Introduction
Glioblastoma is the most aggressive primary brain
tumor that originates from the glial cells in adults or develops
from existing malignant cells (1,2).
Glioblastoma is characterized by the appearance of vascular
proliferation, aggressive invasion and necrosis around human normal
brain tissues (3). Patients with
advanced glioblastoma commonly present with seizures and/or stroke,
which increases the difficulty and risk of clinical treatment
(4). Statistical analysis in
previously published data revealed that glioblastoma accounts for
~75% of all malignant tumors associated with the brain (5). According to characteristics of
pathologic evaluation and infiltrative growth, different malignant
grades result in diverse glioblastoma shapes (6). Therefore, developing treatments for
glioblastoma has been a focus of research.
The majority of conditional treatment schedules for
human cancers remain ineffective and are often toxic for normal
cells (7). Targeted therapy has
demonstrated beneficial potential as it specifically targets cancer
cells and elicits low toxicity to normal human tissues,
demonstrating an increased capacity to eradicate human cancer
(8–11). Targeted therapies often involve the
application of a specific antibody or receptor for tumor molecules,
proteins, peptides, or nucleic acids, as well as efforts that use
adoptive transfer of effector cells that directly target antigens
on tumor cells (12,13). Clinical research has demonstrated
that these novel treatments are effective in treating glioblastoma
(14,15). The present study evaluated the
preclinical outcomes of lenvatinib targeting of receptor tyrosine
kinases for glioblastoma therapy, which promoted cytotoxic T
lymphocyte (CTL) responses and interferon-γ (IFN-γ) release through
stimulating immunization and adoptive transfer of effector cells
that directly target glioblastoma cells in xenograft mice. In
addition, the present study discussed the advantages and efficacy
of lenvatinib and how over the next decade investigators will
attempt to broaden the reach, increase the efficacy and simplify
the application of lenvatinib.
Lenvatinib is a multi-targeted tyrosine kinase
inhibitor that targets fibroblast growth factor receptors 1–4,
vascular endothelial growth factor (VEGF) receptors 1–3, ret
proto-oncogene, v-kit Hardy-Zuckerman 4 feline sarcoma viral
oncogene homolog and platelet-derived growth factor receptor β
(16). Previous studies have
demonstrated that angiogenesis mediated by these receptors is
involved in the tumorigenesis, development and metastasis of
glioblastoma (17,18). Lenvatinib has demonstrated
significant anticancer potential against the majority of human
cancers in clinical trials, mediated by its inhibition of
angiogenesis (19,20). In addition, several reports have
revealed that inhibition of the VEGF pathway presented beneficial
clinical outcomes in cancer therapy, indicating that targeted
therapy using lenvatinib for the treatment of differentiated
thyroid cancer and renal cell carcinoma has significant efficacy
(16,19). Furthermore, as well as the clinical
benefits presented by lenvatinib treatment alone for human cancer
therapy, previous studies have also demonstrated that lenvatinib
combined with everolimus extended overall survival significantly
compared with everolimus alone in patients with metastatic renal
cell cancer (20,21). However, few studies have reported
the therapeutic effects of lenvatinib regarding the treatment of
glioblastoma.
Glioblastoma therapy has attracted scientists and
scholars to research more effective and comprehensive treatments
(22,23). In the present study, the
therapeutic effects of lenvatinib were investigated in a
glioblastoma mouse model. The results indicated that targeted
therapy of lenvatinib significantly suppressed tumor growth and
prolonged the survival of tumor-bearing mice. These results
supported a clinical application for lenvatinib in patients with
glioblastoma.
Materials and methods
Ethics statement
All animal experiments were performed according to
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Bethesda, MD, USA).
The operation was approved by Chinese Association for Laboratory
Animal Sciences, Animal Health Products and the Committee on the
Ethics of Animal Experiments Defense Research. All surgery and
euthanasia were performed under anesthesia with sodium
pentobarbital (50 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). All efforts were made to minimize the suffering of the
experimental mice.
Cell culture and reagents
BV-2, C6, BC3H1 and G422 glioma cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The BV-2 and C6 cells were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; BioWhittaker; Lonza Group, Basel,
Switzerland), 50 µg/ml gentamicin (BioWhittaker; Lonza Group), 3 mM
L-glutamine (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). BC3H1 and G422 cells were cultured in Eagle's
minimum essential medium (Sigma-Aldrich; Merck KGaA) supplemented
with 10% FBS.
Apoptosis assays following lenvatinib
treatment
BV-2, C6, BC3H1 and G422 (1×103) cells
were incubated with lenvatinib in 96-well plates for 48 h in
triplicate for each condition, and PBS was added instead of
lenvatinib as a control. BV-2, C6, BC3H1 and G422cells were grown
at 37°C with 5% CO2 until 80% confluence was reached.
Following incubation for 48 h, apoptosis was assessed by incubation
of these cells with lenvatinib. The BV-2, C6, BC3H1 and G422 cells
were trypsinized and collected into EP tubes. The cells were then
washed with cold PBS three times and adjusted to a concentration of
1×106 cells/ml with PBS. The cells were subsequently
labeled with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (Annexin V-FITC kit; BD Biosciences, San Jose, CA,
USA) and analyzed with a FACScan flow cytometer using WinMDI
software (version 2.9; BD Biosciences).
Flow cytometry analysis
Tumors from experimental mice were ground into
monoplast suspensions and washed three times with PBS for flow
cytometric analysis. Tumor cell suspensions were filtered through a
100 µm nylon strainer and centrifuged (1,000 × g for 5 min at 4°C)
to remove cell debris. The tumor cells were subsequently incubated
with CD3 and CD45-labeled CD4 and CD8 to analyze the degree of CD4
and CD8 cell subsets in the total infiltrated immune cells. The
stained cells were analyzed using a Becton Dickinson FACScan flow
cytometer using WinMDI software (version 2.9; BD Biosciences).
IFN-γ release and CTL response
assays
Spleens were obtained from the euthanized mice,
which had been treated by lenvatinib or PBS. Splenocytes were
subsequently isolated by passing the spleens through 100 µm nylon
mesh filters. The cells were washed three times with PBS and
incubated with mitomycin-inactivated BV-2 cells (mitomycin-C, cat.
no. ab120797; Abcam, Cambridge, UK), and IFN-γ was measured in the
supernatants on day 3 using a sandwich ELISA kit (cat. no.
ab193969; Abcam). In addition, T cells (1×106) from the
splenocytes were purified, as previously described (24) and co-cultured with fresh BV-2 cells
for 4 h at the effector:target ratios of 5:1, 15:1 and 45:1.
Specific CTL responses to the target cells (BV-2) were analyzed
using MTT cytotoxicity assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was isolated using an mRNeasy Extraction
kit (Qiagen, Inc., Valencia, CA, USA). Extracted mRNA (1 µg) was
transcribed into cDNA using a reverse transcription kit (Qiagen,
Inc.). The cDNA (10 ng) was used for qPCR using the SYBR Green
Master Mix system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
for 30 cycles. All the forward and reverse primers were synthesized
by Invitrogen; Thermo Fisher Scientific, Inc. (Table I). PCR amplification followed
preliminary denaturation at 94°C for 2 min, followed by 35 cycles
of 94°C for 30 sec, annealing at 64°C for 30 sec, and 72°C for 10
min. The reaction was performed at a volume of 20 µl, containing 50
ng genomic DNA, 200 µM dNTP, 2.5 units Taq DNA polymerase and 200
µM primers. Relative mRNA expression changes were calculated by
2−ΔΔCq (25). The
results were expressed as n-fold of the expression of β-actin.
 | Table I.Sequences of primers were used in the
present study. |
Table I.
Sequences of primers were used in the
present study.
| Gene name | Sequence |
|---|
| Caspase-3 | F:
5′-AAAGTTTTCAATGACCAAGC-3′ |
|
| R:
5′-TCTGACGAATCTCCTCCAC-3′ |
| Caspase-8 | F:
5′-AGTCTATTTTATTATGGGCTCG-3′ |
|
| R:
5′-TGGATGTTTATGTCACCTTTTC-3′ |
| Caspase-9 | F:
5′-ATGGAGAACACTGAAAACTC-3′ |
|
| R:
5′-TGTGAGCATGGAAACAATAC-3′ |
| Caspase-10 | F:
5′-CTTATCTATGGGACAGACGGGC-3′ |
|
| R:
5′-GCTGCTCCATTTCTTCACAGGTCCGA-3′ |
| β-actin | F:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
|
| R:
5′-CGGAGTCAACGGATTTGGTC-3′ |
Animal experiments
Male BALC/c nude mice (n=100; age, 6–8 weeks; 30–35
g) were purchased from the West China Experimental Animal Center of
Sichuan University (Chengdu, China). All animals were housed in a
temperature-controlled facility at 23±1°C and relative humidity
50±5%, with a 12-h light/dark cycle. BV-2cells (5×106)
in 20 µl PBS were subcutaneously injected into the right forelimb
of nude mice under aseptic conditions (n=100). The
glioblastoma-bearing mice were divided into two groups, and each
group contained 50 mice. Each mouse in the treated group received
0.24 mg lenvatinib by intravenous injection once daily,
administered continuously in 14 day cycles. The mice in the control
groups received normal saline, serving as an injection control.
Tumor dimensions were measured every 2 days for a total of 14
times. The tumor volumes were calculated according to the following
formula: length × width2 × 0.52. Mice were sacrificed
when tumor diameter reached 12 mm. On day 25 following inoculation,
tumors from the mice were used for RT-qPCR assay.
Evaluation of toxicity
The median overall duration of treatment for
dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) was
14 days for the lenvatinib dosing cohorts: 0.08, 0.16, 0.24, 0.32
and 0.40 mg (8 mice/group). Toxicity was graded using the National
Cancer Institute Common Toxicity Criteria (version 3.0) (26). DLT and MTD were defined as any of
the drug-related toxicities described in a previous study (27).
Statistical analysis
All data are reported as the mean ± standard
deviation. The statistical significance of differences between mean
values was assessed by Student's t-test for unpaired data.
Comparisons of data between multiple groups were performed using
one-way analysis of variance followed by the Student-Newman-Keuls
post hoc test. P<0.05 was considered to indicate a statistically
significant difference. Analysis was performed using SPSS software
version 20.0 (IBM Corp., Armonk, NY, USA).
Results
DLT and MTD
The median overall duration of treatment for DLT and
MTD was 14 days for lenvatinib dosing cohorts: 0.08, 0.16, 0.24,
0.32 and 0.40 mg. Treatment with 0.24 mg and 0.40 mg lenvatinib
once daily was identified as the MTD and DLT, respectively. The
lowest dose lenvatinib had the least toxicity in term of
experimental date. In addition, at least one dose of lenvatinib for
all experimental mice for study therapy with post baseline safety
evaluation was included in the safety population. Furthermore, the
most common treatment-related adverse events were hypertension,
vomiting, lethargy, proteinuria, nausea, constipation and fatigue
following the last dose of lenvatinib (Table II).
 | Table II.Treatment-related adverse events of
lenvatinib with an overall incidence ≥10%. |
Table II.
Treatment-related adverse events of
lenvatinib with an overall incidence ≥10%.
| Adverse event | Total (n=50) | 0.08–0.16 mg
(n=20) | 0.24 mg (n=10) | 0.32–0.40 mg
(n=20) |
|---|
| Hypertension | 7 | 2 | 2 | 3 |
| Nausea | 6 | 1 | 2 | 3 |
| Proteinuria | 8 | 2 | 2 | 4 |
| Vomiting | 5 | 1 | 2 | 2 |
| Lethargy | 6 | 1 | 2 | 3 |
| Fatigue | 5 | 1 | 2 | 2 |
| Constipation | 5 | 1 | 2 | 2 |
Lenvatinib effectively induces
apoptosis glioblastoma in vitro
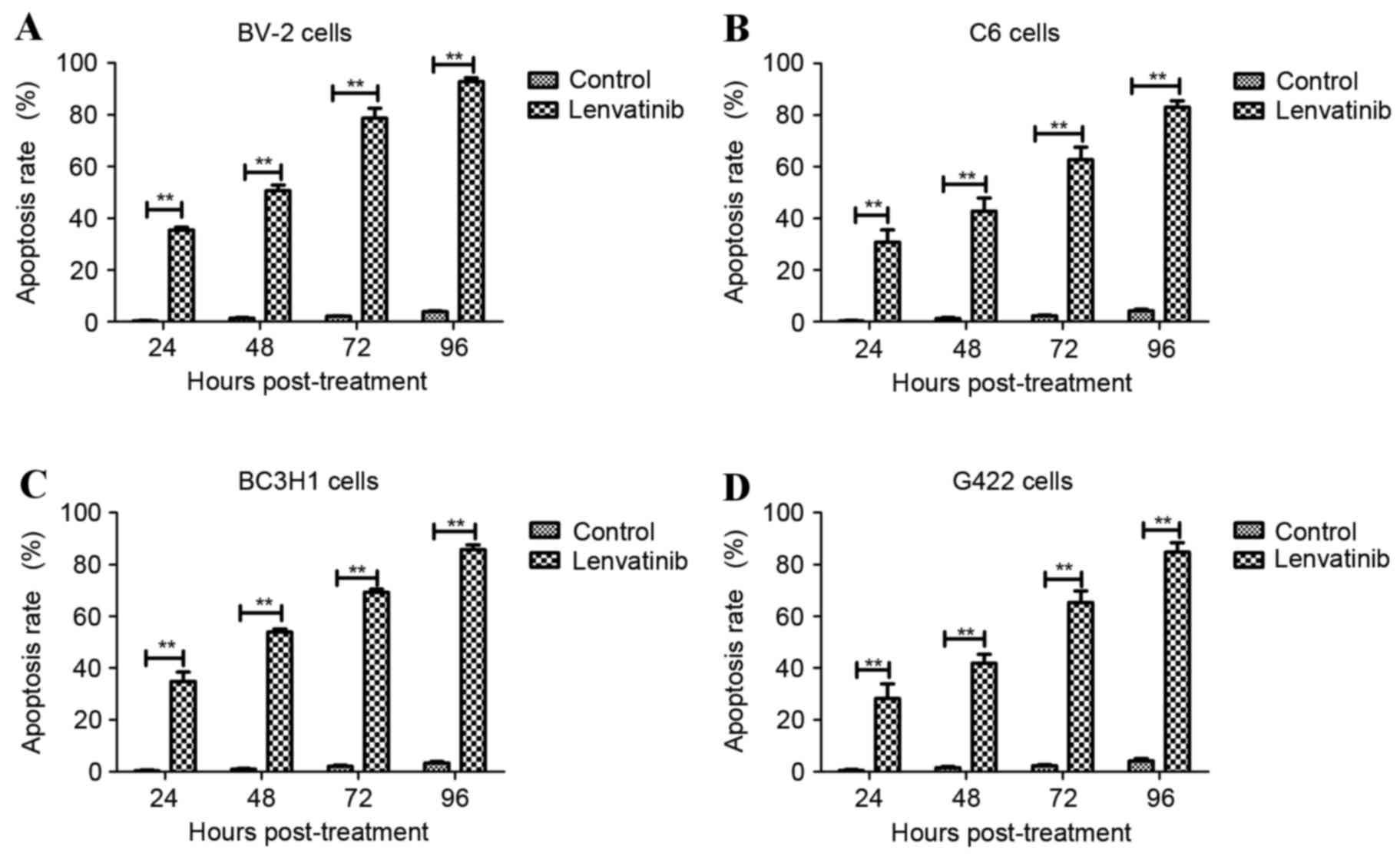
To confirm the efficacy of lenvatinib on glioma
cells, BV-2, C6, BC3H1 and G422 cells were used to detect the
apoptosis rate in vitro induced by lenvatinib (0.24 mg/ml).
The apoptosis rate of BV-2 (Fig.
1A), C6 (Fig. 1B), BC3H1
(Fig. 1C) and G422 cells (Fig. 1D) was significantly upregulated
following treatment with lenvatinib compared with the control
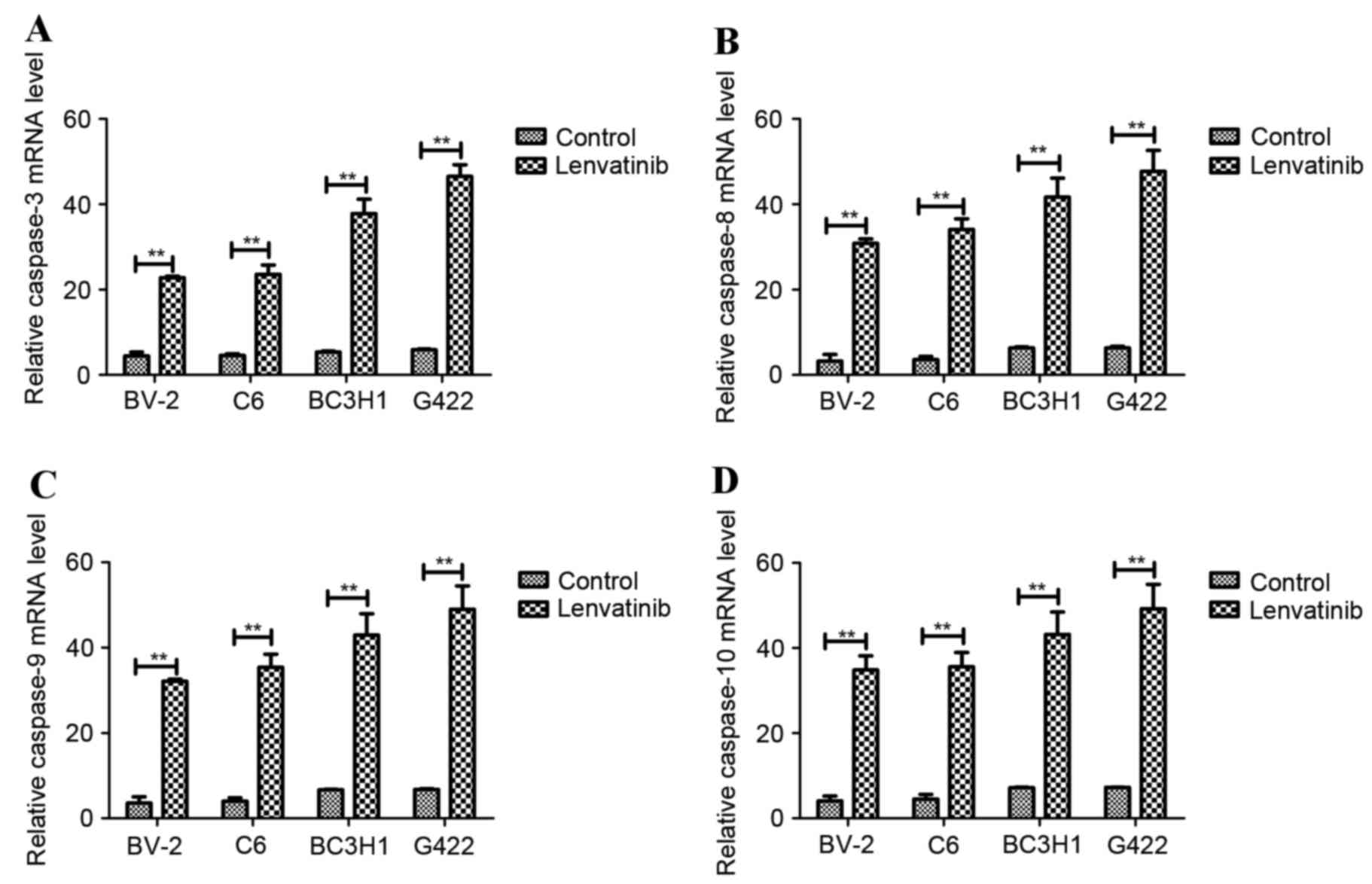
group. In addition, apoptosis-associated gene expression was
analyzed in lenvatinib-treated BV-2, C6, BC3H1 and G422 cell lines
in vitro. The mRNA expression levels of pro-apoptotic genes
were analyzed using RT-qPCR, including caspase-3, −8, −9 and −10.
Expression levels of caspase-3 (Fig.
2A), caspase-8 (Fig. 2B),
caspase-9 (Fig. 2C) and caspase-10
(Fig. 2D) were significantly
increased following 48 h treatment with lenvatinib compared with
the control group. These results suggested that lenvatinib induced
apoptosis and upregulated pro-apoptotic genes inBV-2, C6, BC3H1 and
G422 glioma cell lines.
Lenvatinib significantly inhibited
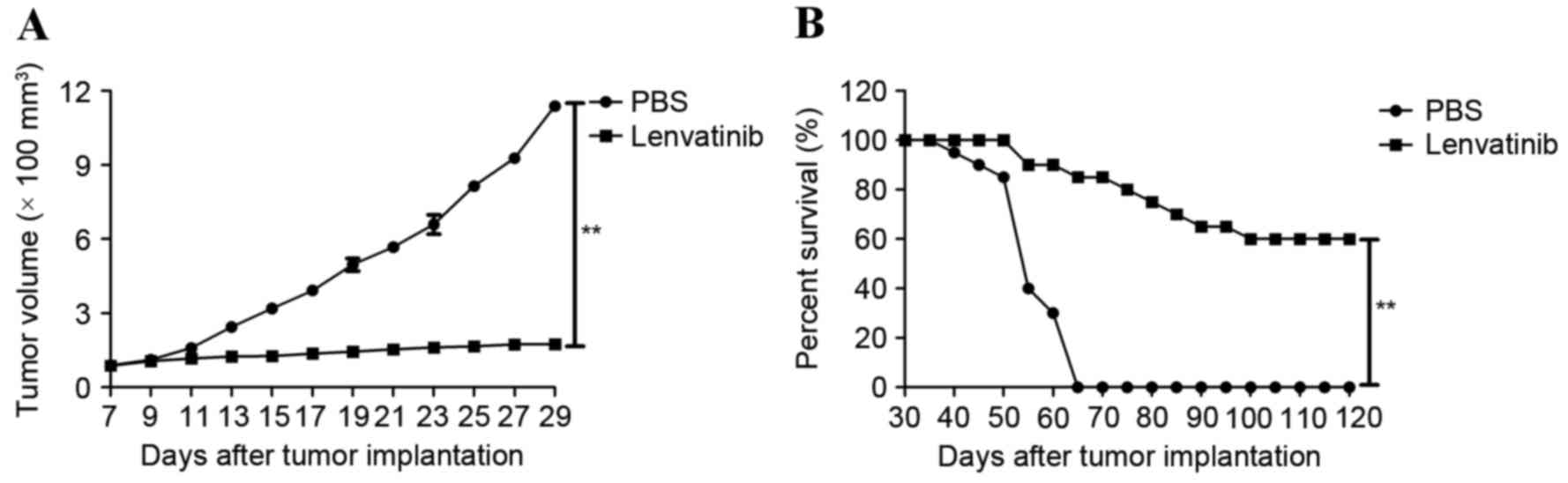
tumor growth in a glioblastoma mouse model
Following the in vitro apoptosis assays, the
present study investigated whether lenvatinib-treated mice
demonstrated tumor growth inhibition. BV-2 cells were
subcutaneously injected into BALB/c nude mice. Lenvatinib treatment
via intravenous injection was initiated when the tumor diameter
reached 5–6 mm on day 7 following the first tumor inoculation. The
treatment was continued for 14 day cycles at frequency of once
daily at the MDT dose (0.24 mg/day). The tumor diameter was
recorded and tumor volume was calculated. Tumor growth was
significantly inhibited in lenvatinib-treated mice (Fig. 3A). In addition, lenvatinib
prolonged the survival of tumor-bearing mice across 120 days of
observation compared with control group (Fig. 3B). These results suggested that the
therapeutic effects of lenvatinib on the BV-2-bearing mice were
strong enough to suppress the glioma tumor growth, which translated
into long-term survival.
Treatment with lenvatinib resulted in
immunological cytotoxicity and a CTL response in glioblastoma
tumors
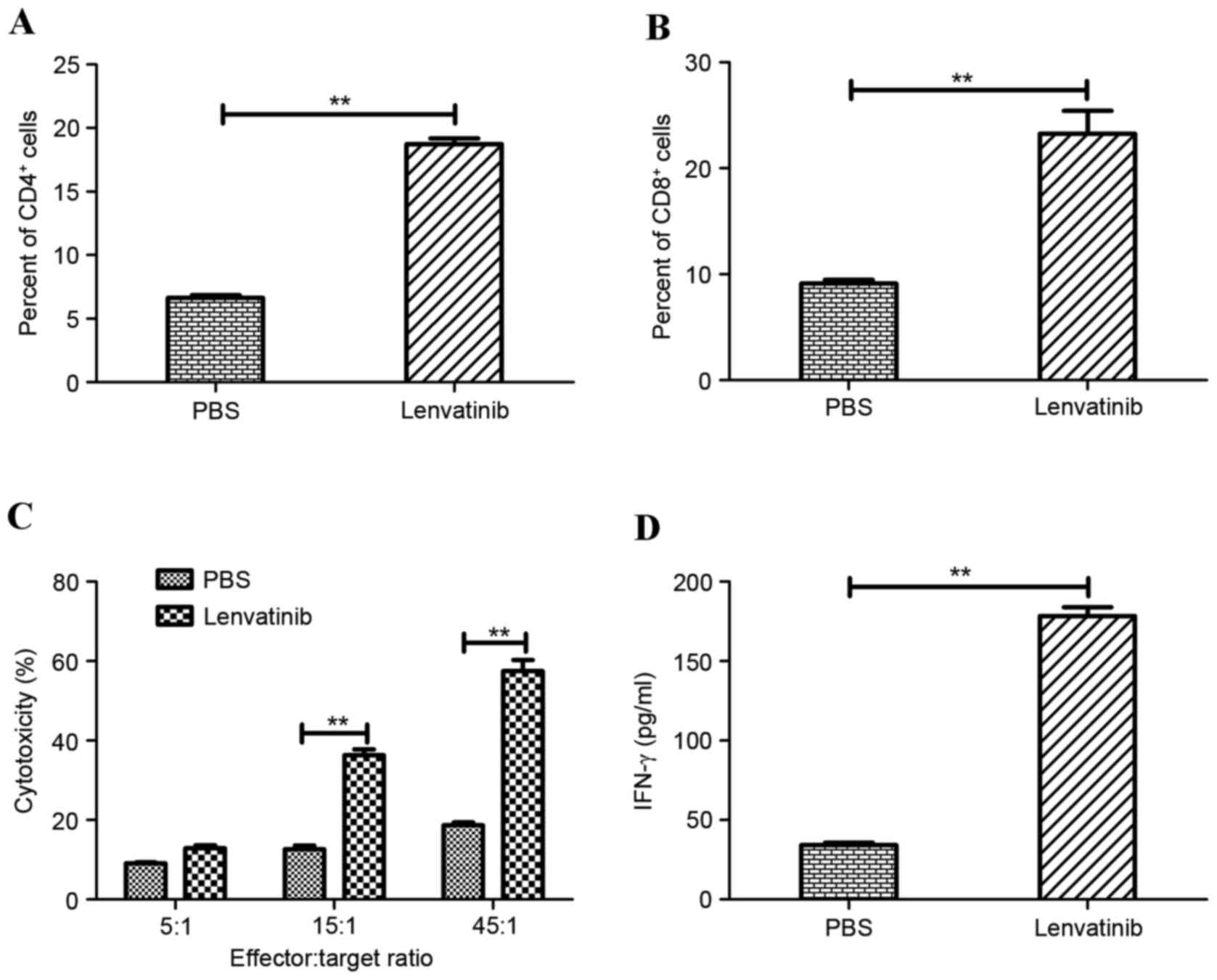
Tumor-bearing mice from the lenvatinib and
PBS-treated groups (n=8 mice/group) were sacrificed on day 30 to
further analysis of beneficial outcomes. Tumors were collected on
day 30 in each group. The tumors were subsequently ground, filtered
and stained for CD4+ and CD8+ expression on
tumors. Tumors from the mice treated with lenvatinib exhibited a
significantly increased degree of CD4+ (Fig. 4A) and CD8+ (Fig. 4B) cell infiltration in the
glioblastoma tumor model, as determined by Student's paired
t-tests. In addition, CTL responses against the BV-2 glioblastoma
cells were assessed on day 30. BV-2-specific CTL activity was
assessed following the purification of T cells co-cultured with
tumor cells. Treatment with lenvatinib resulted in a significant
promotion of CTL activity compared with the PBS-treated group at
the effector:target ratios of 15:1 and 45:1 (Fig. 4C). Furthermore, IFN-γ release assay
was analyzed to identify and explain the long-term survival in
lenvatinib-treated mice. Lenvatinib treatment resulted in
significantly increased IFN-γ release compared with PBS-treated
mice (Fig. 4D). These results
suggested that treatment of tumor-bearing mice with lenvatinib
resulted in the generation of tumor-specific CTL responses and
partial protection of the animals against the tumor cells, which
may contribute to the long-lasting antitumor effects observed in
the BV-2-bearing mice.
Discussion
Targeted therapy has demonstrated marked antitumor
activities in the clinical treatment of several types of human
cancer (16,28,29).
Antineoplastic agents with targeted therapy agents can effectively
target tumor cell-specific recognition domains, either antigens or
receptors (30–33). Different targeted therapy drugs for
cancer treatment are being studied clinically. The targeted therapy
agents frequently use tumor cell-specific recognition to inhibit
tumor angiogenesis, mediated by suppression of tumor-derived
vascular endothelial cells, and have demonstrated encouraging
results in the treatment of certain advanced tumors (34,35).
However, more targeted therapy agents require development to cater
to the needs of an ever-increasing number of cancer patients.
Therapeutic protocols that target VEGF-mediated
pathways have been clinically applied to treat human cancers
(36). However, single therapies
that target VEGF remained challenges in clinical outcomes, and
improvements are required to overcome ineffective drug treatments
(37). Lenvatinib is a
multi-targeted tyrosine kinase inhibitor with anticancer potential,
which improved upon and overcame deficiencies of single anticancer
agents, as well as demonstrated improved therapeutic benefits for
patients with cancer (38,39). However, few studies among these
previous reports studied the efficacy of lenvatinib as a therapy
for glioblastoma. In the present study, an optimal treatment scheme
of lenvatinib for glioblastoma in a murine model has been defined,
and preclinical efficacy has demonstrated low toxicity and positive
outcomes. The data support the use of the effective, multi-targeted
lenvatinib agent for glioblastoma therapy, as it is associated with
improved efficacy of treatment and manageable, lower toxicity
compared with other anticancer agents (40).
Lenvatinib has previously demonstrated favorable
antitumor potential for human cancer therapy (37). Lenvatinib is also well tolerated
without serious treatment-associated adverse events. Despite
targeted therapy providing the advantage of tumor specificity, it
is conceivable that effectively invoked toxicity of immune cells
for tumor cells may contribute to cancer therapy (40). The clinical application of
lenvatinib would be more effective by intravenous injection to
inhibit tumor cell growth and produce tumor-specific killer cells
with improved immunogenicity to stimulate adaptive T cell mediated
anti-tumor immunity (41).
Although previous studies have demonstrated the MTD
of lenvatinib in phase II trial in advanced medullary thyroid
cancer (36). In the present
study, targeted therapy was introduced to treat glioblastoma in
BV-2-bearing BALB/c nude mice. In addition, lenvatinib demonstrated
manageable toxicity at the MTD, and lower-dose cohort and MTD dose
presented an effective treatment for glioblastoma. Furthermore, the
treatment-associated adverse events of lenvatinib treatment were
representative and consisted with previous clinical research
(41).
The responses observed with the present lenvatinib
therapy conferred an advantage relative to other single-target
therapy (42). In the present
primary analysis of glioblastoma therapy, treatment with 0.24 mg
lenvatinib once daily resulted in an increased CTL response,
increased IFN-γ release and improved long-term survival in
glioblastoma-bearing mice.
In conclusion, a novel therapeutic schedule for the
treatment of glioblastoma using the multi-therapy anticancer agent
lenvatinib was introduced as an MTD dose of 0.24 mg at a frequency
of once daily. The treatment-associated adverse events were
consistent with those of multi-targeted tyrosine kinase inhibitors
and were managed effectively by administering continuously in 14
day cycles. However, the beneficial effects of targeted lenvatinib
therapy require further elucidation to improve the clinical value
of this regimen.
References
|
1
|
Lu M, Zhang X, Zhang M, Chen H, Dou W, Li
S and Dai J: Non-model segmentation of brain glioma tissues with
the combination of DWI and fMRI signals. Biomed Mater Eng. 26 Suppl
1:S1315–S1324. 2015.PubMed/NCBI
|
|
2
|
Chow KK, Naik S, Kakarla S, Brawley VS,
Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, et al: T cells
redirected to EphA2 for the immunotherapy of glioblastoma. Mol
Ther. 21:629–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang K, Kievit FM, Jeon M, Silber JR,
Ellenbogen RG and Zhang M: Nanoparticle-mediated target delivery of
TRAIL as gene therapy for glioblastoma. Adv Healthc Mater.
4:2719–2726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goudar RK, Shi Q, Hjelmeland MD, Keir ST,
McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA,
et al: Combination therapy of inhibitors of epidermal growth factor
receptor/vascular endothelial growth factor receptor 2 (AEE788) and
the mammalian target of rapamycin (RAD001) offers improved
glioblastoma tumor growth inhibition. Mol Cancer Ther. 4:101–112.
2005.PubMed/NCBI
|
|
5
|
Delfino KR, Serão NV, Southey BR and
Rodriguez-Zas SL: Therapy-, gender- and race-specific microRNA
markers, target genes and networks related to glioblastoma
recurrence and survival. Cancer Genomics Proteomics. 8:173–183.
2011.PubMed/NCBI
|
|
6
|
Koekkoek JA, Postma TJ, Heimans JJ,
Reijneveld JC and Taphoorn MJ: Antiepileptic drug treatment in the
end-of-life phase of glioma patients: a feasibility study. Support
Care Cancer. 24:1633–1638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powell IJ, Banerjee M, Bianco FJ, Wood DP
Jr, Dey J, Lai Z, Heath M and Pontes EJ: The effect of
race/ethnicity on prostate cancer treatment outcome is conditional:
A review of Wayne State University data. J Urol. 171:1508–1512.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cafarotti S, Lococo F, Froesh P, Zappa F
and Andre D: Target therapy in lung cancer. Adv Exp Med Biol.
893:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sareddy GR and Vadlamudi RK: Cancer
therapy using natural ligands that target estrogen receptor beta.
Chin J Nat Med. 13:801–807. 2015.PubMed/NCBI
|
|
10
|
Hamamoto R and Nakamura Y: Dysregulation
of protein methyltransferases in human cancer: An emerging target
class for anticancer therapy. Cancer Sci. 107:377–384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beck JT: Potential role for mammalian
target of rapamycin inhibitors as first-line therapy in hormone
receptor-positive advanced breast cancer. Onco Targets Ther.
8:3629–3638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson CM, Lim M and Drake CG:
Immunotherapy for brain cancer: Recent progress and future promise.
Clin Cancer Res. 20:3651–3659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi SY, Xue H, Wu R, Fazli L, Lin D,
Collins CC, Gleave ME, Gout PW and Wang Y: The MCT4 gene: A novel,
potential target for therapy of advanced prostate cancer. Clin
Cancer Res. 22:2721–2733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapa C, Linsenmann T, Lückerath K, Samnick
S, Herrmann K, Stoffer C, Ernestus RI, Buck AK, Löhr M and Monoranu
CM: Tumor-associated macrophages in glioblastoma multiforme-a
suitable target for somatostatin receptor-based imaging and
therapy? PloS One. 10:e01222692015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vehlow A and Cordes N: Invasion as target
for therapy of glioblastoma multiforme. Biochim Biophys Acta.
1836:236–244. 2013.PubMed/NCBI
|
|
16
|
Cabanillas ME, Schlumberger M, Jarzab B,
Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner
DL, Topliss D, et al: A phase 2 trial of lenvatinib (E7080) in
advanced, progressive, radioiodine-refractory, differentiated
thyroid cancer: A clinical outcomes and biomarker assessment.
Cancer. 121:2749–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nijaguna MB, Patil V, Urbach S, Shwetha
SD, Sravani K, Hegde AS, Chandramouli BA, Arivazhagan A, Marin P,
Santosh V and Somasundaram K: Glioblastoma-derived macrophage
colony-stimulating factor (MCSF) induces microglial release of
insulin-like growth factor-binding protein 1 (IGFBP1) to promote
angiogenesis. J Biol Chem. 290:23401–23415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krishnan S, Szabo E, Burghardt I, Frei K,
Tabatabai G and Weller M: Modulation of cerebral endothelial cell
function by TGF-β in glioblastoma: VEGF-dependent angiogenesis
versus endothelial mesenchymal transition. Oncotarget.
6:22480–22495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuznar W: Lenvatinib extends survival in
metastatic renal-cell carcinoma. Am Health Drug Benefits.
8:182015.PubMed/NCBI
|
|
20
|
Molina AM, Hutson TE, Larkin J, Gold AM,
Wood K, Carter D, Motzer R and Michaelson MD: A phase 1b clinical
trial of the multi-targeted tyrosine kinase inhibitor lenvatinib
(E7080) in combination with everolimus for treatment of metastatic
renal cell carcinoma (RCC). Cancer Chemother Pharmacol. 73:181–189.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegazi M, Azadi A, Jain D, Redman R and
Perez CA: Pharmacological and clinical profile of lenvatinib
(E-7080) in the treatment of advanced, radioiodine-refractory,
differentiated thyroid cancer. Drugs Today (Barc). 51:689–694.
2015.PubMed/NCBI
|
|
22
|
Iacovelli R, Alesini D, Palazzo A, Trenta
P, Santoni M, De Marchis L, Cascinu S, Naso G and Cortesi E:
Targeted therapies and complete responses in first line treatment
of metastatic renal cell carcinoma. A meta-analysis of published
trials. Cancer Treat Rev. 40:271–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brugarolas J: Renal-cell
carcinoma-molecular pathways and therapies. Brugarolas J.
356:185–187. 2007.
|
|
24
|
Greaves MF and Brown G: Purification of
human T and B lymphocytes. J Immunol. 112:420–423. 1974.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huynh-Le MP, Zhang Z, Tran PT, DeWeese TL
and Song DY: Low interrater reliability in grading of rectal
bleeding using national cancer institute common toxicity criteria
and radiation therapy oncology group toxicity scales: A survey of
radiation oncologists. Int J Radiat Oncol Biol Phys. 90:1076–1082.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehravi B, Alizadeh AM, Khodayari S,
Khodayari H, Ashtari K, Mohseni M, Anaraki NI, Dana EA, Safari S
and Amanlou M: Acute toxicity evaluation of glycosylated
Gd3+-based silica nanoprobe. Mol Imaging Biol.
19:522–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuruoka A, Matsui J, Suzuki T, Koyama N,
Watanabe T and Funahashi Y: Preclinical and clinical researches of
lenvatinib mesylate (Lenvima capsule), a novel antitumor agent
approved for thyroid cancer treatment. Nihon Yakurigaku Zasshi.
146:283–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Midorikawa Y, Sugiyama Y and Aburatani H:
Molecular targets for liver cancer therapy: From screening of
target genes to clinical trials. Hepatol Res. 40:49–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Husain SR, Behari N, Kreitman RJ, Pastan I
and Puri RK: Complete regression of established human glioblastoma
tumor xenograft by interleukin-4 toxin therapy. Cancer Res.
58:3649–3653. 1998.PubMed/NCBI
|
|
31
|
Debinski W, Gibo DM, Obiri NI, Kealiher A
and Puri RK: Novel anti-brain tumor cytotoxins specific for cancer
cells. Nat Biotechnol. 16:449–453. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bera TK, Viner J, Brinkmann E and Pastan
I: Pharmacokinetics and antitumor activity of a bivalent
disulfide-stabilized Fv immunotoxin with improved antigen binding
to erbB2. Cancer Res. 59:4018–4022. 1999.PubMed/NCBI
|
|
33
|
Ghetie MA, Richardson J, Tucker T, Jones
D, Uhr JW and Vitetta ES: Antitumor activity of Fab' and
IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown
in mice with severe combined immunodeficiency disease: Effect on
tumor cells in extranodal sites. Cancer Res. 51:5876–5880.
1991.PubMed/NCBI
|
|
34
|
Sivashankari PR and Prabaharan M: Peptides
to target tumor vasculature and lymphatics for improved
anti-angiogenesis therapy. Curr Cancer Drug Targets. 16:522–535.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collet G, Szade K, Nowak W, Klimkiewicz K,
El Hafny-Rahbi B, Szczepanek K, Sugiyama D, Weglarczyk K,
Foucault-Collet A, Guichard A, et al: Endothelial precursor
cell-based therapy to target the pathologic angiogenesis and
compensate tumor hypoxia. Cancer Lett. 370:345–357. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grun D, Adhikary G and Eckert RL: VEGF-A
acts via neuropilin-1 to enhance epidermal cancer stem cell
survival and formation of aggressive and highly vascularized
tumors. Oncogene. 35:4379–4387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mayor S: Lenvatinib improves survival in
refractory thyroid cancer. Lancet Oncol. 16:e1102015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schlumberger M, Jarzab B, Cabanillas ME,
Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R,
Martins RG, et al: A Phase II trial of the multitargeted tyrosine
kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid
cancer. Clin Cancer Res. 22:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K
and Xiao Y: Safety and efficacy profile of lenvatinib in cancer
therapy: A systematic review and meta-analysis. Oncotarget.
7:44545–44557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lorusso L and Newbold K: Lenvatinib: A new
option for the treatment of advanced iodine refractory
differentiated thyroid cancer? Future Oncol. 11:1719–1727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nair A, Lemery SJ, Yang J, Marathe A, Zhao
L, Zhao H, Jiang X, He K, Ladouceur G, Mitra AK, et al: FDA
approval summary: Lenvatinib for progressive,
radio-iodine-refractory differentiated thyroid cancer. Clin Cancer
Res. 21:5205–5208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Whiteside TL: Inhibiting the inhibitors:
Evaluating agents targeting cancer immunosuppression. Expert Opin
Biol Ther. 10:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|