Introduction
Varicocele is one of the most common causes of male
infertility. In adult males, approximately 30–40% of patients with
primary infertility, and 69–81% of patients with secondary
infertility, suffer from varicocele (1). Varicocele is considered
disadvantageous to spermatogenesis, leading to low sperm count,
abnormal morphology, and poor motility. The academic community has
put forward many hypotheses for varicocele-induced testicular
dysfunction including oxidative stress, increased apoptosis,
hypoxia, and testicular microcirculation abnormalities. However,
the exact mechanism has not yet been fully elucidated (2).
Leptin, secreted mainly by white adipose tissue, is
a protein product of an obese gene. Leptin receptors belong to the
class I cytokine receptor family and have at least six different
subtypes. Leptin which reaches the central or peripheral tissue is
free in the blood or binds to the leptin binding protein (3). Differential leptin receptor
activation conveys different biological activities of leptin
(4). Leptin is a metabolic signal
that connects nutrition and other physiological functions (5). Leptin not only plays an important
role in energy metabolism, but also participates in a series of
important physiological activities such as angiogenesis, immune
regulation, inflammatory reaction and bone formation (6). Previous studies have focused on the
role of leptin in energy metabolism. Recently, an increasing number
of studies have shown that leptin plays an important role in
regulating reproductive function (7,8). It
has been reported that leptin and the leptin receptor exist in
testicular tissue (9), suggesting
that they might be related to spermatogenesis dysfunction caused by
varicocele. In addition, Ishikawa has found that testicular
dysfunction is associated with increased leptin and leptin receptor
expression (10). The purpose of
this study was to elucidate the relationship between
spermatogenesis dysfunction and the expressions of leptin and its
receptor in rats with experimental varicocele. Furthermore, we
examined the roles of leptin and the leptin receptor in the
pathogenesis of varicocele-induced testicular dysfunction.
Materials and methods
Preparation of animals and
tissues
Forty Sprague-Dawley male rats were obtained from
Shanghai SIPPR-BK Laboratory or Animal Co., Ltd. (Shanghai, China).
Weights ranged from 200 to 250 g. Rats were divided into four
groups randomly: Groups 1 (G1) and 3 (G3) underwent the sham
operation as controls; groups 2 (G2) and 4 (G4) underwent
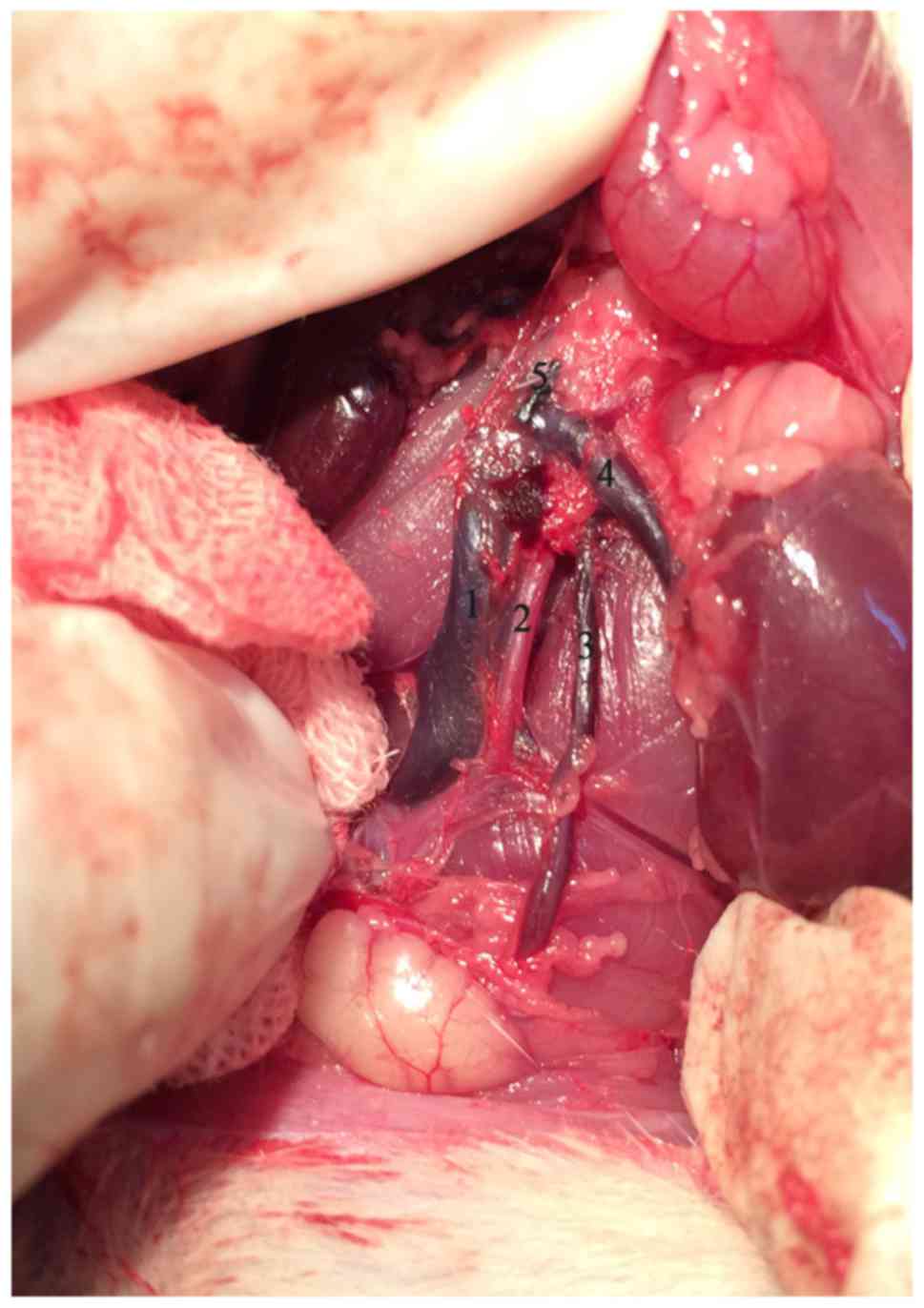
operations to form an experimental left varicocele which was
created by partially narrowing the left renal vein with reference
to Turner's method (11). Briefly,
with a 4-0 silk suture passing under the left renal vein, a 0.7 mm
diameter needle was placed above the left renal vein proximal to
the junction of the left internal spermatic vein. The left renal
vein and the needle were ligated together. The needle was withdrawn
after the knot was secured resulting in partial narrowing of the
vein. We then carefully looked for and ligated branches between the
left internal spermatic and left common iliac veins.
G1 and G3 rats underwent left renal vein isolation
without ligation. The diameters of both the left and right internal
spermatic veins were measured under a microscope with a micrometer
before and 4 or 8 weeks after the operation. Varicocele modeling
was considered successful when the diameter of the left internal
spermatic vein was more than twice the right vein and there was no
renal atrophy (Fig. 1).
G1 and G2 rats were killed 4 weeks after the
operation while G3 and G4 rats were killed at 8 weeks. The left
testis and epididymis were obtained and their weight was recorded.
After washing with phosphate-buffered saline (PBS), the epididymis
was placed into PBS at 37°C for 10 min for semen analysis. Some
testis tissues stored in tubes were placed into liquid nitrogen for
measuring mRNA levels with the real-time polymerase chain reaction
(RT-PCR). Others were fixed in Bouin's solution for histochemical
analysis. Blood was drawn from the abdominal aorta and the
supernatant was collected after centrifugation at 3,500 × g for 15
min at 4°C. Blood samples were obtained to measure hormone levels.
Finally, hypothalamus tissues were obtained and preserved in liquid
nitrogen. The study was approved by the Ethics Committee of
Shanghai Municipal Commission of Health and family Planning
(SWJW005).
Spermatogenesis assessment
Paraffin sections made from fixed testis were
stained with hematoxylin and eosin. Twenty fields were analyzed
randomly under a microscope. Each section of seminiferous tubules
was graded with a Johnsen's score from 1 to 10 as described
previously (12) and an average
score was calculated. In addition, the diameter of the seminiferous
tubules, and the thickness of the seminiferous epithelium, were
analyzed using Image-Pro Plus (Media Cybernetics, Inc., Rockville,
MD, USA). The epididymis was cut into two sections and incubated at
37°C for 10 min in 2 ml PBS to allow sperm to swim out freely. A
blood cell counter was used for sperm counts.
Immunohistochemical staining
A streptavidin-peroxidase kit was used to perform
immunohistochemical staining (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). Hypothalamus was used for
frozen sections and testis was used for paraffin sections. Slides
were incubated for 20 min at room temperature in 3% hydrogen
peroxide to block endogenous peroxidase activity. All slides were
then incubated for 1 h in goat serum, followed by an overnight
incubation at 4°C with primary antibodies against leptin (sc-842;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or the leptin
receptor (sc-8325; Santa Cruz Biotechnology, Inc.) at the dilution
of 1:100. The following day, the slides were incubated with a
biotinylated secondary antibody for 30 min at room temperature, and
then with an enhanced streptavidin horseradish peroxidase conjugate
for 15 min at room temperature. The slides were then washed and
incubated with 3,3′-diaminobenzidine chromogenic reagent for 6 min.
Finally, all slides were counterstained with hematoxylin for 45
sec, destained with acid alcohol for 3 sec, dehydrated with an
ethanol gradient, coated with resin, and covered with a glass
coverslip. Image-Pro Plus was used to perform the quantitative
analysis.
RT-PCR
Total RNA was extracted from cerebral and left
testis tissues using TRIzol® Reagent (Ambion, Inc.,
Austin, TX, USA) according to the manufacturer's instructions.
Total RNA (1 µg) was reverse transcribed using QuantiTect Reverse
Transcription kit (cat. nos. 205310, 205311, 205313, and 205314;
Qiagen GmbH, Hilden, Germany). The primer sequences were
synthesized by Sangon Biotech (Shanghai, China) (Table I). The RT-PCR reaction
(ViiA™ 7 Real-Time PCR; Applied Biosystems, Foster City,
CA, USA) was started at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min. β-actin was used to normalize
the data and differences between the relative expression of the
target genes were calculated according to the 2−ΔΔCq
method. All reactions were performed in triplicate.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence (5′-3′) | Product length
(bp) | Accession number |
|---|
| Leptin-F |
CAATGACATTTCACACACGCAG | 204 | NM_013076 |
| Leptin-R |
AGATGGAGGAGGTCTCGCAG |
|
|
| Leptin
receptor-F |
GTGTCCTTCCTGACTCCGTAG | 119 | NM_012596 |
| Leptin
receptor-R |
GTTATTCTCTGGAAAGACTGGCT |
|
|
| β-actin-F |
GGCTGTATTCCCCTCCATCG | 154 | NM_007393 |
| β-actin-R |
CCAGTTGGTAACAATGCCATGT |
|
|
| KiSS-1-F |
TGGCACCTGTGGTGAACCCTGAAC | 202 | NM_181692 |
| KiSS-1-R |
ATCAGGCGACTGCGGCTGGCACAC |
|
|
| GPR54-F |
TTGGGTCCGAACGTGAGCT | 371 | NM_001301151 |
| GPR54-R |
CATGTGGCTTGCACCGAGA |
|
|
| GnRH-F |
CCGCTGTTGTTCTGTTGACT | 201 | NM_012767 |
| GnRH-R |
GCAGATCCCTAAGAGGTGAA |
|
|
| LH-F |
CCCCATAGTCTCCTTTCCTG | 190 | NM_012858 |
| LH-R |
GGATGGTTAGAACACCTGCT |
|
|
| FSH-F |
CATCCTACTCTGGTGCTTGA | 325 | NM_008045 |
| FSH-R |
TCTTACAGTGCAGTCGGTGC |
|
|
Hormone evaluations
Leptin was measured by an enzyme-linked
immunosorbent assay kit (Shanghai Westang Bio-Tech Co., Ltd.,
Shanghai, China). Follicle-stimulating hormone (FSH), luteinizing
hormone (LH), and testosterone levels were determined by a
radioimmunoassay (Beijing North Institute of Biological Technology,
Beijing, China).
Statistical analysis
Data were analyzed using SPSS 20 (IBM Corp., Armonk,
NY, USA). All values are shown as means ± SEM. Statistical
comparisons were performed using Student's t-test and correlations
were analyzed by using linear regression analysis. P<0.05 was
considered statistically different.
Results
Varicocele model and spermatogenesis
assessment
One rat in the G2 group was excluded because of
death and one in the G4 group was excluded because of unsuccessful
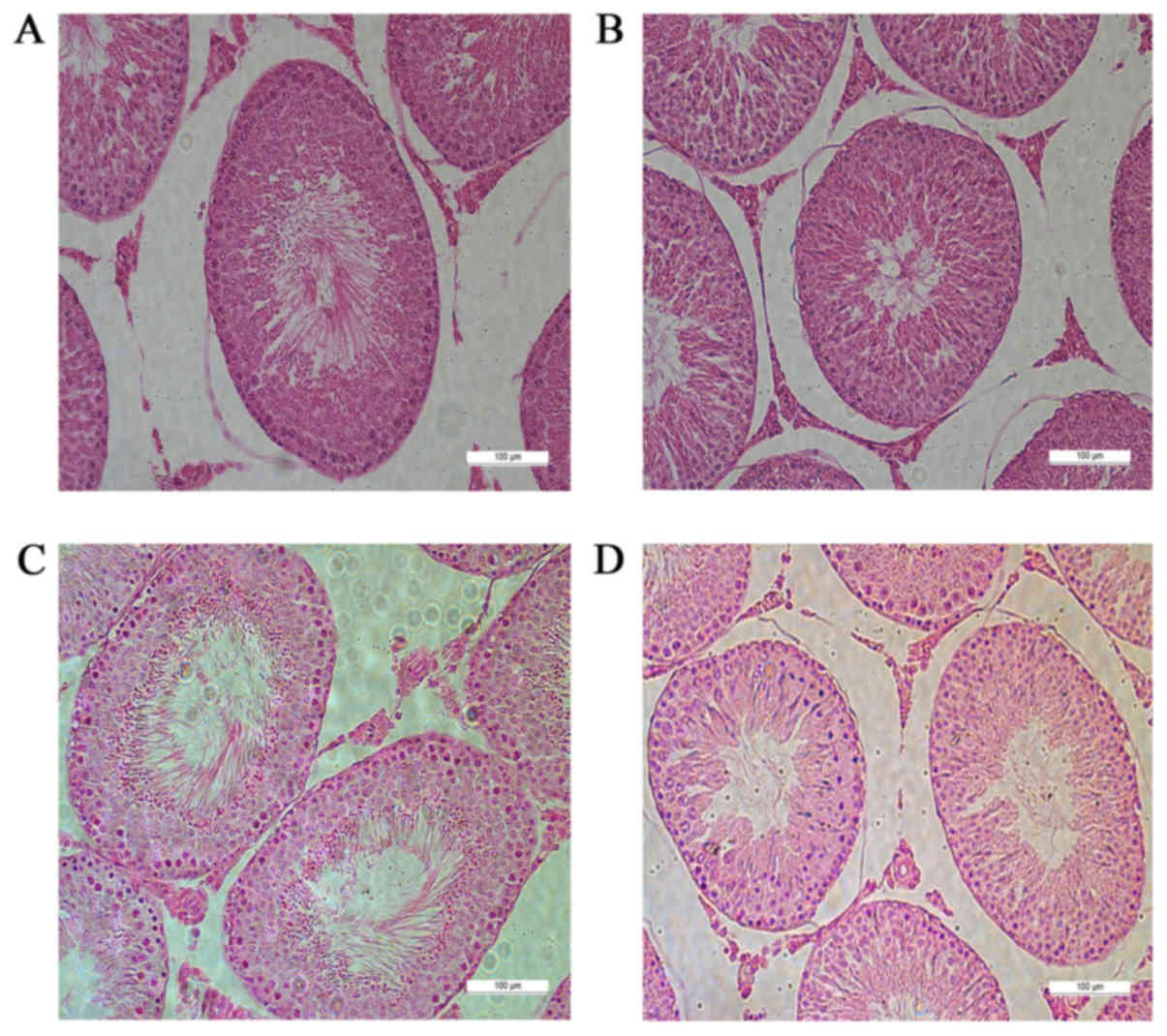
modeling. Hematoxylin and eosin staining showed that the numbers of
mature sperm in the seminiferous epithelium of G2 and G4 rats were
significantly lower than those in G1 and G3 rats. Cells were
relatively loose and disordered, and the number of vacuoles
increased in G2 and G4 rats (Fig.
2).
There was no significant difference among groups in
weights of the left testis and left epididymis. The Johnsen scores
for the left testis of G2 and G4 rats were significantly lower, the
seminiferous epithelium thinner, the seminiferous tubule diameters
smaller, and the sperm counts lower than those in G1 and G3 rats,
respectively (Table II).
 | Table II.Spermatogenesis-associated
indexes. |
Table II.
Spermatogenesis-associated
indexes.
|
| Left testis | Left epididymis |
|---|
|
|
|
|
|---|
| Group | Weight (g) | Johnsen score | Seminiferous tubule
diameter (µm) | Seminiferous
epithelium thickness (µm) | Weight (g) | Sperm count
(×106/g × ml) |
|---|
| G1 |
1.42±0.07 |
9.10±0.28 |
276.21±4.66 |
57.58±1.98 |
0.28±0.005 |
1,552±184 |
| G2 |
1.42±0.06 |
6.44±0.38b |
223.10±9.45a |
48.35±0.99b |
0.27±0.007 |
980±85a |
| G3 |
1.46±0.06 |
9.20±0.25 |
293.25±7.45 |
58.05±0.75 |
0.26±0.009 |
1,356±176 |
| G4 |
1.41±0.05 |
6.67±0.41d |
230.62±10.12c |
47.87±1.42d |
0.27±0.008 |
930±92c |
Leptin and leptin receptor levels in
testis and hypothalamus
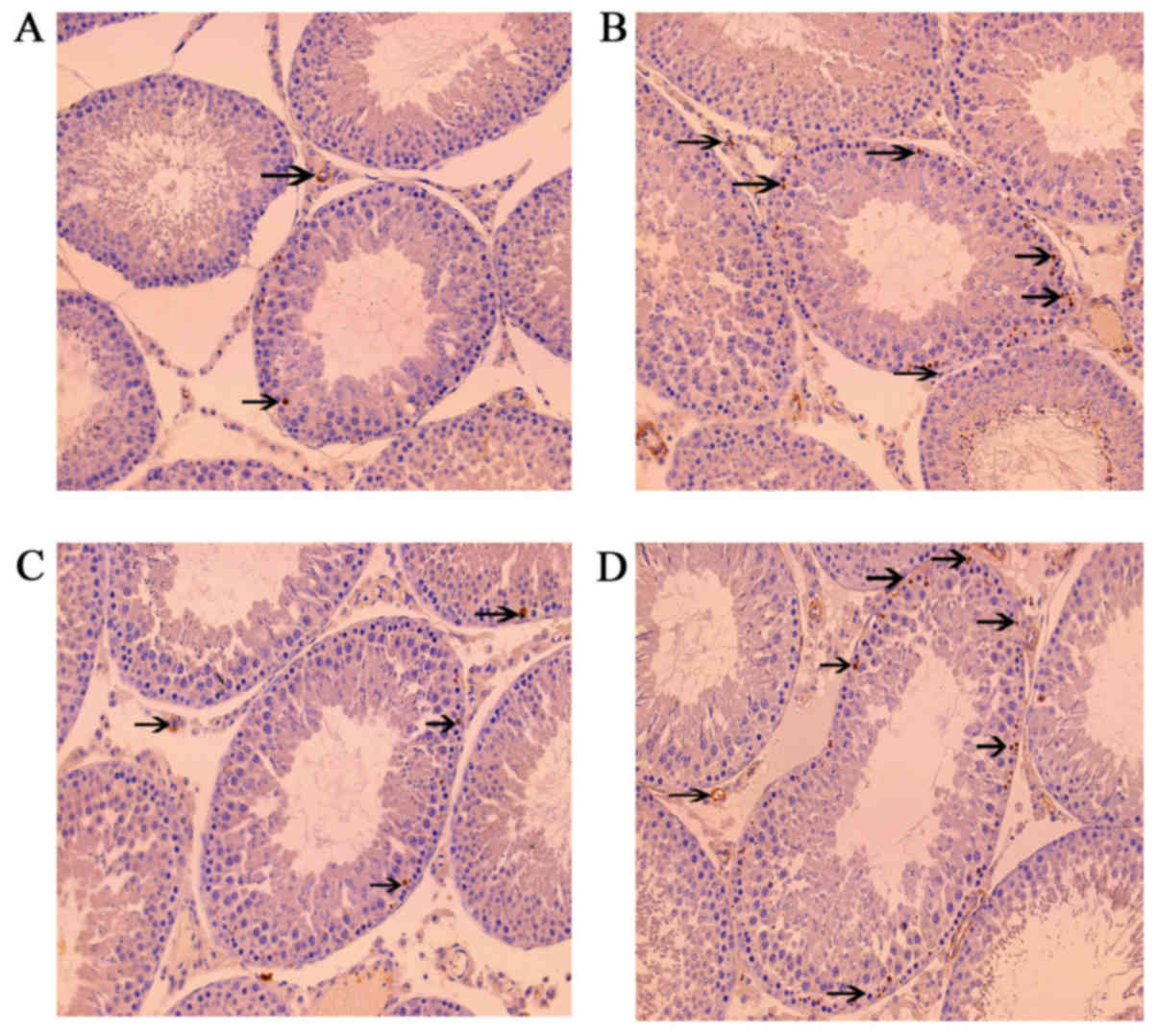
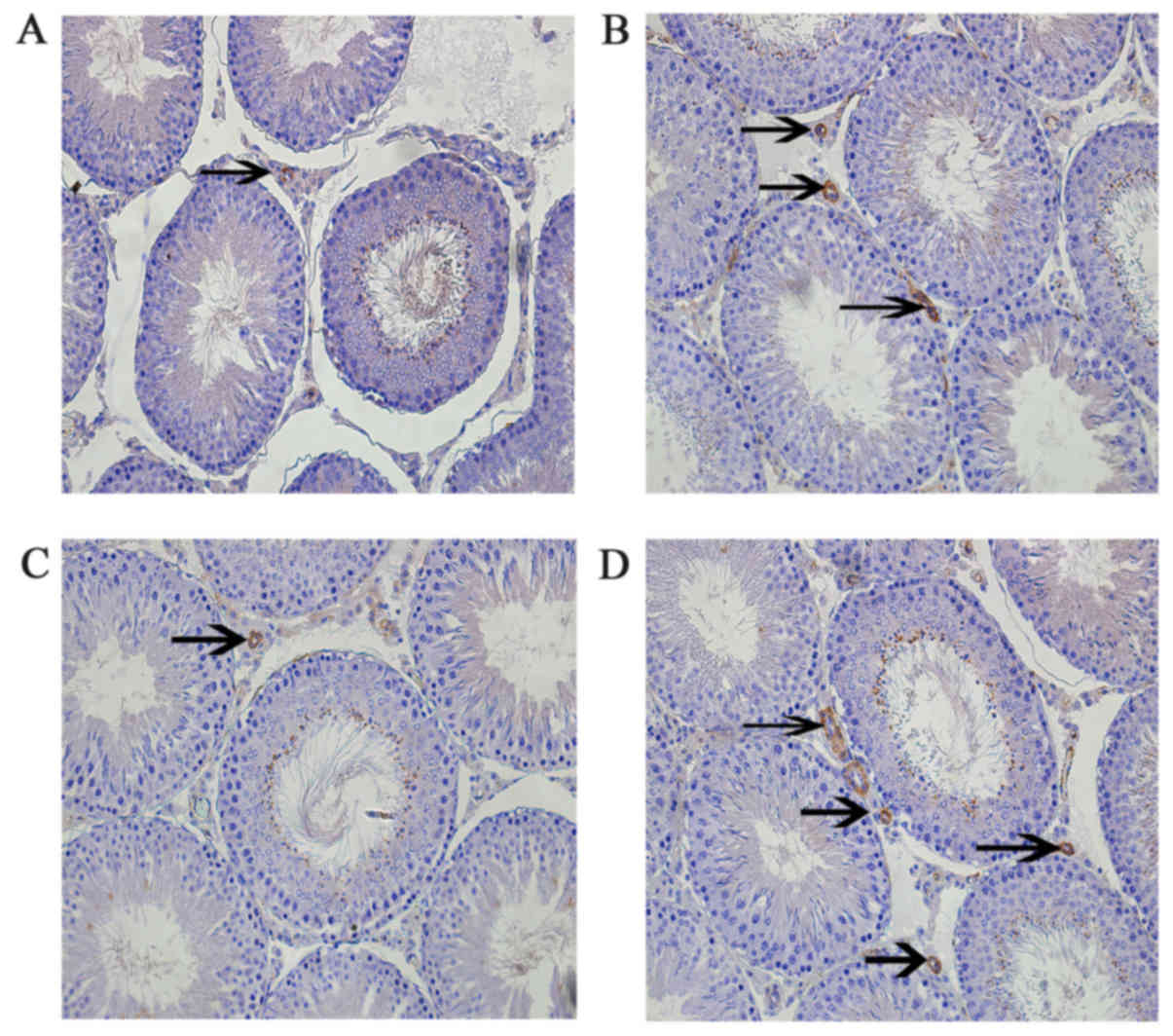
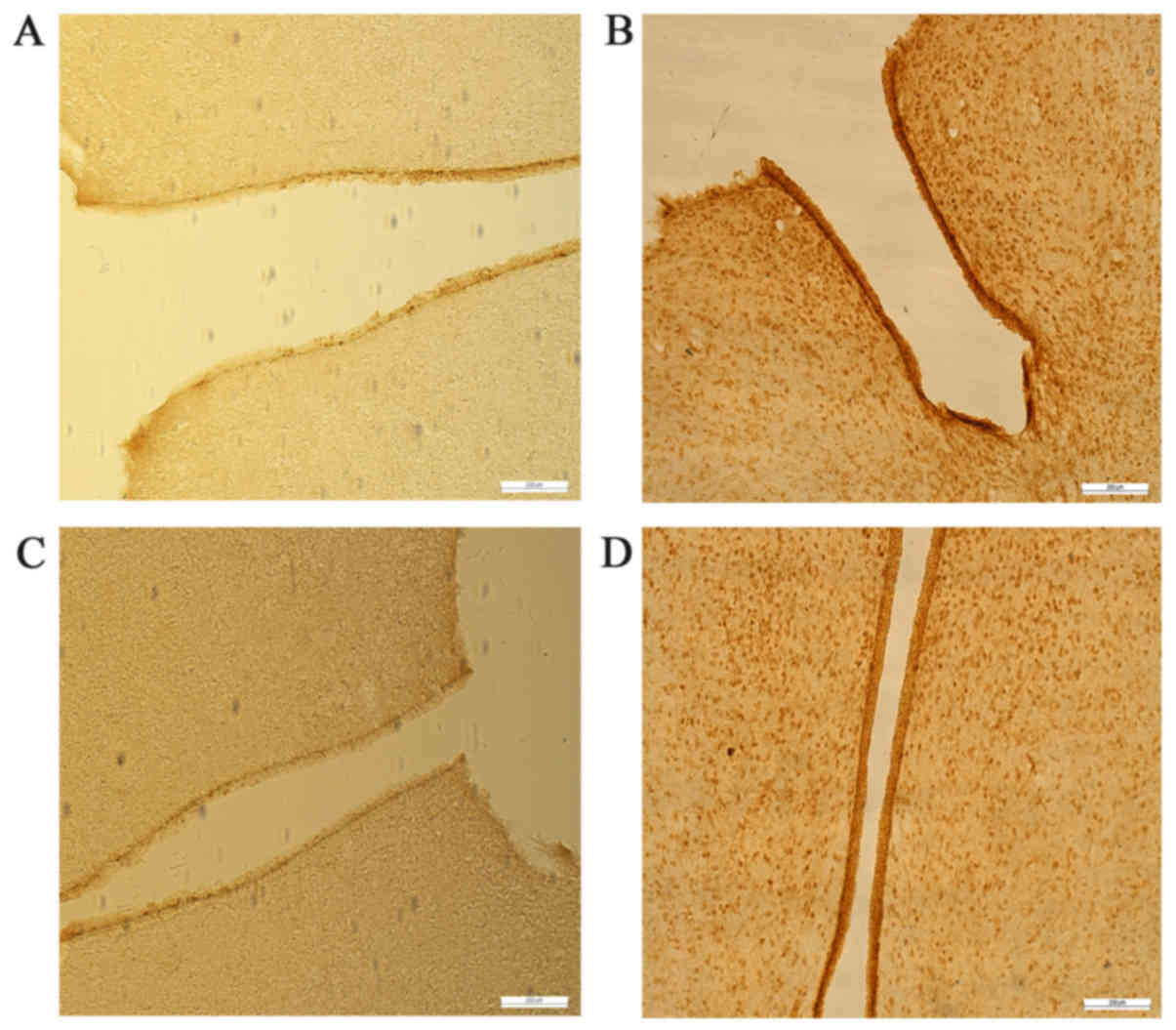
Leptin and leptin receptors were expressed in the
testis of all groups. Leptin was expressed in seminiferous tubules
and intersitium while the leptin receptor was expressed
predominantly in interstitium (Figs.
3 and 4). Expression of leptin
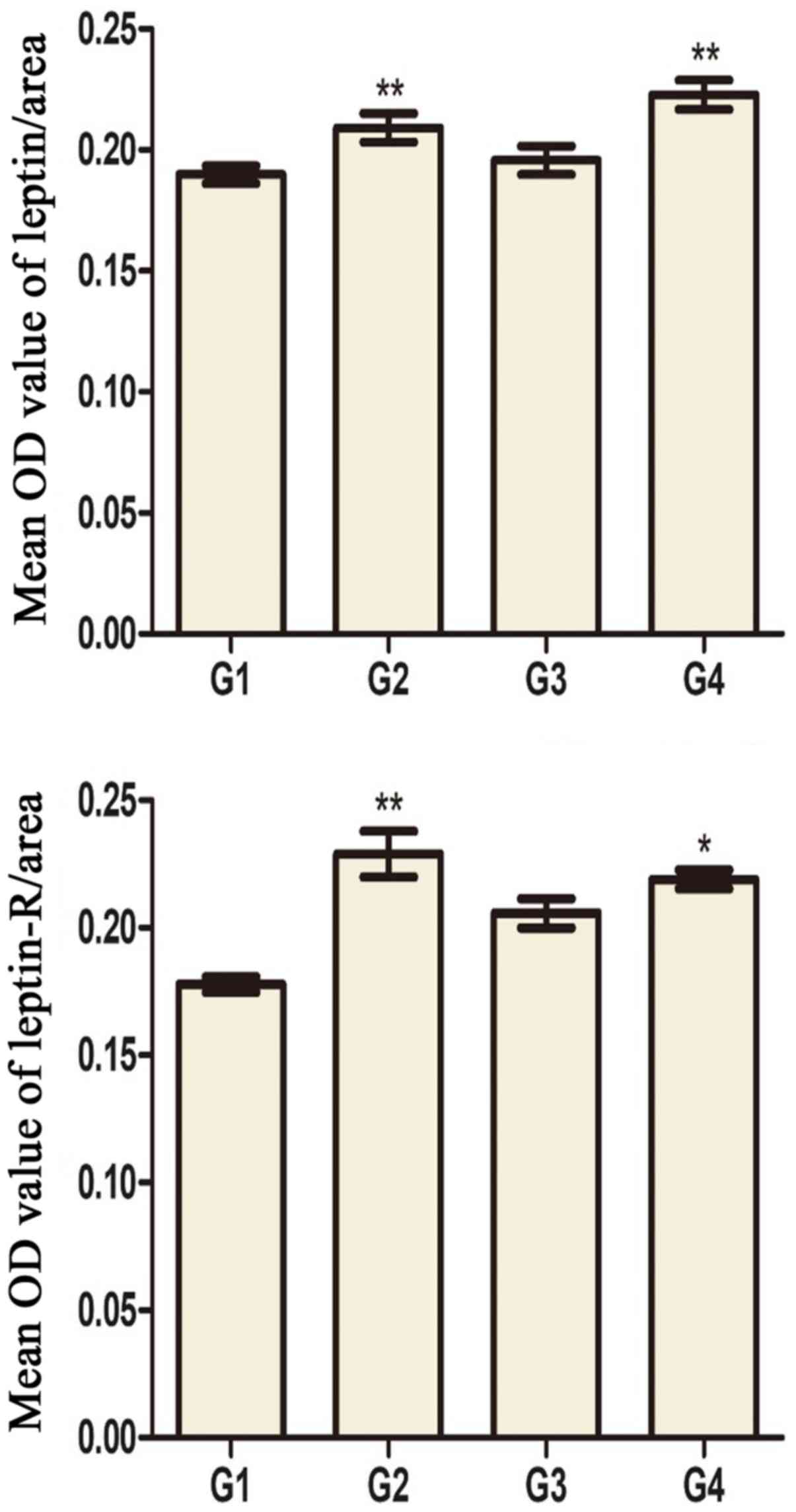
and leptin receptors in the testis of G2 and G4 rats was increased
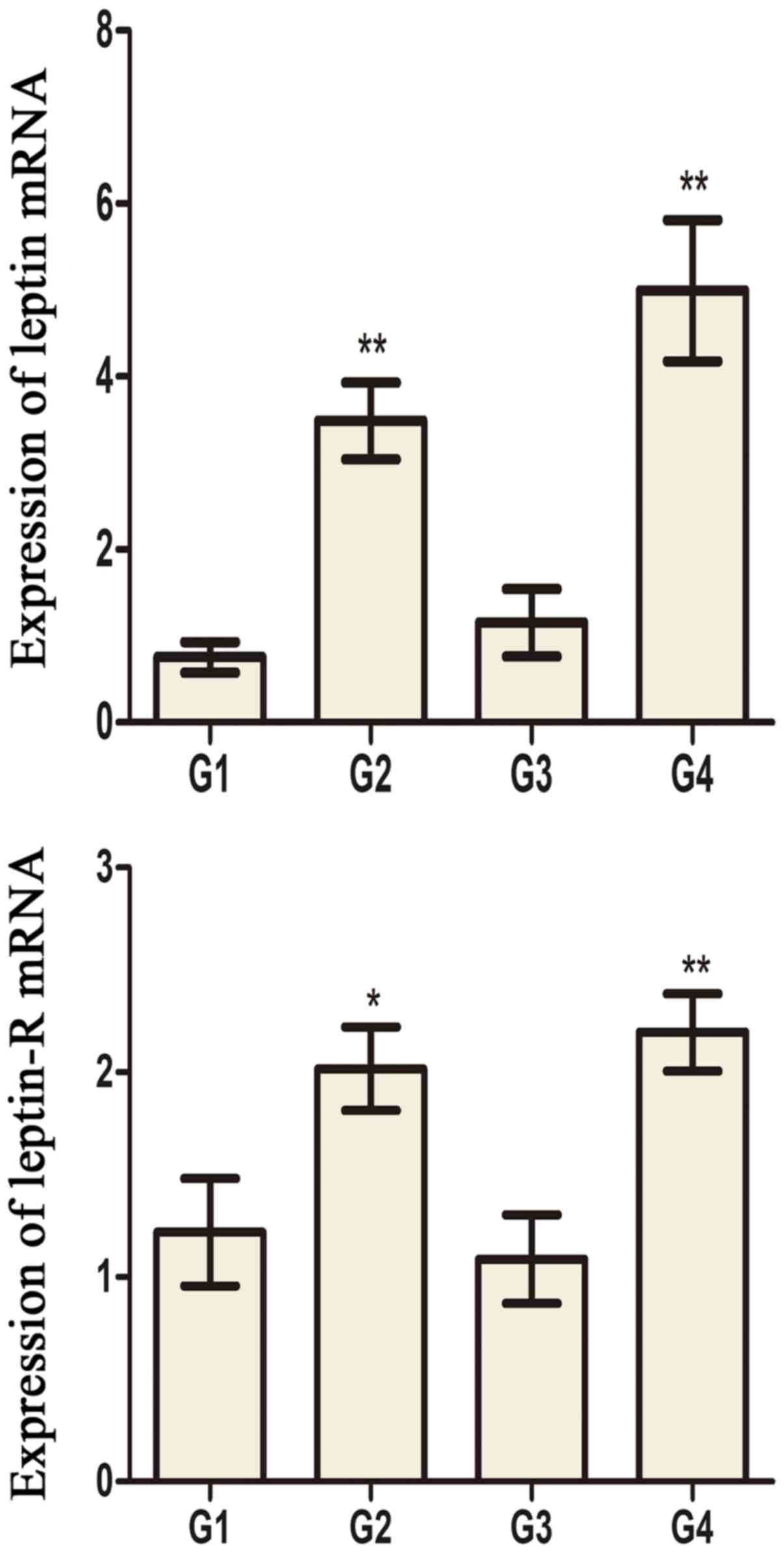
significantly compared to G1 and G3 rats, respectively (Fig. 5). Leptin and leptin receptor mRNA
levels in the testis of G2 and G4 rats were significantly increased
compared to G1 and G3 rats, respectively (Fig. 6). Expression of leptin receptors in
the hypothalamus of G2 and G4 rats was significantly increased
compared to G1 and G3 rats, respectively (Fig. 7).
Expression of kisspeptin (KiSS-1),
G-protein coupled receptor 54 (GPR54), gonadotropin releasing
hormone (GnRH), LH, and FSH mRNA in hypothalamus tissue
KiSS-1, GPR54, GnRH, LH, and FSH mRNA levels in G2
and G4 rats were significantly increased compared to G1 and G3
rats, respectively (Fig. 8).
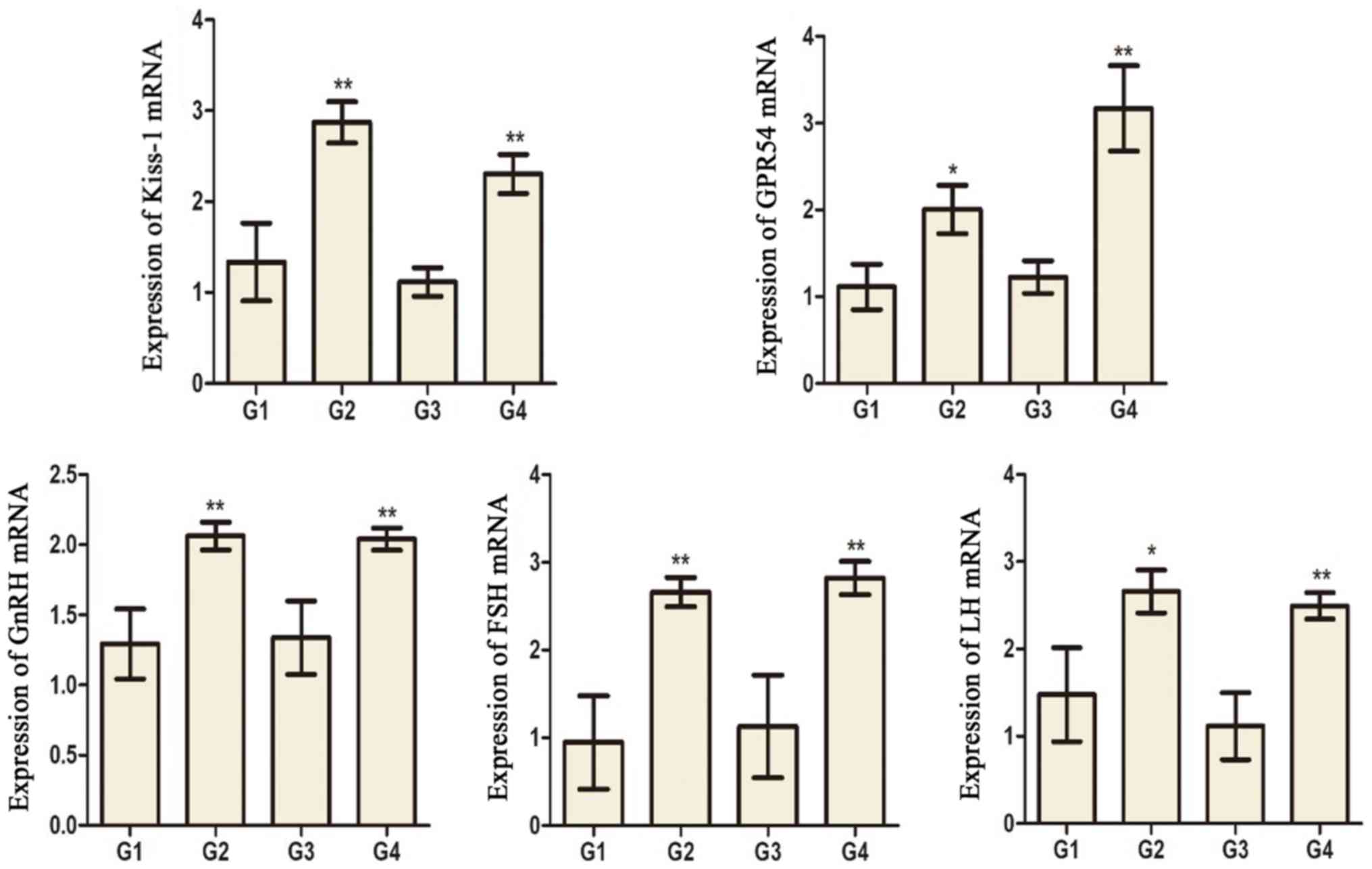 | Figure 8.The mRNA expression of KiSS-1, GPR54,
GnRH, FSH and LH in the hypothalamus tissues of different groups.
*P<0.05 and **P<0.01 vs. the G1 group. ##P<0.01
vs. the G3 group. G1 and G3 are 4 and 8 weeks post the sham
operation, respectively; G2 and G4 are 4 and 8 weeks post
experimental varicocele modelling, respectively. KiSS-1,
kisspeptin; GPR54, G-protein coupled receptor 54; GnRH,
gonadotropin releasing hormone; FSH, follicle-stimulating hormone;
LH, luteinizing hormone; G1, group 1; G2, group 2; G3, group 3; G4,
group 4. |
Hormone levels
Serum testosterone levels in G2 and G4 rats were
significantly lower than those in G1 and G3 rats, respectively.
There was no significant difference in serum levels of FSH, LH, and
leptin (Table III).
 | Table III.Serum hormonal evaluation. |
Table III.
Serum hormonal evaluation.
| Group | T (ng/ml) | FSH (mIU/ml) | LH (mIU/ml) | Leptin (pg/ml) |
|---|
| G1 |
0.95±0.39 |
3.03±0.37 |
4.71±0.33 |
79.18±5.56 |
| G2 |
0.20±0.08a |
3.32±0.63 |
5.57±0.67 |
113.39±18.18 |
| G3 |
1.06±0.46 |
3.18±0.52 |
5.02±0.38 |
88.42±7.86 |
| G4 |
0.24±0.09b |
3.77±0.25 |
5.84±0.38 |
120.80±15.78 |
Correlation analyses
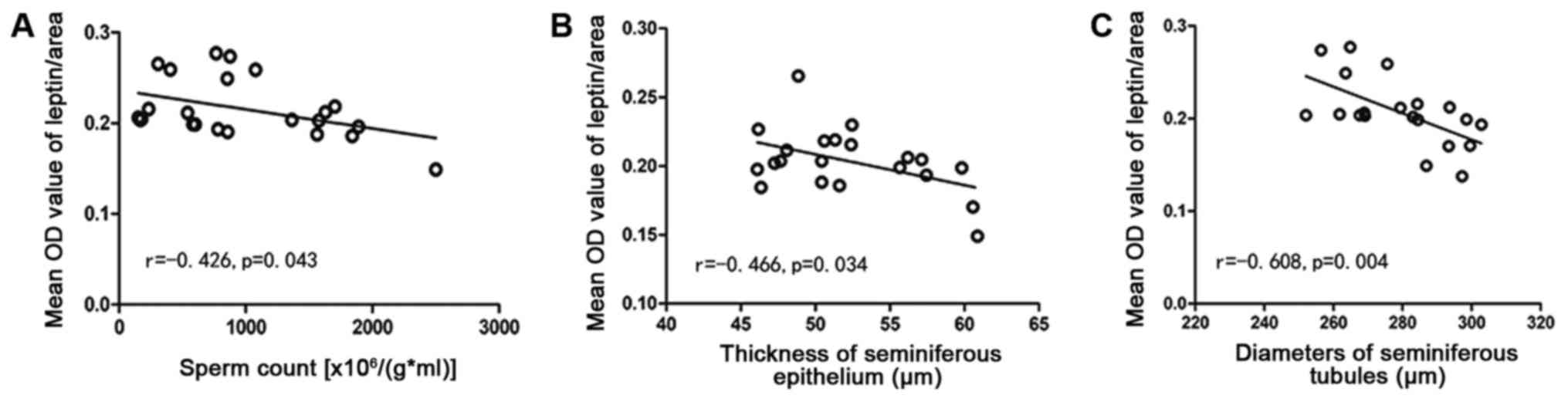
The leptin level was inversely related to sperm
count (r=−0.426, P=0.043), thickness of the seminiferous epithelium
(r=−0.466, P=0.034), and the diameter of seminiferous tubules
(r=−0.608, P=0.004) (Fig. 9).
Discussion
Varicocele is an abnormal elongation, expansion, and
tortuosity of the spermatic vein. Both animal and human studies
have confirmed that varicocele can affect spermatogenesis (13). Our results showed that Johnsen
scores in the left testis of the experimental varicocele groups (G2
and G4) were significantly lower, the seminiferous epithelium was
thinner, the seminiferous tubule diameters were smaller, the sperm
count was lower, and serum testosterone levels were decreased
compared to those in the control groups (G1 and G3). These results
confirm that varicocele causes testicular dysfunction in rats.
Leptin plays an important role in both male and
female reproductive systems. However, the precise relationship
between leptin and male spermatogenesis has not been elucidated.
Leptin receptors are found in hypothalamus, pituitary, testis, and
sperm (10,14). Our study found that leptin was
expressed in seminiferous tubules and interstitium of testicular
tissue, whereas leptin receptors were mainly expressed in the
interstitium, consistent with prior research (15). Ishikawa (10) found overexpression of leptin and
leptin receptors in testicular tissue of patients with varicocele.
Chen et al (15) also found
that the expression of leptin and its receptor in the testis of
experimental varicocele rats increased, and was negatively
correlated with testicular weight, Johnsen score, thickness of
seminiferous epithelium, and diameter of the seminiferous tubules.
Similarly, we found that varicocele increased the expression of
leptin and leptin receptors in rat testis.
Overexpression of leptin and leptin receptors is
closely related to spermatogenesis dysfunction. We found that the
expression of leptin was negatively correlated with sperm count,
thickness of seminiferous epithelium, and diameter of the
seminiferous tubules. However, it is still unknown what pathways
leptin affects in the male reproductive system. Previous studies
have shown that leptin plays a role in both central and peripheral
tissues (6,16). Leptin receptors are expressed on
neurons secreting GnRH in the hypothalamus. Leptin is mainly
involved in regulating GnRH secretion by this tissue by promoting
the pulsatile secretion of GnRH through activating hypothalamic
arcuate nucleus neurons (17). Our
study found that leptin receptors were expressed mainly in Leydig
cells whose major function is to secrete testosterone. This
suggests that leptin and its receptor are likely to affect the
reproductive system by regulating the secretion of testosterone.
Tena-Sempere's study group found that leptin inhibited testosterone
secretion by adult rat tissue in vitro and, in a subsequent
study, found that leptin might inhibit testosterone secretion by
down-regulating mRNA of some elements upstream of the steroidogenic
pathway (18,19).
Fombonne et al found that leptin could
inhibit the division of immature Leydig cells (8). Our results showed that the expression
of leptin and leptin receptors increased, and testosterone
decreased significantly, in the experimental varicocele groups (G2
and G4). Overexpression of leptin and leptin receptors in testis
tissue appears to be related to inhibition of testosterone
secretion. There was a trend toward an increase in serum leptin in
G2 and G4 compared with G1 and G3 rats, respectively, but neither
reached significance. This result can be explained by the fact that
serum leptin is regulated mainly by systemic metabolism and is
related to the body mass index (20).
We found that expression of leptin receptors in the
hypothalamus of rats in the experimental varicocele groups (G2 and
G4) was increased. Furthermore, we also found that the mRNA levels
of KiSS-1 GPR54, GnRH, LH, and FSH increased in the experimental
varicocele groups. KiSS-1 is a coding gene for a tumor metastasis
suppressor. The protein product is also called KiSS-1 and GPR54 is
its receptor. KiSS-1/GPR54 is involved in regulating the
hypothalamic-pituitary-gonadal axis which regulates levels of sex
hormones by activating GnRH (21).
The leptin receptor is present on KiSS-1 hypothalamic neurons, not
on GnRH neurons (22), suggesting
that peripheral leptin levels may regulate the
hypothalamic-pituitary-gonadal axis by acting on the KiSS-1/GPR54
system.
A recent study has shown that leptin not only
promotes the expression of KiSS-1 and GPR54, but also expression of
the leptin and androgen receptors. This suggests that leptin and
androgen may have a positive synergistic effect on regulating the
KiSS-1/GPR54 system (23).
Therefore, we believe that the increased expression of leptin
receptors in the hypothalamus caused by varicocele activated the
KiSS-1/GPR54 system, resulting in upregulation of GnRH mRNA levels
and then upregulation of LH and FSH mRNA levels. The KiSS-1 gene is
a target for regulation by both leptin and testosterone (24). Therefore, the increased expression
of GnRH, LH, and FSH can also be interpreted as negative feedback
regulation of testosterone, or synergistic effects of leptin and
testosterone.
The non-significant trend toward increased serum FSH
and LH levels we observed in the experimental varicocele groups
might be explained by the small sample size or short modeling time.
Based on the results of our study, and the current understanding of
leptin, we cannot fully define the role of leptin and leptin
receptors in the pathogenesis of varicocele-induced testicular
dysfunction. In part, this is because our results are only
observational. Further studies, including both animal and in
vitro cell experiments, are needed to clarify the
mechanism.
Overexpression of leptin and the leptin receptor is
closely related to spermatogenesis dysfunction. The results of our
study indicate that varicocele can increase the expression of
leptin and leptin receptors in rat testis, thus causing
spermatogenesis dysfunction. Leptin and leptin receptors may have a
significant role in the male reproductive system by regulating the
KiSS-1/GPR54 system. Our study may help to further define the
mechanism of how varicocele causes infertility and provide new
ideas for treatment. However, understanding the exact role of
leptin and leptin receptors in the pathogenesis of
varicocele-induced testicular dysfunction requires further research
and we are designing experiments to clarify the role of leptin in
reproductive endocrinology and metabolism.
Acknowledgments
Not applicable.
Funding
This study was funded by the Research Grant for
Health Science and Technology of Shanghai Municipal Commission of
Health and family Planning (grant nos. 201440455 and
ZHYY-ZXYJHZX-201602) and the Key Discipline Construction Project of
Pudong Health and Family Planning Commission of Shanghai (grant no.
PWZxk2017-21).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ performed the research and wrote the paper. PPJ
helped perform the research. MG designed the research and revised
the paper. QTY and RJZ analyzed the data.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Shanghai Municipal Commission of Health and Family Planning
(SWJW005).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lai YW, Hsueh TY, Hu HY, Chiu YC, Chen SS
and Chiu AW: Varicocele is associated with varicose veins: A
population-based case-control study. Int J Urol. 22:972–975. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiraishi K, Matsuyama H and Takihara H:
Pathophysiology of varicocele in male infertility in the era of
assisted reproductive technology. Int J Urol. 19:538–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sáinz N, Barrenetxe J, Moreno-Aliaga MJ
and Martínez JA: Leptin resistance and diet-induced obesity:
Central and peripheral actions of leptin. Metabolism. 64:35–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schulz LC and Widmaier EP: Leptin
receptors. Endocrine Updates. 83:271–361. 2006.
|
|
5
|
Barash IA, Cheung CC, Weigle DS, Ren H,
Kabigting EB, Kuijper JL, Clifton DK and Steiner RA: Leptin is a
metabolic signal to the reproductive system. Endocrinology.
137:3144–3147. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Budak E, Fernández Sánchez M, Bellver J,
Cerveró A, Simón C and Pellicer A: Interactions of the hormones
leptin, ghrelin, adiponectin, resistin, and PYY3-36 with the
reproductive system. Fertil Steril. 85:1563–1581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawwass JF, Summer R and Kallen CB: Direct
effects of leptin and adiponectin on peripheral reproductive
tissues: A critical review. Mol Hum Reprod. 21:617–632. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fombonne J, Charrier C, Goddard I, Moyse E
and Krantic S: Leptin-mediated decrease of cyclin A2 and increase
of cyclin D1 expression: Relevance for the control of prepubertal
rat Leydig cell division and differentiation. Endocrinology.
148:2126–2137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grosfeld A, Andre J, Hauguel-De Mouzon S,
Berra E, Pouyssegur J and Guerre-Millo M: Hypoxia-inducible factor
1 transactivates the human leptin gene promoter. J Biol Chem.
277:42953–42957. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa T, Fujioka H, Ishimura T,
Takenaka A and Fujisawa M: Expression of leptin and leptin receptor
in the testis of fertile and infertile patients. Andrologia.
39:22–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner TT: The study of varicocele through
the use of animal models. Hum Reprod Update. 7:78–84. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnsen SG: Testicular biopsy score
count-a method for registration of spermatogenesis in human testes:
Normal values and results in 335 hypogonadal males. Hormone.
1:2–25. 1970.
|
|
13
|
Agarwal A, Sharma R, Harlev A and Esteves
SC: Effect of varicocele on semen characteristics according to the
new 2010 World Health Organization criteria: A systematic review
and meta-analysis. Asian J Androl. 18:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith MS, True C and Grove KL: The
neuroendocrine basis of lactation-induced suppression of GnRH: Role
of kisspeptin and leptin. Brain Res. 1364:139–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B, Guo JH, Lu YN, Ying XL, Hu K,
Xiang ZQ, Wang YX, Chen P and Huang YR: Leptin and
varicocele-related spermatogenesis dysfunction: Animal experiment
and clinical study. Int J Androl. 32:532–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donato J Jr, Cravo RM, Frazão R and Elias
CF: Hypothalamic sites of leptin action linking metabolism and
reproduction. Neuroendocrinology. 93:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lebrethon MC, Vandersmissen E, Gérard A,
Parent AS, Junien JL and Bourguignon JP: In vitro stimulation of
the prepubertal rat gonadotropin-releasing hormone pulse generator
by leptin and neuropeptide Y through distinct mechanisms.
Endocrinology. 141:1464–1469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tena-Sempere M, Pinilla L, González LC,
Diéguez C, Casanueva FF and Aguilar E: Leptin inhibits testosterone
secretion from adult rat testis in vitro. J Endocrinol.
161:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tena-Sempere M, Manna PR, Zhang FP,
Pinilla L, González LC, Diéguez C, Huhtaniemi I and Aguilar E:
Molecular mechanisms of leptin action in adult rat testis:
Potential targets for leptin-induced inhibition of steroidogenesis
and pattern of leptin receptor messenger ribonucleic acid
expression. J Endocrinol. 170:413–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glander HJ, Lammert A, Paasch U, Glasow A
and Kratzsch J: Leptin exists in tubuli seminiferi and in seminal
plasma. Andrologia. 34:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dungan HM, Clifton DK and Steiner RA:
Minireview: Kisspeptin neurons as central processors in the
regulation of gonadotropin-releasing hormone secretion.
Endocrinology. 147:1154–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skorupskaite K, George JT and Anderson RA:
The kisspeptin-GnRH pathway in human reproductive health and
disease. Hum Reprod Update. 20:485–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morelli A, Marini M, Mancina R, Luconi M,
Vignozzi L, Fibbi B, Filippi S, Pezzatini A, Forti G, Vannelli GB
and Maggi M: Sex steroids and leptin regulate the ‘first Kiss’
(KiSS 1/G-protein-coupled receptor 54 system) in human
gonadotropin-releasing-hormone-secreting neuroblasts. J Sex Med.
5:1097–1113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Popa SM, Clifton DK and Steiner RA: The
role of kisspeptins and GPR54 in the neuroendocrine regulation of
reproduction. Annu Rev Physiol. 70:213–238. 2008. View Article : Google Scholar : PubMed/NCBI
|