Introduction
Cardiovascular disease is one of the most harmful
diseases to human health in the world, with a high incidence.
Although surgery and medication reduce the mortality of patients
with cardiovascular disease, numerous patients develop progressive
myocardial failure, which may be fatal and is a threat to the
quality of life of middle-aged and elderly patients following a
heart attack (1,2). In the USA, a total of 20% of patients
with heart failure succumb after 1 year, and 50% after 5 years
(3). Since adult cardiomyocytes
lose their regenerative ability, necrotic cardiomyocytes may only
be replaced by fibroblasts to form scar tissue, eventually leading
to heart failure (4). A feasible
strategy to prevent heart failure is transplantation of exogenous
cells into the injured myocardium to produce contractile cells.
A number of types of stem cells have been examined
with respect to clinical applications, with the aim of replenishing
necrotic cardiomyocytes or providing a more suitable environment
for cardiac regeneration (5,6).
Among them, mesenchymal stem cells (MSCs) have gained extensive
attention due to their high proliferative ability, low
immunogenicity and fewer ethical considerations. MSCs may be
isolated from various tissues and may differentiate into numerous
types of cells, including cardiocytes, bone cells, cartilage cells,
adipocytes and neurons (7–9).
There are three methods for inducing bone marrow MSC
differentiation into cardiomyocytes in vitro: The first is
drug-induced differentiation, for example 5-azacytidine (5-aza)
(10); the second is coculture
with myocardial cells (11); and
the third is genetic modification (12). Although the efficiency of
differentiation induced by 5-aza requires further improvement, it
remains a widely used model to differentiate MSCs into
cardiomyocytes (13,14).
MicroRNAs are a type of non-coding RNA molecule of
~22 nucleotides in length. MicroRNAs are involved in a number of
physiological processes by binding to the 3′untranslated region
(3′UTR) of target genes, and thus promoting mRNA degradation or
inhibiting the transcription of target genes (15). microRNAs additionally serve
important roles in cardiovascular disease, affecting a number of
facets of cardiac remodeling, including stem cell differentiation,
apoptosis and cardiac contractility (8).
The present study investigated the role of
microRNA-149 (miR-149) in the differentiation of mouse MSCs from
bone marrow into cardiocytes, and examined the underlying mechanism
and signaling pathway. The present study identified a microRNA,
which was able to promote the differentiation of bone marrow MSCs,
and lay the foundation for stem cell transplantation to repair
myocardial injury.
Materials and methods
Culture of cells
MSCs were purchased from Cyagen Biosciences, Inc.
(Santa Clara, CA, USA; cat. no. MUCMX-01001). MSCs were cultured in
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 20% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.), in a
humidified 5% CO2 air incubator at 37°C. The MSCs were
passaged when they reached 80–90% confluence at 1:3 and used at
passage (P)3. 5-aza was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) and dissolved in dimethyl sulfoxide
(Invitrogen; Thermo Fisher Scientific, Inc.). NIH/3T3 cells and
293T cells were purchased from the American Type Culture Collection
(Manassas, VA, USA) and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin, in a humidified 5% CO2 air incubator
at 37°C. XAV-939 was purchased from Selleck Chemicals, Houston, TX,
USA (cat. no. S1180), at working concentration of 10 nM.
5-aza induction of MSCs
MSCs at P3 were seeded into 6-well plates at a
concentration of 5×105 cells/well. At 24 h, MSCs were
treated with 5-aza at a final concentration of 10 µM (day 0). The
induction medium was changed following 24 h and cells were washed
three times with PBS. Cells were cultured in DMEM/F12 containing
10% FBS for a further 6 (day 7) or 20 days (day 21).
Target prediction
Targetscan (http://www.targetscan.org/mamm_31/) and Pictar
(http://www.pictar.org/) were used to determine
potential target genes of miR-149.
Transfection
miR-149 mimics and mimics control were designed and
synthesized by Shanghai Genepharma Co., Ltd. Transfection of
NIH/3T3 and 293T cells was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
NIH/3T3 and 293T cells (2×105) were seeded in 3.5-mm
dishes overnight and transfected with 50 pmol miR-149 or mimic
control the following day. The medium was changed 6 h
post-transfection. The sequence of miR-149 mimics was:
UCUGGCUCCGUGUCUUCACUCCC (+) and GAGUGAAGACACGGAGCCAGAUU (−). The
sequence of mimics control was: UUCUUCGAACGUGUCACGUTT (+) and
ACGUGACACGUUCGGAGAATT (−).
Dual luciferase assay
293T cells were seeded in 24-well plates at a
density of 2×104 cells/well. The 3′UTR of Dab2
containing the binding site of miR-149, was acquired by polymerase
chain reaction (PCR) using MSCs and then cloned into pGL3 (Promega
Corporation, Madison, WI, USA) with XbaI and XhoI
restriction enzymes (New England BioLabs, Inc., Ipswich, MA, USA).
PCR was performed with PrimeSTAR®HS DNA Polymerase
(Takara Bio, Inc., Otsu, Japan) using the following thermocycling
conditions: 30 cycles of 98°C for 10 sec, 55°C for 15 sec and 72°C
for 1 min. The primers used for PCR were as follows: Forward,
5′-GCCCTTTCGGAAATCCTTTTG-3′ and reverse 5′-CTGGGAGAGATCACCAGAAT-3′.
A plasmid with a mutant miR-149 binding site was acquired using a
site-directed mutagenesis kit (Stratagene; Agilent Technologies,
Inc., Santa Clara, CA, USA). Plasmids were transfected into NIH
cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The luciferase activity assay was performed using a Promega
Dual-Glo® Luciferase Assay System (Promega Corporation),
according to the manufacturer's protocol. The luminescence was
measured using a Berthold LB9507 luminometer (Berthold Technologies
GmbH & Co. KG, Bad Wildbad, Germany). Relative luciferase
activity is presented as the ratio of firefly to Renilla
luminescence.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
Total RNA from MSCs was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. For miRNA detection, cDNAs were
synthesized at 37°C for 60 min using the miRcute miRNA First-strand
cDNA kit (Tiangen Biotech Co., Ltd., Beijing, China). For
protein-coding genes, cDNAs were synthesized at 37°C for 60 min
using the Quant Reverse Transcriptase kit (Tiangen Biotech Co.,
Ltd.). The expression level of miR-149 was detected using miRNA
qPCR detection kits (Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol, and was normalized to the expression level
of small nuclear RNA RNU6B (U6). For protein-coding genes, data
were normalized to the expression level of GAPDH. The relative
expression levels of mRNA were calculated using the
2−ΔΔCq method (16).
qPCR was performed using the ABI 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using SYBRGreen in a Super Real Pre
Mix kit (Tiangen Biotech Co., Ltd.). The products were amplified
using the following program: 94°C for 10 min, followed by 40 cycles
of 94°C for 15 sec and 60°C for 30 sec. The sequences of the PCR
primers are listed in Table I.
 | Table I.List of primers for quantitative
polymerase chain reaction analysis in the present study. |
Table I.
List of primers for quantitative
polymerase chain reaction analysis in the present study.
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|
| Mmu-miR-149 |
TCTGGCTCCGTGTCTTCACTCCC | universal (provided
by kit, sequence unavailable) |
| U6 |
CCTGCGCAAGGATGAC |
GTGCAGGGTCCGAGGT |
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCA |
| Nkx2.5 | CGA
CGGAAGCCACGCGTGCT |
CCGCTGTCGCTTGCACTTG |
| GATA4 |
CCCTACCCAGCCTACATGG |
ACATATCGAGATTGGGGTGTCT |
| cTnI |
GTCCTCCTTCTTCACCTGCTTG |
CTCTGCCAACTACCGAGCCTAT |
| CX43 |
GTGCCGGCTTCACTTTCA |
GGAGTAGGCTTGGACCTTGTC |
Lentiviral infection
The lentiviruses overexpressing miR-149 and disabled
homolog 2 (Dab2) were purchased from Shanghai Genepharma Co., Ltd.
(Shanghai, China). Lentiviral infection was performed according to
the manufacturer's protocol. MSCs (5×105 cells/well)
were seeded into 6-well plates and then incubated at 37°C with
lentivirus overexpressing miR-149 or mock lentivirus (Shanghai
Genepharma Co., Ltd.) at a multiplicity of infection (MOI) of 200
for 8 h. For MSCs overexpressing Dab2, the Dab2 lentivirus was
added at an MOI of 100 1 day subsequent to the cells being infected
with the lentivirus overexpressing miR-149, and the DMEM/F12 medium
was changed at 24 h following incubation at 37°C. Gene expression
was separately analyzed using RT-qPCR as previously described on
days 3, 7 and 14 post-infection.
Western blotting
Cells were collected in ice-cold PBS 3 days
post-infection or transfection, and total proteins were extracted
with ice-cold radioimmunoprecipitation assay buffer with protease
inhibitors (Roche Applied Science, Penzberg, Germany) and
quantified with a bicinchoninic acid kit (Thermo Fisher Scientific,
Inc.). Equal amounts of proteins (40 µg) were separated by 10%
SDS-PAGE and transferred to 0.45-µm polyvinylidene fluoride
membranes. The membranes were blocked with 5% fat-free milk in TBS
containing 0.05% Tween-20 at room temperature for 1 h, and probed
with the following primary antibodies overnight at 4°C: Anti-Dab2
(cat. no. ab137866; 1:1,000; Abcam, Cambridge, UK) and anti-β-actin
(cat. no. ab227387; 1:3,000; Abcam). Membranes were subsequently
washed three times with TBST and incubated with
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(cat. no. 32460; 1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Signals were visualized using
enhanced chemiluminescence reagents (Roche Applied Science). Dab2
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Densitometric quantification was performed
using ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All experimental data are presented as the mean ±
standard deviation. Statistical significance was determined by
Student's t-test for two groups, or single factor analysis of
variance followed by the Tukey's multiple comparisons test.
Statistical analysis was performed using GraphPad Prism software
(version 6.01; GraphPad Software Inc., La Jolla, CA, USA). All
experiments were performed in triplicate. P<0.05 was considered
to indicate a statistically significant difference.
Results
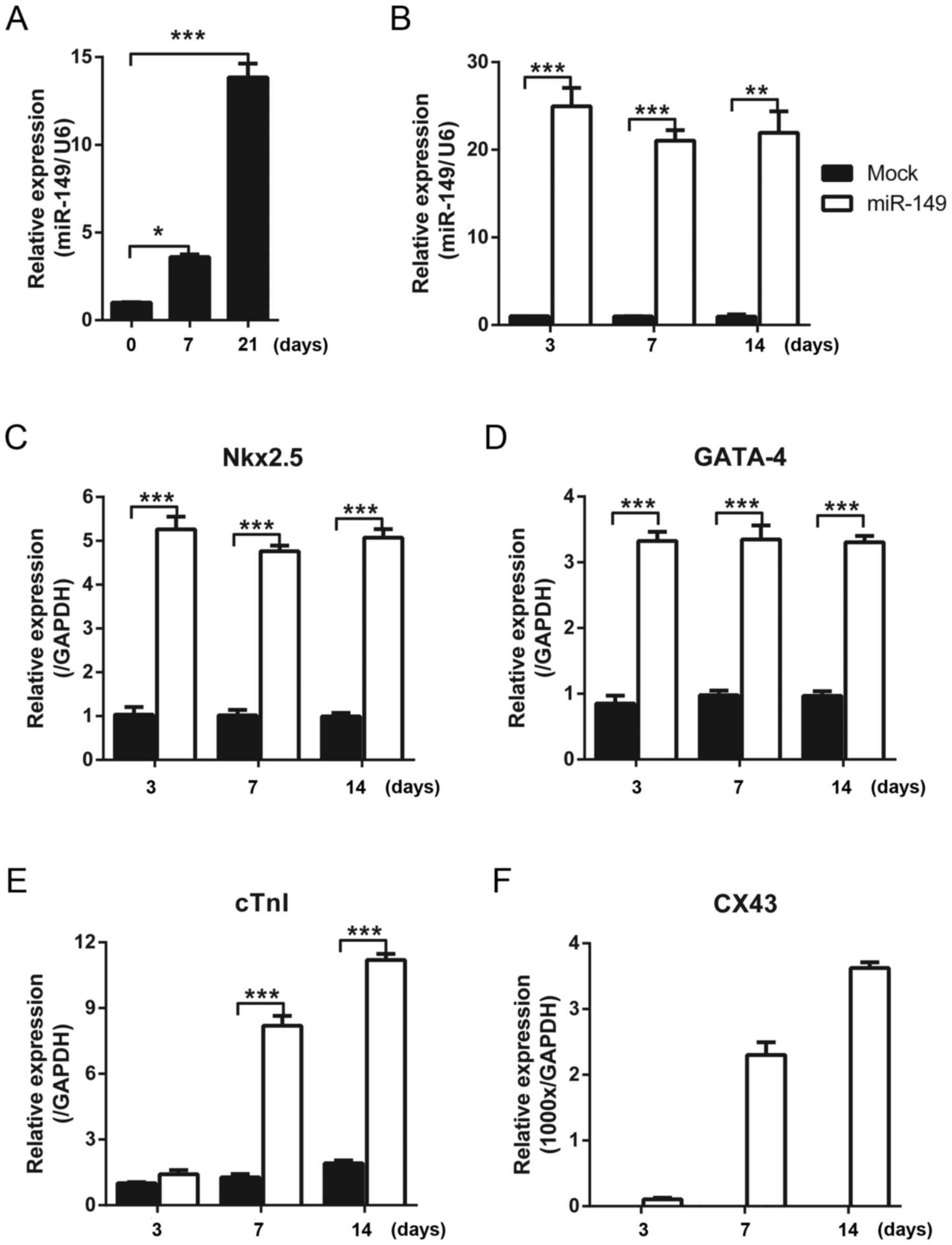
miR-149 promotes the expression of
cardiac differentiation markers
The cardiac differentiation of mouse MSCs from bone
marrow was induced with 5-aza, and the expression of miR-149 was
detected with qPCR at different time points. It was identified that
the expression level of miR-149 was upregulated with increasing
treatment time of 5-aza (Fig. 1A).
Therefore, it was hypothesized that miR-149 may serve a role in the
cardiac differentiation of MSCs. To test this hypothesis, miR-149
was overexpressed in MSCs using a lentivirus and the expression of
cardiac differentiation markers was detected using qPCR. Compared
with the control group which was infected with mock lentivirus,
miR-149 was overexpressed (Fig.
1B). The early markers of cardiac differentiation, Nkx2.5 and
GATA-4, were upregulated when miR-149 was expressed for 3 days and
the high level was maintained between 7 and 14 days (Fig. 1C and D). The expression of the late
markers of cardiac differentiation, cTnI and CX43, continued to
increase over time (Fig. 1E and
F).
Dab2 is the direct target of
miR-149
The present study aimed to elucidate the mechanism
through which miR-149 may affect the cardiac differentiation of
MSCs (Fig. 2). Targetscan and
Pictar were used to analyze the potential target genes of miR-149,
and it was identified that Dab2 may be regulated by miR-149
(17–19). First, the expression level of Dab2
was detected in 5-aza-induced MSCs, and it was observed that the
expression of Dab2 was downregulated at the mRNA and protein level
(Fig. 2A, C and F). Similarly,
overexpressing miR-149 decreased the mRNA expression level of Dab2
at different time points (Fig.
2B). The protein expression level of Dab2 was detected
following overexpression of miR-149 for 3 days in MSCs, and it was
demonstrated that Dab2 was downregulated (Fig. 2D and G). Since Dab2 expression
decreased during the process of cardiac differentiation, to rule
out the influence of cardiac differentiation on Dab2, NIH 3T3 cells
were transfected with miR-149 mimics. It was demonstrated that
miR-149 was able to downregulate the protein expression level of
Dab2 in NIH 3T3 cells (Fig. 2E and
H). A dual luciferase assay was performed to detect whether
Dab2 was the direct target of miR-149. miR-149 was able to decrease
the relative luciferase activity of the wild type 3′UTR of Dab2,
while it had no effects on the mutant 3′UTR of Dab2 (Fig. 2I and J).
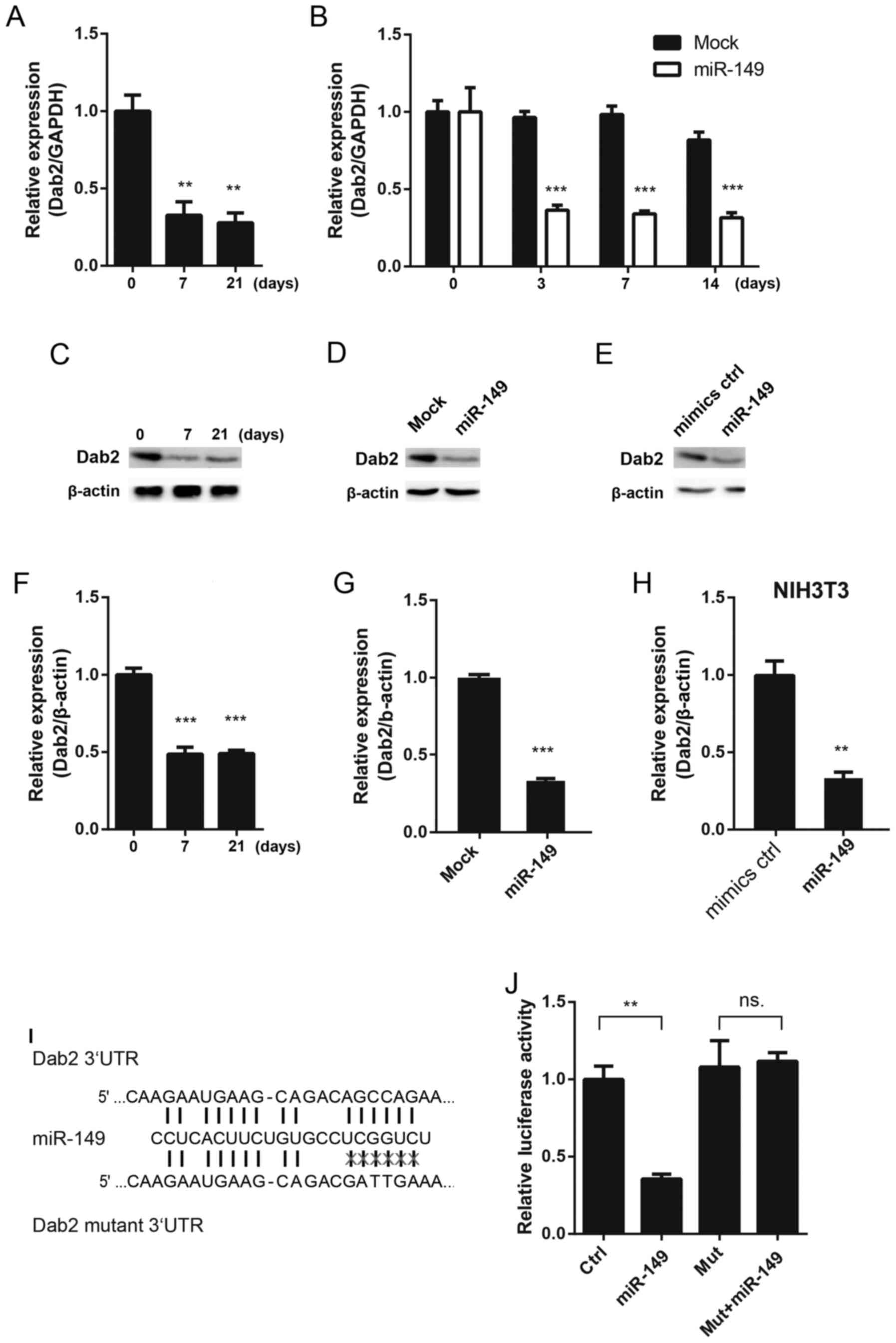 | Figure 2.Dab2 is a direct target of miR-149.
(A) The mRNA expression of Dab2 in MSCs treated with 5-aza at
different time points. (B) The mRNA expression of Dab2 in MSCs
following infection with miR-149-overexpressing lentivirus at
different time points. (C) The protein expression of Dab2 in MSCs
treated with 5-aza at different time points. (D) miR-149 decreased
the protein expression of Dab2 following infection with
miR-149-overexpresseing lentivirus on the 3rd day. (E) miR-149
mimics decreased the protein expression of Dab2 in 3T3 cells
following 3 days' transfection. (F) Quantification of the protein
expression of Dab2 in MSCs at different time points. (G)
Quantification of the protein expression of Dab2 post-lentiviral
infection. (H) Quantification of Dab2 expression in 3T3 cells. (I)
Schematic diagram of the miR-149 binding site in the 3′UTR of Dab2
and the mutant site. (J) Identification of miR-149 in Dab2 with the
luciferase assay. *P<0.05, **P<0.01, ***P<0.001 vs.
respective control. MSCs, mesenchymal stem cells; 5-aza,
5-azacytidine; Dab2, disabled homolog 2; miR-149, microRNA-149; d,
days; UTR, untranslated region; ns, not significant; ctrl, control;
mut, mutant. |
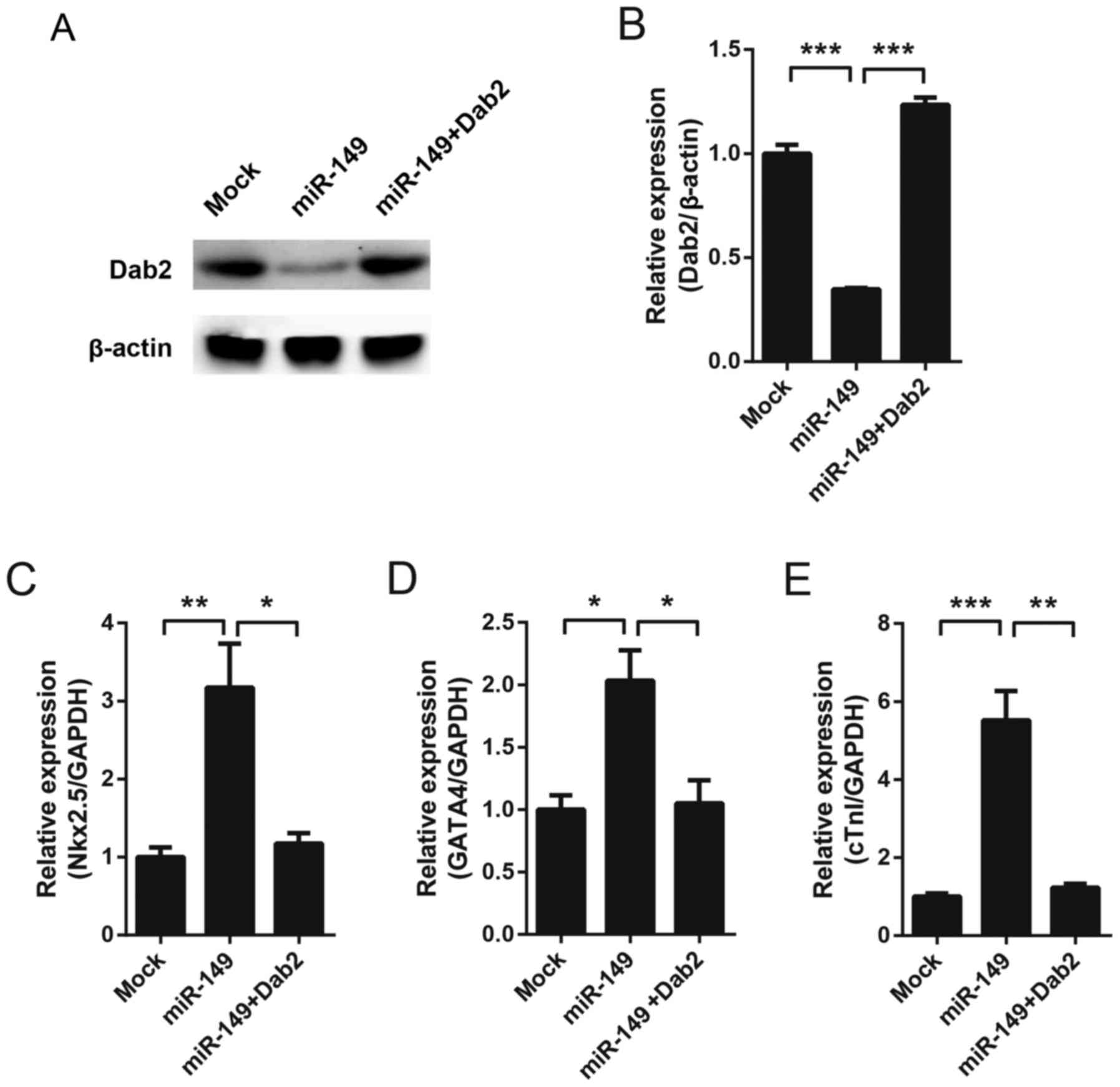
miR-149-induced cardiac
differentiation is mediated by Dab2
To detect whether Dab2 mediates the cardiac
differentiation of MSCs induced by miR-149, Dab2 was overexpression
using a lentivirus in miR-149-overexpressing MSCs. Western blotting
was performed to test the expression of Dab2 on the 7th day. As
hypothesized, miR-149 decreased the expression of Dab2, while
exogenous expression of Dab2 recovered the protein expression level
(Fig. 3A and B). Cardiac
differentiation markers were subsequently detected. Since CX43 was
undetectable at baseline in MSCs (Fig.
1F), and on the 7th day all markers exhibited a detectable
level, Nkx2.5, GATA4 and cTnI were detected on the 7th day. It was
observed that miR-149 increased the expression of these genes,
while overexpression of Dab2 was able to reverse the expression
level of these genes almost to basal levels (Fig. 3C-E).
Effect of the Wnt signaling pathway on
miR-149-induced cardiac differentiation
It has been reported that Dab2 may negatively
regulate the canonical Wnt-β-catenin signaling pathway (20). The results of the present study
demonstrated that overexpressing miR-149 increased the expression
of Dab2-target Wnt pathway genes, including Axin2 and pterin-4
alpha-carbinolamine dehydratase 1 (Fig. 4A and B). In order to assess whether
the effect of miR-149 depended on the Wnt/β-catenin signaling
pathway, overexpressing miR-149 MSCs were treated with XAV-939, a
Wnt/β-catenin signaling pathway inhibitor. It was identified that
XAV-939 reversed the upregulation of cardiac differentiation
markers induced by miR-149 (Fig.
4C-F), which suggested that miR-149 may promote the cardiac
differentiation of MSCs via the Wnt/β-catenin signaling
pathway.
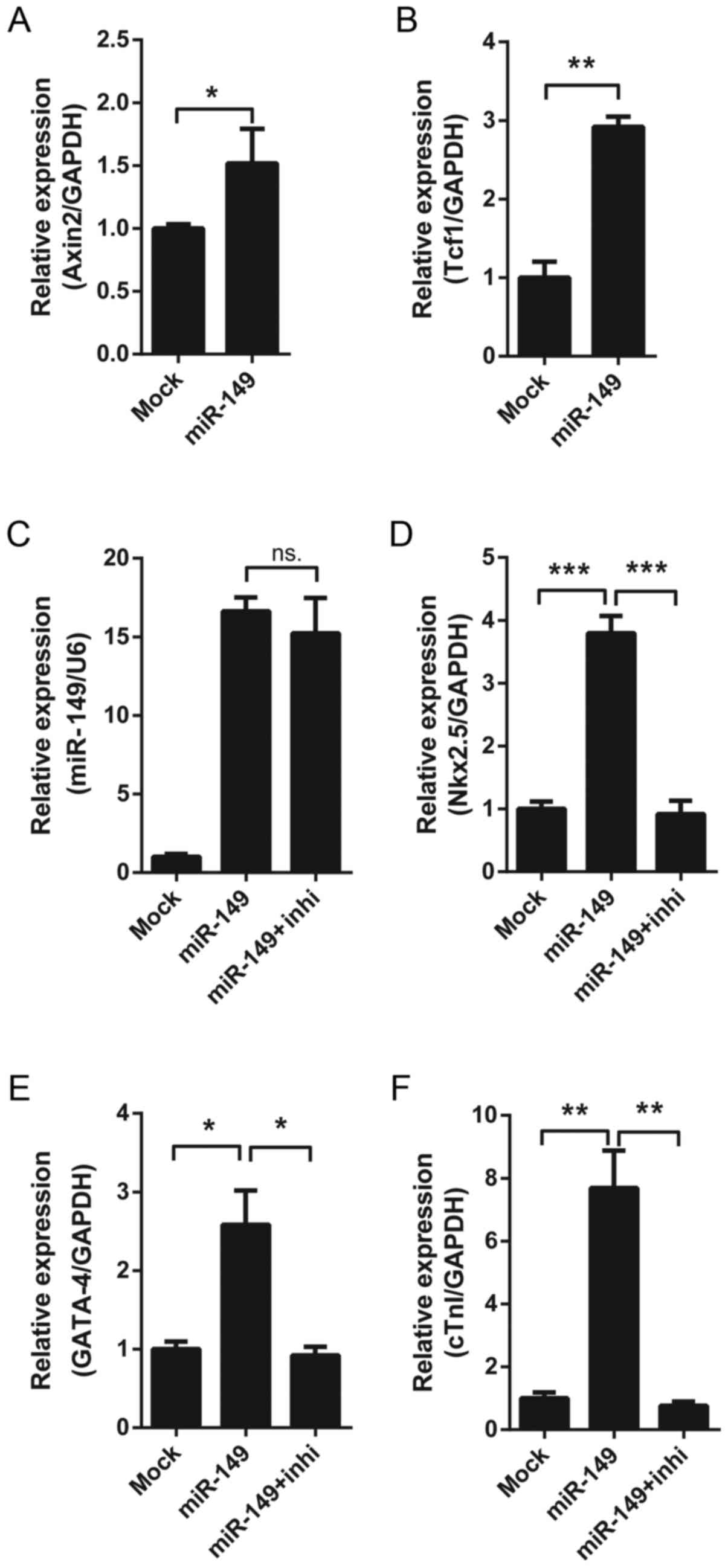 | Figure 4.Effect of the Wnt signaling pathway on
miR-149-induced cardiac differentiation. (A) The mRNA expression of
Axin2 in MSCs following 7 days' lentiviral infection. (B) The mRNA
expression of Tcf1 in MSCs following 7 days' lentiviral infection.
(C) The expression of miR-149 in MSCs following 7 days' lentiviral
infection, with or without treatment with 10 nM XAV-939. The mRNA
expression of the cardiac markers (D) Nkx2.5, (E) GATA-4 and (F)
cTnI in MSCs following 7 days' lentiviral infection, with or
without treatment with 10 nM XAV-939. *P<0.05, **P<0.01,
***P<0.001. miR-149, microRNA-149; MSCs, mesenchymal stem cells;
Tcf1, pterin-4 alpha-carbinolamine dehydratase 1; Nkx2.5, homeobox
protein Nkx2.5; GATA-4, transcription factor GATA-4; cTnI, cardiac
troponin I; ns, not significant; inhi, inhibitor XAV-939. |
Discussion
The present study demonstrated that expression of
miR-149 in mouse MSCs from bone marrow promoted the expression of
cardiac phenotypic markers, suggesting that miR-149 may serve a
role in the induction of differentiation from MSCs into
cardiocytes. Furthermore, it was observed that Dab2 was a direct
target gene of miR-149, and that exogenous expression of Dab2 was
able to reverse the upregulation of cardiac markers induced by
miR-149. Further mechanistic analysis demonstrated that miR-149
likely regulated cardiac differentiation through the Wnt/β-catenin
signaling pathway.
Increasing microRNAs have been reported to serve
important roles in cardiac differentiation and myocardial repair
following a heart attack (8,15).
MSCs are able to secrete exosomes and microvessels rich in
microRNAs, in order to shape the microenvironment to promote
myocardial regeneration following a myocardial infarction (21). Therefore, it is important to
elucidate the potential function of microRNAs to comprehensively
understand the process of cardiac differentiation.
miR-149 has been extensively studied in tumor
biology, and was demonstrated to be involved in different processes
of tumorigenesis and development, including proliferation,
migration, invasion and epithelial-mesenchymal transition (22,23).
In addition, miR-149 serves important roles in a number of other
diseases, including stroke, type II diabetes and non-alcoholic
fatty liver disease (24–28). A recent study reported that the
circulating level of miR-149 decreased in mouse models with severe
heart failure (29). An earlier
study in 2013 demonstrated that the alteration of the binding site
of miR-149 located in the 3′UTR of methylenetetrahydrofolate
reductase due to a single-nucleotide polymorphism was associated
with coronary heart disease susceptibility (30). These reports suggested that miR-149
may have important roles in heart disease. However, the function
and mechanism of miR-149 in heart disease remained unclear. The
results of the present study revealed a novel function of miR-149
in cardiac differentiation from bone marrow MSCs.
The present study further identified the target gene
of miR-149, Dab2, which mediated the cardiac differentiation
induced by miR-149. Dab2 is a scaffold protein with multiple
modules. It has important roles in signaling transduction and
affects numerous biological processes, including cell growth,
vesicle trafficking, cell interaction, macrophages polarization and
platelet activation (31–33). Certain previous studies
demonstrated that Dab2 is important for the expression of cardiac
markers, and decreased expression of Dab2 was able to promote the
transforming growth factor-β-stimulated cardiac differentiation of
MSCs and improve cardiac function following MSC transplantation
(20,34). These results support the present
findings that downregulation of Dab2 by miR-149 promoted cardiac
differentiation. However, whether these cells may differentiate to
mature cardiocytes in vivo and promote the impaired
cardiocyte repair requires further study.
The Wnt/β-catenin signaling pathway serves important
roles during the process of heart development and regeneration. In
the present study, it was observed that the reduction in Dab2
increased the expression of β-catenin target genes, which suggested
that Dab2 may be a negative regulator of the Wnt/β-catenin
signaling pathway. Through treatment with a Wnt/β-catenin
inhibitor, it was illustrated that the regulatory effect of miR-149
on the expression of cardiac markers is dependent upon the
Wnt/β-catenin signaling pathway. This finding is supported by a
previous study that demonstrated that the deletion of Dab2 in
zebrafish led to an abnormal cardiomyocyte number and increased
Wnt/β-catenin signaling (20).
These results demonstrated that miR-149 may regulate cardiac
differentiation through the Dab2/Wnt/β-catenin signaling pathway.
Whether other signaling pathways are involved in this process
requires investigation in the future.
In conclusion, the results of the present study
demonstrated that miR-149 promoted the cardiac differentiation of
mouse MSCs from bone marrow in vitro, which depended on Dab2
and the Wnt/β-catenin signaling pathway. The present study provides
a potential molecular target for the cardiac differentiation of
MSCs, and a foundation for further study with animals and in the
clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Guangdong Provincial Department of Science and Technology (grant
no. 2017B020247042).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and SY designed the experiments. ML, LX, MW, TG,
FL and NS performed the experiments. ML, LX and TC analyzed the
data and organized the figures. ML, SY and TC wrote and revised the
manuscript. SY and TC supervised the work.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carvalho E, Verma P, Hourigan K and
Banerjee R: Myocardial infarction: Stem cell transplantation for
cardiac regeneration. Regen Med. 10:1025–1043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho GS, Fernandez L and Kwon C:
Regenerative medicine for the heart: Perspectives on stem-cell
therapy. Antioxid Redox Signal. 21:2018–2031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American Heart Association. Circulation.
131:e29–e322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poglajen G and Vrtovec B: Stem cell
therapy for chronic heart failure. Curr Opin Cardiol. 30:301–310.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oliveira MS, Saldanha-Araujo F, Goes AM,
Costa FF and de Carvalho JL: Stem cells in cardiovascular diseases:
Turning bad days into good ones. Drug Discov Today. 22:1730–1739.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wegener M, Bader A and Giri S: How to mend
a broken heart: Adult and induced pluripotent stem cell therapy for
heart repair and regeneration. Drug Discov Today. 20:667–685. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carvalho PH, Daibert AP, Monteiro BS,
Okano BS, Carvalho JL, Cunha DN, Favarato LS, Pereira VG, Augusto
LE and Del Carlo RJ: Differentiation of adipose tissue-derived
mesenchymal stem cells into cardiomyocytes. Arq Bras Cardiol.
100:82–89. 2013.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen Z, Zheng S, Zhou C, Yuan W, Wang J and
Wang T: Bone marrow mesenchymal stem cells for post-myocardial
infarction cardiac repair: microRNAs as novel regulators. J Cell
Mol Med. 16:657–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halleux C, Sottile V, Gasser JA and Seuwen
K: Multi-lineage potential of human mesenchymal stem cells
following clonal expansion. J Musculoskelet Neuronal Interact.
2:71–76. 2001.PubMed/NCBI
|
|
10
|
Li J, Zhu K, Wang Y, Zheng J, Guo C, Lai H
and Wang C: Combination of IGF-1 gene manipulation and 5-AZA
treatment promotes differentiation of mesenchymal stem cells into
cardiomyocyte-like cells. Mol Med Rep. 11:815–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Xu Z, Jiang W and Ma A:
Cell-to-cell contact induces mesenchymal stem cell to differentiate
into cardiomyocyte and smooth muscle cell. Int J Cardiol.
109:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arminán A, Gandía C, Bartual M,
García-Verdugo JM, Lledó E, Mirabet V, Llop M, Barea J, Montero JA
and Sepúlveda P: Cardiac differentiation is driven by NKX2.5 and
GATA4 nuclear translocation in tissue-specific mesenchymal stem
cells. Stem Cells Dev. 18:907–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen X, Pan B, Zhou H, Liu L, Lv T, Zhu J,
Huang X and Tian J: Differentiation of mesenchymal stem cells into
cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway.
J Biomed Sci. 24:292017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosca AM and Burlacu A: Effect of
5-azacytidine: Evidence for alteration of the multipotent ability
of mesenchymal stem cells. Stem Cells Dev. 20:1213–1221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hofsteen P, Robitaille AM, Chapman DP,
Moon RT and Murry CE: Quantitative proteomics identify DAB2 as a
cardiac developmental regulator that inhibits WNT/β-catenin
signaling. Proc Natl Acad Sci USA. 113:1002–1007. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen TS, Lai RC, Lee MM, Choo AB, Lee CN
and Lim SK: Mesenchymal stem cell secretes microparticles enriched
in pre-microRNAs. Nucleic Acids Res. 38:215–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cimpeanu RA, Popescu DM, Burada F, Cucu
MG, Gheonea DI, Ioana M and Rogoveanu I: miR-149 rs2292832 C>T
polymorphism and risk of gastric cancer. Rom J Morphol Embryol.
58:125–129. 2017.PubMed/NCBI
|
|
23
|
Ow SH, Chua PJ and Bay BH: miR-149 as a
potential molecular target for cancer. Curr Med Chem. Jul
18–2017.(Epub ahead of print).
|
|
24
|
Alipoor B, Meshkani R, Ghaedi H, Sharifi
Z, Panahi G and Golmohammadi T: Association of miR-146a rs2910164
and miR-149 rs2292832 variants with susceptibility to type 2
diabetes. Clin Lab. 62:1553–1561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An X, Yang Z and An Z: MiR-149 compromises
the reactions of liver cells to fatty acid via its polymorphism and
increases Non-alcoholic fatty liver disease (NAFLD) risk by
targeting methylene tetrahydrofolate reductase (MTHFR). Med Sci
Monit. 23:2299–2307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du J, Cui C, Zhang S, Yang X and Lou J:
Association of MicroRNA-146a and MicroRNA-149 polymorphisms with
strokes in asian populations: An updated meta-analysis. Angiology.
68:863–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu JY, Hu F, Du W, Ma XL and Yuan K: Study
of the association between five polymorphisms and risk of
hepatocellular carcinoma: A meta-analysis. J Chin Med Assoc.
80:191–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng L, Zhuang C, Zhao J and Ming L:
Functional miR-146a, miR-149, miR-196a2 and miR-499 polymorphisms
and the susceptibility to hepatocellular carcinoma: An updated
meta-analysis. Clin Res Hepatol Gastroenterol. 41:664–676. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaneko M, Satomi T, Fujiwara S, Uchiyama
H, Kusumoto K and Nishimoto T: AT1 receptor blocker azilsartan
medoxomil normalizes plasma miR-146a and miR-342-3p in a murine
heart failure model. Biomarkers. 22:253–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu C, Gong Y, Sun A, Zhang Y, Zhang C,
Zhang W, Zhao G, Zou Y and Ge J: The human MTHFR rs4846049
polymorphism increases coronary heart disease risk through
modifying miRNA binding. Nutr Metab Cardiovasc Dis. 23:693–698.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finkielstein CV and Capelluto DG:
Disabled-2: A modular scaffold protein with multifaceted functions
in signaling. Bioessays. 38 Suppl 1:S45–S55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adamson SE, Griffiths R, Moravec R,
Senthivinayagam S, Montgomery G, Chen W, Han J, Sharma PR, Mullins
GR, Gorski SA, et al: Disabled homolog 2 controls macrophage
phenotypic polarization and adipose tissue inflammation. J Clin
Invest. 126:1311–1322. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Chen Y, Tang J and Xie X:
Frequent loss expression of dab2 and promotor hypermethylation in
human cancers: A meta-analysis and systematic review. Pak J Med
Sci. 30:432–437. 2014.PubMed/NCBI
|
|
34
|
Hannigan A, Smith P, Kalna G, Lo Nigro C,
Orange C, O'Brien DI, Shah R, Syed N, Spender LC, Herrera B, et al:
Epigenetic downregulation of human disabled homolog 2 switches
TGF-beta from a tumor suppressor to a tumor promoter. J Clin
Invest. 120:2842–2857. 2010. View
Article : Google Scholar : PubMed/NCBI
|