Introduction
Experimental transient cerebral ischemia (TCI)
induced by the reduction of blood supply to the brain by the
occlusion of the bilateral common carotid arteries results in
irreversible neuronal damage in some sensitive brain regions, such
as the hippocampus (1–3). The region I of hippocampus proper
(CA1), in particular, is well known for the grading of the
susceptibility to transient global cerebral ischemia (4,5). The
loss of neurons in the adult hippocampal CA1 region progressively
occurs from day 3 to 4 after a transient ischemic injury, which is
referred to as ‘delayed neuronal death’ (DND) (6). It is well known that the process of
DND is different according to age. For instance, it has been
reported that young animals are partly resistant to
ischemia-induced neuronal damage (7). Also, our previous studies clearly
showed that after 5 min of TCI DND occurred from day 7 to 10 in the
young gerbil, which was considerably more delayed and less severe
than that in the adult gerbils (7,8).
The growth hormone insulin-like growth factor 1
(IGF-1) is a well-known growth factor with well-defined effects on
many organs, including the brain (9). IGF-1 is highly expressed throughout
the brain, including the cortex and hippocampus (10,11).
Many researchers have reported that IGF-1 participates in neuronal
survival and glucose utilization in the hippocampus (12,13).
It is also reported that IGF-1 could regulate neuronal integration
into the synaptic circuits of the hippocampus (14,15).
In addition, IGF-1 was found to promote synapse formation and
prevent the death of neurons in neurodegenerative diseases
(15,16). Additionally, blunting IGF-1 was
reported to cause an obvious decrease in the survival of neurons
under basal conditions or after hypoxia/ischemia (17). Most of the IGF-1 actions are
primarily mediated through its receptor (IGF-1R). Indeed, IGF-1 is
the main ligand for IGF-1R (18),
which is a multifunctional transmembrane tyrosine kinase (19,20).
In the nervous system, IGF-1 exerts its biological function by
binding with IGF-1R, which can integrate with the enhancement of
protein synthesis, cell survival and cell proliferation (21,22).
Recent research demonstrated that the IGF-1R also plays an
essential role in neuroprotection following hypoxia/ischemia
(17,23).
The expression of IGF-1 and IGF-1R is significantly
changed after cerebral ischemia injury (24). Also, some researchers have reported
that IGF-1 and IGF-1R, which are expressed in the hippocampus, are
associated with the loss of neurons (25,26).
In addition, it has also been reported that the expression levels
of IGF-1 and IGF-1R decrease in an age-dependent manner (27). However, few studies have focused on
the effect of the age-dependent change of IGF-1 and IGF-1R
expression in the brain following TCI. Accordingly, in the present
study, we examined the change in the expression of IGF-1 and IGF-1R
in the hippocampal CA1 region of young gerbil (postnatal 1 month)
after TCI and compared them with those in the adult gerbil
(postnatal 6 month) after TCI (28).
Materials and methods
Experimental animals
The male Mongolian gerbils (Meriones
unguiculatus) were bought from the Experimental Animal Center
of the Kangwon National University (Chuncheon, South Korea).
Mongolian gerbils aged 1-month old (body weight, 25–30 g) were
divided into the young group and those aged 6 months old (body
weight, 65–75 g) were divided into the adult group. The animals
were housed in pathogen-free conditions at 23±2°C and 58±2%
relative humidity, with a 12-h light/12-h dark cycle, and had free
access to food and water. Procedures involving animals handling and
their care were conformed to current international laws and
policies (Guide for the Care and Use of Laboratory Animals, the
National Academies Press, 8th Edition, 2011). All efforts were made
to minimize animal suffering and the number of experimental
animals. The animal protocol was approved based on ethical
procedures and scientific care by the Yangzhou
University-Institutional Animal Care and Use Committee
(YIACUC-14-0013).
Induction of TCI
Transient global cerebral ischemia was induced as
follows, which was described previously (29). The bilateral common carotid artery
of gerbil was occluded using non-traumatic aneurysm clips under
anesthesia by inhalation of 2.5% isoflurane in 33% oxygen and 67%
nitrous oxide. The complete interruption of blood flow was
confirmed by observing the central artery in retinae using an
ophthalmoscope. After 5 min of occlusion, the aneurysm clips were
removed from the common carotid arteries. The restoration of blood
flow (reperfusion) was observed directly using the ophthalmoscope.
The body (rectal) temperature under free-regulating or normothermic
(37±0.5°C) conditions was monitored with a rectal temperature
probe. Meanwhile, the body temperature was maintained by a
thermometric blanket before, during and after the surgery until the
animals completely recovered from anesthesia. Thereafter, animals
were kept on the thermal incubator to maintain the body temperature
until the animals were euthanized. Sham-operated animals were
subjected to the same surgical procedures except that the common
carotid arteries were not occluded.
Tissue processing for histology
Tissues were collected as previously described
(30), sham-(n=7 at each time
point) and ischemia-operated young (n=7 at each time point) and
adult (n=7 at each time point) gerbils at designated times (1, 4
and 7 days after reperfusion) were sacrificed. The animals were
anesthetized with pentobarbital sodium and perfused transcardially
with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4%
paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains
were removed and postfixed in the same fixative for 6 h. The brain
tissues were cryoprotected by infiltration with 30% sucrose
overnight. Thereafter, frozen tissues were serially sectioned on a
cryostat (Leica Microsystems GmbH, Wetzlar, Germany) into 30-µm
coronal sections, and they were then collected into 6-well plates
containing PBS.
Immunohistochemistry
Immunohistochemistry was performed according to a
previously published procedure (30). To examine the change of Neuronal
nuclei (NeuN), IGF-1 and its receptors in the CA1 after
ischemia-reperfusion, we carried out immunohistochemical staining
with rabbit anti-NEUN (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-IGF-1 and IGF-1R (1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and biotinylated goat
anti-rabbit IgG (1:250; Vector Laboratories, Inc., Burlingame, CA,
USA) for secondary antibody. In order to establish the specificity
of the immunostaining, a negative control test was carried out with
pre-immune serum instead of primary antibody. The negative control
resulted in the absence of immunoreactivity in all structures.
Densities of NEUN, IGF-1 and IGF-1R immunoreactive
structures were measured as previously described (11), Digital images of the hippocampal
subregions were captured with an image analyzing system equipped
with a computer-based microscope (Nikon Corporation, Tokyo, Japan).
Cell counts were obtained by averaging the counts from the sections
taken from each animal. In addition, the staining intensity of
IGF-1 and IGF-1R immunoreactive structures was evaluated on the
basis of an optical density (OD), which was obtained after the
transformation of the mean gray level using the formula: OD=log
(256/mean gray level). The OD of background was taken from areas
adjacent to the measured area. After the background density was
subtracted, a ratio of the OD of image file was calibrated as %
(relative OD, ROD) using Adobe Photoshop version 8.0 and then
analyzed using NIH Image 1.59 software. All measurements were
performed in order to ensure objectivity in blind conditions, by
two observers for each experiment, carrying out the measures of
experimental samples under the same conditions.
Western blot analysis
In order to examine the protein levels of IGF-1 and
its receptors in the ischemic CA1 region, the animals (n=7 at each
time point) were used for western blot analysis sham, 4, 7 days
after the ischemic surgery in the young and adult group. Western
blot analysis was performed out according to a previously published
procedure (31). After sacrificing
them and removing the brains, they were serially and transversely
cut into a thickness of 400 µm on a vibratome, and the hippocampus
was dissected with a surgical blade. They were preprocessed by
Whole Cell Lysis Assay kit (KeyGEN, Nanjing, China)/Total Protein
Extraction kit. Protein concentrations were determined using a
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Equal amounts of protein (30 µg) were separated
by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to nitrocellulose membranes (EMD
Millipore, Bedford, MA, USA). In order to incubate antibodies, the
same nitrocellulose membranes striped were used. To reduce
background staining, the membranes were incubated with 5% BSA in
TBS containing 0.1% Tween-20 for 60 min, followed by incubation
with rabbit anti-IGF-1, IGF-1R (1:1,000; Santa Cruz Biotechnology,
Inc.) and β-actin (1:1,000; Cell Signaling Technology, Inc.)
overnight at 4°C and subsequently exposed to secondary goat
anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature and the SuperSignal West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.) was used for protein
detection. The result of western blot analysis was scanned, and
densitometric analysis for the quantification of the bands was done
using Quantity One Analysis Software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), which was used to count relative optical
density (ROD): A ratio of the ROD was calibrated as %, with
sham-group designated as 100%. Each blot shown is representative of
at least three similar independent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hippocampus of brain
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Then, total RNA
(2 µg) was purified with the purification kit (Thermo Fisher
Scientific, Inc.), and reversely transcribed into cDNA using the
PrimeScript™ II 1st Strand cDNA synthesis kit (Takara
Bio, Inc., Dalian, Japan) according to the manufacturer's
instructions. RT-qPCR was performed using the PrimeScript™ RT
Master Mix (Takara Bio, Inc.) on a LightCycler® 96
Real-Time PCR System (Roche Diagnostics, Basel, Switzerland). The
2-step PCR was performed: 95°C for 10 sec and 40 cycles of 95°C for
5 sec and 60°C for 30 sec. The RNA U6 mRNA was used as internal
controls. All the reactions were conducted in triplicate. Primers
for U6 were: 5′-GGAACGATACAGAGAAGATTAGC-3′ (forward) and
5′-TGGAACGCTTCACGAATTTGCG-3′ (reverse). Primers for IGF-1 were
5′-CTGGACCAGAGACCCTTTGC-3′ (forward) and
5′-GGACGGGGACTTCTGAGTCTT-3′ (reverse); Primers for IGF-1R were
5′-ACACGCAACGAGACTAATCG-3′ (forward) and 5′-TTAGAGACTGAGCGGCATCC-3′
(reverse). Data are shown as the fold-change.
Statistical analysis
Data are expressed as the mean ± SEM. The data were
evaluated by a one-way ANOVA SPSS program, and the means assessed
using Duncan's multiple-range test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Neuronal damages
In the adult sham-group, NeuN immunoreactive neurons
were easily observed in the striatum pyramidale (SP) of the
hippocampus including CA1-3 region (Fig. 1A-C). However, seven day after
ischemia/reperfusion (I/R), a few NeuN immunoreactive neurons were
found in the SP of the hippocampal CA1 not CA2-3 region (Fig. 1G-I).
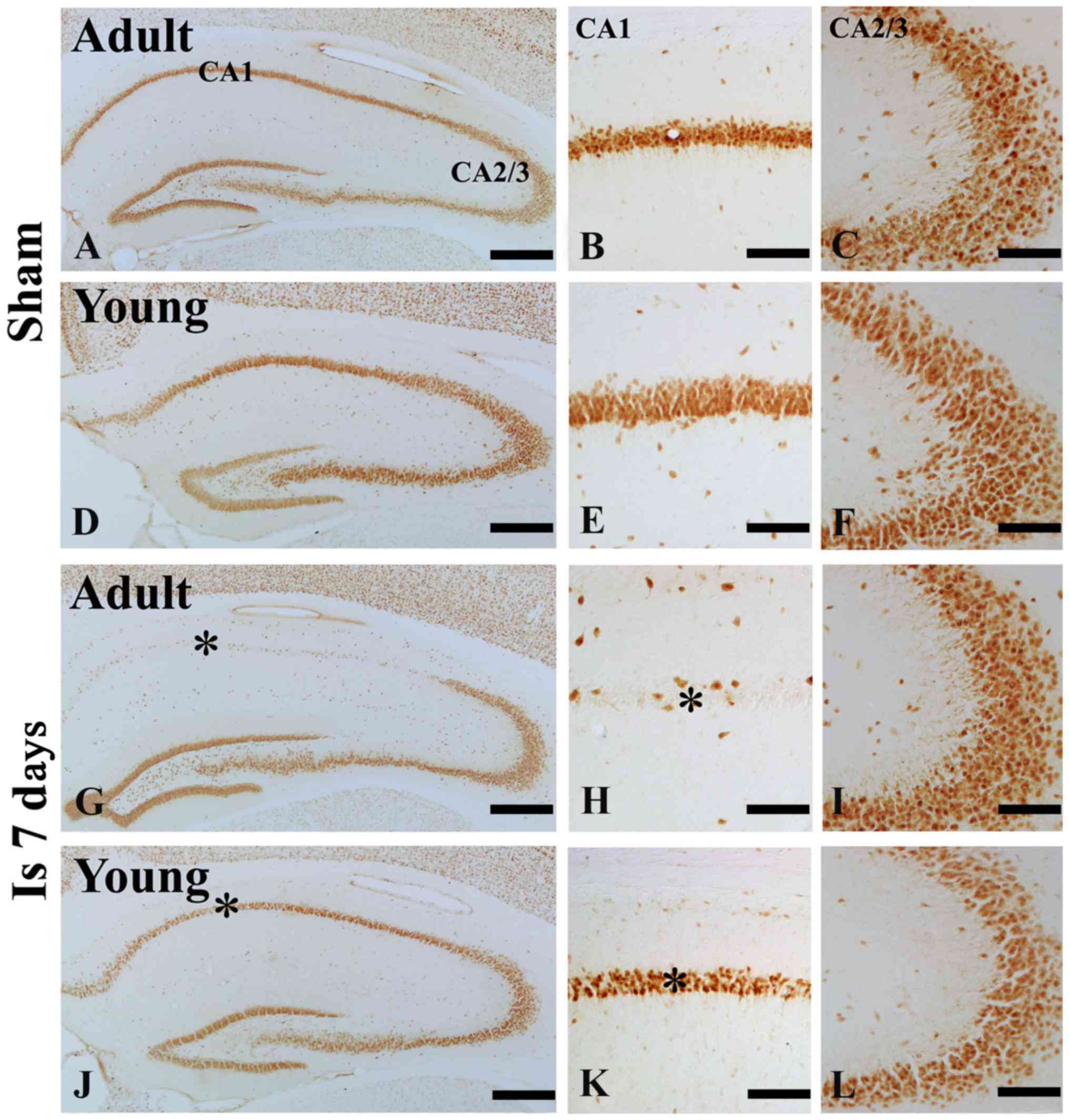 | Figure 1.NeuN immunohistochemistry in the
hippocampal CA1 and CA 2/3 region of the (A-F) sham and (G-L) 7 day
post-ischemia groups in the adult- and young gerbils. The number of
NeuN immunoreactive cells in the 7 day post ischemia group of young
gerbils was greater than that observed in the adults. Scale
bars=200 µm (A, D, G and J) and 50 µm (B, C, E, F, H, I, K and L).
The * on each image indicates the expression of NeuN. NeuN,
neuronal nuclei; CA, cornus ammonis; Is 7 d, 7 day post-ischemia
group. |
In the young sham-group, NeuN immunoreactive neurons
in the SP of the hippocampus were also well observed (Fig. 1D-F). In the 7 days after I/R, the
number of NeuN immunoreactive neurons in the SP was significantly
much more than that in the adult group (Fig. 1J-L).
IGF-1 immunoreactivity
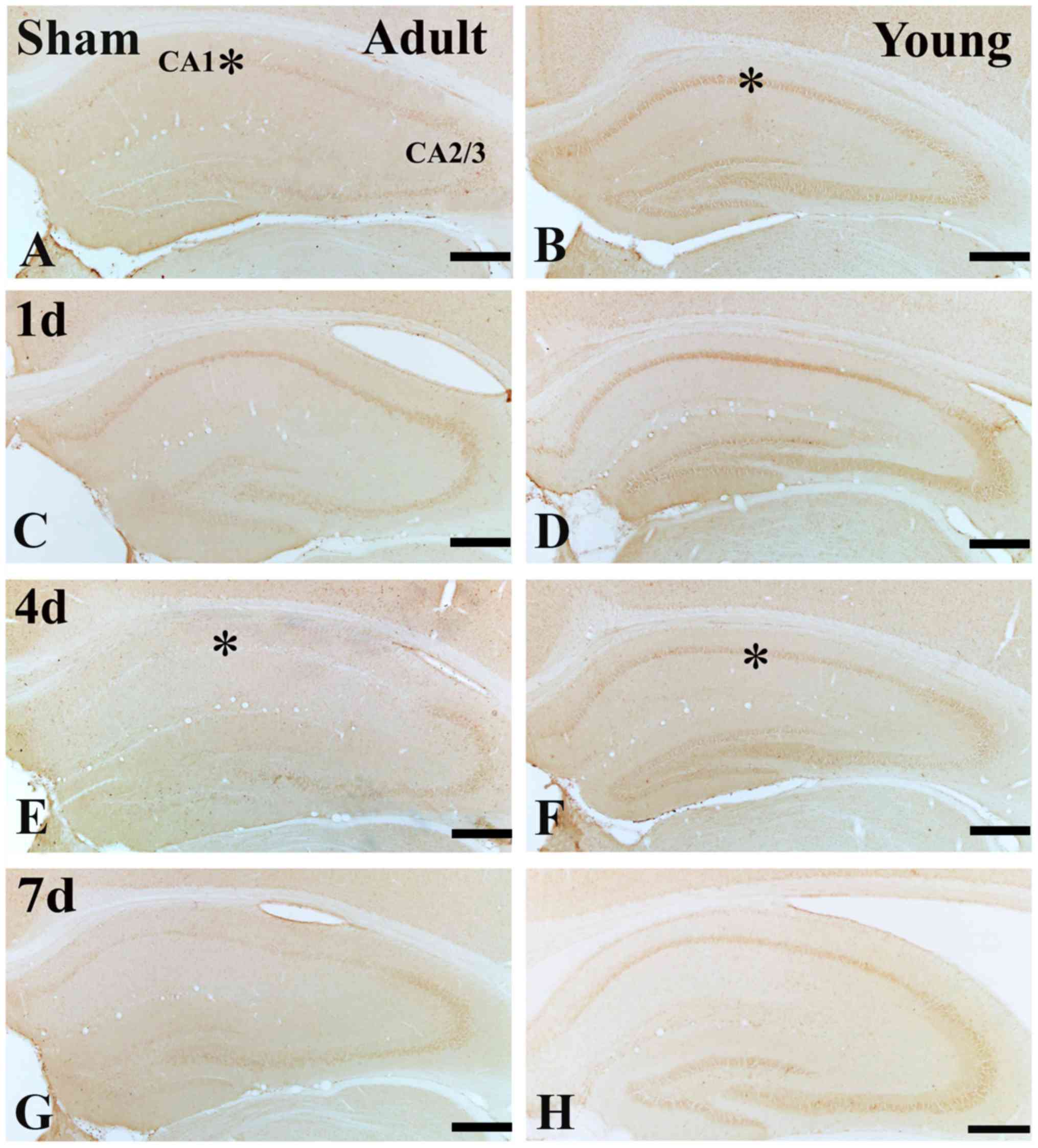
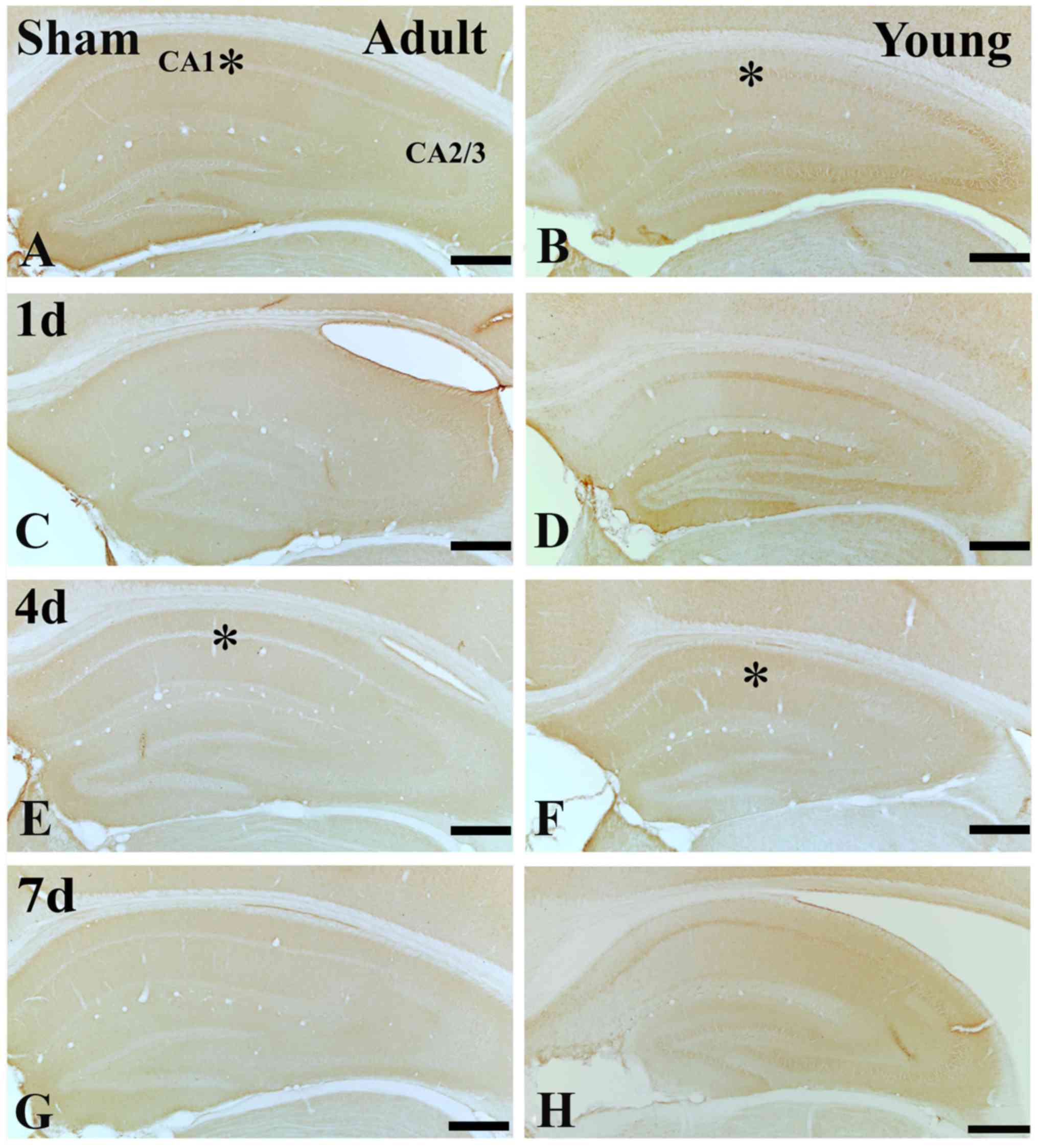
In the adult sham-group, IGF-1 immunoreactivity in
the SP was much lower than that of the young sham-group (Figs. 2A and B; and 3A, B and I). One day after I/R, IGF-1
immunoreactivity in the SP was increased (Figs. 2C; and 3C and I), its immunoreactivity was
apparently decreased at 4 days after I/R (Figs. 2E; and 3E and I). Seven days after I/R, the
immunoreactivity was similar to that at 4 days after I/R. (Figs. 2B; and 3B and I).
In the young sham-group, strong IGF-1
immunoreactivity was found in the SP of the CA1 region (Figs. 2B and 3B). IGF-1 immunoreactivity was maintained
until 4 days post-ischemia in the SP of the CA1 region (Figs. 2D and F; and 3D and F). Seven days after I/R, IGF-1
immunoreactivity in the SP was obviously decreased (Figs. 2H; and 3H and I).
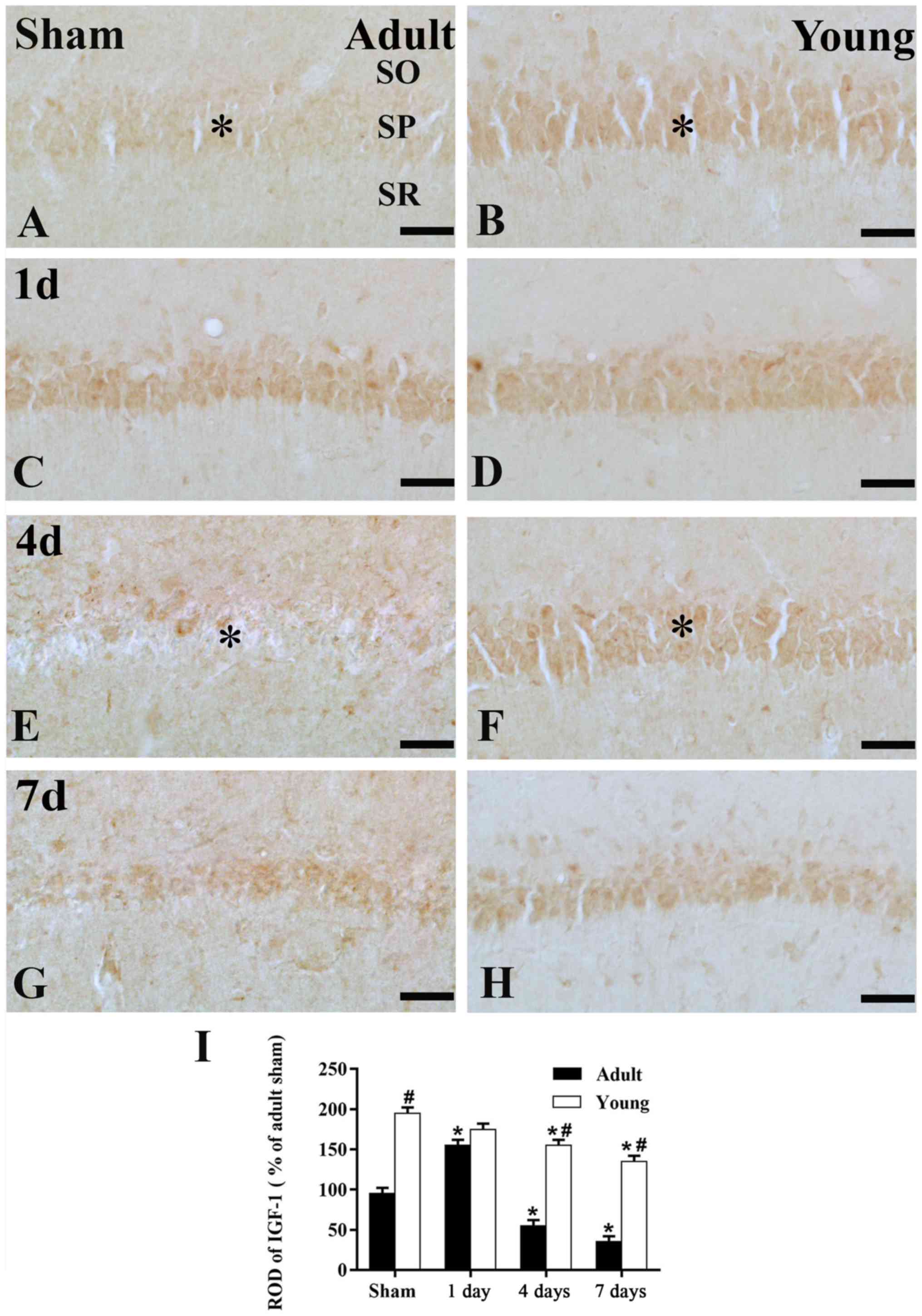
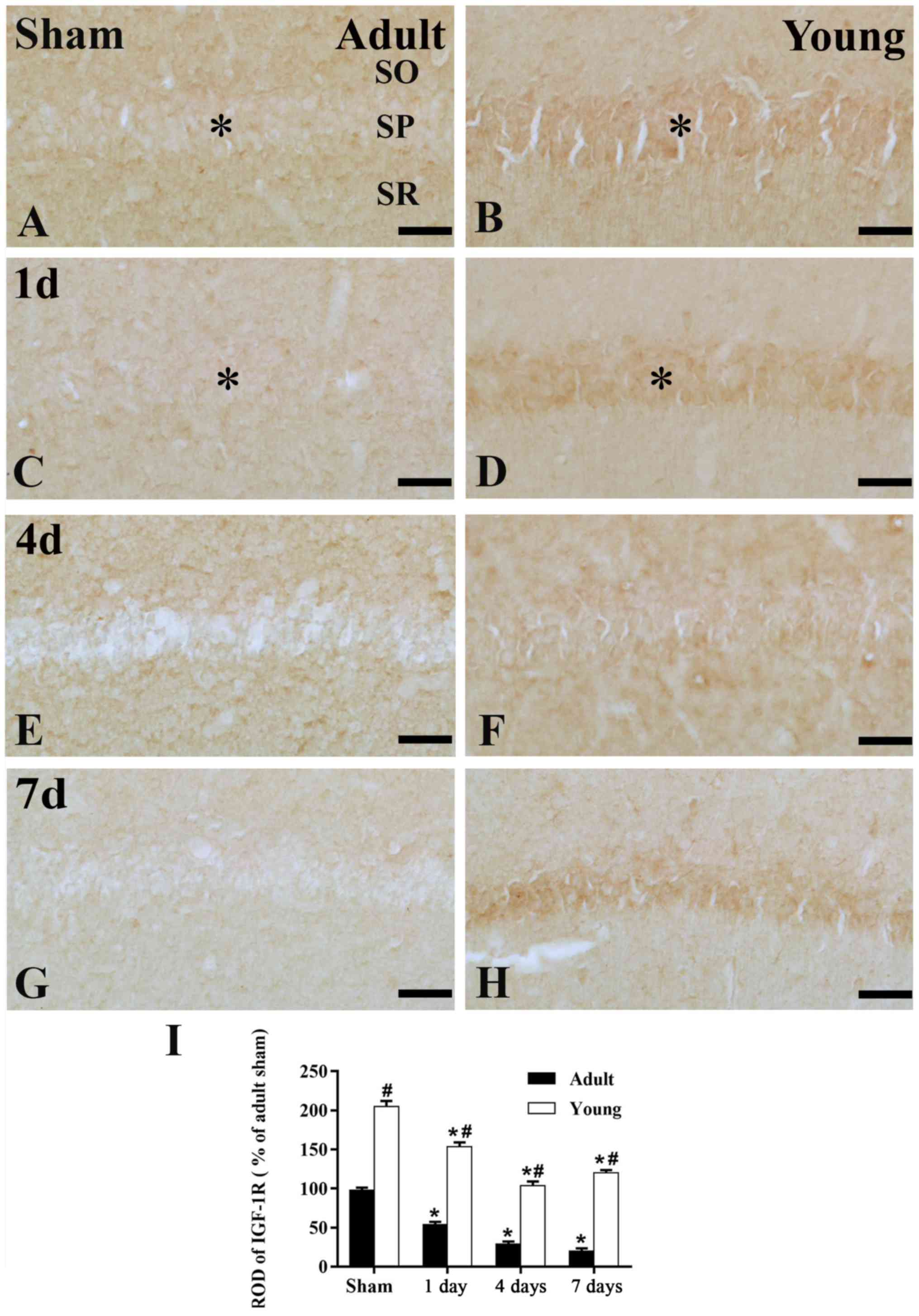
IGF-1R immunoreactivity
In the adult sham-group, IGF-1R immunoreactivity in
the CA1 region was much lower than that in the young sham-group
(Figs. 4A, B and E; and 5A, B and
E). One day after I/R, IGF-1R immunoreactivity was decreased in SP
(Figs. 4C; and 5C and I). Four days after I/R, IGF-1R
immunoreactivity was hardly detected in SP of the CA1 region
(Figs. 4E and 5E). Thereafter, IGF-1R immunoreactivity
in the SP was weakly detected in the CA1 region (Figs. 4G and 5G).
In the young sham-group, IGF-1R immunoreactivity in
the CA1 region was strongly detected in the SP of CA1 region.
(Figs. 4B and 5B). All groups after I/R, IGF-1R
immunoreactivity in the SP of young was much higher than that in
the adult (Figs. 4D, F and H; and
5F, H and I).
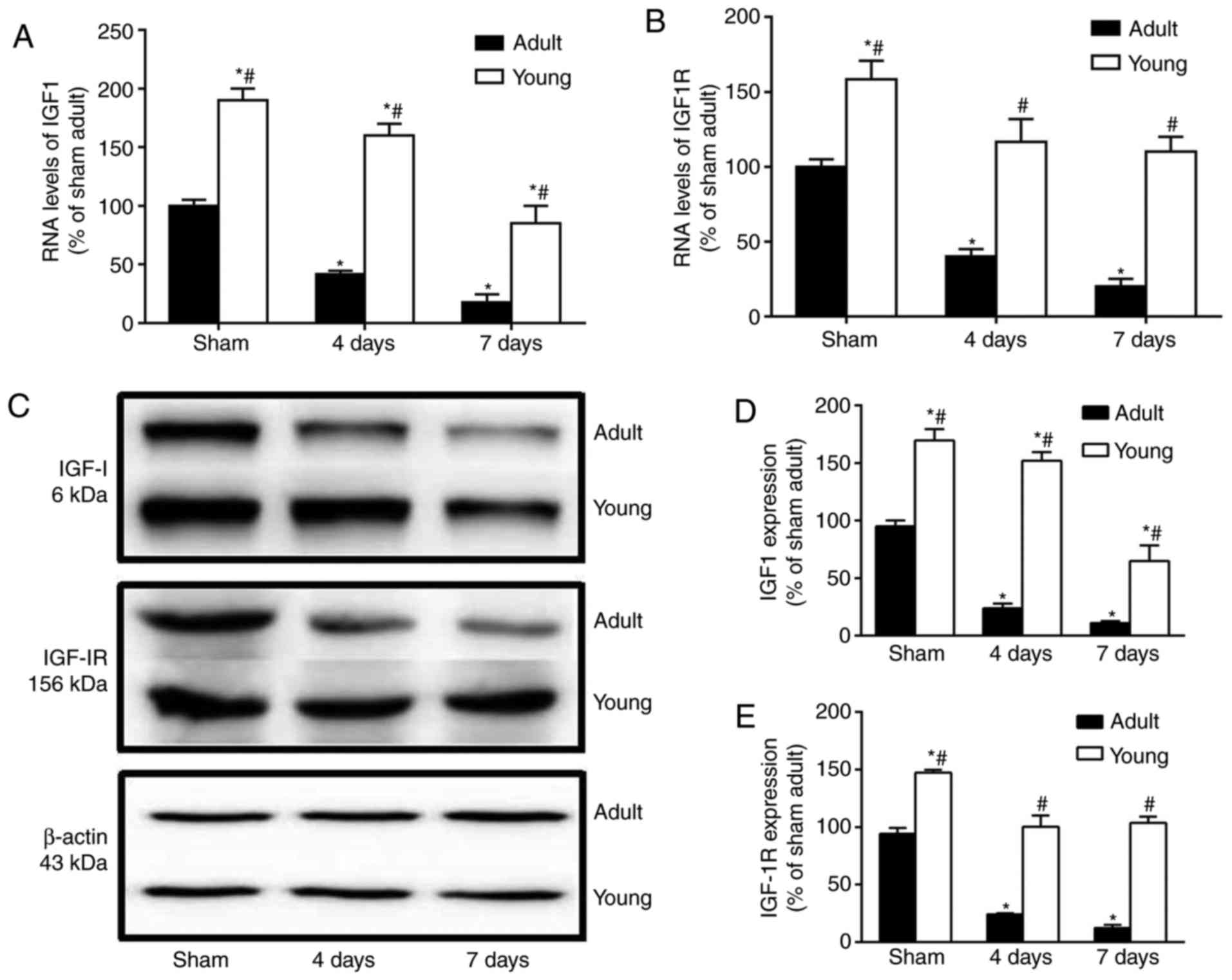
Changes in mRNA and protein levels of
IGF-1/IGF-1R
The mRNA and protein levels of IGF-1 and IGF-1R were
much higher in the young sham-group than those in the adult
sham-group (Fig. 6). Four and
seven days after I/R, the mRNA and protein levels of IGF-1 and
IGF-1R were particularly decreased in the adult group. However, in
the young group at this time after I/R, They were significantly
higher than those in the corresponding adult-group (Fig. 6A-E).
Discussion
Among the multiple risk factors for ischemic stroke,
age plays a key role in the brain injury induced by cerebral
ischemic stroke (8). Our previous
studies have demonstrated that young gerbils have a stronger to
anti-cerebral ischemia activity than the adult gerbils under the
same condition (7,32). In addition, it has been reported
that in the young gerbil neuronal death occurs in the hippocampal
CA1 region 7 days after TCI, which is later than it occurs in the
adult gerbil (33).
In this study, we found that the immunoreactivity
and their RNA and protein expression levels of IGF-1 and IGF-1R in
the young gerbil were much higher than those in the adult gerbil
under physiological conditions. This finding is consistent with a
previous study that showed that the immunoreactivity and protein
levels of IGF-1 and IGF-1R were significantly decreased with age
(27). Many researchers have
reported that IGF-1 and IGF-1R act as the key modulator of brain
glucose to participate in neuroprotection (12,34).
Glucose is the major source of metabolic energy for the central
nervous system (CNS), thus it is very important to provide
sufficient glucose to the brain (35). It is well known that impairment of
the energy metabolism in the brain is one of the important
mechanisms of cerebral ischemic injury (36). GLUT-1, the predominant glucose
transporter specifically expressed by capillary endothelial cells
of the brain, is of great importance in regulating the
transportation and level of glucose in the brain, and its
expression reflects the rate of cerebral glucose utilization
(1,37,38).
Park et al reported that the higher expression of GLUT-1in
the hippocampal CA1 region of the young gerbils after TCI may
contribute to less and more delayed neuronal death in the young
gerbil (1). Some studies have
reported that IGF-1 and IGF-1R contributed to increase glucose
metabolism, which indicated that the elevated expression of IGF-1
and IGF-1R expression was associated with neuroprotection after TCI
(21,39).
We additionally compared changes of IGF-1and IGF-1R
in the CA1 region between adult and young gerbils after
ischemia-reperfusion. The IGF-1 immunoreactivity and its protein
level in the CA1 region of adult hippocampus were increase at
earlier time and then dramatically decreased. Hwang et al
have demonstrated that the expression of IGF-1 was transiently
increased in the hippocampus and cerebral cortex after I/R injury,
which may be associated with the short resistance to DND after
ischemic insult (40). However, in
the young gerbil after ischemia-reperfusion, the immunoreactivity
and mRNA and protein expression levels of IGF-1 was sustained until
day 4 after ischemia-reperfusion in the hippocampal CA1 region.
Certain researchers reported that endogenous IGF-1 and IGF-1R were
involved in the neuroprotective effect against ischemic damage in
the brain (41–44). Activation of IGF-1/IGF-1R
stimulates the PI-3K/Akt pathway and inhibits the GSK-3β pathway,
to exert their effect on the antioxidant defense of neuron-,
metabolism of glucose- and synthesis of anti-apoptotic-associated
proteins, which result in the protective effect and ultimately in
neuronal survival (21,45). It is particularly noteworthy that
the Akt signaling pathway, as an important upstream signaling
pathway, plays an important role in the survival and repair of
neuronal cells after cerebral ischemia (46,47).
The activation of Akt can control multiple intracellular signals,
such as the mTOR signaling pathway, GSK-3β signaling pathway etc.
Then, the downstream signaling pathways can promote proliferation
and survival after cerebral ischemia (48–50).
In addition, some studies reported that treatment with IGF-1 after
an ischemic stroke partially improved the ischemic damage of
neurons induced by ischemia-reperfusion injury (51,52).
Therefore, the reduced neuronal death in the hippocampal CA1 region
of the young gerbils after TCI compared to that in the adults may
be associated with the higher and sustained expression of IGF-1 and
IGF-1R. Furthermore, the relevant molecular biological mechanisms
may be associated with the Akt signaling pathway.
In conclusion, our present findings indicated that
the expression levels of IGF-1 and IGF-1R in the hippocampal CA1
region in the normal young gerbils were much higher than those in
the normal adult. Additionally, their sustained expression levels
in the hippocampal CA1 region after ischemia-reperfusion may serve
as the evidence to explain the reason for the more delayed and
reduced neuronal death/damage in the young gerbil. Also, it could
be hypothesized that increasing the levels of IGF-1/IGF-1R has
potential as an alternative target for the prevention of ischemic
damage in the brain.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81401005), The Natural Science
Foundation of Jiangsu Province of China (no. BK20140494), Key
University Science Research Project of Jiangsu Province (no.
16KJA310006), China Postdoctoral Science Foundation (no.
2014M561720) and Postdoctoral Science Foundation of Jiangsu
Province (no. 1401155C).
References
|
1
|
Park SM, Lee JC, Chen BH, Shin BN, Cho JH,
Kim IH, Park JH, Won MH, Ahn JH, Tae HJ, et al: Difference in
transient ischemia-induced neuronal damage and glucose
transporter-1 immunoreactivity in the hippocampus between adult and
young gerbils. Iran J Basic Med Sci. 19:521–528. 2016.PubMed/NCBI
|
|
2
|
Bae EJ, Chen BH, Yan BC, Shin BN, Cho JH,
Kim IH, Ahn JH, Lee JC, Tae HJ, Hong S, et al: Delayed hippocampal
neuronal death in young gerbil following transient global cerebral
ischemia is related to higher and longer-term expression of p63 in
the ischemic hippocampus. Neural Regen Res. 10:944–950. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JC, Tae HJ, Chen BH, Cho JH, Kim IH,
Ahn JH, Park JH, Shin BN, Lee HY, Cho YS, et al: Failure in
neuroprotection of remote limb ischemic postconditioning in the
hippocampus of a gerbil model of transient cerebral ischemia. J
Neurol Sci. 358:377–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Araki T, Kato H and Kogure K: Selective
neuronal vulnerability following transient cerebral ischemia in the
gerbil: Distribution and time course. Acta Neurol Scand.
80:548–553. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Domoráková I, Burda J, Mechírová E and
Feriková M: Mapping of rat hippocampal neurons with NeuN after
ischemia/reperfusion and Ginkgo biloba extract (EGb 761)
pretreatment. Cell Mol Neurobiol. 26:1193–1204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oguro K, Miyawaki T, Yokota H, Kato K,
Kamiya T, Katayama Y, Fukaya M, Watanabe M and Shimazaki K:
Upregulation of GluR2 decreases intracellular Ca2+ following
ischemia in developing gerbils. Neurosci Lett. 364:101–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan BC, Park JH, Ahn JH, Lee YJ, Lee TH,
Lee CH, Cho JH, Kim MJ, Kim TY, Kang IJ and Won MH: Comparison of
the immunoreactivity of Trx2/Prx3 redox system in the hippocampal
CA1 region between the young and adult gerbil induced by transient
cerebral ischemia. Neurochem Res. 37:1019–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kartal Ö, Aydınöz S, Kartal AT, Kelestemur
T, Caglayan AB, Beker MC, Karademir F, Süleymanoğlu S, Kul M, Yulug
B and Kilic E: Time dependent impact of perinatal hypoxia on growth
hormone, insulin-like growth factor 1 and insulin-like growth
factor binding protein-3. Metab Brain Dis. 31:827–835. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tarantini S, Tucsek Z, Valcarcel-Ares MN,
Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM,
et al: Circulating IGF-1 deficiency exacerbates
hypertension-induced microvascular rarefaction in the mouse
hippocampus and retrosplenial cortex: Implications for
cerebromicrovascular and brain aging. Age (Dordr). 38:273–289.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CH, Ahn JH, Park JH, Yan BC, Kim IH,
Lee DH, Cho JH, Chen BH, Lee JC, Cho JH, et al: Decreased
insulin-like growth factor-I and its receptor expression in the
hippocampus and somatosensory cortex of the aged mouse. Neurochem
Res. 39:770–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bondy CA and Cheng CM: Insulin-like growth
factor-1 promotes neuronal glucose utilization during brain
development and repair processes. Int Rev Neurobiol. 51:189–217.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng CM, Cohen M, Tseng V and Bondy CA:
Endogenous IGF1 enhances cell survival in the postnatal dentate
gyrus. J Neurosci Res. 64:341–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieto-Estévez V, Defterali Ç and
Vicario-Abejón C: IGF-I: A key growth factor that regulates
neurogenesis and synaptogenesis from embryonic to adult stages of
the brain. Front Neurosci. 10:522016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu W, Ye P, O'Kusky JR and D'Ercole AJ:
Type 1 insulin-like growth factor receptor signaling is essential
for the development of the hippocampal formation and dentate gyrus.
J Neurosci Res. 87:2821–2832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carro E, Trejo JL, Gomez-Isla T, LeRoith D
and Torres-Aleman I: Serum insulin-like growth factor I regulates
brain amyloid-beta levels. Nat Med. 8:1390–1397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, D'Ercole JA and Ye P: Blunting type
1 insulin-like growth factor receptor expression exacerbates
neuronal apoptosis following hypoxic/ischemic injury. BMC Neurosci.
12:642011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts CT Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czech MP: Signal transmission by the
insulin-like growth factors. Cell. 59:235–238. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gazit N, Vertkin I, Shapira I, Helm M,
Slomowitz E, Sheiba M, Mor Y, Rizzoli S and Slutsky I: IGF-1
receptor differentially regulates spontaneous and evoked
transmission via mitochondria at hippocampal synapses. Neuron.
89:583–597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng CM, Reinhardt RR, Lee WH, Joncas G,
Patel SC and Bondy CA: Insulin-like growth factor 1 regulates
developing brain glucose metabolism. Proc Natl Acad Sci USA. 97:pp.
10236–10241. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madathil SK and Saatman KE: IGF-1/IGF-R
Signaling in Traumatic Brain Injury: Impact on cell survival,
neurogenesis and behavioral outcomeBrain Neurotrauma: Molecular,
Neuropsychological and Rehabilitation Aspects. Kobeissy FH: CRC
Press/Taylor & Francis; Boca Raton, FL: 2015
|
|
23
|
Gualco E, Wang JY, Del Valle L, Urbanska
K, Peruzzi F, Khalili K, Amini S and Reiss K: IGF-IR in
neuroprotection and brain tumors. Front Biosci. 14:352–375. 2009.
View Article : Google Scholar
|
|
24
|
Song Y, Pimentel C, Walters K, Boller L,
Ghiasvand S, Liu J, Staley KJ and Berdichevsky Y: Neuroprotective
levels of IGF-1 exacerbate epileptogenesis after brain injury. Sci
Rep. 6:320952016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Li X, Sun Q, He B, Jia Y, Cai D
and Zhao R: Folate deprivation induces cell cycle arrest at G0/G1
phase and apoptosis in hippocampal neuron cells through
down-regulation of IGF-1 signaling pathway. Int J Biochem Cell
Biol. 79:222–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Werner H and LeRoith D: Insulin and
insulin-like growth factor receptors in the brain: Physiological
and pathological aspects. Eur Neuropsychopharmacol. 24:1947–1953.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sonntag WE, Lynch CD, Bennett SA, Khan AS,
Thornton PL, Cooney PT, Ingram RL, McShane T and Brunso-Bechtold
JK: Alterations in insulin-like growth factor-1 gene and protein
expression and type 1 insulin-like growth factor receptors in the
brains of ageing rats. Neuroscience. 88:269–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pentón-Rol G, Marin-Prida J, Pardo-Andreu
G, Martínez-Sánchez G, Acosta-Medina EF, Valdivia-Acosta A,
Lagumersindez-Denis N, Rodríguez-Jiménez E, Llópiz-Arzuaga A,
López-Saura PA, et al: C-Phycocyanin is neuroprotective against
global cerebral ischemia/reperfusion injury in gerbils. Brain Res
Bull. 86:42–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan BC, Park JH, Ahn JH, Kim IH, Lee JC,
Yoo KY, Choi JH, Hwang IK, Cho JH, Kwon YG, et al: Effects of
high-fat diet on neuronal damage, gliosis, inflammatory process and
oxidative stress in the hippocampus induced by transient cerebral
ischemia. Neurochem Res. 39:2465–2478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan BC, Ohk TG, Ahn JH, Park JH, Chen BH,
Lee JC, Lee CH, Shin MC, Hwang IK, Moon SM, et al: Differences in
neuronal damage and gliosis in the hippocampus between young and
adult gerbils induced by long duration of transient cerebral
ischemia. J Neurol Sci. 337:129–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen H, Wang J, Jiang D, Xu P, Zhu X,
Zhang Y, Yu X, Won MH, Su PQ and Yan BC: Topiramate improves
neuroblast differentiation of hippocampal dentate gyrus in the
D-galactose-induced aging mice via its antioxidant effects. Cell
Mol Neurobiol. 37:869–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kusumoto M, Arai H, Mori K and Sato K:
Resistance to cerebral ischemia in developing gerbils. J Cereb
Blood Flow Metab. 15:886–891. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan BC, Park JH, Lee CH, Yoo KY, Choi JH,
Lee YJ, Cho JH, Baek YY, Kim YM and Won MH: Increases of
antioxidants are related to more delayed neuronal death in the
hippocampal CA1 region of the young gerbil induced by transient
cerebral ischemia. Brain Res. 1425:142–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernandez-Garzón E, Fernandez AM,
Perez-Alvarez A, Genis L, Bascuñana P, Fernandez de la Rosa R,
Delgado M, Angel Pozo M, Moreno E, McCormick PJ, et al: The
insulin-like growth factor I receptor regulates glucose transport
by astrocytes. Glia. 64:1962–1971. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Attwell D and Laughlin SB: An energy
budget for signaling in the grey matter of the brain. J Cereb Blood
Flow Metab. 21:1133–1145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu J, Lin B, Liu W, Huang J, Shang G, Lin
Y, Wang L, Chen L and Tao J: Roles of electro-acupuncture in
glucose metabolism as assessed by 18F-FDG/PET imaging and AMPKα
phosphorylation in rats with ischemic stroke. Int J Mol Med.
40:875–882. 2017.PubMed/NCBI
|
|
37
|
Simpson IA, Carruthers A and Vannucci SJ:
Supply and demand in cerebral energy metabolism: The role of
nutrient transporters. J Cereb Blood Flow Metab. 27:1766–1791.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farrell CL and Pardridge WM: Blood-brain
barrier glucose transporter is asymmetrically distributed on brain
capillary endothelial lumenal and ablumenal membranes: An electron
microscopic immunogold study. Proc Natl Acad Sci USA. 88:pp.
5779–5783. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duarte AI, Santos P, Oliveira CR, Santos
MS and Rego AC: Insulin neuroprotection against oxidative stress is
mediated by Akt and GSK-3beta signaling pathways and changes in
protein expression. Biochim Biophys Acta. 1783:994–1002. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hwang IK, Yoo KY, Park SK, An SJ, Lee JY,
Choi SY, Kang JH, Kwon YG, Kang TC and Won MH: Expression and
changes of endogenous insulin-like growth factor-1 in neurons and
glia in the gerbil hippocampus and dentate gyrus after ischemic
insult. Neurochem Int. 45:149–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wadowska M, Woods J, Rogozinska M and
Briones TL: Neuroprotective effects of enriched environment housing
after transient global cerebral ischaemia are associated with the
upregulation of insulin-like growth factor-1 signalling.
Neuropathol Appl Neurobiol. 41:544–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bergstedt K and Wieloch T: Changes in
insulin-like growth factor 1 receptor density after transient
cerebral ischemia in the rat. Lack of protection against ischemic
brain damage following injection of insulin-like growth factor 1. J
Cereb Blood Flow Metab. 13:895–898. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui H, Meng Y and Bulleit RF: Inhibition
of glycogen synthase kinase 3beta activity regulates proliferation
of cultured cerebellar granule cells. Brain Res Dev Brain Res.
111:177–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Johnston BM, Mallard EC, Williams CE and
Gluckman PD: Insulin-like growth factor-1 is a potent neuronal
rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin
Invest. 97:300–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang LH, Yuan XL, Yang NY, Ren L, Zhao
FM, Luo BX, Bian YY, Xu JY, Lu DX, Zheng YY, et al: Daucosterol
protects neurons against oxygen-glucose
deprivation/reperfusion-mediated injury by activating IGF1
signaling pathway. J Steroid Biochem Mol Biol. 152:45–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu D, Shi J, Elmadhoun O, Duan Y, An H,
Zhang J, He X, Meng R, Liu X, Ji X and Ding Y: Dihydrocapsaicin
(DHC) enhances the hypothermia-induced neuroprotection following
ischemic stroke via PI3K/Akt regulation in rat. Brain Res.
1671:18–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang L, Zhang Y, Yan Z and Tian F: The
role of mTOR signaling pathway on cognitive functions in cerebral
ischemia-reperfusion. Exp Ther Med. 14:2839–2844. 2017.PubMed/NCBI
|
|
49
|
Li X, Ren C, Li S, Han R, Gao J, Huang Q,
Jin K, Luo Y and Ji X: Limb remote ischemic conditioning promotes
myelination by upregulating PTEN/Akt/mTOR signaling activities
after chronic cerebral hypoperfusion. Aging Dis. 8:392–401.
2017.PubMed/NCBI
|
|
50
|
Zhuang Q, Dai C, Yang L, Wen H, Wang H,
Jiang X and Zhang Y: Stimulated CB1 cannabinoid receptor inducing
ischemic tolerance and protecting neuron from cerebral ischemia.
Cent Nerv Syst Agents Med Chem. 17:141–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gluckman P, Klempt N, Guan J, Mallard C,
Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C and Nikolics
K: A role for IGF-1 in the rescue of CNS neurons following
hypoxic-ischemic injury. Biochem Biophys Res Commun. 182:593–599.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang JM, Hayashi T, Zhang WR, Sakai K,
Shiro Y and Abe K: Reduction of ischemic brain injury by topical
application of insulin-like growth factor-I after transient middle
cerebral artery occlusion in rats. Brain Res. 859:381–385. 2000.
View Article : Google Scholar : PubMed/NCBI
|