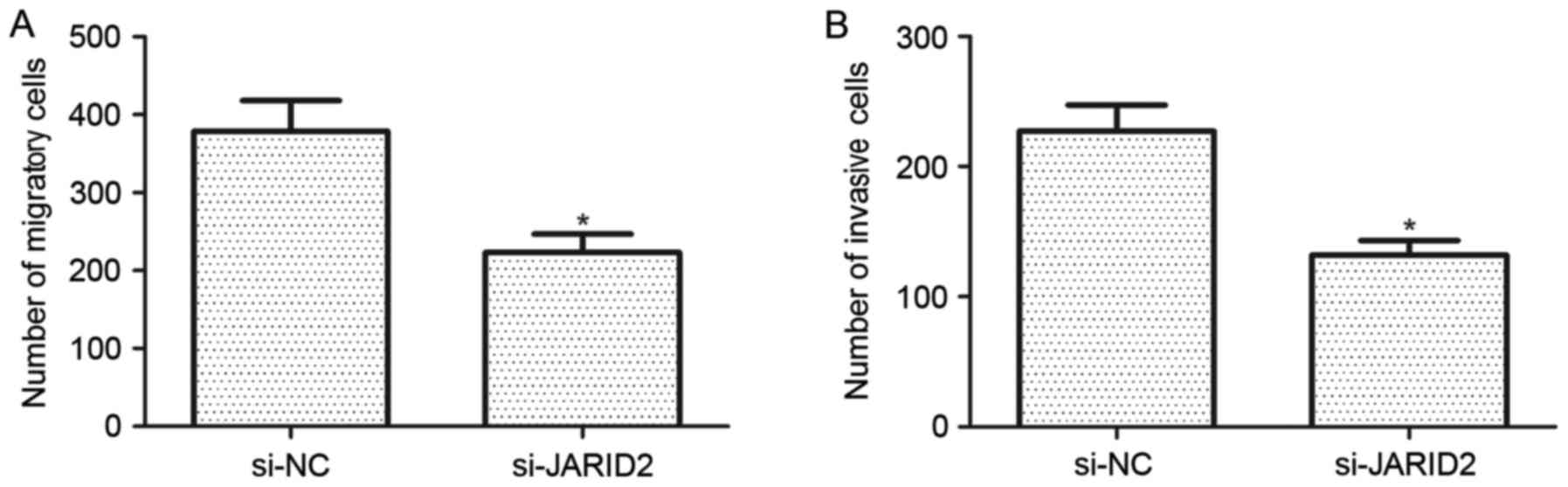
Downregulation of JARID2 inhibits the
migration and invasion of ovarian cancer cells
The effect of JARID2 on the migration and invasion
of ovarian cancer cells was investigated. Compared with the si-NC
group, SKOV3 cells transfected with si-JARID2 exhibited a
significant reduction in cell migration (Fig. 3A). Furthermore, the results of the
Matrigel invasion assay demonstrated that downregulation of JARID2
reduced the number of invasive SKOV3 cells when compared with the
si-NC group (Fig. 3B).
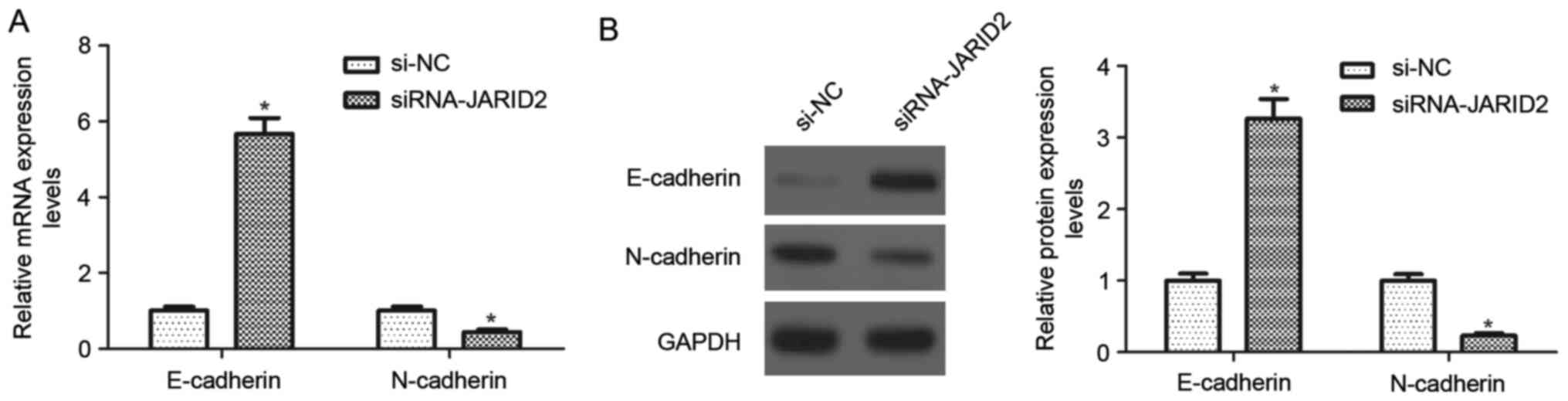
Downregulation of JARID2 inhibits the
epithelial-mesenchymal transition (EMT in ovarian cancer cells
To better investigate the effects of JARID2 on
ovarian cancer cell migration and invasion, the expression of
EMT-associated markers was evaluated using RT-qPCR and western blot
analysis. As indicated in Fig. 4A,
the mRNA expression of E-cadherin was significantly upregulated in
SKOV3 cells transfected with si-JARID2, whereas, the mRNA
expression of N-cadherin was downregulated, compared with the si-NC
group Similarly, downregulation of JARID2 caused a 3.2-fold
increase in E-cadherin protein expression and a decrease in
N-cadherin protein expression in SKOV3 cells, as compared with the
si-NC group (Fig. 4B).
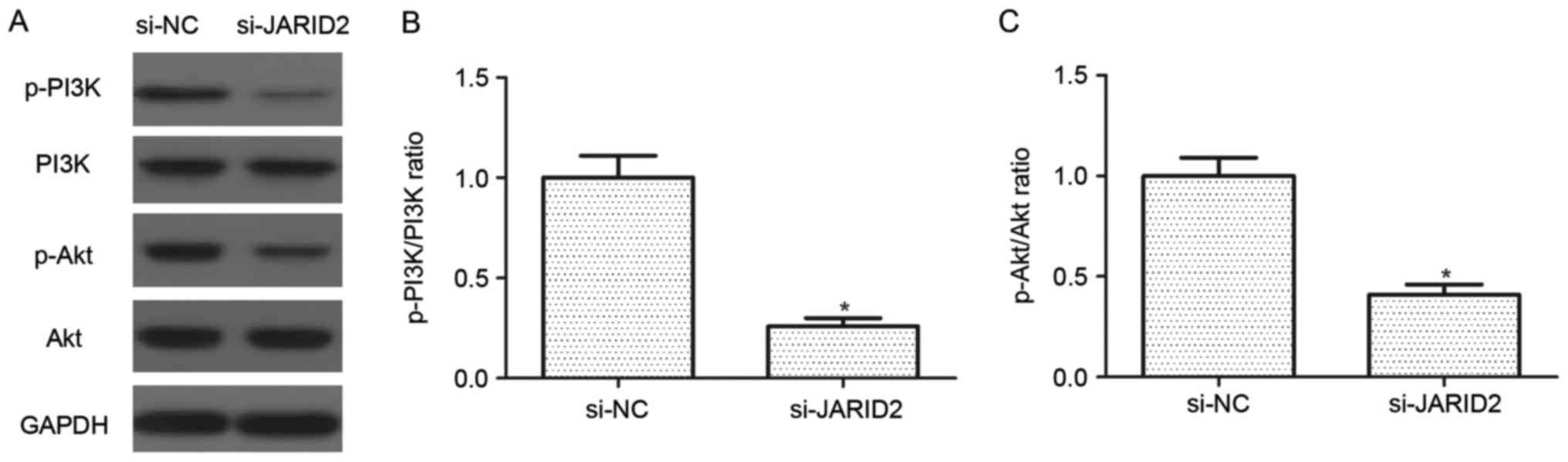
Downregulation of JARID2 inhibits the
activation of the PI3K/Akt signaling pathway in ovarian cancer
cells
The PI3K/Akt signaling pathway serves an important
role in the development and progression of ovarian cancer.
Therefore, the effect of JARID2 on the activation of the PI3K/Akt
signaling pathway was investigated in SKOV3 cells. As indicated in
Fig. 5, knockdown of JARID2
suppressed the protein expression levels of p-PI3K and p-Akt in
SKOV3 cells, as compared with the si-NC group.
Discussion
In the present study, it was demonstrated that the
expression of JARID2 is upregulated in human ovarian cancer cell
lines. Furthermore, downregulation of JARID2 significantly
suppressed the proliferation, migration, invasion and EMT in human
ovarian cancer cells. Mechanistically, downregulation of JARID2
decreased the protein expression levels of p-PI3K and p-Akt in
ovarian cancer cells.
Several studies reported that JARID2 serves a
critical role in cancer development and progression. Walters et
al (11) reported that JARID2
is overexpressed in rhabdomyosarcomas. Knockdown of JARID2 resulted
in reduced cell proliferation coupled with increased myogenic
differentiation (11). Another
study confirmed that the expression of JARID2 was significantly
upregulated in HCC tissues (14).
In accordance with previous studies, in the current study, it was
observed that JARID2 is expressed at high levels in human ovarian
cancer cell lines. Downregulation of JARID2 significantly
suppressed the proliferation of human ovarian cancer cells. These
data suggested that JARID2 is involved in the carcinogenesis of
ovarian cancer.
Ovarian cancer is the most lethal gynecological
malignancy due to its high metastatic ability (16). The increased motility and
invasiveness of malignant tumor cells is associated with the EMT
phenotype. Ovarian cancer is characterized by the loss of
epithelial differentiation and acquisition of mesenchymal-like
cellular competence of tumor cells. During this process, epithelial
cells downregulate the expression of cell adhesion molecules,
including E-cadherin, dissolve cell-cell junctions, lose their
apical-basal polarity, and enhance migratory and invasive
properties (17,18). In the present study, it was
observed that downregulation of JARID2 suppressed the migration and
invasion of ovarian cancer cells. In addition, it was observed that
downregulation of JARID2 increased the expression of E-cadherin and
decreased the expression of N-cadherin in ovarian cancer cells.
These results suggested that JARID2 may be an important contributor
to EMT progression, thus facilitating the migration and invasion of
ovarian cancer cells.
Increasing evidence has reported that the PI3K/Akt
signaling pathway serves important roles in the progression of
ovarian cancer (19–21). PI3K is activated by oncogenes, and
activated PI3K promotes cancer cell growth and invasion (22). Akt, a downstream effector of PI3K,
is implicated in various cellular processes, including cell
proliferation, cell invasion, metabolism and EMT (23). It has been reported that the
activation of Akt enhances EMT, downregulates E-cadherin
transcription, and increases cancer cell migration and invasion
(24). PI3K/Akt signaling pathway
activation is associated with higher invasive and migratory
capacities in human ovarian cancer cells (25). Therefore, inhibiting this pathway
may be beneficial for the treatment of ovarian cancer. It was
observed that knockdown of JARID2 downregulated the protein
expression levels of p-PI3K and p-Akt in ovarian cancer cells.
These results suggested that knockdown of JARID2 inhibits cell
proliferation and migration through the inactivation of the
PI3K/Akt signaling pathway.
In conclusion, the results of the present study
demonstrate that JARID2 may serve important roles in ovarian cancer
cell proliferation, invasion and EMT. Therefore, JARID2 may be a
potential therapeutic target for the treatment of
ovariancancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402139).
References
|
1
|
Kuznia AL and Roett MA: Genital cancers in
women: Ovarian cancer. FP Essent. 438:24–30. 2015.PubMed/NCBI
|
|
2
|
Nicoletto MO, Artioli G, Donach M, Sileni
VC, Monfardini S, Talamini R, Veronesi A, Ferrazzi E, Tumolo S,
Visonà E, et al: Elderly ovarian cancer: Treatment with
mitoxantrone-carboplatin. Gynecol Oncol. 80:221–226. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rustin GJ, van der Burg ME, Griffin CL,
Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C,
Qian W, et al: Early versus delayed treatment of relapsed ovarian
cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet.
376:1155–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harries M and Gore M: Part II:
Chemotherapy for epithelial ovarian cancer-treatment of recurrent
disease. Lancet Oncol. 3:537–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sundar S, Neal RD and Kehoe S: Diagnosis
of ovarian cancer. BMJ. 351:h44432015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian Q, Yan Y, Yang J, Cao D, Zhu Z, Wu M,
Chen J, Lang J and Shen K: Management and prognosis of patients
with ovarian sex cord tumor with annular tubules: A retrospective
study. BMC Cancer. 15:2702015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirato H, Ogawa S, Nakajima K, Inagawa M,
Kojima M, Tachibana M, Shinkai Y and Takeuchi T: A jumonji (Jarid2)
protein complex represses cyclin D1 expression by methylation of
histone H3-K9. J Biol Chem. 284:733–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanulli S, Justin N, Teissandier A,
Ancelin K, Portoso M, Caron M, Michaud A, Lombard B, da Rocha ST,
Offer J, et al: Jarid2 Methylation via the PRC2 complex regulates
H3K27me3 deposition during cell differentiation. Mol Cell.
57:769–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landeira D, Sauer S, Poot R, Dvorkina M,
Mazzarella L, Jørgensen HF, Pereira CF, Leleu M, Piccolo FM,
Spivakov M, et al: Jarid2 is a PRC2 component in embryonic stem
cells required for multi-lineage differentiation and recruitment of
PRC1 and RNA Polymerase II to developmental regulators. Nat Cell
Biol. 12:618–624. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng JC, Valouev A, Swigut T, Zhang J,
Zhao Y, Sidow A and Wysocka J: Jarid2/Jumonji coordinates control
of PRC2 enzymatic activity and target gene occupancy in pluripotent
cells. Cell. 139:1290–1302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walters ZS, Villarejo-Balcells B, Olmos D,
Buist TW, Missiaglia E, Allen R, Al-lazikani B, Garrett MD, Blagg J
and Shipley J: JARID2 is a direct target of the PAX3-FOXO1 fusion
protein and inhibits myogenic differentiation of rhabdomyosarcoma
cells. Oncogene. 33:1148–1157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tange S, Oktyabri D, Terashima M, Ishimura
A and Suzuki T: JARID2 is involved in transforming growth
factor-beta-induced epithelial-mesenchymal transition of lung and
colon cancer cell lines. PLoS One. 9:e1156842014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Z, Tang LS, Xu L, Qin X, Mao S, Song
Y, Liu L, Li F, Liu P, Yi L, et al: Genome-wide association study
identifies new susceptibility loci for adolescent idiopathic
scoliosis in Chinese girls. Nat Commun. 6:83552015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei X, Xu JF, Chang RM, Fang F, Zuo CH and
Yang LY: JARID2 promotes invasion and metastasis of hepatocellular
carcinoma by facilitating epithelial-mesenchymal transition through
PTEN/AKT signaling. Oncotarget. 7:40266–40284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Wang L, Zhang W, Tang B, Zhang J,
Song H, Yao D, Tang Y, Chen X, Yang Z, et al: Correlation of serum
VEGF levels with clinical stage, therapy efficacy, tumor metastasis
and patient survival in ovarian cancer. Anticancer Res.
24:1973–1979. 2004.PubMed/NCBI
|
|
17
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metast Rev.
28:15–33. 2009. View Article : Google Scholar
|
|
19
|
Meng Q, Xia C, Fang J, Rojanasakul Y and
Jiang BH: Role of PI3K and AKT specific isoforms in ovarian cancer
cell migration, invasion and proliferation through the p70S6K1
pathway. Cell Signal. 18:2262–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Zhang L, Greshock J, Colligon TA,
Wang Y, Ward R, Katsaros D, Lassus H, Butzow R, Godwin AK, et al:
Frequent genetic abnormalities of the PI3K/AKT pathway in primary
ovarian cancer predict patient outcome. Genes Chromosomes Cancer.
50:606–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
25
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar : PubMed/NCBI
|