Introduction
Glial cells are macrophage cells in brain that are
able to perform the phagocytosis and protect neurons in the central
nervous system (CNS) (1,2). They act as phagocytic cells and are
the primary immune cells in CNS. Additionally, glial cells function
as a debris scavengers, regulate the innate immunity and
participate in the adaptive immune responses in neural tissues
(3).
The pro-inflammatory responses have important
functions in the pathogenesis of several CNS-associated disorders,
including Alzheimer's disease, multiple sclerosis and Parkinson's
disease (4–6). Activated glial cells, including both
microglia and astrocytes are the primary sources for
proinflammatory mediators, such as cytokines, chemokines and nitric
oxide (NO) (1,7). Therefore, more attention has been
directed towards considering these cells as the putative targets
for treatment of inflammatory disorders.
However, over-activate glial cells may also produce
excessive inflammatory substances such as NO, various cytokines and
prostaglandins (1,7). Previous studies revealed that
suppression of inflammatory responses from glial cells may
alleviate these pathological conditions (8,9).
Therefore, it is possible that anti-inflammatory agents may have
neuroprotective functions. It should be noted that
anti-inflammatory agents have been previously investigated for
targeting particular proinflammatory mediators and selectively
deactivating glial cells (8,9).
Migri-Heal® as a novel herbal remedy,
which was introduced as potential treatment of migraine headaches
based on anecdotal evidence in traditional Iranian medicine. This
drug has been patented by the Invention and Patent Registration
Office of I.R. of Iran (IRC1228143083). It has been previously
reported that Migri-Heal® reduces NO levels in
endothelial cell culture (10).
Therefore, the evaluation of the possible anti-inflammatory effects
of Migri-Heal® may be useful to illustrate other
therapeutic aspects of this herbal remedy in neuroinflammatory
diseases.
To the best of our knowledge the present study is
the first to investigate the effect of Migri-Heal® on
expression and secretion of inflammatory mediators from microglia
cells stimulated with LPS for the first time.
Materials and methods
Reagents
Fetal bovine serum (FBS), Dulbecco's modified
Eagle's medium (DMEM) and Griess reagent were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Bacterial LPS
(cat. no. E5:055), MTT assay kit (cat. no. M2128 500MG),
antibiotics (streptomycin and penicillin) and trypsin were
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Dimethyl sulfoxide (DMSO) (cat. no. 1.02952.1000) was purchased
from Merck Millipore and Migri-Heal® was prepared by Dr
Mohammad Ansari (Tehran University of Medical Sciences, Tehran,
Iran) (11). This drug has been
patented by the Invention and Patent Registration Office of I.R. of
Iran (IRC1228143083).
Migri-Heal® dilution
A total of 100 g Migri-Heal® powder was
dissolved in 250 ml water. Following 10 min, boiling water (powder
and water) extract was passed through Whatman No. 1 filter paper
and dried by freeze dryer. This extract was 11%
Migri-Heal® powder. The dried extract of
Migri-Heal® (1 mg/ml) was dissolved in DMEM and filtered
using 0.22 µm filter (Orange Scientific, Braine-l'Alleud, Belgium).
It is of note that Migri-Heal® should be freshly
prepared.
Cell culture
The primary mixed glial cultures were prepared from
whole brains of 1 to 3 days-old Wistar rats (National Institute of
Genetic Engineering and Biotechnology, Tehran, Iran) (n=10; 5
males, 5 females; weight, between 4 and 5 g). Rats were housed at a
constant temperature of 23±2°C with a 12 h light/dark cycle (lights
on at 7 am) and received standard rat chow and water ad
libitum.
The study was reviewed and approved by the Bioethics
Committee of the Health Ministry (Tehran, Iran; permit no.
IR.NIGEB.EC.1395.4.1.C). Isolated cells were cultured according to
previous studies with some modifications (3,12–14).
Briefly, brains were excised aseptically from the skull, the
meninges and blood vessels were carefully removed, and mechanically
disrupted by trituration in DMEM. Then the suspended cells were
transferred inside a flask prepared with DMEM supplemented with 100
UI/ml penicillin G, 100 µg/ml streptomycin and 10% of
heat-inactivated FBS. Cultures were incubated at 37°C with 95%
humidity and 5% CO2. The medium was replenished on day 1
after plating and every third or fourth day thereafter with medium
supplemented with 10% of heat-inactivated FBS and the
aforementioned antibiotics. In this study, cells after two passages
were used.
Treatment with
Migri-Heal®
Treatment with Migri-Heal® was done in
two ways, as, in some studies (15,16),
stimulation with LPS was performed prior to drug treatment, whereas
in other studies (11,17), stimulation with LPS was performed
following drug treatment; therefore, the two methods were
investigated in the present study. The first included 10 µg/ml LPS
was added to primary mixed glial cells, incubated in 37°C for 1 h.
Subsequently, cells were treated with Migri-Heal® (25,
50, 100, 150, 200, 250 and 300 µg/ml) in fresh DMEM containing 1%
FBS and cells were incubated in 37°C for 48 h. The second method
included primary mixed glial cell cultures were pretreated with
Migri-Heal® (25, 50, 100, 150, 200, 250 and 300 µg/ml)
in fresh DMEM containing 1% FBS for 1 h. Subsequently, 10 µg/ml LPS
was added and cells were incubated in 37°C for 48 h.
Cell viability assay
The cell viability was evaluated by an MTT assay as
previously described (18).
Following various treatments, 1 mg/ml MTT solution was added 10% of
medium for 3 h at 37°C. After 3 h, the medium was removed and the
cells were lysed in 100 µl of DMSO, which can release the blue
product. The level of MTT formazan was determined by measuring
absorbance at 580 nm using a Multiskan RC microplate reader.
Nitrate assay
The Griess nitrite assay, which may be used as an
index of NO production, was used to estimate NO production in the
cultured glial cells (7,14). Serial diluted NaNO2
(Sigma-Aldrich, Merck Millipore) solutions were freshly made and
served as standards (0–100 mol/l) for the assay. Briefly, samples
were collected 48 h after stimulation with LPS. Following
centrifugation (1,000 × g, 5 min, room temperature), 50 µl culture
medium (supernatant) was transferred to 96-well plates (BD
Biosciences, Franklin Lakes, NJ, USA) and mixed with an equal
volume of Griess reagent. The plate was placed in darkness for 15
min and the quantity of nitrite was calculated by measuring
absorbance at 540 nm using a micro plate reader.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from treated cell cultures
with Easy BLUE® (iNtRON Biotechnology, Inc., Daejeon,
South Korea) according to the manufacturer's protocol.
Concentration of the total RNA was determined by NanoDrop
spectrophotometer at 260 nm. Identical quantity of RNA (1 µg) was
reverse transcribed for 1 h at 42°C in a reaction mixture
containing 20 U RNase inhibitor (Fermentas; Thermo Fisher
Scientific, Inc.), 1 mM dNTP (CinaGen, Tehran, Iran), 1× reverse
transcriptase buffer, and 5 U reverse transcriptase (Fermentas;
Thermo Fisher Scientific, Inc.). The cDNA was amplified by PCR with
using the following primers: INOS forward (F)
5′-GACATCGACCAGAAGCTGTC-3′ and reverse (R)
5′-GGGCTCTGTTGAGGTCTAAAG-3′; TNFα F 5′-GCTCCCTCTCATCAGTTCCA-3′ and
R 5′-TTGGTGGTTTGCTACGACG-3′; GAPDH F 5′-CCCCCAATGTATCCGTTGTG-3′ and
R 5′-TAGCCCAGGATGCCCTTTAGT-3′. PCR was conducted by using the
following conditions for 33 cycles: Denaturation at 95°C for 30
sec, annealing at 60°C for 30 sec and extension at 72°C for 45 sec.
PCR products were separated by electrophoresis on a 2% for iNOS and
3% for TNFα agarose gels. The products were visualized by staining
with ethidium bromide (MR7729; CinnaGen, Tehran, Iran) and detected
under UV light. Densitometric analysis of the data was normalized
to GAPDH. The intensity of bands was determined using the TotalLab
version 1.1 (TotalLab, Ltd., Newcastle upon Tyne, UK).
Total protein extraction
Protein levels were determined in primary mixed
glial cells 48 h after treatment. After a cold PBS wash, the cells
were scraped and recovered in 100 µl per sample of NP-40 lysis
buffer (tris-HCl 50 mM, pH 8, solution with 1% Triton X-100, NaCl
150 mM). After pipetting the content of the wells was pooled and
centrifuged at 9,800 × g at 4°C for 10 min and stored at −20°C.
Protein content was assayed colorimetrically using the Bradford
method (Abcam, Cambridge, MA, USA).
Western blotting
A total of 45 µg denatured total protein extracts
(2.5 mM DTT, 100°C for 5 min) were subjected to 12.5% SDS-PAGE and
transferred to a PVDF Western Blotting membranes (Roche Applied
Science, Penzberg, Germany; cat. no. 03010040001). Then, the
transferred membrane was blocked with a blocking solution [1X TBS,
0.1% Tween-20 with 5% w/v dry milk (Merck Millipore; cat. no.
1153630500)] for 1 h and incubated with the following primary
antibodies overnight at 4°C: NF-κB p65 (catalog no. sc-71675;
dilution, 1:250; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
Nurr1 (catalog no. sc-991; dilution, 1:250; Santa Cruz
Biotechnology, Inc.) and monoclonal mouse anti β actin (dilution,
1:10,000; catalog no. A1978; Sigma-Aldrich; Merck Millipore),
diluted in immunoblot buffer (TBS containing 0.05% Tween-20 and 5%
non-fat dry milk). Then, the membranes were washed twice in 0.05%
Tween-20 in TBS for 15 min and incubated with horseradish
peroxidase (HRP)-labelled secondary antibodies; goat anti-mouse
(1:5,000; cat. no. sc-2055; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Following extensive washes in 0.05% Tween-20
in TBS, the cells were incubated in ECL-Plus (cat. no. RPN2132; GE
Healthcare, Chicago, IL, USA) for 5 min. The membranes were the
exposed by X-ray film in darkroom. Densitometry analysis of the
bands was performed by TotalLab version 1.10.
Statistical analysis
The statistical analysis in the present study was
performed using SPSS version 16 (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance followed by a Fisher's least
significant difference post-hoc test was used to determine the
statistical differences among groups. P<0.05 was considered to
indicate a statistically significant difference compared with the
LPS-treated group without Migri-Heal®.
Results
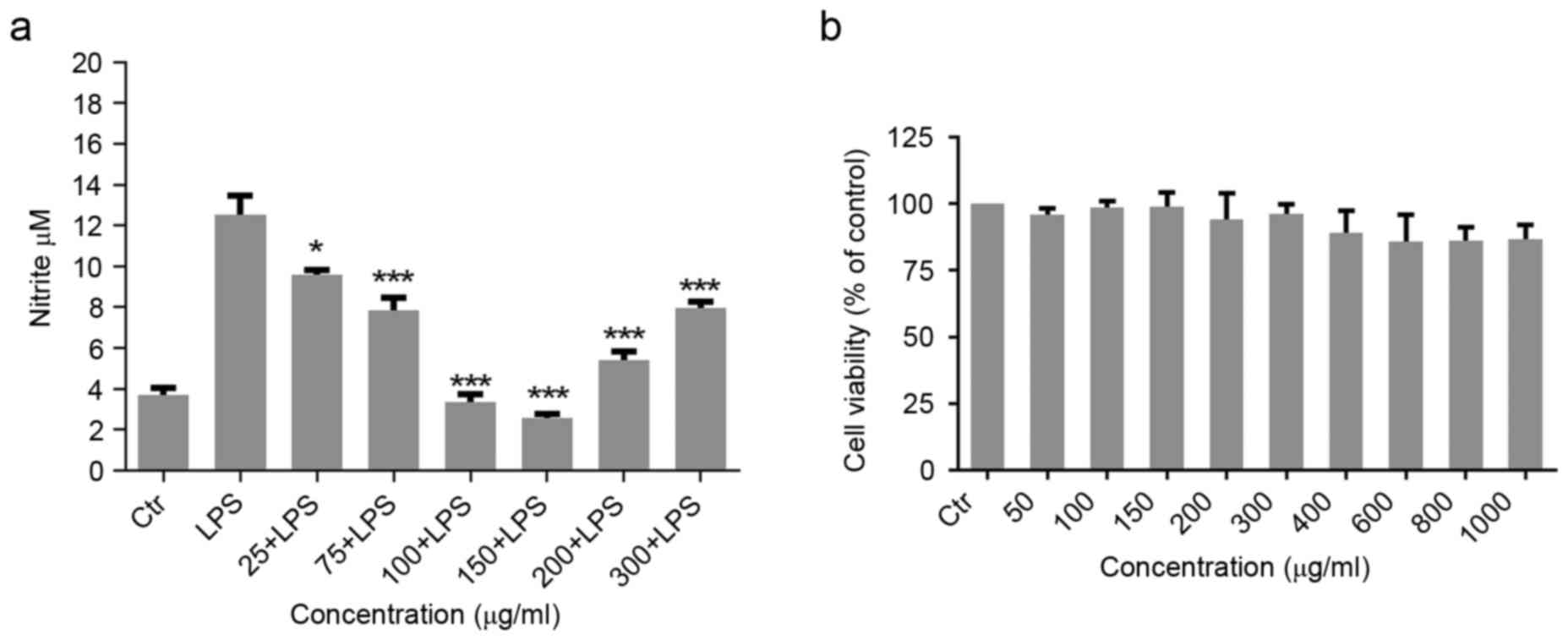
Effect of Migri-Heal® on NO
production in LPS-induced glial cells
To investigate the possible effect of
Migri-Heal® in modulation of immune functions of glial
cells, the present study examined the effects of 25–300 µg/ml
Migri-Heal® on 10 µg/ml LPS-induced production of NO in
rat primary mixed glial cell cultures. Glial cells were stimulated
with 10 µg/ml LPS and after 1 h, the cells were treated with
different concentrations of Migri-Heal®. The findings
revealed that Migri-Heal®, had no effect on glial cells
treated with LPS and there was no reduction in nitrite release of
these cells (data not shown). Then, primary mix glial cells were
exposed to various concentrations of Migri-Heal® and
after 1 h were treated with 10 µg/ml LPS. Migri-Heal® at
150 µg/ml significantly reduced NO which was produced by cells in a
dose-dependent manner. Migri-Heal® inhibited the
LPS-induced production of NO with a U-shaped concentration-response
effect. As presented in Fig. 1A,
the maximal inhibition of NO secretion was observed at 150 µg/ml of
Migri-Heal®.
Assessment of toxicity of
Migri-Heal® on glial cell viability
In order to assess the toxicity of the
Migri-Heal®, cell viability was investigated using MTT
assay. Formation of formazan crystals in treated glial cells with
different concentrations of Migri-Heal® revealed that
this compound had no cytotoxic effect on glial cells in different
doses and particularly when used at a dose of 150 µg/ml (Fig. 1B).
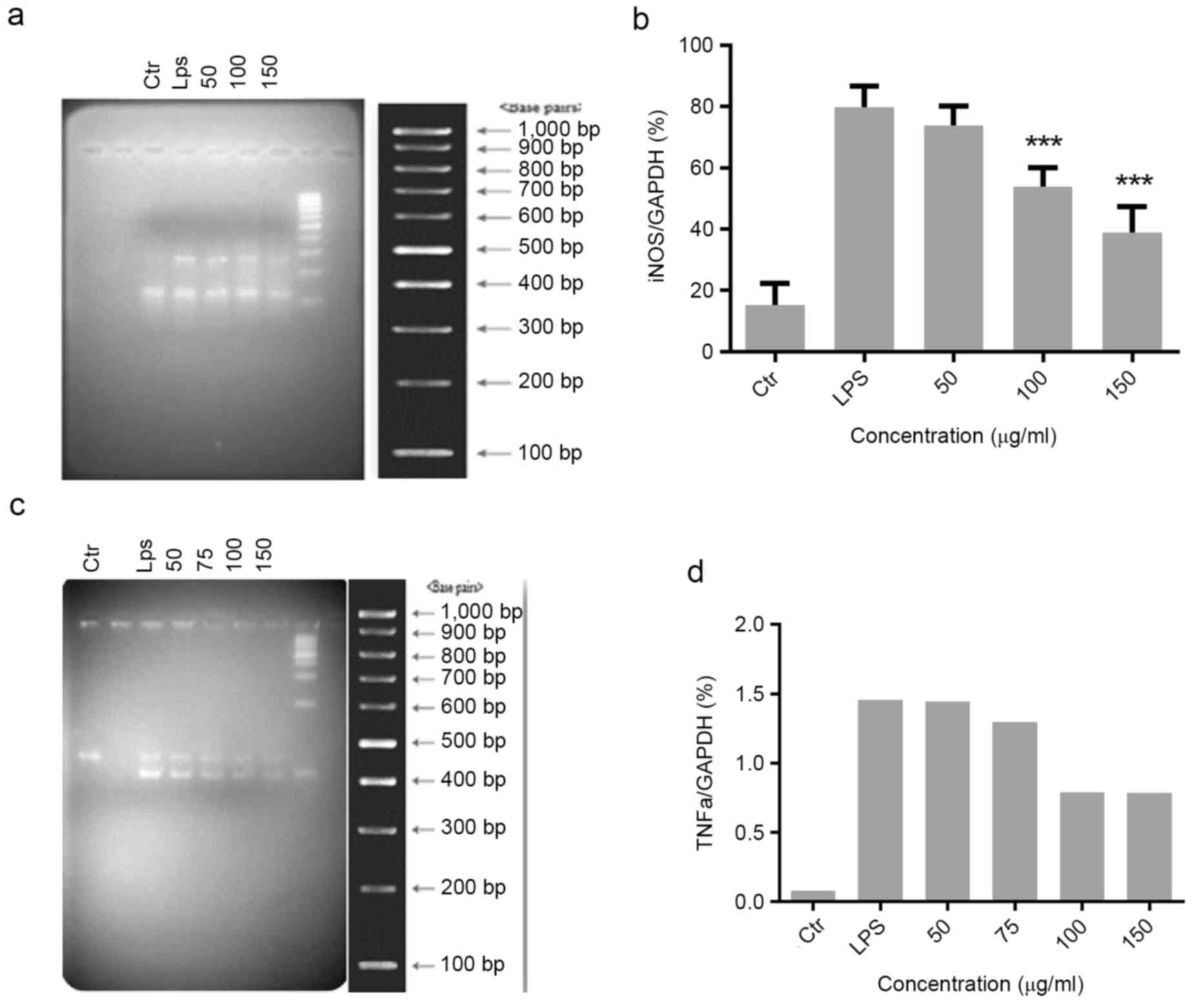
Effect of Migri-Heal® on
iNOS and TNFα expression in glial cells treated with LPS
iNOS and TNFα have essential roles in the
progression of inflammatory processes. The present study evaluated
the effect of different concentrations of Migri-Heal® on
the expression of the iNOS and TNFα genes in LPS-treated glial
cells. The expression of iNOS and TNFα was markedly increased when
compared with controls following treatment of cells with LPS. For
the iNOS expression, cells were pretreated with various
concentrations of Migri-Heal® (50, 100 and 150 µg/ml)
(Fig. 2A and B) and with 50, 75,
100 and 150 µg/ml Migri-Heal® for evaluation of TNFα
expression (Fig. 2C and D).
Migri-Heal® 150 µg/ml was identified to have the maximum
anti-inflammatory effect.
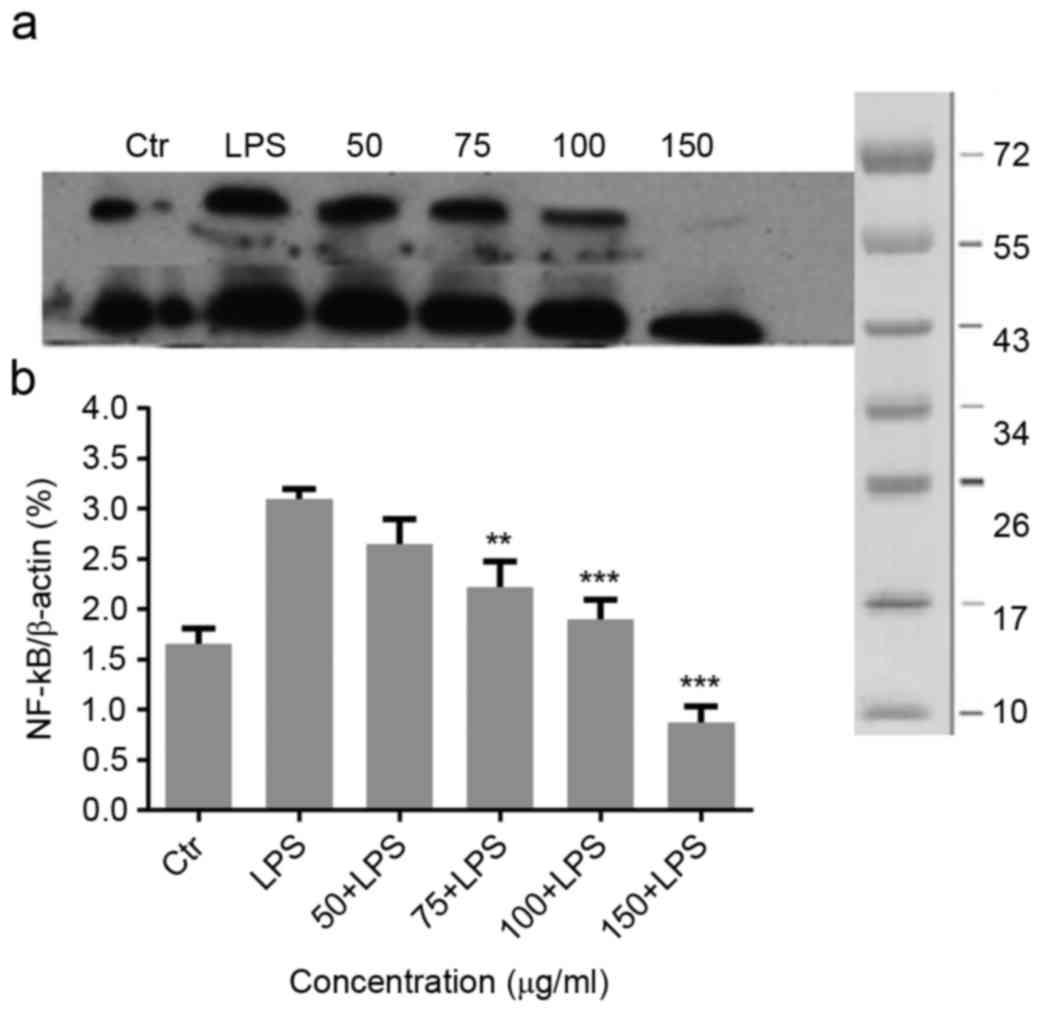
Effect of Migri-Heal® on
LPS-induced NF-κB in mixed glial cells
To investigate the molecular mechanism underlying
the anti-inflammatory effects of Migri-Heal®, the
present study examined the its effect on NF-κB, as a key
transcription factors modulating the gene expressions of
pro-inflammatory molecules, such as iNOS and TNF-α in glial cells
(19). As presented in Fig. 3A, LPS treatment increased NF-κB
expression and Migri-Heal® decreased the NF-κB
expression. The present findings were confirmed by normalized
values presented in Fig. 3B.
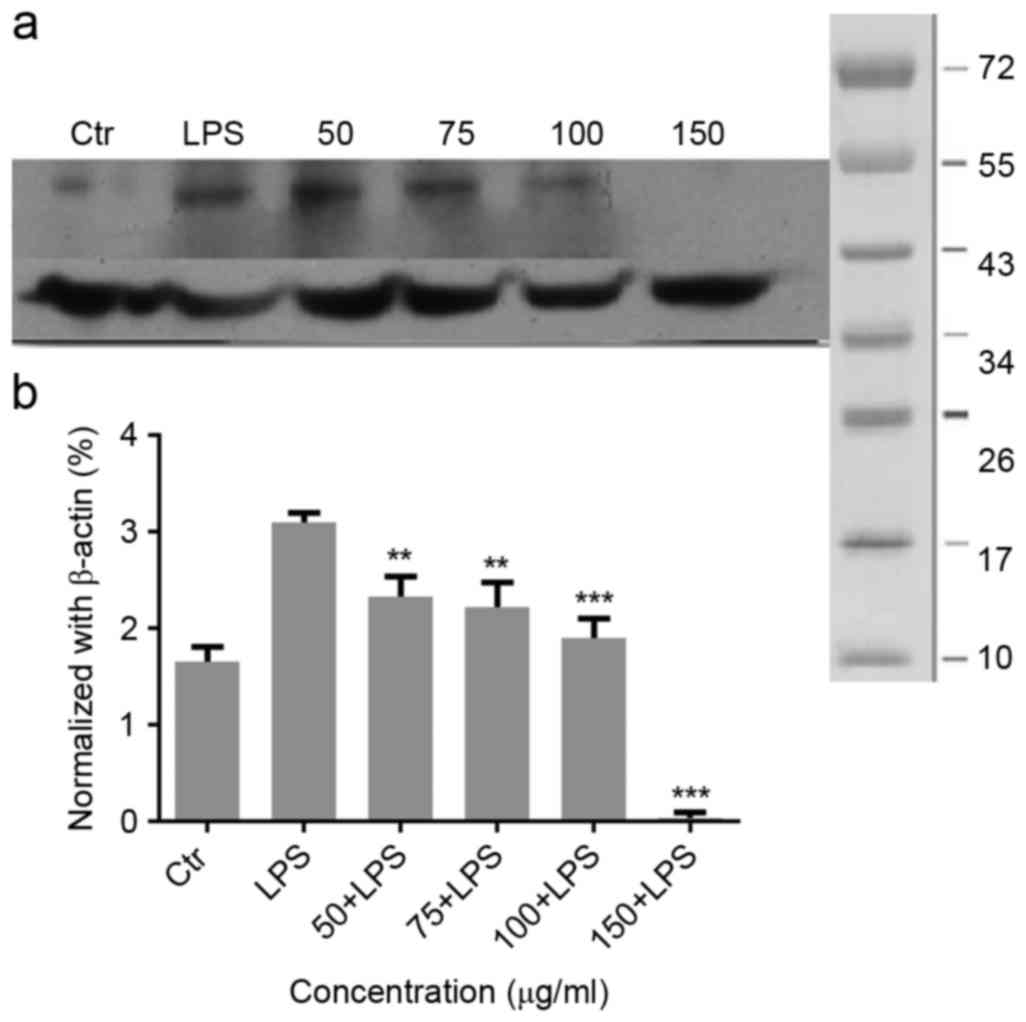
Expression of nuclear receptor
related-1 (Nurr1) in the inflammatory process
Nurr1 exhibits different mechanisms that functions
to resolve inflammatory responses. As presented in Fig. 4A, LPS treatment significantly
increased Nurr1 and inhibited the NF-κB DNA binding. Therefore, the
inhibitory effects of Nurr1 on NF-κB may be one of the factors
contributing to the anti-inflammatory effect observed in
LPS-stimulated glial cells. Migri-Heal® at 100 µg/ml and
150 µg/ml was able to significantly reduce Nurr1 expression in
cells treated with LPS in comparison with LPS-only stimulated
cells. These findings were confirmed by the normalized values
Fig. 4B.
Discussion
Inflammation is a response of vascular tissues
against aggressive agents such as pathogens, irritants, or damaged
cells. It has been previously established that neuroinflammation in
the CNS contributed to the pathogenesis and progression of several
neurodegenerative diseases (4,5).
Glial cells, such as astrocytes, microglia and oligodendrocytes,
are more numerous than neurons in the CNS (20). Glial cells have an essential role
in CNS and modulate homeostasis in the CNS. These cells were also
activated by various inflammatory stimuli, which in turn lead to
the production of cytokines and other pro-inflammatory mediators
that contribute to neuronal damage (21). Hence, considerable efforts have
been made to alleviate inflammation by targeting the glial cells
through the introduction of novel therapeutic agents. There has
been more interest in the development of novel therapeutic agents
that may selectively attenuate neuroinflammation, specifically
through the inhibition of glial cell activation.
Migri-Heal® is an emerging drug and
investigation on the underlying molecular mechanism remains to be
fully elucidated; therefore, interpretation of the findings of the
present study and comparison with previous studies are difficult.
It is of note, that the molecular mechanisms underlying the
anti-inflammatory effects of Migri-Heal® in glial cells
are not fully understood. Therefore, the present study aimed to
investigate the anti-inflammatory effect of Migri-Heal®
on glial stimulated with LPS.
The effect of LPS stimulation suggests that the
activation of the NF-κB is a critical step in inflammatory pathways
(22,23), which ultimately leads to the
induction of iNOS and NO production (24,25).
Increased production of NO and iNOS in monocytes during migraines
occurs following the increase in NF-κB activity. We previously
reported that NO production was inhibited with
Migri-Heal® (11,26).
It was previously revealed that the aqueous extract fractions and
the essence of these herbs may have a dose-dependent reduction of
NO in the endothelial cell supernatant.
The essence analysis of Migri-Heal® by
gas chromatography mass spectrometry and composition of aqueous
extracts by high-performance liquid chromatography (27) indicated the chemical compounds in
Migri-Heal®. The most abundant compounds in the essence
of Migri-Heal® were monoterpenoids, specifically cineol
and thymol. Additionally, the analysis of aqueous extracts of some
plants in Migri-Heal® revealed that it is rich of
melatonin (27). A previous study
revealed 9 the role of melatonin in NO scavenging, inhibition of
iNOS expression and inhibition of the NF-κB activity (28). Accordingly, the low levels of
nocturnal melatonin in patients with migraines may be one of the
possible reasons for the importance of melatonin in migraine
therapy (29,30).
It is of note that Migri-Heal®
significantly reduced the expression of NF-κB, which is an
important transcription factor in regulation of inflammatory
pathways, iNOS and TNF-α which are two important target genes of
NF-κB in LPS-stimulated glial cells. Therefore, the present
findings suggest that Migri-Heal® may exert beneficial
effects on alleviation of inflammation.
Previous studies demonstrated that monoterpenoid
compounds such as thymol and cineol inhibit expression of
inflammatory factors such as NF-κB and iNOS (31). The general effect of NO and iNOS
inhibition were reported in some combinations of these herbs,
including bromelain and Nigella sativa L. (17,32).
Therefore, due to the high content of these compounds in
Migri-Heal® they may be involved in underlying mechanism
by which Migri-Heal® regulates inflammatory pathways.
However, further studies are required to confirm these
findings.
In response to over expression of NF-κB, Nurr1 binds
to the p65 subunit and blocks the activity of NF-κB (33). There is evidence that the orphan
nuclear receptor Nurr1 acts via a negative feedback mechanism,
modulating NF-κB activity and its target genes. It exerts
anti-inflammatory activity by docking to NF-κB-p65 on target
inflammatory gene promoters. Subsequently, Nurr1 recruits the
CoREST corepressor complex, which in turn leads to clearance of
NF-κB-p65 and transcriptional repression (33). The findings of the present study
revealed that Migri-Heal® at concentrations of 100 and
150 µg/ml may significantly reduce Nurr1 expression level when
compared with cells stimulated with LPS.
In conclusion, it appears that
Migri-Heal® may exert beneficial effects on inflammatory
pathways and reduce cell damage; however, comprehensive studies,
including in vivo assays with other signaling pathways, are
required.
Acknowledgements
The present study was supported by the National
Institute of Genetic Engineering and Biotechnology (grant no.
398).
References
|
1
|
Allan SM and Rothwell NJ: Cytokines and
acute neurodegeneration. Nat Rev Neurosci. 2:734–744. 2001.
View Article : Google Scholar
|
|
2
|
Nguyen MD, Julien JP and Rivest S: Innate
immunity: The missing link in neuroprotection and
neurodegeneration? Nat Rev Neurosci. 3:216–227. 2002. View Article : Google Scholar
|
|
3
|
Shibakawa YS, Sasaki Y, Goshima Y, Echigo
N, Kamiya Y, Kurahashi K, Yamada Y and Andoh T: Effects of ketamine
and propofol on inflammatory responses of primary glial cell
cultures stimulated with lipopolysaccharide. Br J Anaesth.
95:803–810. 2005. View Article : Google Scholar
|
|
4
|
Polazzi E and Monti B: Microglia and
neuroprotection: From in vitro studies to therapeutic applications.
Prog Neurobiol. 92:293–315. 2010. View Article : Google Scholar
|
|
5
|
Hirsch EC, Hunot S, Damier P and Faucheux
B: Glial cells and inflammation in Parkinson's disease: A role in
neurodegeneration? Ann Neurol. 44 3 Suppl 1:S115–S120. 1998.
View Article : Google Scholar
|
|
6
|
Miller DW, Cookson MR and Dickson DW:
Glial cell inclusions and the pathogenesis of neurodegenerative
diseases. Neuron Glia Biol. 1:13–21. 2004. View Article : Google Scholar :
|
|
7
|
Chang RC, Hudson P, Wilson B, Haddon L and
Hong JS: Influence of neurons on lipopolysaccharide-stimulated
production of nitric oxide and tumor necrosis factor-alpha by
cultured glia. Brain Res. 853:236–244. 2000. View Article : Google Scholar
|
|
8
|
Tikka T, Fiebich BL, Goldsteins G,
Keinanen R and Koistinaho J: Minocycline, a tetracycline
derivative, is neuroprotective against excitotoxicity by inhibiting
activation and proliferation of microglia. J Neurosci.
21:2580–2588. 2001.
|
|
9
|
Wu DC, Jackson-Lewis V, Vila M, Tieu K,
Teismann P, Vadseth C, Choi DK, Ischiropoulos H and Przedborski S:
Blockade of microglial activation is neuroprotective in the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of
Parkinson disease. J Neurosci. 22:1763–1771. 2002.
|
|
10
|
Ansari M, Rafiee K, Emamgholipour S and
Fallah MS: Migraine: Molecular basis and herbal medicine. Chen KS:
Advanced Topics in Neurological Disorders: InTech. 185–214.
2012.
|
|
11
|
Rafiee K, Ansaria M, Mahdian R, Paknejad
M, Fallah MS and Azizi M: In vitro effects of a herbal remedy for
migraine treatment, migriHeal®, on basal and LPS-induced
nitric oxide. J Basic Appl Sci Res. 3:206–211. 2013.
|
|
12
|
Obuchowicz E, Kowalski J, Labuzek K,
Krysiak R, Pendzich J and Herman ZS: Amitriptyline and
nortriptyline inhibit interleukin-1 release by rat mixed glial and
microglial cell cultures. Int J Neuropsychopharmacol. 9:27–35.
2006. View Article : Google Scholar
|
|
13
|
Zanassi P, Paolillo M, Montecucco A,
Avvedimento EV and Schinelli S: Pharmacological and molecular
evidence for dopamine D(1) receptor expression by striatal
astrocytes in culture. J Neurosci Res. 58:544–552. 1999. View Article : Google Scholar
|
|
14
|
Cao L, Fei L, Chang TT and DeLeo JA:
Induction of interleukin-1beta by interleukin-4 in
lipopolysaccharide-treated mixed glial cultures:
Microglial-dependent effects. J Neurochem. 102:408–419. 2007.
View Article : Google Scholar
|
|
15
|
Amiraslani B, Sabouni F, Abbasi S, Nazem H
and Sabet M: Recognition of betaine as an inhibitor of
lipopolysaccharide-induced nitric oxide production in activated
microglial cells. Iran Biomed J. 16:84–89. 2012.
|
|
16
|
Javadian S, Sabouni F and Haghbeen K: O
riganum V ulgare L.: Extracts versus thymol: An anti-inflammatory
study on activated microglial and mixed glial cells. J Food
Biochem. 40:100–108. 2016. View Article : Google Scholar
|
|
17
|
Habashi Abbasi S, Sabouni F, Moghimi A and
Majd Ansari S: Modulation of lipopolysaccharide stimulated nuclear
factor kappa B mediated iNOS/NO production by bromelain in rat
primary microglial cells. Iran Biomed J. 20:33–40. 2016.
|
|
18
|
Mossman BT, Jean L and Landesman JM:
Studies using lectins to determine mineral interactions with
cellular membranes. Environ Health Perspect. 51:23–25. 1983.
View Article : Google Scholar :
|
|
19
|
Smale ST: Selective transcription in
response to an inflammatory stimulus. Cell. 140:833–844. 2010.
View Article : Google Scholar :
|
|
20
|
Doherty GH: Nitric oxide in
neurodegeneration: Potential benefits of non-steroidal
anti-inflammatories. Neurosci Bull. 27:366–382. 2011. View Article : Google Scholar :
|
|
21
|
Craft JM, Watterson DM, Frautschy SA and
Van Eldik LJ: Aminopyridazines inhibit beta-amyloid-induced glial
activation and neuronal damage in vivo. Neurobiol Aging.
25:1283–1292. 2004. View Article : Google Scholar
|
|
22
|
Bogdan C: Nitric oxide and the immune
response. Nat Immunol. 2:907–916. 2001. View Article : Google Scholar
|
|
23
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar
|
|
24
|
Sarchielli P, Floridi A, Mancini ML, Rossi
C, Coppola F, Baldi A, Pini LA and Calabresi P: NF-kappaB activity
and iNOS expression in monocytes from internal jugular blood of
migraine without aura patients during attacks. Cephalalgia.
26:1071–1079. 2006. View Article : Google Scholar
|
|
25
|
Stirparo G, Zicari A, Favilla M, Lipari M
and Martelletti P: Linked activation of nitric oxide synthase and
cyclooxygenase in peripheral monocytes of asymptomatic migraine
without aura patients. Cephalalgia. 20:100–106. 2000. View Article : Google Scholar
|
|
26
|
Hassani M, Sabouni F, Emamgholipour S,
Rafiee MH, Fallah MS, Abbasi SS and Ansari M: The effect of
Migri-Heal® on nitric oxide production in an in vitro
inflammatory model of primary microglial cells. Arch Med Lab Sci.
2:54–61. 2016.
|
|
27
|
Ansari M, Rafiee Kh, Yasa N, Vardasbi S,
Naimi SM and Nowrouzi A: Measurement of melatonin in alcoholic and
hot water extracts of Tanacetum parthenium, Tripleurospermum
disciforme and Viola odorata. Daru. 18:173–178. 2010.
|
|
28
|
Gilad E, Wong HR, Zingarelli B, Virág L,
O'Connor M, Salzman AL and Szabó C: Melatonin inhibits expression
of the inducible isoform of nitric oxide synthase in murine
macrophages: Role of inhibition of NFkappaB activation. FASEB J.
12:685–693. 1998.
|
|
29
|
Peres MF, Masruha MR, Zukerman E,
Moreira-Filho CA and Cavalheiro EA: Potential therapeutic use of
melatonin in migraine and other headache disorders. Expert Opin
Investig Drugs. 15:367–375. 2006. View Article : Google Scholar
|
|
30
|
Fooladsaz K, Ansari M and Rasaie MJ:
Evaluation and comparison of serum melatonin determination in
normal individuals and migraine patients. Tehran Univ Med J.
62:37–43. 2004.
|
|
31
|
Kavoosi G, da Silva Teixeira JA and
Saharkhiz MJ: Inhibitory effects of Zataria multiflora essential
oil and its main components on nitric oxide and hydrogen peroxide
production in lipopolysaccharide-stimulated macrophages. J Pharm
Pharmacol. 64:1491–1500. 2012. View Article : Google Scholar
|
|
32
|
Alemi M, Sabouni F, Sanjarian F, Haghbeen
K and Ansari S: Anti-inflammatory effect of seeds and callus of
Nigella sativa L. extracts on mix glial cells with regard to
their thymoquinone content. AAPS Pharm Sci Tech. 14:160–167. 2013.
View Article : Google Scholar
|
|
33
|
Saijo K, Winner B, Carson CT, Collier JG,
Boyer L, Rosenfeld MG, Gage FH and Glass CK: A Nurr1/CoREST pathway
in microglia and astrocytes protects dopaminergic neurons from
inflammation-induced death. Cell. 137:47–59. 2009. View Article : Google Scholar :
|