Introduction
Neural stem cells (NSCs) are self-renewing,
multipotent and undifferentiated precursors that retain the ability
to differentiate into glial and neuronal lineages, which offer
potential for use in cell-based therapy strategies for neurological
disorders, including Alzheimer's disease, Parkinson's disease,
Huntington's disease and nerve damage (1–3).
Given the importance of NSCs in the development of the nervous
system, it has been proposed that the use of NSCs for the treatment
of neurodevelopmental disorders may be a promising approach to
rescue impaired neuronal plasticity (4,5).
Additionally, neurogenesis is tightly regulated at multiple levels
by extrinsic and intrinsic factors and uncovering the molecular
mechanisms that underlie neurogenesis is crucial to understand the
functions and plasticity of brain development, and to prevent such
pathologies. Recent studies achieved substantial progress, which
has been made in elucidating the regulatory mechanisms underlying
NSC proliferation, differentiation and functional integration in
neural circuits (6,7).
It has been previously reported that microRNAs
(miRNAs), a subset of small, noncoding RNAs, can directly bind to
the 3′ untranslated region (3′UTR) of mRNAs to regulate gene
expression post-transcriptionally resulting in translational
repression or mRNA degradation (8). miRNAs are involved in a considerable
variety of biological process, including apoptosis, proliferation,
differentiation and survival. Increasing evidence suggests that
miRNAs have important roles in neuronal differentiation,
maturation, and synaptic function, including neural stem cell
proliferation and differentiation (9). As demonstrated in previous studies,
aberrant expression of miRNAs is closely associated with brain
disease, and ectopic expression of specific miRNAs may modulate
NSCs biological function (10).
However, the precise regulatory mechanisms of miRNAs in NSCs remain
largely unexplored.
The aim of the present study was to determine the
role of miRNA (miR)-138-5p in NSCs proliferation and
differentiation. The expression of miR-138-5p was examined during
neuronal differentiation of NSCs in vitro and evaluated the
function and the induction mechanism.
Materials and methods
Cell culture
By using the Percoll gradient method described
previously, NSCs were stemmed from adult (8–10 week old) male
C57BL/6 mouse forebrain, supplied by the Laboratory Animal Center
of Tongji University (Shanghai, China) (11). The cells were cultured in DMEM/F12
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 1 mM L-glutamine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 1% N2 supplement (Gibco; Thermo
Fisher Scientific, Inc.), 20 ng/ml epidermal growth factor (EGF;
PeproTech, Inc., Rocky Hill, NJ, USA), 20 ng/ml basic fibroblast
growth factor (bFGF; PeproTech, Inc.), 50 ng/ml heparin
(Sigma-Aldrich; Merck KGaA) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) in humidified air at
37°C and 5% CO2. For neural differentiation, NSCs were
cultured in an environment with 1 mM retinoic acid (Sigma-Aldrich;
Merck KGaA) and 0.5% foetal bovine serum (FBS) for 3 days in 0.5%
N2 with Euromed-N medium (Invitrogen; Thermo Fisher
Scientific, Inc.) to induce neural differentiation. This study was
approved by the Ethics Committee of East Hospital, Tongji
University School of Medicine (no. EHTJ2016022; Shanghai,
China).
Cell transfection
The miR-138-5p
(5′-AGCUGGUGUUGUGAAUCAGGCCG-3′)/anti-miR-138-5p mimics
(5′-CGGCCTGATTCACAACACCAGCT-3′) and its control mimics
(5′-ATTTAGCCGGTACATCAGGCC-3′) were purchased from Shanghai
GenePharma Co., Ltd (Shanghai, China). NSCs were transfected with
the miRNA mimic (50 nM) using Dharmafect 1 (GE Dharmacon; GE
Healthcare Life Sciences, Lafayette, CO, USA) according to the
manufacturer's instructions following seeding of
1×105/well in 6-well plates and cultured to 70%
confluence. Cells were collected for further analyses 48 h after
transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNAs including mRNAs and small RNAs from NSCs
cells were extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Complementary DNA (cDNA) was generated
using TaqMan microRNA RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) for miRNAs and SuperScript VILO cDNA synthesis
kit (Thermo Fisher Scientific, Inc.) for mRNAs. qPCR was performed
using SYBR Green PCR kit (Qiagen China Co., Ltd., Shanghai, China)
on a 7900 Real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR was performed with the following
thermocycling conditions: 25 cycles of 10 sec at 98°C, 10 sec at
55°C and 20 sec at 72°C. The primers used were as follows: Nestin,
forward 5′-GATCTAAACAGGAAGGAAATCCAGG-3′ and reverse
5′-TCTAGTGTCTCATGGCTCTGGTTTT-3′; GFAP, forward
5′-CAACGTTAAGCTAGCCCTGGACAT-3′ and reverse
5′-CTCACCATCCCGCATCTCCACAGT-3′; neuronal class III β-tubulin
(Tuj1), forward 5′-CGCCATGTTCAGACGCAAG-3′ and reverse
5′-CTCGGACACCAGGTCGTTCA-3′; GAPDH, forward
5′-ATTCCATGGCACCGTCAAGGCTGA-3′ and reverse
5′-TTCTCCATGGTGGTGAAGACGCCA-3′; miR-138-5p, forward
5′-AGCTGGTGTTGTGAATCAGGCCG-3′ and reverse 5′-TGGTGTCGTGGAGTCG-3′;
U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAAYYYGCGT-3′. The usage of the 2−ΔΔCq
method (12) calculated the
relative quantification. By using GAPDH/U6 as a control for mRNA
and miRNA respectively, a series of data has been standardized.
Immunofluorescence
NSCs were fixed in 4% paraformaldehyde for 24 h at
room temperature and then permeabilized by using 0.2% Triton-X for
1 h at room temperature. Following blocking with 10% goat serum
(Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, cells were treated with rabbit anti-Ki67 (cat. no.
612254; 1:250; BD Biosciences, Franklin Lakes, NJ, USA) and
anti-thyroid hormone receptor interactor 6 (TRIP6; cat. no. 612254;
1:250; BD Bioscience, Franklin Lakes, NJ, USA) antibodies at 4°C
overnight, followed by incubation with DyLight-549 goat anti-rabbit
antibody (cat. no. 012-500-003; 1:500; Jackson ImmunoResearch, West
Grove, PA) according to the manufacturer's instructions. Sections
were washed with PBS and incubated with 0.5 µg/ml
4,6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc.,
Burlingame, CA, USA) solution for 30 min in the dark at room
temperature; DAPI was used for nuclear staining in order to
maintain an analogous background. Cells were imaged using an
inverted fluorescence microscope (Leica DMI3000; Leica
Microsystems, Inc.). The labelled nuclei and fluorescence intensity
were analysed in an average of 5 high-powered fields
(magnification, ×200) using ImageJ software (version 1.48; National
Institutes of Health, Bethesda, MD, USA).
Western blot assays
Cells were lysed on ice using 1X SDS lysis buffer
[100 mM 2-ME, 50 mM Tris-HCl (pH 6.8), 2% w/v SDS, 10% glycerol].
Protein concentration was determined using the bicinchoninic acid
method. Protein (30 µg) was separated by 10–12% SDS-PAGE and
transferred to nitrocellulose membrane (GE Healthcare Life
Sciences). Membranes were probed with anti-GAPDH antibody (cat. no.
#sc-25778; 1:2,000; Santa Cruz Biotechnology, Inc.) and anti-TRIP6
antibody (cat. no. 612254; 1:500; BD Bioscience) overnight at 4°C
and then incubated with the horseradish peroxidase-conjugated
secondary antibodies (cat. no. 1662408; 1:3,000; Bio-Rad
Laboratories Inc., Hercules, CA, USA) at 37°C for 1 h. The bands
were visualized with the ChemiDoc XRS system (Bio-Rad Laboratories,
Inc.).
miRNA target analysis
Genes containing the miR-binding sites in the UTR
were identified using TargetScan Release 7.1 (http://www.targetscan.org/vert_71/).
Luciferase reporter assays
The full-length 3′-untranslated region of TRIP6 was
synthesized by PCR from human cDNA [obtained from the U87 cell
line; American Type Culture Collection (ATCC), Manassas, VA, USA].
The primers were as follows: Forward, 5′-GTCTTCCTAGAAGTACC-3′;
reverse, 5′-CGAGGGATTATTATTTC-3′. The PCR product was cloned into
the pmirGLO vector luciferase reporter (Promega Corporation,
Madison, WI, USA). For point mutation, site-directed mutagenesis of
potential target site in the TRIP6 3′UTR was performed using a
QuikChange Site-Directed Mutagenesis kit (Promega Corporation;
primers: Forward, 5′-GATCTGGGCTGCGACGGCC-3′; reverse,
5′-GGCCGTCGCAGCCCAGATC-3′). The recombinant plasmids containing
wildtype/mutant 3′-UTR of TRIP6 were confirmed by sequencing. The
NSCs cells were cultured to 70–80% confluence in 24-well plates and
co-transfected with a firefly luciferase reporter vector containing
the TRIP6 3′UTR or mutant 3′UTR and miR-138-5p/anti-miR-138-5p or
control mimics (50 nM) using Lipofectamine™ 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
control luciferase gene was also on this empty vector. Luciferase
activity was analysed 48 h after co-transfection using a
dual-luciferase reporter system (Promega Corporation).
Plasmid construction
For the TRIP6-overexpressing construct, a human cDNA
(U87 cell line; ATCC) sequence of mouse TRIP6 was inserted into the
T expressing vector with CMV promoter (Promega Corporation, primer,
forward, 5′-ATGTCGGGGCCCACCTGGCT-3′; reverse,
5′-TCAGCAGTCAGTGGTGACGGT-3′). TRIP6-targeting short hairpin RNA
(shTRIP6; GCC TGG ACG CCG AGA TAG A) was synthesized by Shanghai
GenePharma Co., Ltd and inserted in pSUPER vector (OligoEngine,
Seattle, WA, USA). NSCs were seeded at a density of 70% confluence.
NSCs were transfected with the plasmid (50 nM) using Dharmafect 1
(GE Dharmacon; GE Healthcare Life Sciences) according to the
manufacturer's instructions. In addition, NSCs were co-transfected
with the miR-138-5p+ctrl vector, miR-138-5p+TRIP6 plasmid,
anti-miR-138-5p+sh-ctrl vector or anti-miR-138-5p+sh-TRIP6 plasmid
at 37°C for 48 h. Cells were collected for further analyses
performed 48 h after transfection.
Statistical analysis
Statistical analyses were processed using SPSS
version 11.5 (SPSS Inc., Chicago, IL, USA). The data are presented
as the mean ± standard deviation of at least three independent
experiments. Significant differences were analysed using Student's
t-test between two groups, and one-way analysis of variance was
used for multiple comparisons. A Tukey post hoc test was used to
compare the differences between three groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-138-5p is downregulated during
NSCs differentiation
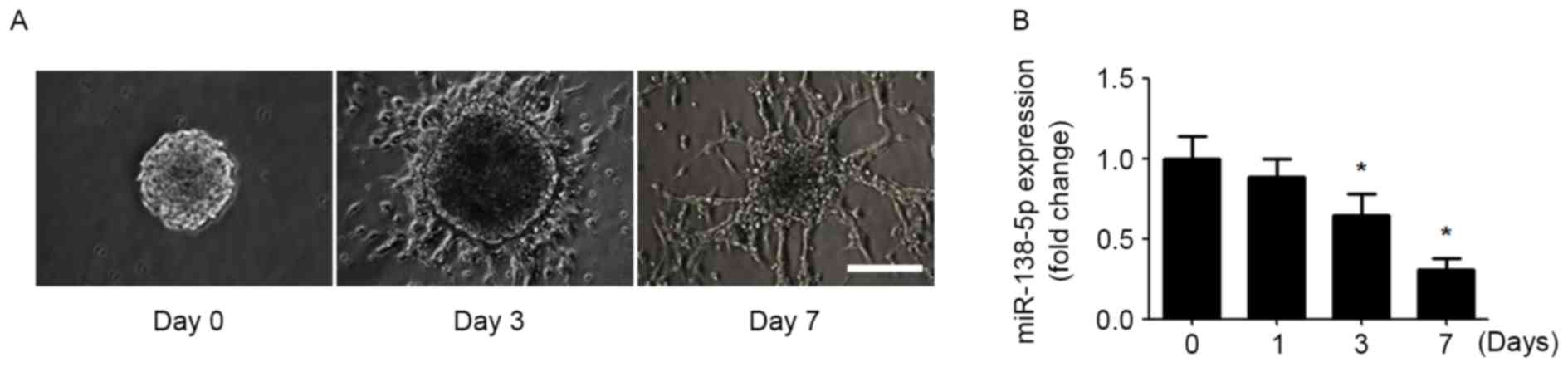
In order to determine the involvement of miR-138-5p
in regulating NSC differentiation, an in vitro model of NSC
differentiation was established. NSCs obtained from mouse forebrain
formed neurospheres in the presence of EGF and bFGF. Dissociated
neurospheres were re-seeded and differentiated in EGF- and
bFGF-free medium. After 7 day of differentiation, the NSCs
developed neuronal morphology with long and branched neurites
(Fig. 1A). Subsequently,
miR-138-5p expression was detected during NSC differentiation.
During NSC differentiation, the expression of miR-138-5p was
gradually decreased over time (Fig.
1B).
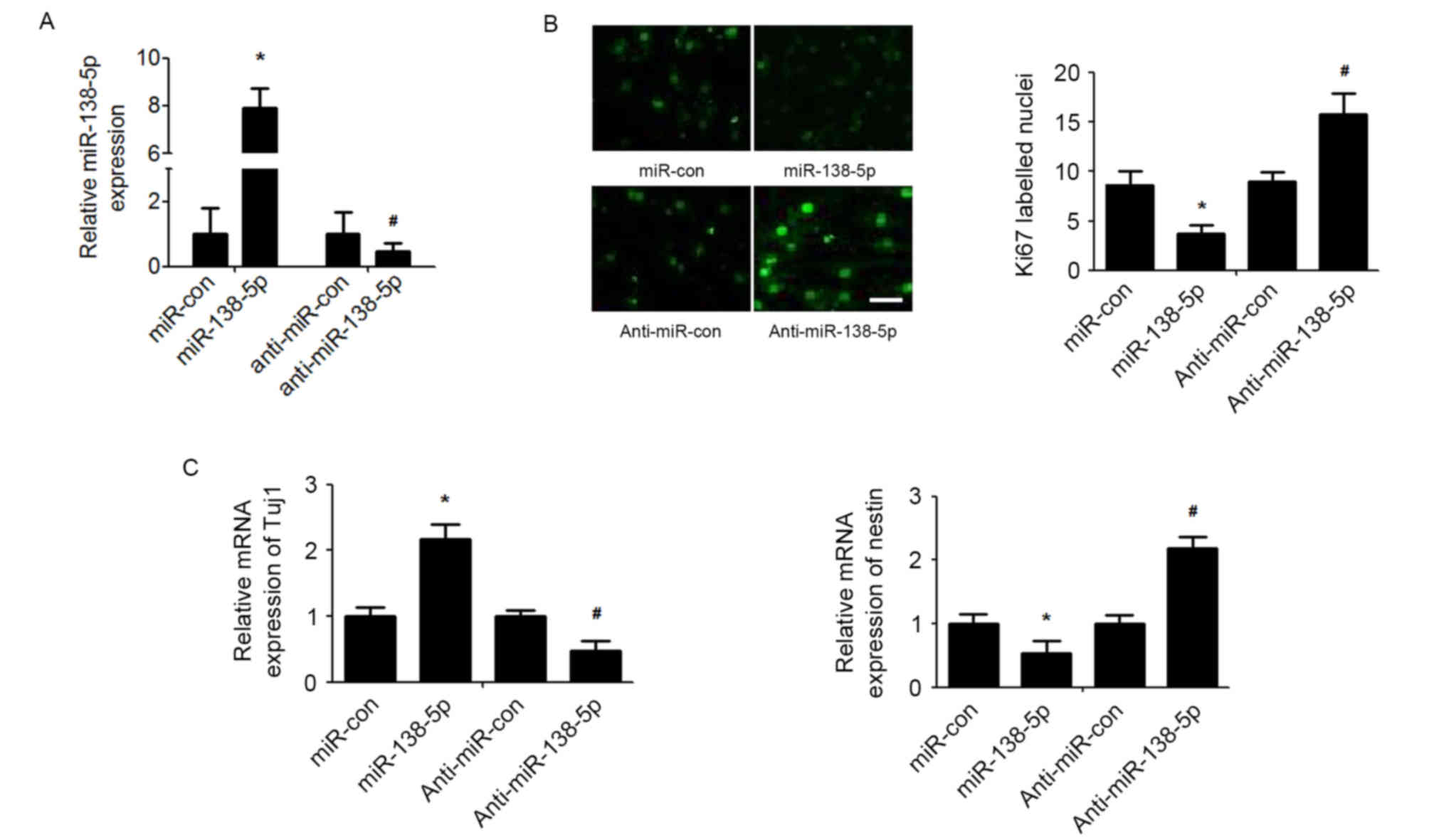
miR-138-5p regulates the proliferation
and differentiation of NSCs
To investigate the functional implications of
miR-138-5p in NSCs, the effect of miR-138-5p overexpression or
silencing on NSCs proliferation and differentiation was determined
(Fig. 2A). As detected by the Ki67
immunofluorescence assay, the results demonstrated that
overexpression of miR-138-5p reduced NSC proliferation, whereas
suppression of miR-138-5p enhanced NSCs proliferation (Fig. 2B). Furthermore, miR-138-5p
overexpression significantly promoted expression of the neuronal
marker Tuj1 and decreased expression of nestin, a neural stem cell
marker, in NSCs induced to differentiate with retinoic acid and FBS
(Fig. 2C). By contrast,
suppression of miR-378 presented exactly the opposite effect on
NSCs differentiation (Fig.
2C).
TRIP6 is a direct target of miR-138-5p
in NSCs
Recent studies have demonstrated that TRIP6
regulates neural stem cell maintenance and serves as a new marker
for NSCs (13,14). Western blot analysis showed that
the expression of TRIP6 was increased the same time as post NSCs
differentiation (Fig. 3A).
Bioinformatic algorithms (TargetScan) indicated that TRIP6 is a
potential target of miR-138-5p. Correlation analysis demonstrated
that miR-138-5p expression was inversely correlated with TRIP6
level during NSCs differentiation on days 0, 1, 3 and 7 (Fig. 3B). Immunofluorescence indicated
that increased miR-138-5p reduced TRIP6 expression, and
downregulation of miR-138-5p level elevated TRIP6 expression
(Fig. 3C). To verify whether
miR-138-5p could directly bind the 3′UTR of TRIP6, we analysed the
potential seed sequence for miR-138-5p in the 3′UTR region of TRIP6
mRNA and cloned the wild type and mutant TRIP6 3′UTR fragments into
a luciferase reporter gene system (Fig. 3D). The miR-138-5p/anti-miR-138-5p
mimic and the redesigned luciferase reporter plasmid were then
co-transfected into NSCs cells. Luciferase activity from the wild
type vector was increased by knockdown of miR-138-5p and reduced by
overexpression of miR-138-5p. By contrast, the activity of the
luciferase reporter gene linked to the mutant TRIP6 3′UTR altered
by the miR-138-5p mimic or anti-miR-138-5p (Fig. 3E).
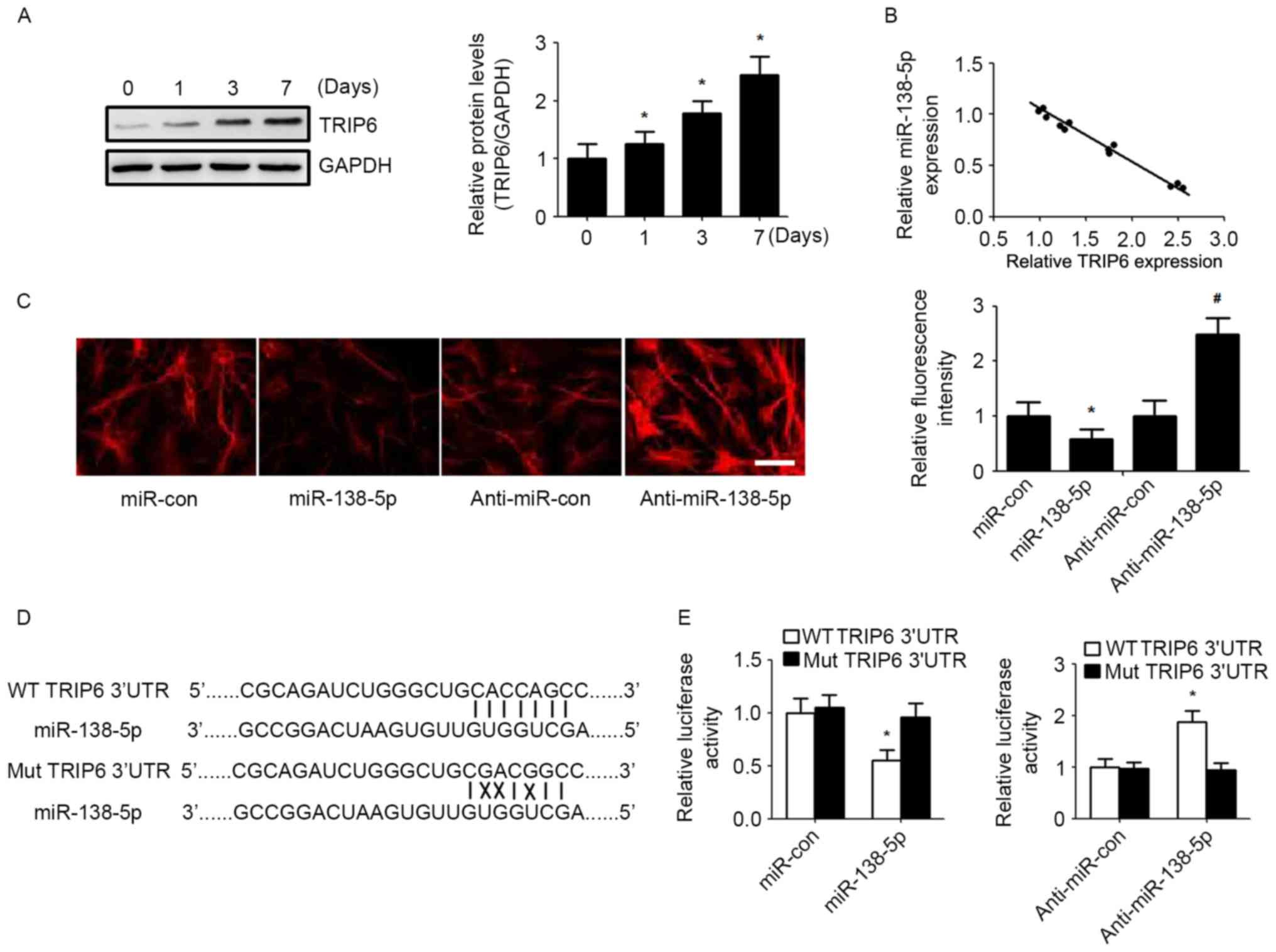 | Figure 3.miR-138-5p regulates TRIP6 expression
by directly binding its 3′UTR. (A) TRIP6 protein level was detected
by western blot in NSCs during differentiation. Quantification of
relative protein levels (TRIP6/GAPDH) according to band intensity.
The data are expressed as the mean ± SD. *P<0.05 vs. day 0, n=5.
(B) Spearman's correlation analysis was used to determine the
correlation between the expression levels of TRIP6 protein and
miR-138-5p. Spearman's correlation, r=−0.7589 (n=20). (C)
Immunofluorescence assay detected the expression of TRIP6 in NSCs
(scale bar, 50 µm) and TRIP6 fluorescence intensity was quantified.
*P<0.05 vs. miR-con group, #P<0.05 vs.
anti-miR-con group. (D) Schematic of miR-138-5p interaction with WT
and Mut TRIP6 3′UTR. (E) Luciferase reporter assays in NSCs
co-transfected with WT/Mut TRIP6 3′UTR reporter plasmid, along with
miR-138-5p/anti-miR-138-5p or control mimic/inhibitor. The data are
expressed as the mean ± SD. *P<0.05 vs. Mut TRIP6 3′UTR, n=5.
NSCs, neural stem cells; SD, standard deviation; TRIP6, thyroid
hormone receptor interacting protein 6; miR, microRNA; miR-con,
control miR; WT, wild type; Mut, mutant. |
miR-138-5p exerts its biological
effect via TRIP6 expression
TRIP6 expression was upregulated and downregulated
using a TRIP6 overexpression vector and sh-TRIP6, respectively, to
determine whether TRIP6 is a functional target of miR-138-5p in
NSCs. As presented in Fig. 4A, in
NSCs transfected with miR-138-5p TRIP6 protein expression was
increased by co-transfection with TRIP6-overexpressing plasmid,
whereas reduced TRIP6 protein expression was observed in NSCs
transfected with anti-miR-138-5p co-transfected shTRIP6 (Fig. 4A). Functionally, restoration of
TRIP6 expression overturned the effect of miR-138-5p mimic,
resulting in an increase in cell proliferation (Fig. 4B) and decrease of cell
differentiation (Fig. 4C). By
contrast, transfection with shTRIP6 reversed the effect of
anti-miR-138-5p on NSCs proliferation (Fig. 4B) and differentiation (Fig. 4C).
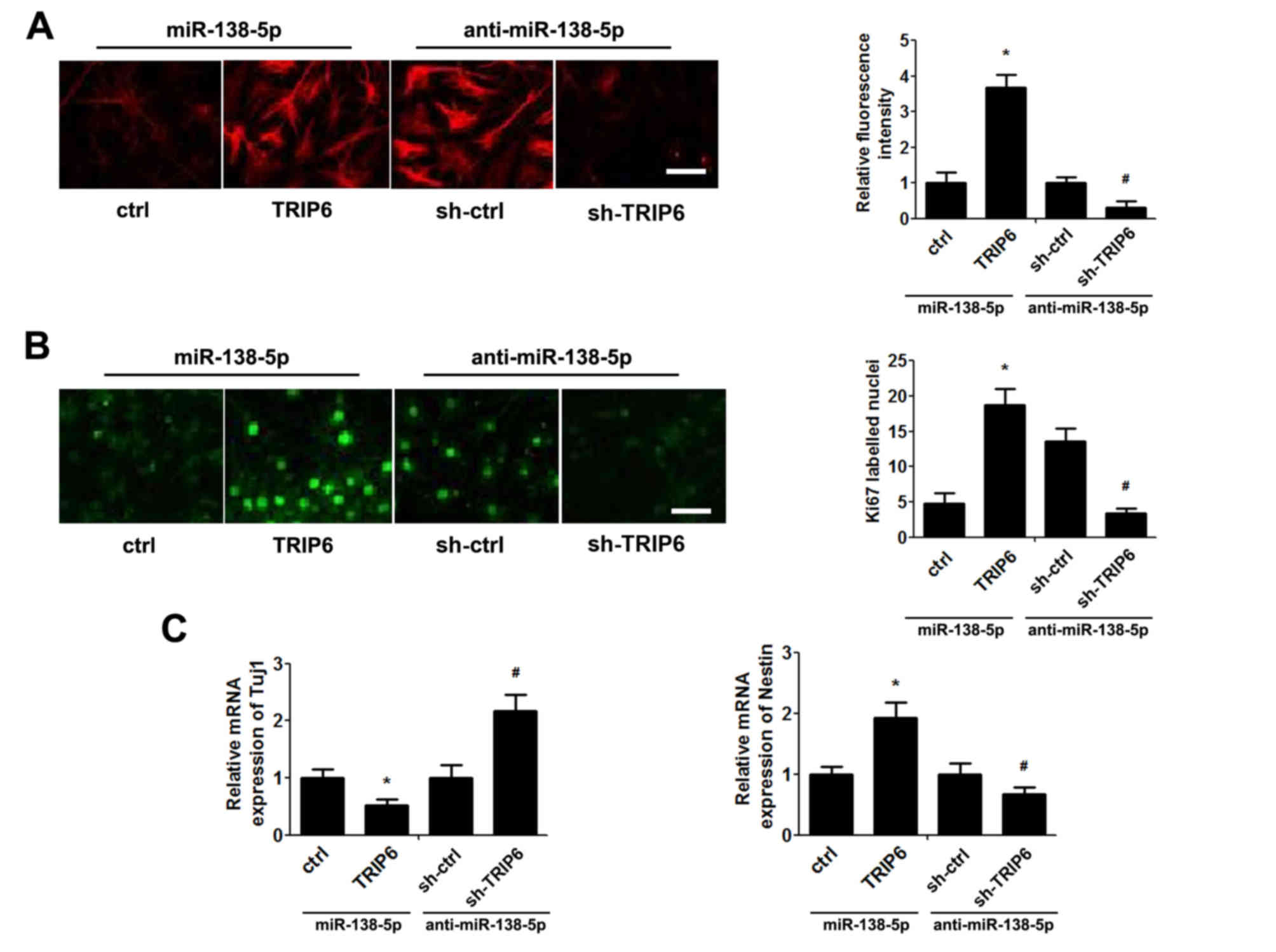 | Figure 4.miR-138-5p exerts its biological
effect via TRIP6. NSCs were co-transfected with miR-138-5p+ctrl
vector, miR-138-5p+TRIP6 plasmid, anti-miR-138-5p+sh-ctrl vector or
anti-miR-138-5p+sh-TRIP6 plasmid for 48 h. (A) Immunofluorescence
assay detected the expression of TRIP6 in NSCs (scale bar, 50 µm)
and quantification of TRIP6 fluorescence intensity. The ctrl and
sh-ctrl groups were both set as 1. (B) Proliferation of NSCs was
detected by the Ki67 immunofluorescence (scale bar, 50 µm) and
quantification of the number of Ki67-labelled nuclei by counting
five high-power fields. (C) Quantitative polymerase chain reaction
analysis of Tuj1 and nestin expression. The ctrl and sh-ctrl groups
were both set as 1. The data are expressed as the mean ± standard
deviation. *P<0.05 vs. ctrl, #P<0.05 vs. sh-ctrl,
n=5. NSCs, neural stem cells; miR, microRNA; TRIP6, thyroid hormone
receptor interacting protein 6; sh, short hairpin RNA; Tuj1, Tuj1,
class III β-tubulin. |
Discussion
In recent years, research has focused on the
profound importance of miRNAs in regulating neurogenesis.
Increasing evidence supports that miRNAs are associated with the
regulation of NSC proliferation and differentiation, and
manipulating miRNAs in NSCs may be useful for the development of
novel interventions for the treatment of certain neurological
disorders (15,16). The precise network of miRNAs that
regulate neuronal proliferation and differentiation remains
unclear.
Up or downregulation of miR-138 is important for
regulation of the growth and/or apoptosis of various cancer types,
including lung cancer (17),
hepatocellular carcinoma (18) and
leukaemia (19). miR-138-5p, the
most common human isoform of miR-138, was previously demonstrated
to be significantly downregulated in primary human pancreatic
cancer and human pancreatic cancer-derived cell lines (20,21).
Exogenous overexpression of miR-138-5p inhibits pancreatic cancer
cell growth (21). miR-138 is
particularly well investigated in neuroscience, and has been
suggested to have a potential role in mammalian brain function
(22,23). It was also reported that miR-138-5p
acts as a molecular regulator of human memory function and
dendritic spines, and regulates phosphorylation of tau protein,
which may improve cognition and Alzheimer disease (24). However, the further association of
miR-138-5p and proliferation and differentiation of NSCs has not
yet been identified. In the current study, it was demonstrated that
there was a gradual decline in the expression of miR-138-5p during
the NSC differentiation. Suppression of miR-138-5p induced
proliferation and reduced differentiation of NSCs, and
overexpression of miR-138-5p reduced NSCs proliferation and
promoted NSCs differentiation. These results indicated an important
role of miR-138-5p in NSCs proliferation and differentiation.
Subsequently, it was elucidated that miR-138-5p
targets and regulates TRIP6. TRIP6 was originally identified as an
interacting protein of the nuclear thyroid hormone receptor in a
yeast two-hybrid system, and as a member of the zyxin family of LIM
proteins (25,26). TRIP6 is a focal adhesion molecule
with the ability to shuttle between the cell surface and nucleus,
which is involved in the regulation of actin dynamics and signal
transduction during in cell adhesion and migration (27). Accumulating evidence supports that
TRIP6 is expressed in hippocampal neurons and modulates
neurological biological function (14,28).
A previous study demonstrated that TRIP6 is necessary and
sufficient for the self-renewal and proliferation of NSCs, but
inhibited their differentiation (13). The results of the current study are
in line with this previous conclusion, indicating that TRIP6
regulates NSC maintenance and it may be a novel marker for NSCs. In
addition, the effect of miR-138-5p on proliferation and
differentiation of NSCs was demonstrated be reversed by up- or
downregulation of TRIP6 in the present study, which suggested that
miR-138-5p regulates NSCs proliferation and differentiation, at
least in part through modulating TRIP6 expression. These data also
imply that TRIP6 is crucial for regulation of the balance between
proliferation and differentiation of NSCs. Indeed, regarding the
treatment of neurodevelopmental disorders, TRIP6 may be as a
promising target for modulating NSCs.
In conclusion, the current study regards miR-138-5p
as an important miRNA involved in the regulation of NSC
proliferation and differentiation via targeting TRIP6. Therefore,
altering miR-138-5p and TRIP6 regulation may be a promising
treatment for dealing with neurogenesis and neurodegenerative
diseases.
Acknowledgements
This project was supported by the National Natural
Science Foundation (grant no. 31401258) and the Natural Science
Foundation of Shandong Province, China (grant no. ZR2012BM006).
References
|
1
|
Liu S, Yin F, Zhang J, Wicha MS, Chang AE,
Fan W, Chen L, Fan M and Li Q: Regulatory roles of miRNA in the
human neural stem cell transformation to glioma stem cells. J Cell
Biochem. 115:1368–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaker Z, Codega P and Doetsch F: A mosaic
world: Puzzles revealed by adult neural stem cell heterogeneity.
Wiley Interdiscip Rev Dev Biol. 5:640–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung SY, Kishinevsky S, Mazzulli JR,
Graziotto J, Mrejeru A, Mosharov EV, Puspita L, Valiulahi P, Sulzer
D, Milner TA, et al: Parkin and PINK1 patient iPSC-derived midbrain
dopamine neurons exhibit mitochondrial dysfunction and α-synuclein
accumulation. Stem Cell Reports. 7:664–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng A, Hou Y and Mattson MP:
Mitochondria and neuroplasticity. ASN Neuro. 2:e000452010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao P, Benito E and Fischer A: MicroRNAs
as biomarkers for CNS disease. Front Mol Neurosci. 6:392013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi X, Yan C, Liu B, Yang C, Nie X, Wang
X, Zheng J, Wang Y and Zhu Y: miR-381 regulates neural stem cell
proliferation and differentiation via regulating Hes1 expression.
PLoS One. 10:e01389732015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryu JR, Hong CJ, Kim JY, Kim EK, Sun W and
Yu SW: Control of adult neurogenesis by programmed cell death in
the mammalian brain. Mol Brain. 9:432016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meza-Sosa KF, Pedraza-Alva G and
Pérez-Martínez L: microRNAs: Key triggers of neuronal cell fate.
Front Cell Neurosci. 8:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer TD, Markakis EA, Willhoite AR,
Safar F and Gage FH: Fibroblast growth factor-2 activates a latent
neurogenic program in neural stem cells from diverse regions of the
adult CNS. J Neurosci. 19:8487–8497. 1999.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai YJ, Li MY, Yang CY, Huang KH, Tsai JC
and Wang TW: TRIP6 regulates neural stem cell maintenance in the
postnatal mammalian subventricular zone. Dev Dyn. 243:1130–1142.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv K, Chen L, Li Y, Li Z, Zheng P, Liu Y,
Chen J and Teng J: Trip6 promotes dendritic morphogenesis through
dephosphorylated GRIP1-dependent myosin VI and F-actin
organization. J Neurosci. 35:2559–2571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Liu X and Wang Y: MicroRNA-378
regulates neural stem cell proliferation and differentiation in
vitro by modulating Tailless expression. Biochem Biophys Res
Commun. 466:214–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tandon PN and Seth P: Cell therapy for
neurological disorders: The elusive goal. Neurol India. 64:612–623.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao
CX, Cui JJ, Zhang W, Zhou HH, Yin JY and Liu ZQ: MicroRNA-138 acts
as a tumor suppressor in non small cell lung cancer via targeting
YAP1. Oncotarget. 7:40038–40046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Zhang W, Liu K, Liu S, Ji B and
Wang Y: miR-138 suppresses cell proliferation and invasion by
inhibiting SOX9 in hepatocellular carcinoma. Am J Transl Res.
8:2159–2168. 2016.PubMed/NCBI
|
|
19
|
Xu C, Fu H, Gao L, Wang L, Wang W, Li J,
Li Y, Dou L, Gao X, Luo X, et al: BCR-ABL/GATA1/miR-138 mini
circuitry contributes to the leukemogenesis of chronic myeloid
leukemia. Oncogene. 33:44–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian S, Guo X, Yu C, Sun C and Jiang J:
miR-138-5p suppresses autophagy in pancreatic cancer by targeting
SIRT1. Oncotarget. 8:11071–11082. 2017.PubMed/NCBI
|
|
21
|
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X,
Deng Y, Jiang J and Sun C: MicroRNA-138-5p regulates pancreatic
cancer cell growth through targeting FOXC1. Cell Oncol (Dordr).
38:173–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schroder J, Ansaloni S, Schilling M, Liu
T, Radke J, Jaedicke M, Schjeide BM, Mashychev A, Tegeler C,
Radbruch H, et al: MicroRNA-138 is a potential regulator of memory
performance in humans. Front Hum Neurosci. 8:5012014.PubMed/NCBI
|
|
23
|
Castaneda P, Muñoz M, Garcia-Rojo G, Ulloa
JL, Bravo JA, Márquez R, García-Pérez MA, Arancibia D, Araneda K,
Rojas PS, et al: Association of N-cadherin levels and downstream
effectors of Rho GTPases with dendritic spine loss induced by
chronic stress in rat hippocampal neurons. J Neurosci Res.
93:1476–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Tan L, Lu Y, Peng J, Zhu Y, Zhang
Y and Sun Z: MicroRNA-138 promotes tau phosphorylation by targeting
retinoic acid receptor alpha. FEBS Lett. 589:726–729. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin VT and Lin FT: TRIP6: An adaptor
protein that regulates cell motility, antiapoptotic signaling and
transcriptional activity. Cell Signal. 23:1691–1697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Willier S, Butt E, Richter GH, Burdach S
and Grunewald TG: Defining the role of TRIP6 in cell physiology and
cancer. Biol Cell. 103:573–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grunewald TG, Willier S, Janik D, Unland
R, Reiss C, da Costa Prazeres O, Buch T, Dirksen U, Richter GH,
Neff F, et al: The Zyxin-related protein thyroid receptor
interacting protein 6 (TRIP6) is overexpressed in Ewing's sarcoma
and promotes migration, invasion and cell growth. Biol Cell.
105:535–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoffman LM, Nix DA, Benson B, Boot-Hanford
R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler
FB, et al: Targeted disruption of the murine zyxin gene. Mol Cell
Biol. 23:70–79. 2003. View Article : Google Scholar : PubMed/NCBI
|