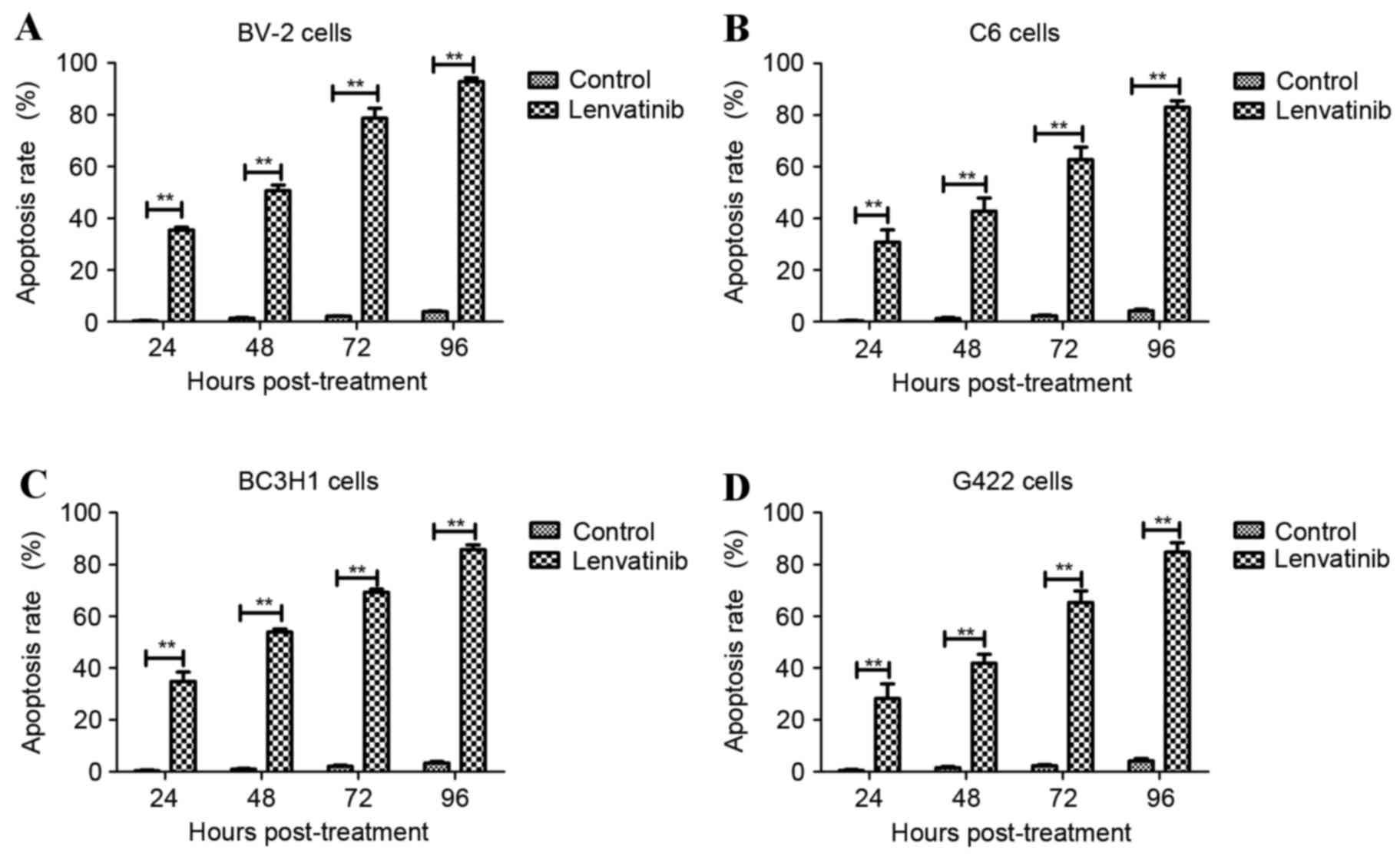
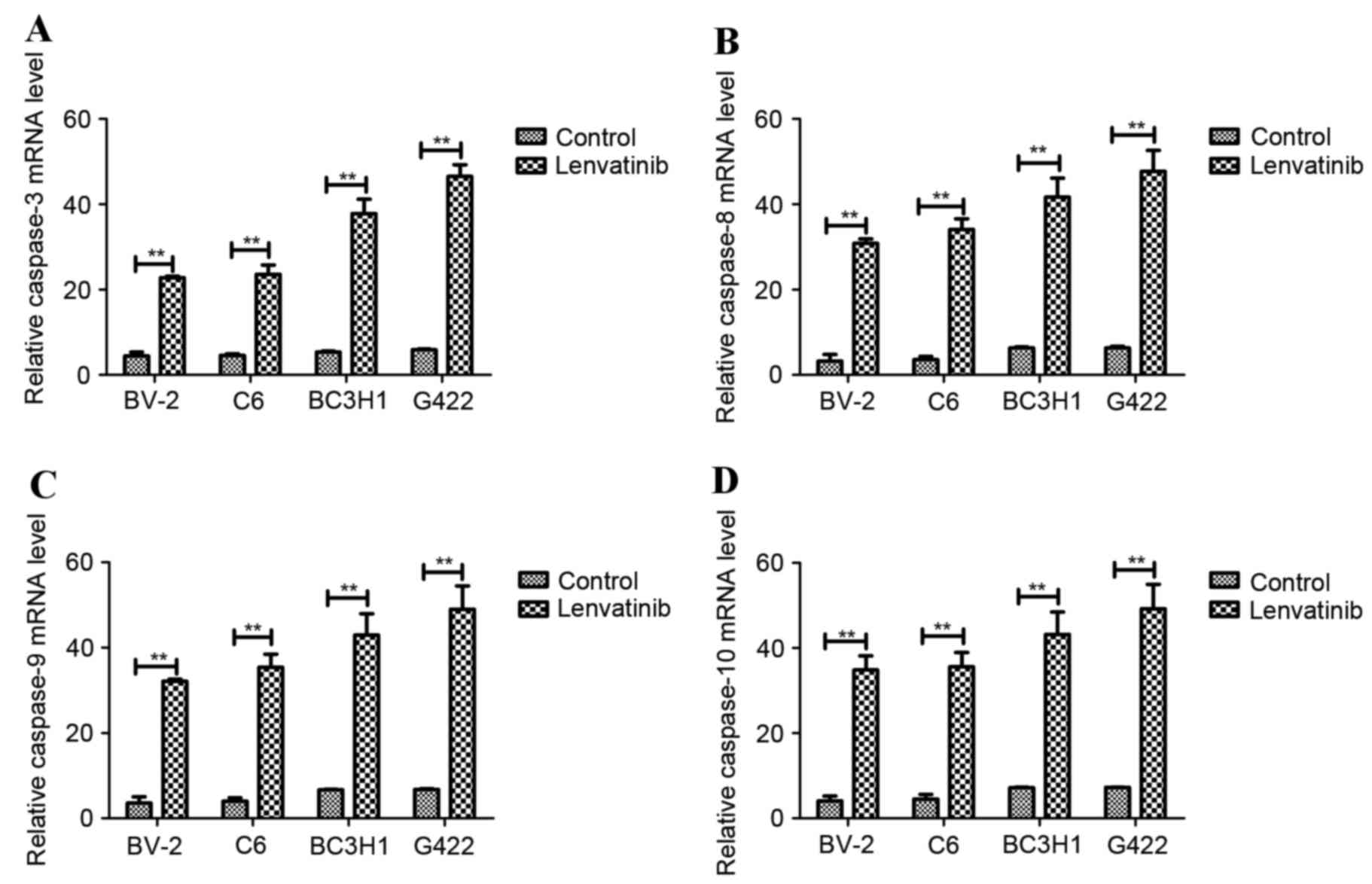
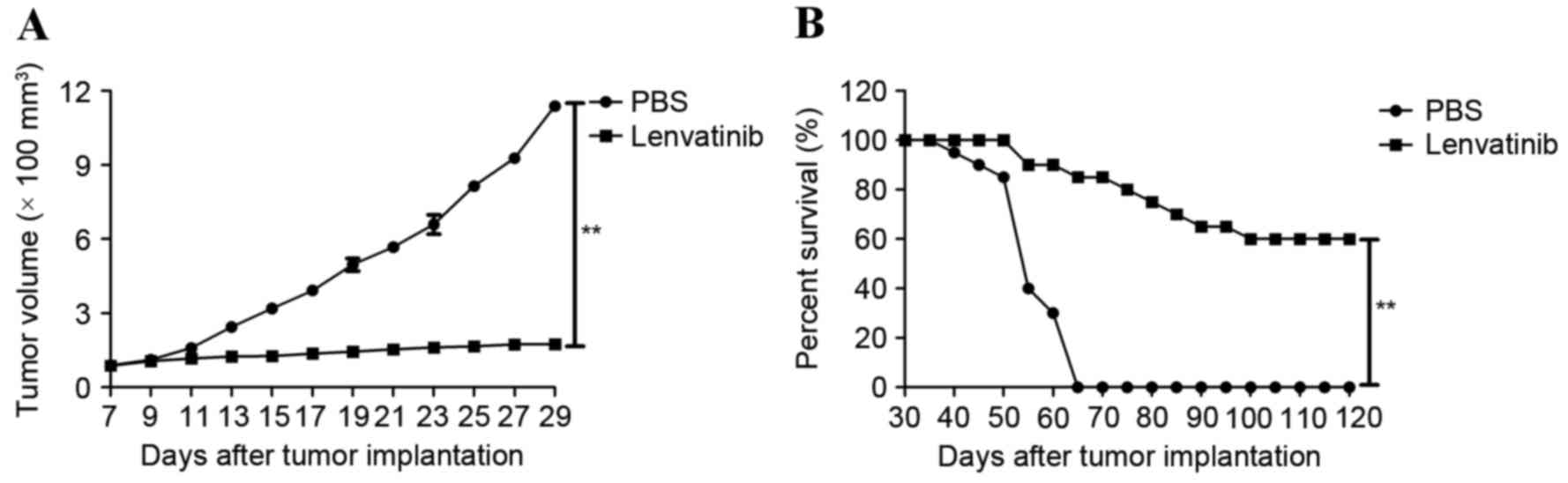
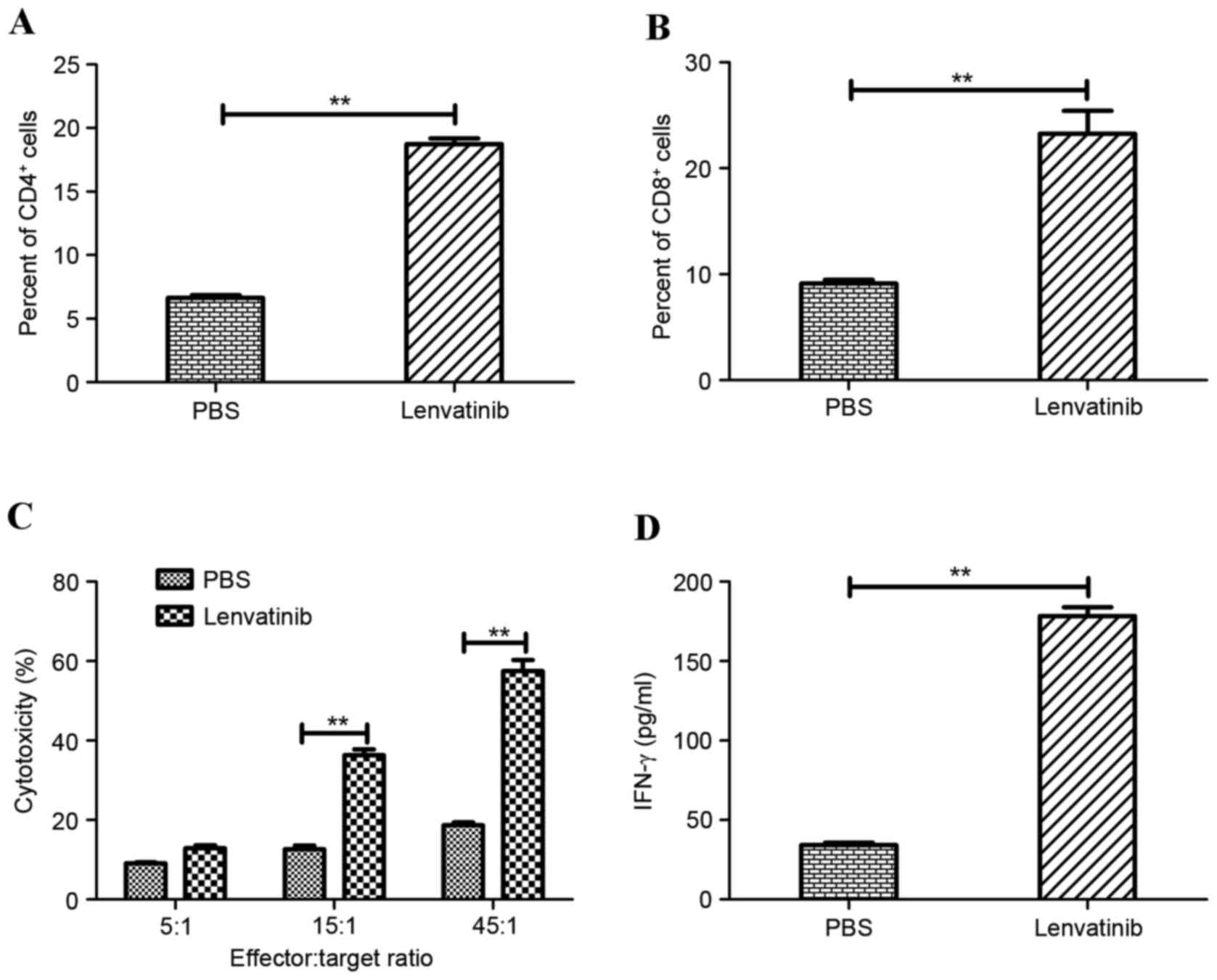
|
1
|
Lu M, Zhang X, Zhang M, Chen H, Dou W, Li
S and Dai J: Non-model segmentation of brain glioma tissues with
the combination of DWI and fMRI signals. Biomed Mater Eng. 26 Suppl
1:S1315–S1324. 2015.PubMed/NCBI
|
|
2
|
Chow KK, Naik S, Kakarla S, Brawley VS,
Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, et al: T cells
redirected to EphA2 for the immunotherapy of glioblastoma. Mol
Ther. 21:629–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang K, Kievit FM, Jeon M, Silber JR,
Ellenbogen RG and Zhang M: Nanoparticle-mediated target delivery of
TRAIL as gene therapy for glioblastoma. Adv Healthc Mater.
4:2719–2726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goudar RK, Shi Q, Hjelmeland MD, Keir ST,
McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA,
et al: Combination therapy of inhibitors of epidermal growth factor
receptor/vascular endothelial growth factor receptor 2 (AEE788) and
the mammalian target of rapamycin (RAD001) offers improved
glioblastoma tumor growth inhibition. Mol Cancer Ther. 4:101–112.
2005.PubMed/NCBI
|
|
5
|
Delfino KR, Serão NV, Southey BR and
Rodriguez-Zas SL: Therapy-, gender- and race-specific microRNA
markers, target genes and networks related to glioblastoma
recurrence and survival. Cancer Genomics Proteomics. 8:173–183.
2011.PubMed/NCBI
|
|
6
|
Koekkoek JA, Postma TJ, Heimans JJ,
Reijneveld JC and Taphoorn MJ: Antiepileptic drug treatment in the
end-of-life phase of glioma patients: a feasibility study. Support
Care Cancer. 24:1633–1638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powell IJ, Banerjee M, Bianco FJ, Wood DP
Jr, Dey J, Lai Z, Heath M and Pontes EJ: The effect of
race/ethnicity on prostate cancer treatment outcome is conditional:
A review of Wayne State University data. J Urol. 171:1508–1512.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cafarotti S, Lococo F, Froesh P, Zappa F
and Andre D: Target therapy in lung cancer. Adv Exp Med Biol.
893:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sareddy GR and Vadlamudi RK: Cancer
therapy using natural ligands that target estrogen receptor beta.
Chin J Nat Med. 13:801–807. 2015.PubMed/NCBI
|
|
10
|
Hamamoto R and Nakamura Y: Dysregulation
of protein methyltransferases in human cancer: An emerging target
class for anticancer therapy. Cancer Sci. 107:377–384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beck JT: Potential role for mammalian
target of rapamycin inhibitors as first-line therapy in hormone
receptor-positive advanced breast cancer. Onco Targets Ther.
8:3629–3638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson CM, Lim M and Drake CG:
Immunotherapy for brain cancer: Recent progress and future promise.
Clin Cancer Res. 20:3651–3659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi SY, Xue H, Wu R, Fazli L, Lin D,
Collins CC, Gleave ME, Gout PW and Wang Y: The MCT4 gene: A novel,
potential target for therapy of advanced prostate cancer. Clin
Cancer Res. 22:2721–2733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapa C, Linsenmann T, Lückerath K, Samnick
S, Herrmann K, Stoffer C, Ernestus RI, Buck AK, Löhr M and Monoranu
CM: Tumor-associated macrophages in glioblastoma multiforme-a
suitable target for somatostatin receptor-based imaging and
therapy? PloS One. 10:e01222692015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vehlow A and Cordes N: Invasion as target
for therapy of glioblastoma multiforme. Biochim Biophys Acta.
1836:236–244. 2013.PubMed/NCBI
|
|
16
|
Cabanillas ME, Schlumberger M, Jarzab B,
Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner
DL, Topliss D, et al: A phase 2 trial of lenvatinib (E7080) in
advanced, progressive, radioiodine-refractory, differentiated
thyroid cancer: A clinical outcomes and biomarker assessment.
Cancer. 121:2749–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nijaguna MB, Patil V, Urbach S, Shwetha
SD, Sravani K, Hegde AS, Chandramouli BA, Arivazhagan A, Marin P,
Santosh V and Somasundaram K: Glioblastoma-derived macrophage
colony-stimulating factor (MCSF) induces microglial release of
insulin-like growth factor-binding protein 1 (IGFBP1) to promote
angiogenesis. J Biol Chem. 290:23401–23415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krishnan S, Szabo E, Burghardt I, Frei K,
Tabatabai G and Weller M: Modulation of cerebral endothelial cell
function by TGF-β in glioblastoma: VEGF-dependent angiogenesis
versus endothelial mesenchymal transition. Oncotarget.
6:22480–22495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuznar W: Lenvatinib extends survival in
metastatic renal-cell carcinoma. Am Health Drug Benefits.
8:182015.PubMed/NCBI
|
|
20
|
Molina AM, Hutson TE, Larkin J, Gold AM,
Wood K, Carter D, Motzer R and Michaelson MD: A phase 1b clinical
trial of the multi-targeted tyrosine kinase inhibitor lenvatinib
(E7080) in combination with everolimus for treatment of metastatic
renal cell carcinoma (RCC). Cancer Chemother Pharmacol. 73:181–189.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegazi M, Azadi A, Jain D, Redman R and
Perez CA: Pharmacological and clinical profile of lenvatinib
(E-7080) in the treatment of advanced, radioiodine-refractory,
differentiated thyroid cancer. Drugs Today (Barc). 51:689–694.
2015.PubMed/NCBI
|
|
22
|
Iacovelli R, Alesini D, Palazzo A, Trenta
P, Santoni M, De Marchis L, Cascinu S, Naso G and Cortesi E:
Targeted therapies and complete responses in first line treatment
of metastatic renal cell carcinoma. A meta-analysis of published
trials. Cancer Treat Rev. 40:271–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brugarolas J: Renal-cell
carcinoma-molecular pathways and therapies. Brugarolas J.
356:185–187. 2007.
|
|
24
|
Greaves MF and Brown G: Purification of
human T and B lymphocytes. J Immunol. 112:420–423. 1974.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huynh-Le MP, Zhang Z, Tran PT, DeWeese TL
and Song DY: Low interrater reliability in grading of rectal
bleeding using national cancer institute common toxicity criteria
and radiation therapy oncology group toxicity scales: A survey of
radiation oncologists. Int J Radiat Oncol Biol Phys. 90:1076–1082.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehravi B, Alizadeh AM, Khodayari S,
Khodayari H, Ashtari K, Mohseni M, Anaraki NI, Dana EA, Safari S
and Amanlou M: Acute toxicity evaluation of glycosylated
Gd3+-based silica nanoprobe. Mol Imaging Biol.
19:522–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuruoka A, Matsui J, Suzuki T, Koyama N,
Watanabe T and Funahashi Y: Preclinical and clinical researches of
lenvatinib mesylate (Lenvima capsule), a novel antitumor agent
approved for thyroid cancer treatment. Nihon Yakurigaku Zasshi.
146:283–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Midorikawa Y, Sugiyama Y and Aburatani H:
Molecular targets for liver cancer therapy: From screening of
target genes to clinical trials. Hepatol Res. 40:49–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Husain SR, Behari N, Kreitman RJ, Pastan I
and Puri RK: Complete regression of established human glioblastoma
tumor xenograft by interleukin-4 toxin therapy. Cancer Res.
58:3649–3653. 1998.PubMed/NCBI
|
|
31
|
Debinski W, Gibo DM, Obiri NI, Kealiher A
and Puri RK: Novel anti-brain tumor cytotoxins specific for cancer
cells. Nat Biotechnol. 16:449–453. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bera TK, Viner J, Brinkmann E and Pastan
I: Pharmacokinetics and antitumor activity of a bivalent
disulfide-stabilized Fv immunotoxin with improved antigen binding
to erbB2. Cancer Res. 59:4018–4022. 1999.PubMed/NCBI
|
|
33
|
Ghetie MA, Richardson J, Tucker T, Jones
D, Uhr JW and Vitetta ES: Antitumor activity of Fab' and
IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown
in mice with severe combined immunodeficiency disease: Effect on
tumor cells in extranodal sites. Cancer Res. 51:5876–5880.
1991.PubMed/NCBI
|
|
34
|
Sivashankari PR and Prabaharan M: Peptides
to target tumor vasculature and lymphatics for improved
anti-angiogenesis therapy. Curr Cancer Drug Targets. 16:522–535.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collet G, Szade K, Nowak W, Klimkiewicz K,
El Hafny-Rahbi B, Szczepanek K, Sugiyama D, Weglarczyk K,
Foucault-Collet A, Guichard A, et al: Endothelial precursor
cell-based therapy to target the pathologic angiogenesis and
compensate tumor hypoxia. Cancer Lett. 370:345–357. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grun D, Adhikary G and Eckert RL: VEGF-A
acts via neuropilin-1 to enhance epidermal cancer stem cell
survival and formation of aggressive and highly vascularized
tumors. Oncogene. 35:4379–4387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mayor S: Lenvatinib improves survival in
refractory thyroid cancer. Lancet Oncol. 16:e1102015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schlumberger M, Jarzab B, Cabanillas ME,
Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R,
Martins RG, et al: A Phase II trial of the multitargeted tyrosine
kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid
cancer. Clin Cancer Res. 22:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K
and Xiao Y: Safety and efficacy profile of lenvatinib in cancer
therapy: A systematic review and meta-analysis. Oncotarget.
7:44545–44557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lorusso L and Newbold K: Lenvatinib: A new
option for the treatment of advanced iodine refractory
differentiated thyroid cancer? Future Oncol. 11:1719–1727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nair A, Lemery SJ, Yang J, Marathe A, Zhao
L, Zhao H, Jiang X, He K, Ladouceur G, Mitra AK, et al: FDA
approval summary: Lenvatinib for progressive,
radio-iodine-refractory differentiated thyroid cancer. Clin Cancer
Res. 21:5205–5208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Whiteside TL: Inhibiting the inhibitors:
Evaluating agents targeting cancer immunosuppression. Expert Opin
Biol Ther. 10:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|