Introduction
Isoproterenol (ISO) is a β-adrenoceptor agonist and
synthetic catecholamine. Treatment with a high dose of ISO can
result in myocardial infarction (MI) and the development of
necrotic lesions in the myocardium of experimental animals
(1,2). The majority of these undesirable
consequences in patients with MI are associated with ventricular
remodeling (VR), which occurs post-infarction (3). The mechanism underlying VR following
MI remains to be elucidated, despite the advances in medical
treatment over previous decades. VR is associated with an increased
risk of cardiovascular death and heart failure (HF) (4,5). VR
occurs in a similar terminal sequence of molecular, biochemical and
mechanical events that lead to HF. Therefore, inhibition of VR in
post-MI patients is beneficial. VR, a myocardium-associated
response to noxious, hemodynamic, metabolic and inflammatory
stimuli, is associated with mortality in patients with acute
coronary syndromes (6).
Furthermore, myocyte hypertrophy and loss following necrosis or
apoptosis, interstitial cell growth and fibroblast proliferation,
which in turn leads to myocardial fibrosis, are associated with VR
(7). VR is also affected by
preload and afterload activation of the neurohumoral system, and
other factors that further adversely influence the remodeling
process (8). The net result of
these events is the development of left ventricular hypertrophy
with or without fibrosis, which ultimately progresses to left
ventricular dilation and systolic failure (9).
Atractylodis macrocephalae rhizoma (AMR), the
dry rhizome of Atractylodes macrocephala koidz, is an edible
Chinese medicinal herb. In traditional Chinese medicine, herbal
medicinal compounds containing AMR are frequently administered in
oral treatments for a number of diseases, including congestive HF.
For example, Ling Gui Zhu Gan Tang, composed of four herbal
components, Hoelen, Cinnamomi cortex, AMR and Glycyrrhizae
radix, is frequently used for the treatment of diseases
associated with edema, including chronic bronchitis, congestive HF
and chronic nephritis (10).
However, the protective effect of AMR, as a single drug therapy in
the cardiovascular system, has not been extensively studied. The
present study investigated the effect of AMR treatment on
ISO-induced VR in order to provide experimental evidence for
potential future clinical treatments.
Materials and methods
Animals
Healthy male Sprague Dawley rats (n=37, 180–200 g,
aged 6–7 weeks) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. (Shanghai, China). The animals were individually housed
at the ambient temperature of 22–24°C and humidity of 30–50% with a
12-h light/dark cycle, and free access to standard food and water.
All rats received humane care and animal experiments were performed
in accordance with the guidelines of the Animal Care and Use
Committee of Shanghai University of Traditional Chinese Medicine
and conformed to the Guide for the Care and Use of Laboratory
Animals, published by the US National Institute of Health (11) (NIH publication number: 85–23,
revised in 1996). Ethical approval for all animal experiments
performed in the present study was obtained from the Medical Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(Shanghai, China; reference no. SZY201504022).
Materials
AMR was purchased from Kangqiao Traditional Chinese
Medicine Co., Ltd. (Shanghai, China) and decocted in water to the
concentration of 0.24 g crude drug/ml. Isoproterenol hydrochloride
(99.0% purity; lot number: MI5VB-DI) was purchased from TCI
(Shanghai) Development Co., Ltd. (Shanghai, China).
Experimental protocols
All rats were randomly allocated to the normal
control group (n=7) or ISO-induced group (n=30). Rats in the normal
control and ISO-induced groups were subcutaneously injected with
physiological saline (4 ml/kg, the solvent for ISO) and ISO 85
mg/kg/day, respectively, for two consecutive days. Following ISO
treatment, 18 rats survived in the ISO-induced group and were
randomly allocated to the AMR and ISO-induced groups (n=9). Rats in
the normal control and ISO-induced groups were administered
intragastrically with drinking water (10 ml/kg/day), and rats in
the AMR group were administered a AMR decoction at a volume of 10
ml/kg/day for 4 weeks from the second day following the
administration of ISO.
Following 4 weeks of experimentation, all rats were
anesthetized with an intraperitoneal injection of urethane (1.0
g/kg; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Once
body weight (BW) and hemodynamic parameters were measured, blood
samples were collected and centrifuged at 4°C and 1,780 × g for 10
min to recover the serum. After blood sample collection, rats were
sacrificed and hearts were removed immediately and washed in
chilled physiological saline. The left ventricles (LVs) were
separated from the atria, aorta and adipose tissue. The upper part
of the LV was fixed in 10% formalin at 22–24°C for one week and
embedded in paraffin wax, and the lower part of the LV and serum
were stored at −80°C until further analysis.
Measurement of hemodynamic
parameters
The right carotid artery was separated and
cannulated with a polyethylene 90 catheter filled with 80 U/ml
heparin saline, connected to a pressure transducer for the
measurement of systolic blood pressure (SBP), diastolic BP (DBP),
mean arterial BP (MABP), pulse pressure (PP), LV systolic pressure
(LVSP), LV end-diastolic pressure (LVEDP), and the maximum rate of
LV pressure increase and decline (+dP/dtmax and
-dP/dtmax, respectively). All parameters were
continuously recorded using a multichannel biological signal
analysis system (RM6240C; Chengdu Technology & Market Co.,
Ltd., Chengdu, China).
Determination of heart weight index
(HWI), LV weight index (LVWI) and histopathological
observations
Following the excision of hearts, excluding
connective tissue and large blood vessels, heart weight (HW) and LV
weight (LVW) were measured, and the HWI and LVWI were estimated by
calculating the ratios of HW to BW and LVW to BW.
The aforementioned fixed parts of the LVs were
dehydrated in ethanol (70–100%), cleared in xylene, and embedded in
paraffin. Each specimen was cut into 5 µm thick sections and heated
overnight in a 60°C incubator. The sections were stained with
hematoxylin and eosin (H&E) and Masson stain at room
temperature for one day. Images of each sample were captured
(magnification, ×400) under a light microscope (UB202i; Chongqing
COIC Industrial Co., Ltd., Chongqing, China). A total of three
random fields in each H&E stained sample were examined and 30
myocardial cells from each field were selected to calculate the
mean cross section area of cardiomyocytes. The interstitial
collagen volume fraction (ICVF), in the selected myocardium
sections stained with Masson stain, was calculated as a percentage
of the collagen area/field. Three sections of each sample were
randomly selected to calculate the above parameters using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Measurement of the level of N-terminal
prohormone of brain natriuretic peptide (NT-proBNP) in serum
The levels of NT-proBNP in sera were determined
using a rat NT-proBNP ELISA kit according to the manufacturer's
protocol (cat no. JL15585; Shanghai Jianglai Biotechnology Co.,
Ltd., Shanghai, China).
Measurement of malondialdehyde (MDA)
content and the activities of antioxidant enzymes in the
myocardium
LV tissue (100 mg) was homogenized with 1 ml chilled
physiological saline and centrifuged at 4°C, 1,780 × g for 15 min
to collect the supernatant. Myocardium protein and MDA levels, and
the activity of superoxide dismutase (SOD) and glutathione
peroxidase (GSH-Px) were measured using the Coomassie Brilliant
Blue method, thiobarbituric acid method, xanthine oxidase method
and a rate assay, respectively, using kits manufactured by Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). The MDA level
and activities of antioxidant enzymes in the myocardium were
expressed as their contents/mg protein of ventricular tissue.
Measurement of angiotensin II (Ang II)
and aldosterone (ALD) levels in the myocardium
The levels of Ang II and ALD in the myocardium were
measured by radioimmunoassays with an Iodine [125I]
Angiotensin II Radioimmunoassay kit (cat no. D02PZB) and Iodine
[125I] Aldosterone Radioimmunoassay kit (cat no.
D03PZB). All measurements were performed according to the
manufacturer's protocol (Northern Biotechnology Research Institute,
Beijing, China). The contents of Ang II and ALD were expressed as
their mass/mg total protein in ventricular tissue and mass/ml
serum, respectively.
Statistical analysis
Data were analyzed using one-way analysis of
variance and presented as the mean ± standard error of the mean. A
Student-Newman-Keuls post hoc test was performed for multiple
comparisons. A rank-sum test was used as an alternative test for
variance heterogeneity. Statistical analysis was performed using
SPSS software (version 21.0; IBM Corp., Armonk, NY, USA).
Experiments were repeated ≥5 times. P<0.05 was considered to
indicate a statistically significant difference.
Results
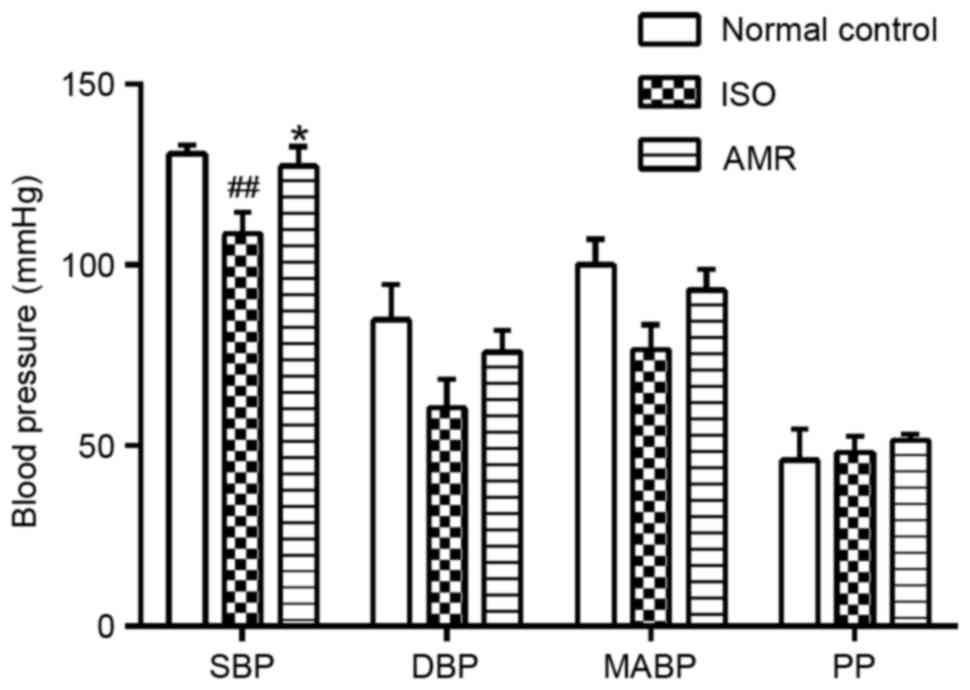
Hemodynamic parameters
SBP levels significantly decreased in the
ISO-induced group compared with the normal control group (Fig. 1; P<0.01). Administration of AMR
significantly increased SBP levels when compared with the
ISO-induced group (P<0.05), presenting levels similar to those
of the normal control group (Fig.
1). Compared with the ISO-induced group, DBP increased in the
AMR group, however, the difference was not statistically
significant. There were no differences in MABP and PP values
between all groups.
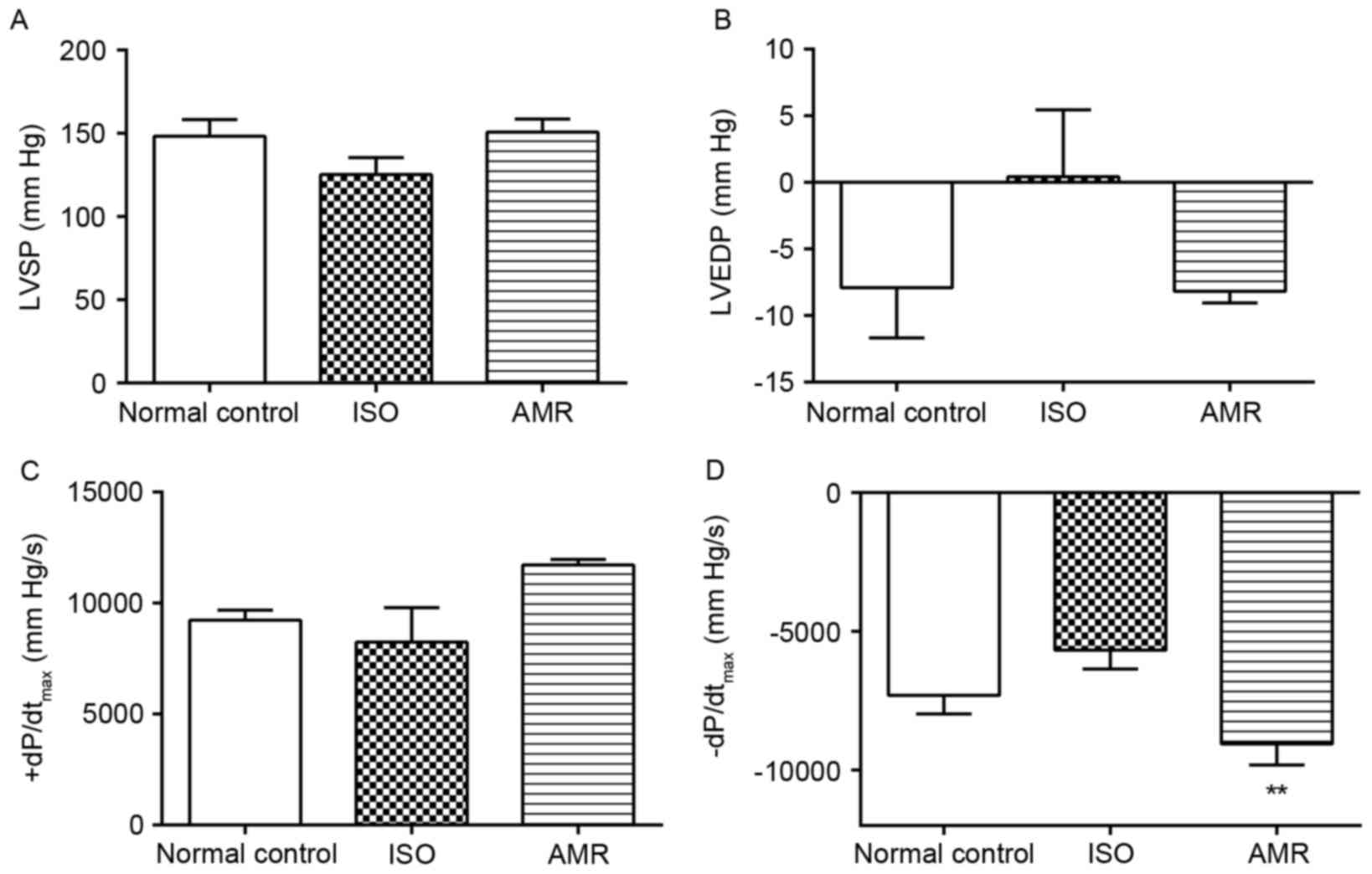
The levels of -dP/dtmax were not
significantly different in the ISO-induced group compared with the
normal control group; however, they significantly increased in the
AMR group when compared with the ISO-induced group (Fig. 2; P<0.01). LVSP, LVEDP and
+dP/dtmax levels did not significantly differ between
the groups.
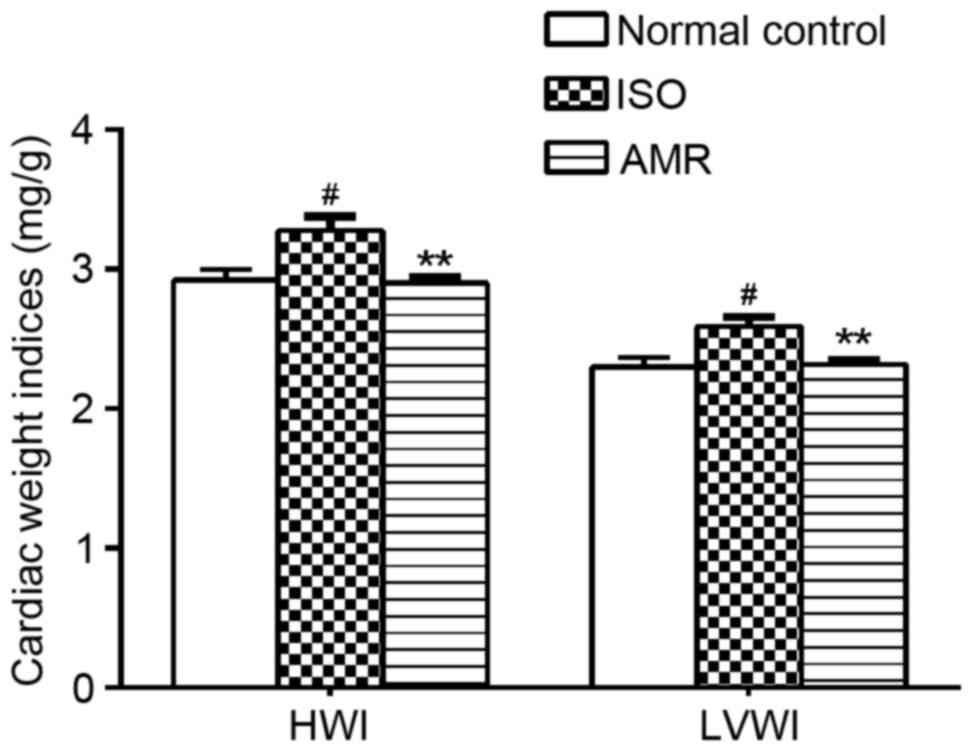
Cardiac weight indices and
histopathology
In the present study, LVWI and HWI significantly
increased in the ISO-induced group when compared with the normal
control group (both P<0.05; Fig.
3). Following 4 weeks of AMR treatment, LVWI and HWI levels in
the AMR group significantly decreased, compared with the
ISO-induced group (P<0.01).
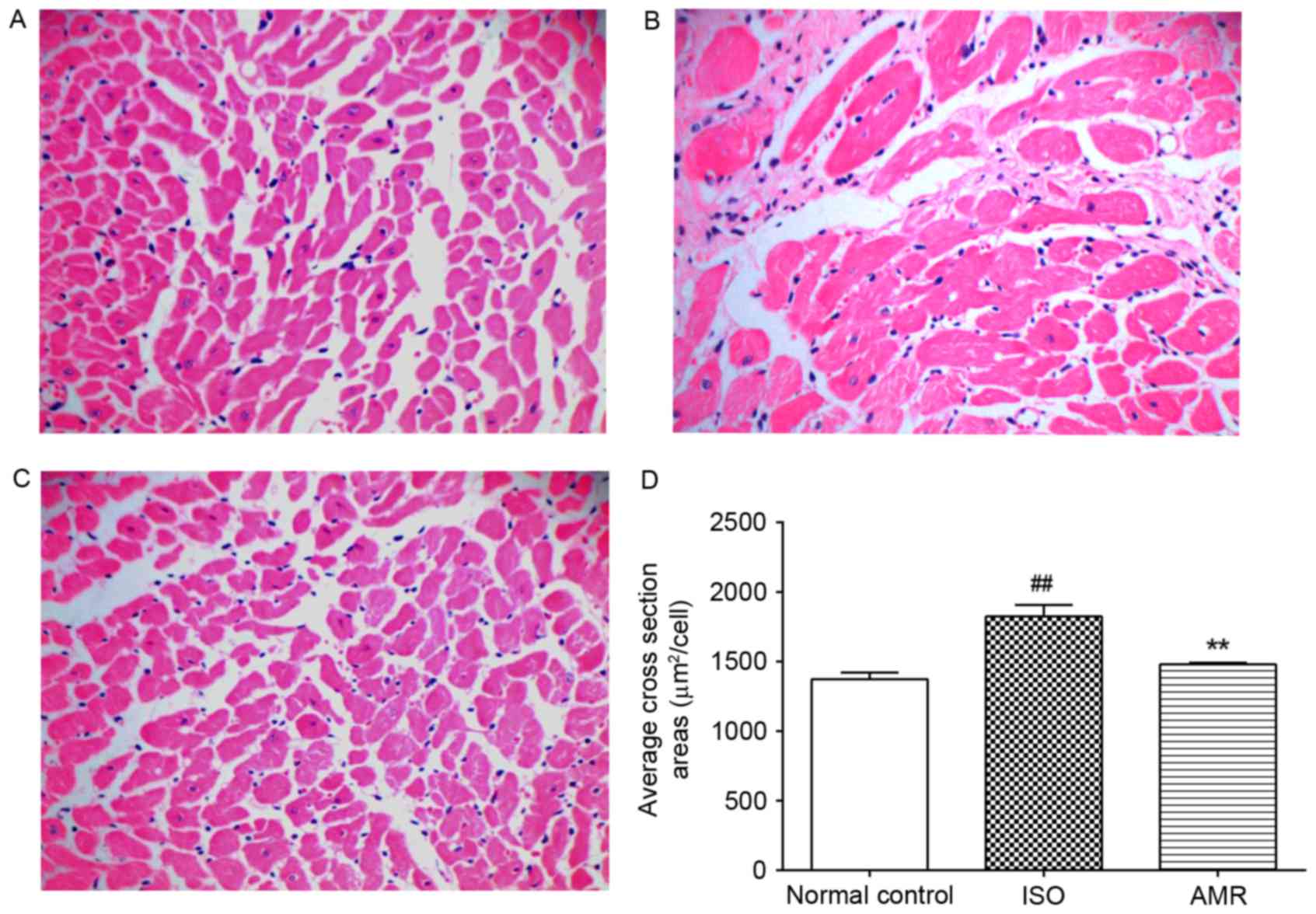
In the ISO-induced group, the average cross section
area markedly increased compared with the normal control group
(P<0.01); however, it markedly decreased in the AMR group
(P<0.01; Fig. 4). In the
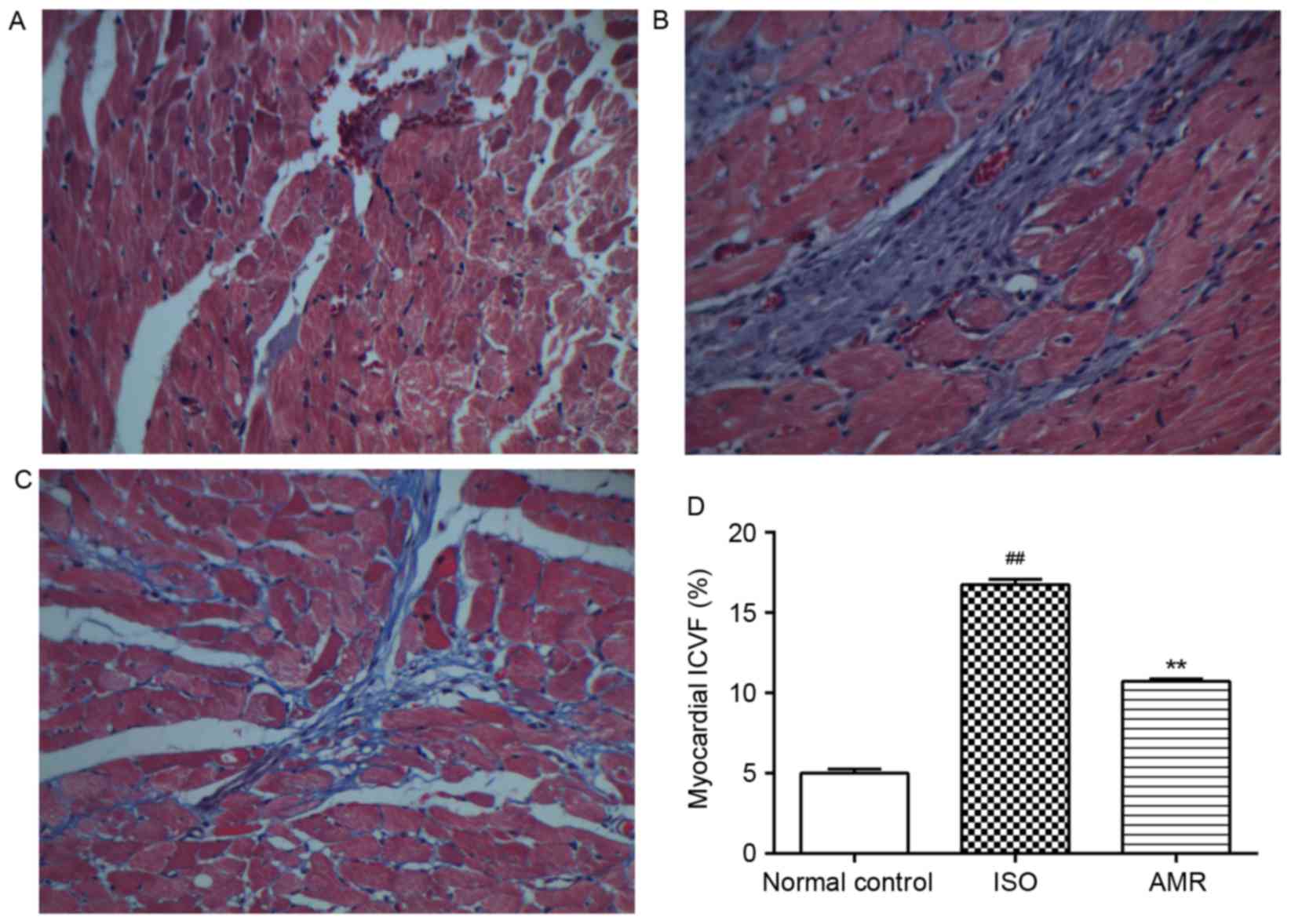
myocardium of the normal control group, a low level of collagen was
identified in the interstitial space. There was a significant
increase in the accumulation of collagen in the ventricle of the
ISO-induced group (P<0.01; Fig.
5). Decreased levels of collagen deposition were identified in
the AMR group compared with the ISO-induced group (P<0.01). The
above results were confirmed by quantification of the ICVF
(Fig. 5).
Levels of NT-proBNP in the
myocardium
Compared with the normal control group, treatment
with ISO resulted in a significant increase in the levels of
NT-proBNP in the myocardium (P<0.01; Fig. 6). Administration of AMR decreased
the levels of NT-proBNP to similar levels to those observed in the
normal control group (Fig. 6).
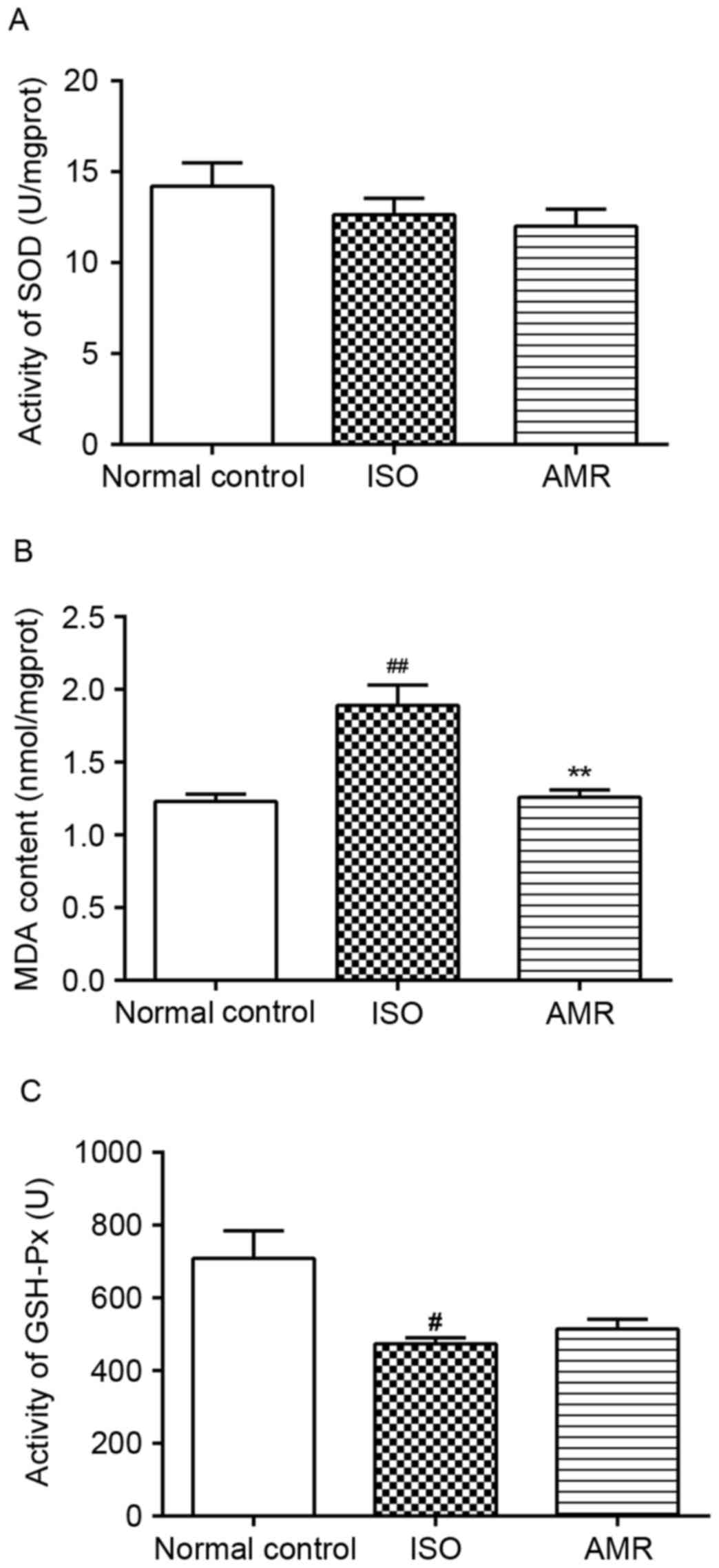
Levels of MDA, SOD and GSH-Px in the
myocardium
The levels of MDA significantly increased and the
activity of GSH-Px significantly decreased in the myocardium of the
ISO-induced group when compared with the normal control group. In
the AMR group, the level of MDA significantly decreased compared
with the ISO-induced group (P<0.01; Fig. 7). All other comparisons were not
significantly different.
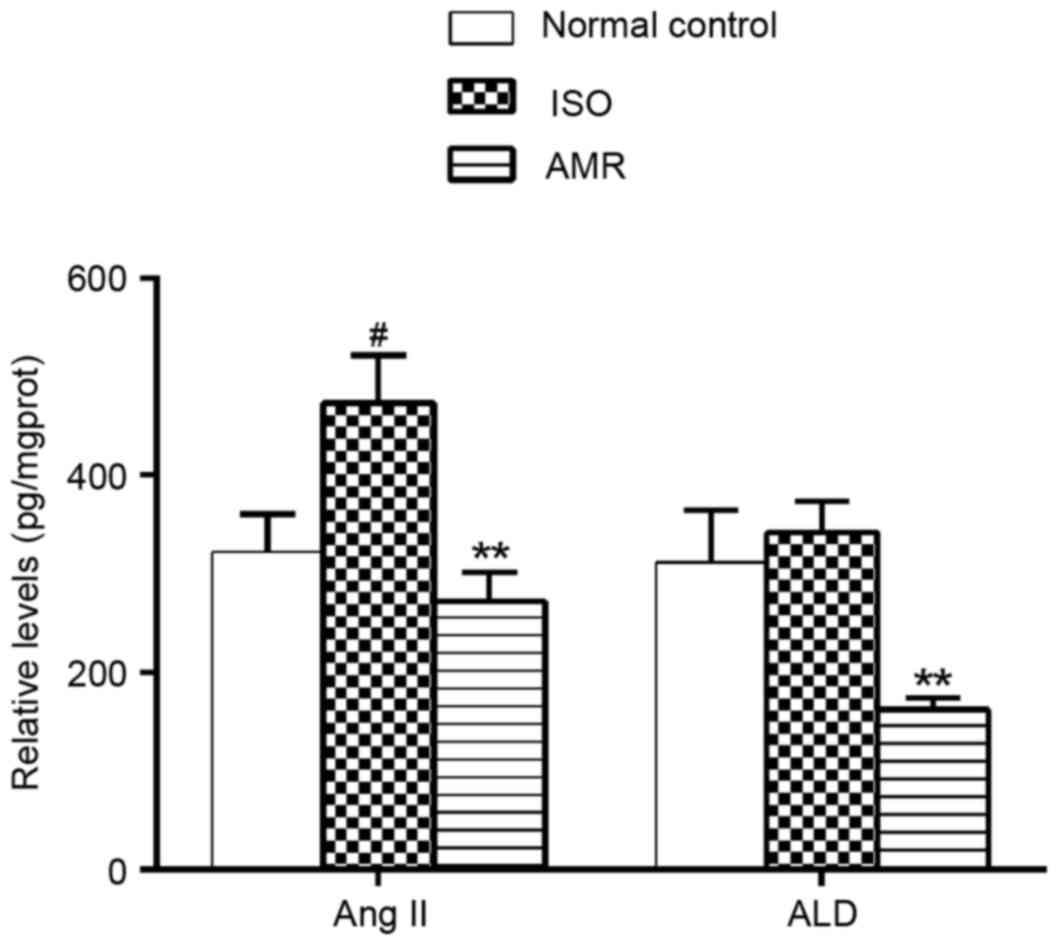
Levels of Ang II and ALD in the
myocardium
In the present study, the levels of Ang II in the
myocardium significantly increased in ISO-induced rats (P<0.05).
Administration of AMR significantly decreased the levels of Ang II
and ALD, when compared with the respective ISO-induced groups (both
P<0.01; Fig. 8).
Discussion
VR is associated with alterations in the structure
and function of the myocardium, which include cardiac dilatation,
myocardial hypertrophy, interstitial fibrosis and a reduction in
contractility and relaxation of the heart (12,13).
VR frequently occurs as a result of MI and hypertension (14,15).
Administration of a supramaximal dose of ISO can induce
cardiomyocyte necrosis, and has been used to induce MI in animal
experimental research through catecholamine toxicity (16,17)
and the subsequent stimulated VR and myocardial hypertrophy as a
result of infarct expansion. In VR animal models, cardiac WI and
the average cross section area of cardiomyocytes are indicators
used to measure the severity of cardiac hypertrophy (18,19).
In the present study, ISO treatment resulted in myocardial
hypertrophy, demonstrated by elevated levels of HWI and LVWI, and
an increased average cross section area of cardiomyocytes in rats.
These results suggested that AMR may limit the extent of myocardial
hypertrophy induced by ISO.
VR is not only measured by the extent of myocardial
hypertrophy, but also by the alterations in myocardial interstitial
composition. In cardiac tissues, as well as in other organs,
myofibroblasts are hypothesized to serve a role as predominant
cellular mediators of fibrosis (20). Myofibroblasts, the primary source
of cardiac fibrosis, can secrete extracellular matrix components
including collagen, fibronectin and laminin to promote the
development of fibrosis (21,22).
An increase in collagen secretion and aggregation can lead to
alterations in cardiac structure and the occurrence of VR or
ventricular dysfunction (23,24).
Therefore, inhibition of myocardial fibrosis can improve cardiac
function. In the present study, treatment with AMR markedly reduced
the accumulation of intercellular collagen. It can be hypothesized
that AMR may delay and inhibit fibrosis by reducing the synthesis
and secretion of collagen, and subsequently delay or inhibit the
process of VR and myocardial failure initiated by myocardial
hypertrophy or fibrosis.
In the present study, hemodynamic parameters were
used to evaluate VR. Characteristics of ISO-induced cardiac
dysfunction include diastolic and systolic dysfunction (25,26),
which may result from cardiac apoptosis and disruption of
myofibrils. Validation of effective screening tools for the
identification of patients with early stage VR (diagnostic markers)
is therefore required. In the present study, AMR inhibited the
increase in LVEDP and the reduction of SBP, DBP, LVSP and
±dP/dtmax induced by ISO. These results indicated that
following 4 weeks of treatment, AMR can alleviate LV
dysfunction.
BNP is synthesized and stored as a full-length
prohormone and cleaved to yield equimolar amounts of NT-proBNP.
NT-proBNP is secreted into the blood from cardiac ventricles in
response to excessive stretching of cardiomyocytes and volume
overload (27,28). The level of NT-proBNP is a
biomarker for the diagnosis and monitoring of VR (29). In the present study, rats treated
with AMR demonstrated a decrease in the level of NT-proBNP,
suggesting that AMR may protect the heart from ISO-induced VR and
heart failure.
ROS accumulate to promote oxidative stress (OS) and
lipid membrane peroxidation (30,31).
Accumulation of quinone metabolites induced by ISO may result in
OS, which reacts with oxygen to produce superoxide anions
(O2−), hydroxyl radicals (OH−) and hydrogen
peroxide (H2O2) species, and interfere with
antioxidant enzymes (32). ROS
degrade polyunsaturated lipids, leading to the formation of MDA as
a final product of lipid peroxidation (31). MDA causes toxic stress in cells and
it is used as a biomarker to measure the level of OS (33). Antioxidant enzymes, including SOD
and GSH-Px, can remove ROS to inhibit OS-associated injury and
protect organisms from the release of free radicals (32). In the present study, the activity
of GSH-Px markedly decreased and MDA content increased in
ISO-induced rats. AMR inhibited the increase in MDA levels induced
by ISO. These results suggested that AMR may alleviate OS injury
induced by ISO following 4 weeks.
The rennin-angiotensin-aldosterone system (RAAS) is
an endocrine system that serves a role in the development and
progression of cardiovascular diseases. Activation of RAAS is
associated with VR (34,35). Ang II serves a role in promoting
cardiac hypertrophy, fibrosis, the production of ALD, retention of
water and sodium, and the activation of the sympathetic nervous
system (36). Therefore, constant
elevated levels of Ang II and ALD in the myocardium can induce
deleterious effects on the cardiovascular system. Treatment with
AMR can reduce the levels of Ang II and ALD. These results
indicated that AMR can reduce the activation of RAAS in the
myocardium.
In conclusion, AMR may prevent VR induced by ISO in
rats, by inhibiting cardiac hypertrophy, myocardial fibrosis,
NT-proBNP levels and by improving the levels of hemodynamic
parameters. It can be hypothesized that AMR-induced mitigation of
VR is associated with its anti-oxidative effect and prevention of
activation of RAAS. The results may be helpful to fundamental
pharmacology research and AMR may be an effective treatment for
patients with VR and improve the clinic therapy strategies.
References
|
1
|
Laky D, Constantinescu S, Filipescu G,
Ratea E and Zeană C: Morphophysiological studies in experimental
myocardial aggressions with isoproterenol. Note I. Morphological
aspects. Morphol Embryol (Bucur). 29:273–277. 1983.PubMed/NCBI
|
|
2
|
Kung HF and Blau M: Subcutaneous
isoproterenol: A convenient rat model for early detection of
myocardial necrosis. J Nucl Med. 19:948–951. 1978.PubMed/NCBI
|
|
3
|
Parikh NI, Gona P, Larson MG, Fox CS,
Benjamin EJ, Murabito JM, O'Donnell CJ, Vasan RS and Levy D:
Long-term trends in myocardial infarction incidence and case
fatality in the National Heart, Lung, and Blood Institute's
Framingham Heart study. Circulation. 119:1203–1210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sutton MG and Sharpe N: Left ventricular
remodeling after myocardial infarction: Pathophysiology and
therapy. Circulation. 101:2981–2988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gajarsa JJ and Kloner RA: Left ventricular
remodeling in the post-infarction heart: A review of cellular,
molecular mechanisms, and therapeutic modalities. Heart Fail Rev.
16:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon HJ, Jeong MH, Jeong Y, Kim KH, Song
JE, Cho JY, Jang SY, Jeong HC, Lee KH, Park KH, et al: Progressive
dilation of the left atrium and ventricle after acute myocardial
infarction is associated with high mortality. Korean Circ J.
43:731–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olivetti G, Abbi R, Quaini F, Kajstura J,
Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski
S, et al: Apoptosis in the failing human heart. N Engl J Med.
336:1131–1141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heusch G, Libby P, Gersh B, Yellon D, Böhm
M, Lopaschuk G and Opie L: Cardiovascular remodelling in coronary
artery disease and heart failure. Lancet. 383:1933–1943. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuznetsova T, Herbots L, Jin Y,
Stolarz-Skrzypek K and Staessen JA: Systolic and diastolic left
ventricular dysfunction: From risk factors to overt heart failure.
Expert Rev Cardiovasc Ther. 8:251–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song ZH, Dai SJ, Li HQ, Bi KS and Feng D:
Study on the compatibility of composite herbal medicines of the
lingguizhugan decoction. Zhongguo Zhong Yao Za Zhi. 27:760–762.
2002.(In Chinese). PubMed/NCBI
|
|
11
|
Council NR: Guide for the Care and Use of
Laboratory Animals. The National Academies Press; Washington, DC:
1996
|
|
12
|
Remme WJ: Pharmacological modulation of
cardiovascular remodeling: A guide to heart failure therapy.
Cardiovasc Drugs Ther. 17:349–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swynghedauw B: Molecular mechanisms of
myocardial remodeling. Physiol Rev. 79:215–262. 1999.PubMed/NCBI
|
|
14
|
Mitsi AC, Hatzistergos KE, Niokou D, Pappa
L, Baltogiannis GG, Tsalikakis DG, Papalois A, Kyriakides ZS,
Malamou-Mitsi V and Kolettis TM: Early, intracoronary growth
hormone administration attenuates ventricular remodeling in a
porcine model of myocardial infarction. Growth Horm IGF Res.
16:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maisch B: Ventricular remodeling.
Cardiology. 87 Suppl 1:S2–S10. 1996. View Article : Google Scholar
|
|
16
|
Rona G, Chappel CI, Balazs T and Gaudry R:
An infarct-like myocardial lesion and other toxic manifestations
produced by isoproterenol in the rat. AMA Arch Pathol. 67:443–455.
1959.PubMed/NCBI
|
|
17
|
Tisné-Versailles J, Constantin M, Lamar JC
and Pourrias B: Cardiotoxicity of high doses of isoproterenol on
cardiac haemodynamics and metabolism in SHR and WKY rats. Arch Int
Pharmacodyn Ther. 273:142–154. 1985.PubMed/NCBI
|
|
18
|
Ji K, Minakawa M, Fukui K, Suzuki Y and
Fukuda I: Increased superoxide radical with a decrease in vascular
endothelial growth factor and inducible nitric oxide synthase level
leads to the progression of left ventricular hypertrophy in a
pressure-overload rat heart model. Ann Thorac Cardiovasc Surg.
14:210–217. 2008.PubMed/NCBI
|
|
19
|
Hohimer AR, Davis LE and Hatton DC:
Repeated daily injections and osmotic pump infusion of
isoproterenol cause similar increases in cardiac mass but have
different effects on blood pressure. Can J Physiol Pharmacol.
83:191–197. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krzesinski JM, Rorive G and Van
Cauwenberge H: Hypertension and left ventricular hypertrophy. Acta
Cardiol. 51:143–154. 1996.PubMed/NCBI
|
|
24
|
Weber KT and Brilla CG: Pathological
hypertrophy and cardiac interstitium. Fibrosis and
renin-angiotensin-aldosterone system. Circulation. 83:1849–1865.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teerlink JR, Pfeffer JM and Pfeffer MA:
Progressive ventricular remodeling in response to diffuse
isoproterenol-induced myocardial necrosis in rats. Circ Res.
75:105–113. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HJ, Wang W, Cornish KG, Rozanski GJ
and Zucker IH: Cardiac sympathetic afferent denervation attenuates
cardiac remodeling and improves cardiovascular dysfunction in rats
with heart failure. Hypertension. 64:745–755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Semenov AG, Seferian KR, Tamm NN,
Artem'eva MM, Postnikov AB, Bereznikova AV, Kara AN, Medvedeva NA
and Katrukha AG: Human pro-B-type natriuretic peptide is processed
in the circulation in a rat model. Clin Chem. 57:883–890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dogan H, Sarikaya S, Neijmann ST, Uysal E,
Yucel N, Ozucelik DN, Okuturlar Y, Solak S, Sever N and Ayan C:
N-terminal pro-B-type natriuretic peptide as a marker of blunt
cardiac contusion in trauma. Int J Clin Exp Pathol. 8:6786–6792.
2015.PubMed/NCBI
|
|
29
|
Tziakas DN, Chalikias GK, Hatzinikolaou
EI, Stakos DA, Tentes IK, Kortsaris A, Hatseras DI and Kaski JC:
N-terminal pro-B-type natriuretic peptide and matrix
metalloproteinases in early and late left ventricular remodeling
after acute myocardial infarction. Am J Cardiol. 96:31–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parthasarathy A, Gopi V, Devi K, M S,
Balaji N and Vellaichamy E: Aminoguanidine inhibits ventricular
fibrosis and remodeling process in isoproterenol-induced
hypertrophied rat hearts by suppressing ROS and MMPs. Life Sci.
118:15–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pryor WA and Stanley JP: Letter: A
suggested mechanism for the production of malonaldehyde during the
autoxidation of polyunsaturated fatty acids. Nonenzymatic
production of prostaglandin endoperoxides during autoxidation. J
Org Chem. 40:3615–3617. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rathore N, John S, Kale M and Bhatnagar D:
Lipid peroxidation and antioxidant enzymes in isoproterenol induced
oxidative stress in rat tissues. Pharmacol Res. 38:297–303. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sayer G and Bhat G: The
renin-angiotensin-aldosterone system and heart failure. Cardiol
Clin. 32:21–32, vii. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gregori M, Tocci G, Marra A, Pignatelli G,
Santolamazza C, Befani A, Ciavarella GM, Ferrucci A and Paneni F:
Inadequate RAAS suppression is associated with excessive left
ventricular mass and systo-diastolic dysfunction. Clin Res Cardiol.
102:725–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neves MF, Amiri F, Virdis A, Diep QN and
Schiffrin EL; CIHR Multidisciplinary Research Group on
Hypertension, : Role of aldosterone in angiotensin II-induced
cardiac and aortic inflammation, fibrosis, and hypertrophy. Can J
Physiol Pharmacol. 83:999–1006. 2005. View
Article : Google Scholar : PubMed/NCBI
|