Introduction
Cardiopulmonary arrest is common seen in emergency
practice. With the standardization of cardiopulmonary resuscitation
(CPR), the rate of resuscitation is increased (1). However, ~60% of patients experience
cerebral resuscitation failure and 2–3% of patients that survive
suffer from severe nerve function deficits (1). The prognosis of resuscitated patients
depends on the levels of cerebral ischemic injuries (2). Reperfusion injury following complete
cerebral ischemia is the major cause of cerebral injury. Following
CPR, patients experience ischemia-reperfusion injury (3). It is established that the mechanisms
of ischemia-reperfusion injury are associated with oxygen radicals
and calcium overload (4).
Following CPR, various inflammatory cells are activated and produce
cytokines that participate in the damaging effects of
ischemia-reperfusion (5). At the
early stage of CPR, the increase of tumor necrosis factor-α (TNF-α)
and interleukin-6 (IL-6) indicates that the inflammatory response
system is activated following CPR. The release of cytokines can be
considered as a stress response to cardiac arrest (6).
The uniform conduction of electrical activity
depends on electrical coupling among myocardial cells and is
influenced by the extracellular matrix (ECM) (7). Under normal physiological conditions,
the synthesis and degradation of ECM is in dynamic equilibrium
(8). Its excessive synthesis or
abnormal degradation may alter the mechanical properties of
myocardium and electrophysiological structures, which may affect
its uniform conduction (9).
Previous studies revealed that the regulation of matrix
metalloproteinases (MMPs) have an important role in the synthesis
and degradation of ECM (7,10).
B-cell specific moloney leukemia virus insertion
site 1 (Bmi-1) is an important member of the Polycomb-group protein
family (11). It has essential
actions in repairing DNA damage, cell cycle control, the stability
of chromatin, the activation of genetic transcription and apoptosis
(12).
Patients with cardiac arrest have obvious central
lesions following CPR. Following complete cerebral
ischemia-reperfusion injury, hydromechanics, pathomorphology and
metabolic disorders may form encephaledema, and severity influences
patient prognosis (13). Clinic
trials demonstrated that even when autonomic circulation recovered
following CPR, some patients still experienced loss of
consciousness (14).
Quercetin is a polyhydroxy flavonoid widely present
in flowers, leaves and fruits, with various biological activities
and high pharmaceutical value (15). Quercetin is present in >100
types of Chinese herbal medicine, including sophora flower bud,
sophora flower, the root bark of the peony tree, chrysanthemum,
Hypericum japonicum, Parasemia plantaginis, parasitic
Loranthus, hairyvein agrimony, Perfoliate knotweed herb,
Gynostemma pentaphyllum, maythorn, Hypericum
perforatum, semen cuscutae, ginkgo leaf, Aesculus
wilsonii rehd, Oldenlandia diffusa, cockscomb,
Houttuynia cordata, emblic leafflower fruit, Saururus
chinensis, Psidium guajava leaf, phyllan lhus mat shilllirae,
Chinese violet and Sedum sarmentosum (16–19).
The results of the current study demonstrated that quercetin
inflammation, MMP-2 activation and apoptosis induction in a rat
model of CPR and investigated the mechanism of the protective
effect of quercetin following CPR.
Materials and methods
Experimental animals
The protocol of animal experiments was approved by
the University Laboratory Animal Research Committee of The First
Hospital of Jilin University (Changchun, China). Adult
Sprague-Dawley (SD) rats (250±30 g; n=30) were purchased from
Qinghai Experimental Animal Center (Qinghai, China) and maintained
in a specific pathogen-free environment. The CPR model was
established by an asphyxia method and animals anaesthetized with
intraperitoneal injection of chloral hydrate (Shanghai Ruiqi
Biological Engineering Research Center, Shanghai, China). Tracheal
intubation was closed at the end of expiration for 5 min. SD rats
were randomly divided into sham operation group (sham; n=6),
quercetin group (QCT; n=6), Model group (Model, n=8) and Model
+quercetin group (Model + QCT; n=8). In the QCT group and Model +
quercetin group, normal or CPR model SD rats were treated with
intragastric injection of 50 mg/kg quercetin once a day for 5 days,
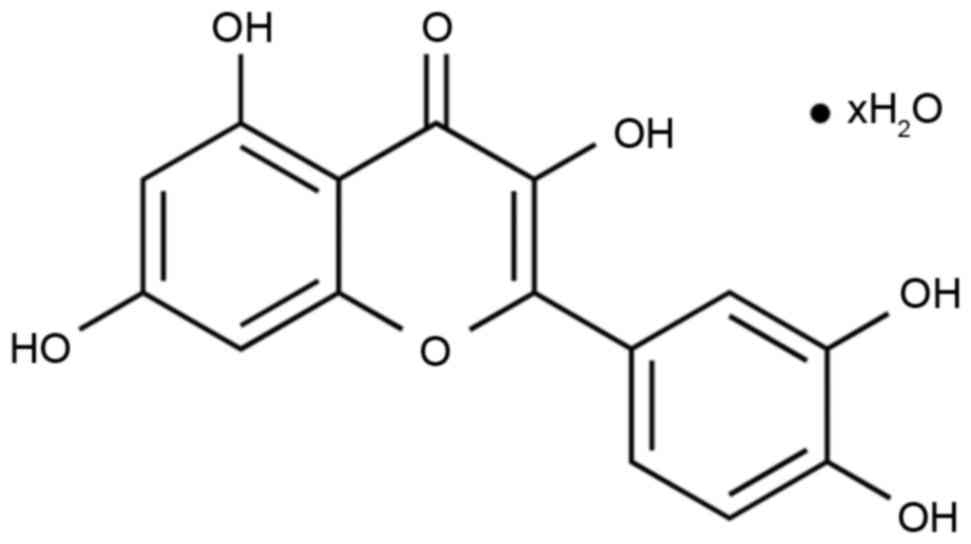
respectively. The chemical structure of quercetin is presented in
Fig. 1 and was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The rats of Model
and QCT group were anaesthetized with 35 mg/kg pentobarbital
sodium, tracheal intubation was closed at the end of expiration for
5 min and cardiopulmonary resuscitation was performed. The rats of
sham group were anaesthetized with 35 mg/kg pentobarbital sodium
without cardiopulmonary resuscitation.
Hemodynamic changes
After treatment with quercetin, surgical procedures
were performed on all SD rats to measure hemodynamic parameters.
Left ventricular dysfunction systolic (LVDs), left ventricular
dysfunction diastolic (LVDd), stroke volume (SV) and cardiac output
(CO). Ejection fraction (EF%) and left ventricular shortening
fraction (FS%) was calculated using the same area-length method as
previously described (19).
ELISA
Serum was separated from venous blood of each rat
following intragastric injection treatment with quercetin and used
to measure reactive oxygen species (ROS) generation (S0033), IL-6
(PI328) and TNF-α (PT516) activities using assay kits according to
the manufacturer's instructions (Beyotime Institute of
Biotechnology, Haimen, China). Caspase-3 activity was measured
using a caspase-3 activity detection kit (C1116, Beyotime Institute
of Biotechnology, Haimen, China).
Left ventricle weight/body weight
After 4 weeks, body weight was measured and
recorded. Subsequently, left ventricle weight was acquired. Left
ventricle weight/body weight was calculated.
Western blotting analysis
Heart tissue samples were homogenized in
radioimmunoprecipitation assay buffer (10 µg/ml, Beyotime Institute
of Biotechnology) in the presence of protease inhibitors (EMD
Millipore, Billerica, MA, USA). The supernatant was collected to
measure total proteins using the bicinchoninic acid method
(Beyotime Institute of Biotechnology). Total proteins (50 µg) were
separated by 12% SDS-PAGE and transferred to nitrocellulose
membranes. Following blocking with 5% non-fat milk in TBS-Tween at
37°C for 1 h, membranes were incubated with anti-MMP-2 (sc-10736,
1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-Bmi-1 (sc-10745, 1:2,000; Santa Cruz Biotechnology, Inc.),
anti-inducible nitric oxide synthase (iNOS;sc-649, 1:3,000; Santa
Cruz Biotechnology, Inc.) and β-actin (sc-7210, 1:2,000; Santa Cruz
Biotechnology, Inc.) at 4°C overnight, followed by incubation with
goat anti-rabbit IgG-HRP secondary antibodies (sc-2004, 1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The
blot images were observed using BeyoECL Moon kit (P0018F; Beyotime
Institute of Biotechnology) and analyzed with Image_Lab version 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The values are presented as the mean ± standard
error. One-way analysis of variance followed by a Tukey post hoc
test was used to analyze the differences between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Quercetin protects LVDs and LVDd in a
rat model of CPR
CPR rat were treated with 50 mg/kg quercetin, and
LVDs and LVDd levels were measured to evaluate that quercetin
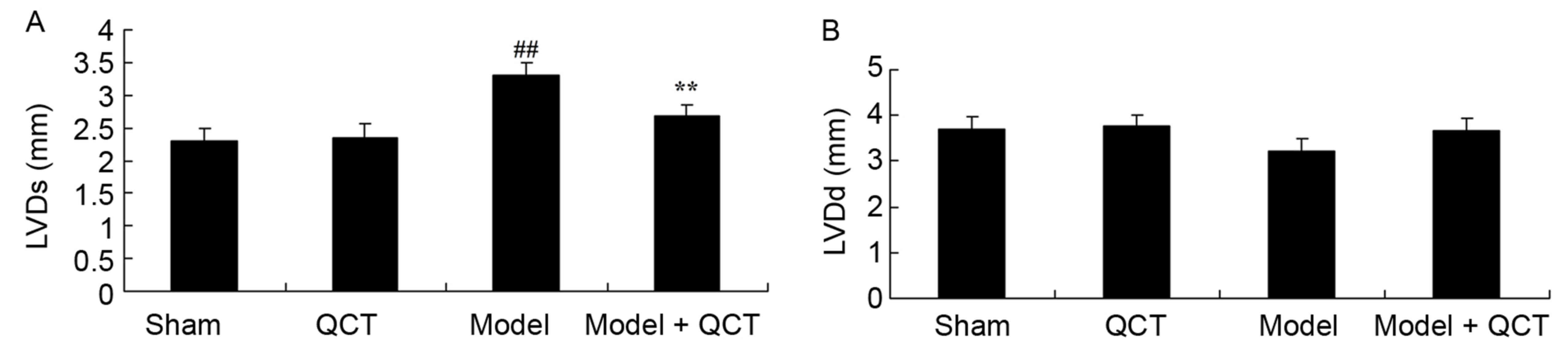
protects against CPR. As presented in Fig. 2A, the LVDs of CPR model rats was
higher than that of the sham group. However, quercetin
significantly inhibited the LVDs of CPR model rats (Fig. 2A). There was no significant
difference in the LVDd between the sham, QCT, CPR model group and
model + QCT groups (P>0.05; Fig.
2B).
Quercetin protects the EF, FS, SV and
CO in a rat model of CPR
It was evaluated whether quercetin protects EF, FS,
SV and CO in the rat model of CPR. There was no significant
difference between the sham group and QCT group (Fig. 3). However, the levels of EF, FS, SV
and CO in the CPR model rats were significantly lower than those of
the sham group (Fig. 3). Treatment
with quercetin significantly increased the EF, FS, SV and CO levels
in CPR model rats (Fig. 3).
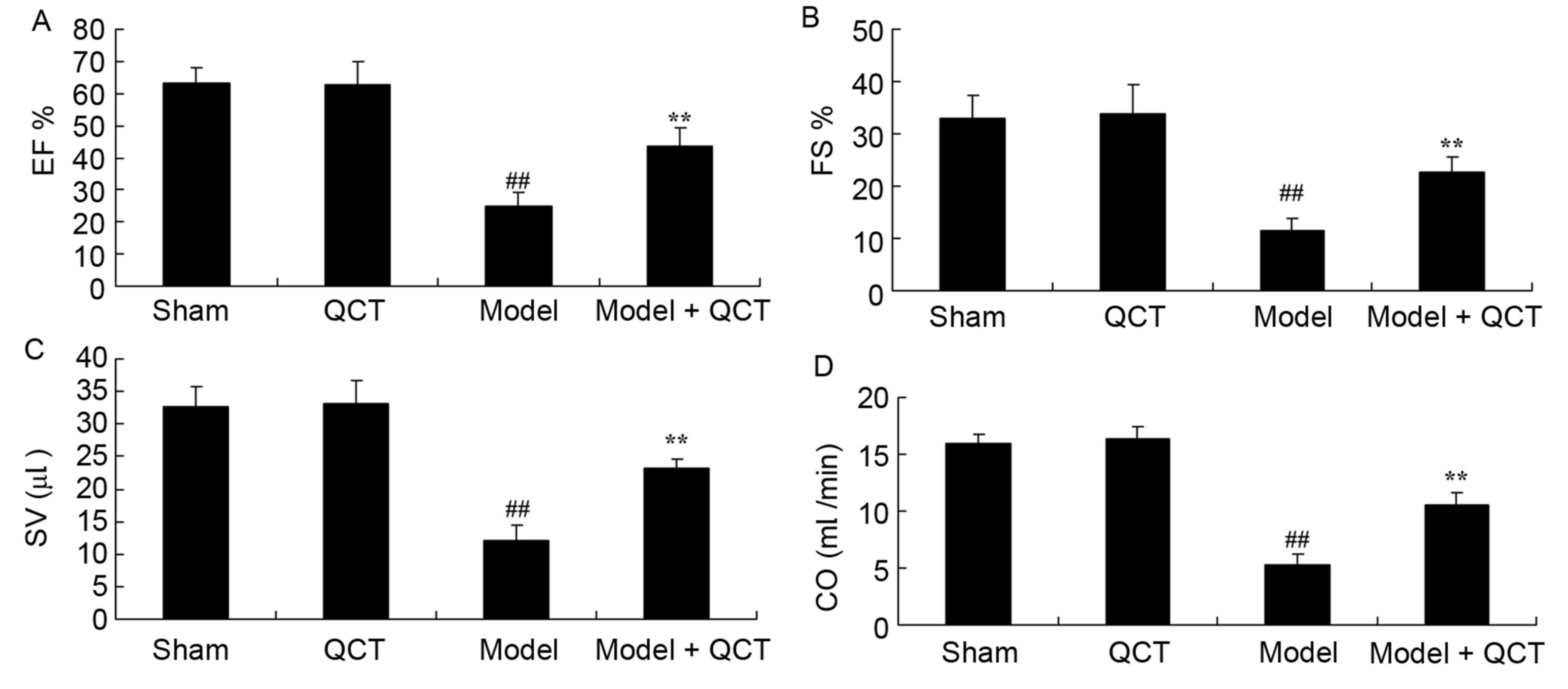 | Figure 3.Quercetin protects EF, FS, SV and CO
in rat model of CPR. (A) EF, (B) FS, (C) SV and (D) CO in rat model
of cardiopulmonary resuscitation. ##P<0.01 vs. sham
group; **P<0.01 vs. model group. EF, ejection fraction; QCT,
quercetin group; Model, CPR model; Model + QCT, CPR model +
quercetin group; FS, left ventricular shortening fraction; SV,
stroke volume; CO, cardiac output; CPR, cardiopulmonary
resuscitation. |
Quercetin protects the left ventricle
weight/body weight in a rat model of CPR
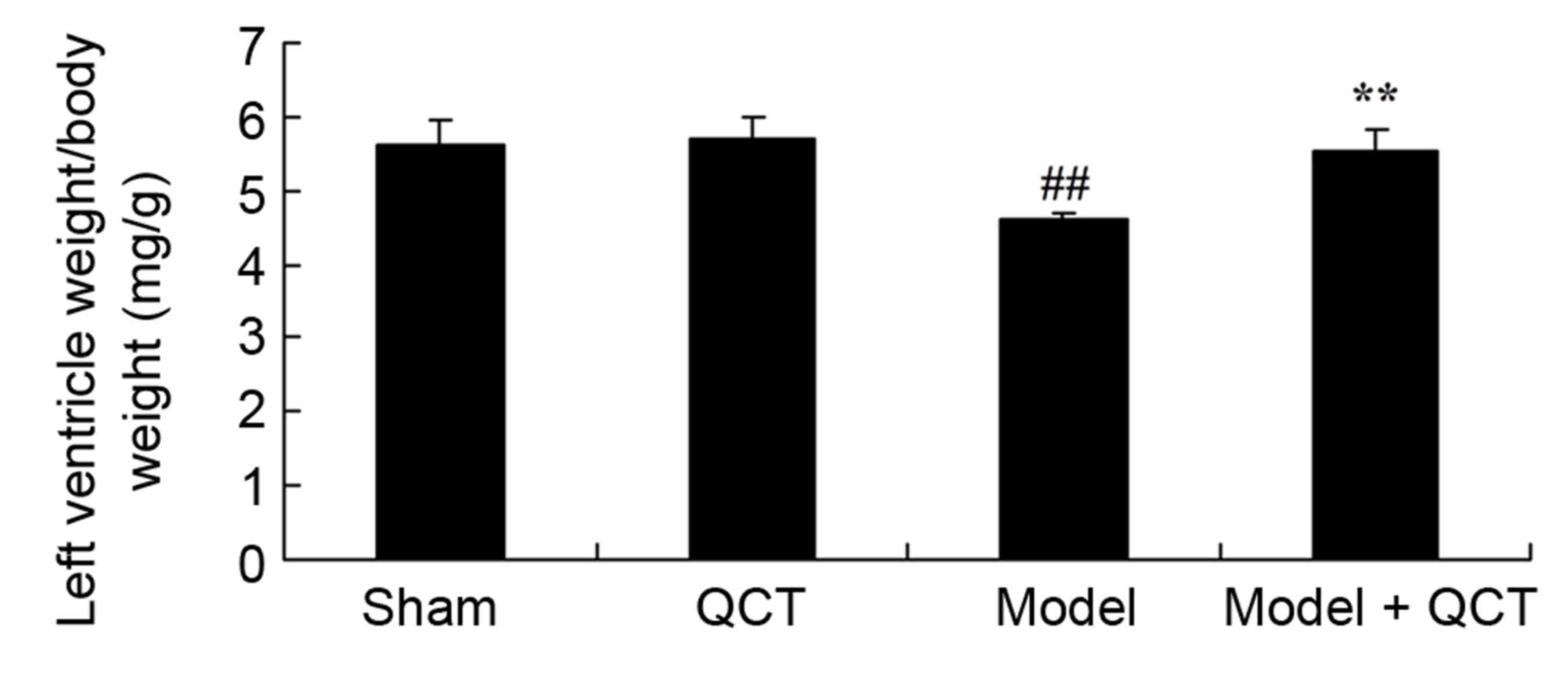
Subsequently, it was verified whether quercetin
protects the left ventricle weight/body weight in a rat model of
CPR. As presented in Fig. 4, there
was no significant difference in left ventricle weight/body weight
between sham group and QCT group. The left ventricle weight/body
weight in the CPR model group was significantly lower than that of
the sham group (Fig. 4). Treatment
with quercetin significantly enhanced left ventricle weight/body
weight in CPR model rats (Fig.
4).
Quercetin protects against ROS
generation in rat model of cardiopulmonary resuscitation
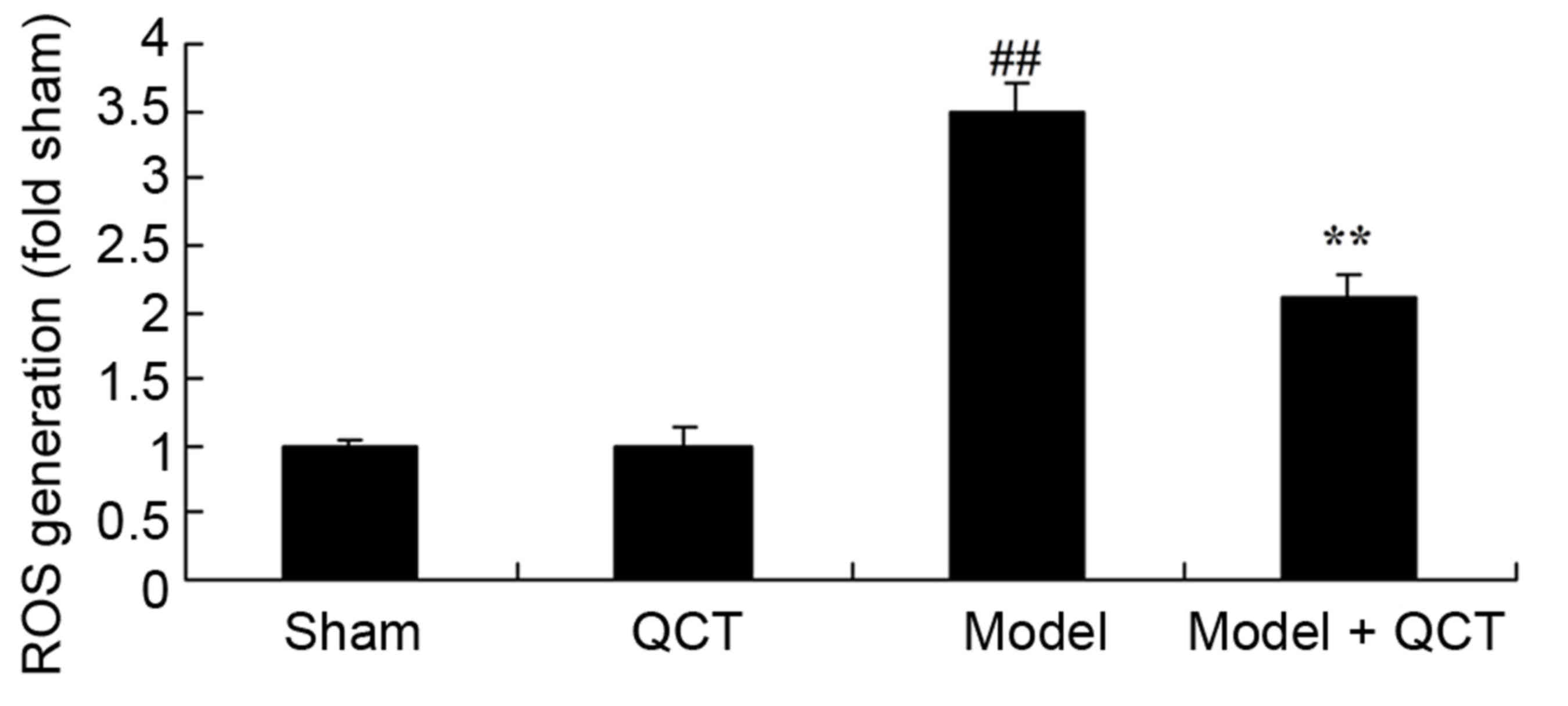
The results demonstrated that there was no
significant change in ROS generation between the sham group and QCT
group (P>0.05; Fig. 5). The CPR
model significantly induced ROS generation in rats compared with
the sham group (Fig. 5). Treatment
with quercetin significantly suppressed ROS generation in CPR model
rats (Fig. 5).
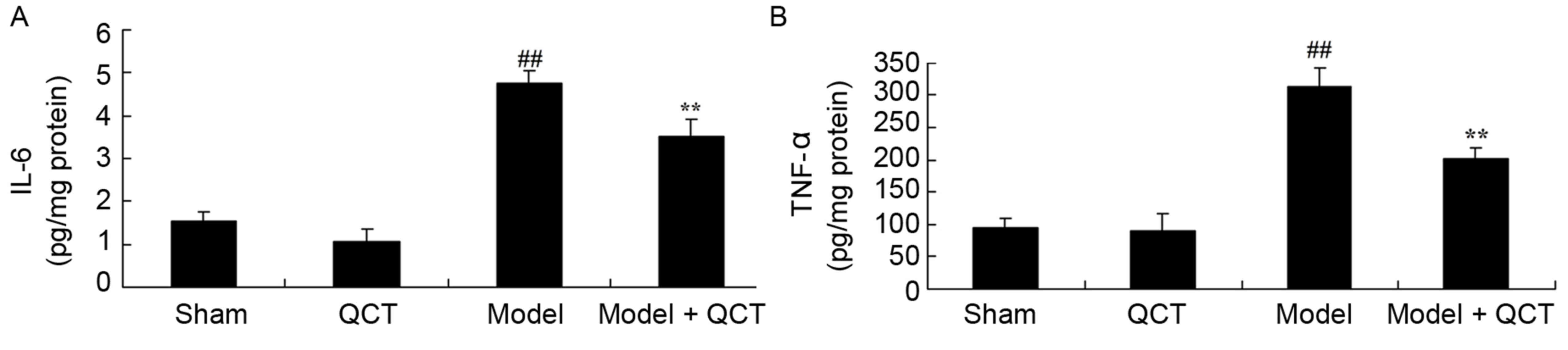
Quercetin protects against
inflammation in rat model of cardiopulmonary resuscitation
To determine the anti-inflammatory effect of
quercetin in CPR model rats, IL-6 and TNF-α levels were measured.
IL-6 and TNF-α were similar in the sham group and QCT group
(P>0.05; Fig. 6). IL-6 and
TNF-α levels were significantly induced in the CPR rat model group
compared with the sham group (Fig.
6). Treatment with quercetin significantly inhibited the
activation of IL-6 and TNF-αin CPR model rats (Fig. 6).
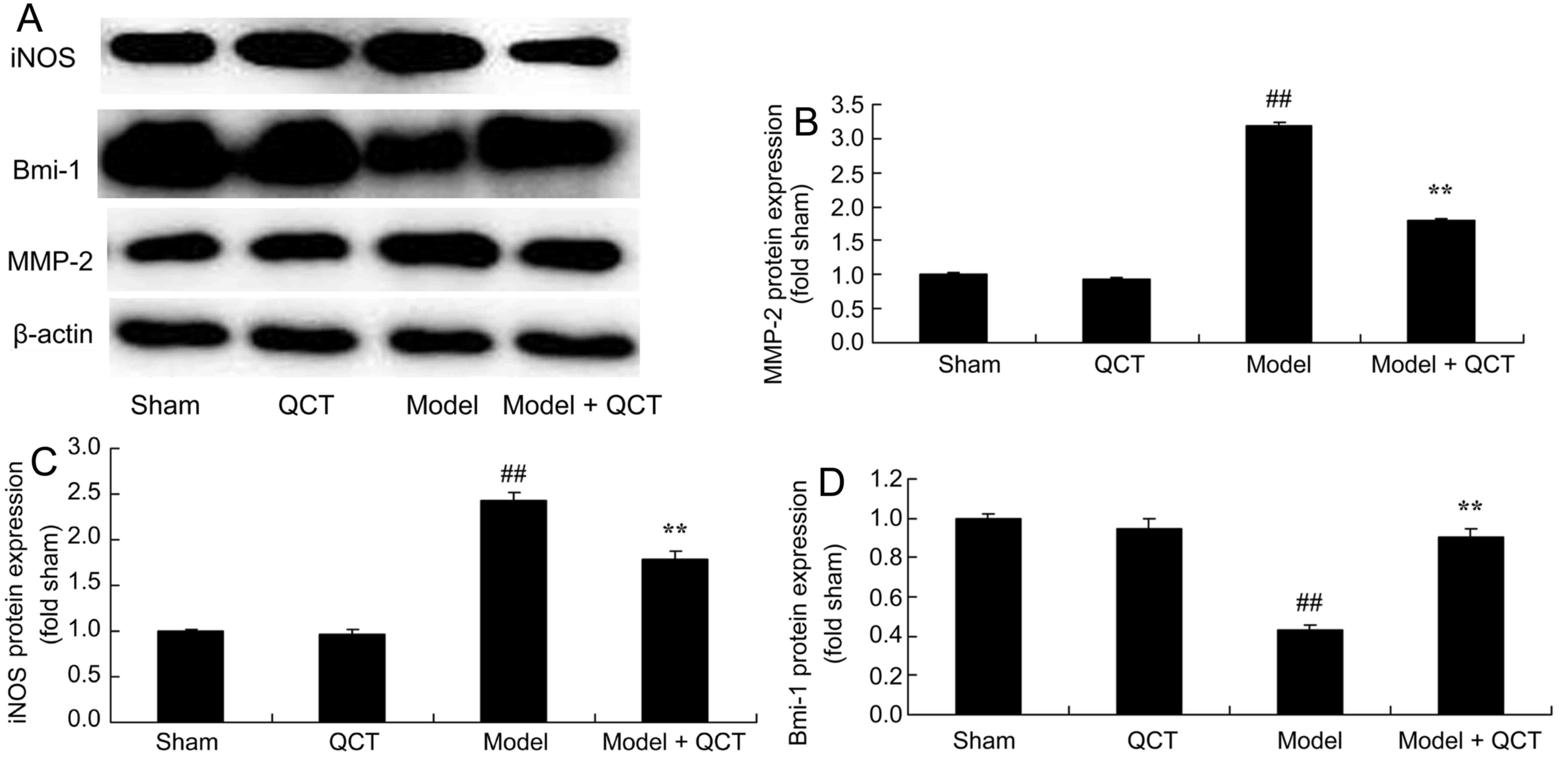
Quercetin reduces MMP-2 and iNOS
protein expression and induces Bmi-1 protein expression in a rat
model of CPR
It was evaluated whether quercetin protects against
increased MMP-2, iNOS and Bmi-1 protein expression in a rat model
of CPR using western blotting analysis. There was no significant
difference in MMP-2, iNOS and Bmi-1 protein expression between the
sham group and QCT group (Fig. 7).
However, CPR significantly induced MMP-2 and iNOS protein
expression and suppressed Bmi-1 protein expression in compared with
the sham group (Fig. 7). Quercetin
treatment significantly inhibited MMP-2 and iNOS protein
expression, and induced Bmi-1 protein expression in cardiopulmonary
resuscitation rat (Fig. 7).
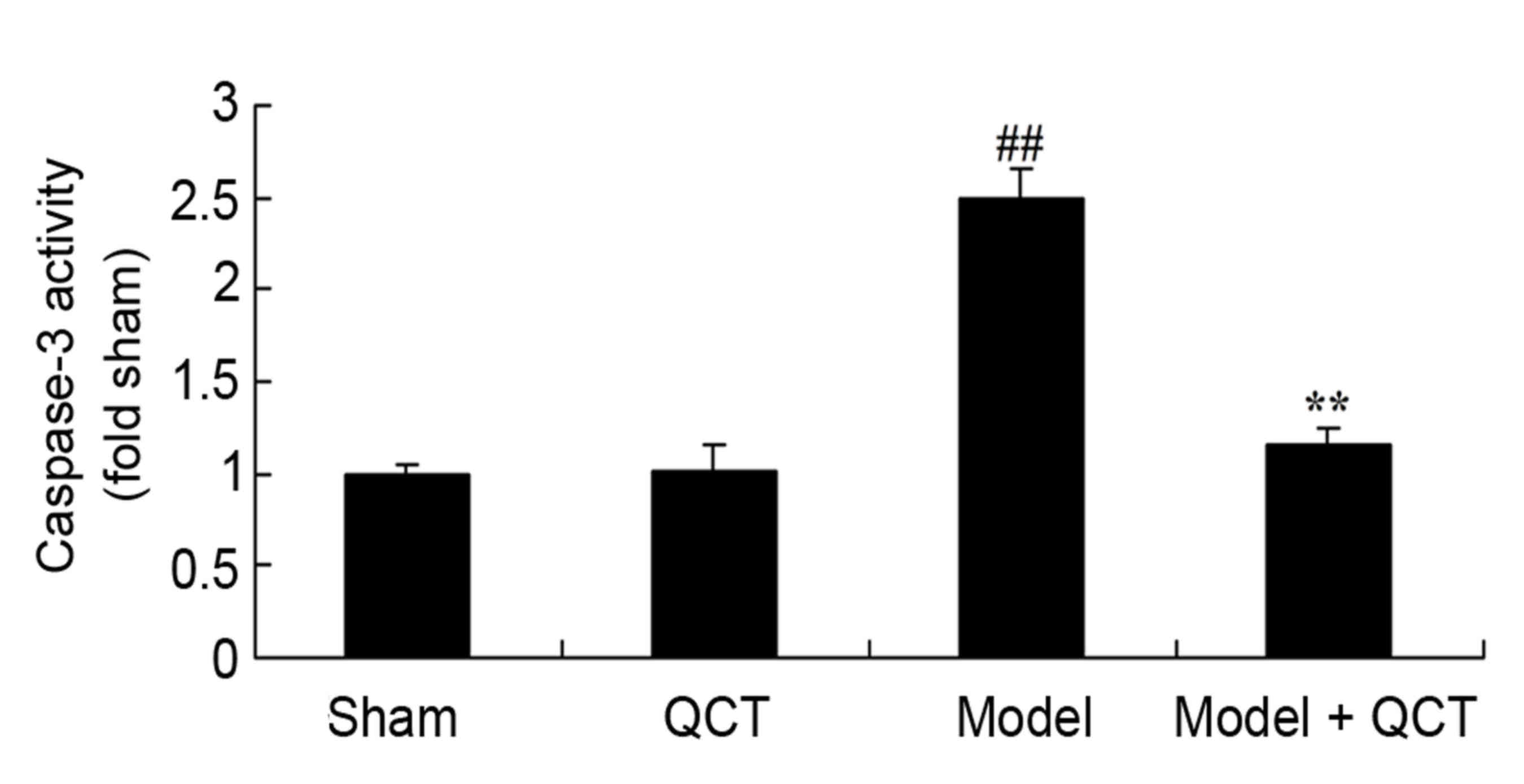
Quercetin protects against caspase-3
activity in rat model of CPR
The current results revealed that there was no
significant difference in caspase-3 activity between the sham group
and QCT group (P>0.05; Fig. 8).
Caspase-3 activity was significantly increased in the CPR model
group compared with the sham group (Fig. 8). Quercetin treatment significantly
inhibited caspase-3 activity in CPR model rats (Fig. 8).
Discussion
CPR causes hypoxia-ischemia. Effective and timely
CPR may restore the heart beat for the majority of patients
(20). However, cerebral injury
following resuscitation remains difficult issue for complete
rehabilitation (21). Following
successful CPR, the autonomic circulation recovery of the cerebral
blood flow causes cerebral-reperfusion injury and may cause further
aggravation of the prognosis of cerebral functions and mortality
(22). In present study, quercetin
significantly reduced the LVDs, increased the EF, FS, SV and CO,
and enhanced left ventricle weight/body weight in CPR model
rats.
Following cardiac arrest, organisms are in severe
and general hypoxic-ischemic states, which lead to reperfusion
injury and secondary lesions of visceral organs (23). During CPR, organisms produce stress
reactions. Under strong pathological stimuli, cytokines are
produced and cascade reactions are triggered. Reperfusion injury
following cardiac arrest is closely associated with
pro-inflammatory cytokines, including TNF-α, IL-1 and IL-6
(21). The current study
demonstrated that treatment with quercetin significantly inhibited
the activation of IL-6 and TNF-α release and suppressed ROS
generation in CPR model rats. Liu et al (24) reported that quercetin suppressed
insulin-mediated glucose disposal during inflammatory conditions in
skeletal muscle tissue/cells.
In cardiac muscle tissues, MMPs and TIMPs are
tightly balanced. If the level of MMPs increases, the balance is
disrupted, which may cause the remodeling of ECM (25). Previous studies reported that MMPs
have an important role in left atrioventricular remodeling
following acute myocardial infarction, chronic heart failure,
hypertension, diastolic cardiomyopathy and atrial fibrillation
(25,26). MMPs also participate in
pathophysiological processes, including platelet aggregation,
angiotasis regulation, inflammation and ischemia-reperfusion injury
(27). However, studies on the
association between MMPs and ventricular fibrillation are rare. In
the current study, quercetin treatment significantly inhibited
MMP-2 protein expression in CPR model rats. Barteková et al
(16) demonstrated that quercetin
improves post-ischemic recovery of heart function through
suppression of MMP-2 and anti-apoptosis.
Reperfusion following CPR can cause server cerebral
anoxia and ultimately result in dysneuria. Randomized clinical
trial confirmed that low temperature therapy is effective for coma
patients following CPR, with ventricular fibrillation to improve
dysneuria. It has been reported that fast cooling of the head when
conducting CPR can improve survival rates and nervous system
functions, while studies on the endogenous protective mechanisms
involved are rare (29).
Biological behaviors and external information transmission are
produced by a series of signal transduction and regulation
mechanisms. Signal transduction systems have an essential role in
cell differentiation, growth, apoptosis and gene expression
(30). In the current study,
quercetin treatment significantly inhibited caspase-3 activity in
CPR model rats.
It is generally established that Bmi-1 is highly
expressed in hematopoietic stem cells and neural stem cells
(31). Bmi-1 is involved in
maintaining the self-renewal capacities of stem cells and has an
important role in stem cell growth (32). In the current study, treatment with
quercetin significantly increased the protein expression of Bmi-1
and suppressed the protein expression of iNOS in CPR model rats.
Dong et al (32)
demonstrated that quercetin attenuates doxorubicin cardiotoxicity
through activation of Bmi-1 expression. Zhang et al
(15) suggested that quercetin
protected endothelial NOS expression in cavernous endothelial
cells.
In summary, quercetin significantly inhibited the
LVDs, increased EF, FS, SV and CO, and enhanced left ventricle
weight/body weight in a rat CPR model. Additionally, quercetin
protected against inflammation, MMP-2 activation, iNOS expression
and apoptosis, and modulated Bmi-1 expression in rat model of
CPR.
Acknowledgements
Not applicable.
Funding
Natural Science Foundation of China (grant no.
81471830).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
X-LL and NZ conceived and designed the experiment;
DW, XL, X-MJ and CY performed the experiments; X-LL and NZ wrote
the paper. DW, X-LL and NZ analyzed the data.
Ethics approval and consent to
participate
The protocol of animal experiments was approved by
the University Laboratory Animal Research Committee of The First
Hospital of Jilin University (Changchun, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Capuano F, Lechiancole A, Angeloni E,
Goracci M, Bianchin R, Roscitano A, Comito C, Melina G and Sinatra
R: Miniaturized versus conventional cardiopulmonary bypass in
patients undergoing coronary artery bypass surgery: Impact on
lymphocyte depletion and sternal wound healing. J Cardiothorac
Surg. 10 Suppl 1:A3222015. View Article : Google Scholar :
|
|
2
|
Wang XT, Liu DW, Zhang HM and Chai WZ:
Integrated cardiopulmonary sonography: A useful tool for assessment
of acute pulmonary edema in the intensive care unit. J Ultrasound
Med. 33:1231–1239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Souza SS, Intelisano TR, De Biaggi CP,
Moura CA, Selmi AL, Dias RA and Cortopassi SR: Cardiopulmonary and
isoflurane-sparing effects of epidural or intravenous infusion of
dexmedetomidine in cats undergoing surgery with epidural lidocaine.
Vet Anaesth Analg. 37:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Ji B, Zhang Y, Zhu X, Liu J, Long
C and Zheng Z: Comparison of the effects of three cell saver
devices on erythrocyte function during cardiopulmonary bypass
procedure-a pilot study. Artif Organs. 36:931–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhutta AT, Schmitz ML, Swearingen C, James
LP, Wardbegnoche WL, Lindquist DM, Glasier CM, Tuzcu V, Prodhan P,
Dyamenahalli U, et al: Ketamine as a neuroprotective and
anti-inflammatory agent in children undergoing surgery on
cardiopulmonary bypass: A pilot randomized, double-blind,
placebo-controlled trial. Pediatr Crit Care Med. 13:328–337. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun YJ, Song DD, Diao YG, Zhou J and Zhang
TZ: Penehyclidine hydrochloride preserves the intestinal barrier
function in patients undergoing cardiopulmonary bypass. J Thorac
Cardiovasc Surg. 146:179–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garner JR, Stroud RE, Finklea L,
Ikonomidis JS, Dorman BH and Spinale FG: The effects of leukocyte
reduction on matrix metalloproteinase release in cardiopulmonary
bypass. J Extra Corpor Technol. 36:185–190. 2004.PubMed/NCBI
|
|
8
|
Smith CR, Stamou SC, Boeve TJ and Hooker
RC: Repair of a penetrating ascending aortic ulcer with localized
resection and extracellular matrix patch aortoplasty. Ann Thorac
Surg. 94:988–989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asberg AE and Videm V: Neutrophil
dysfunction after biomaterial contact in an in vitro model of
cardiopulmonary bypass. Eur J Cardiothorac Surg. 30:744–748. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chowdhury B, Hemming R, Hombach-Klonisch
S, Flamion B and Triggs-Raine B: Murine hyaluronidase 2 deficiency
results in extracellular hyaluronan accumulation and severe
cardiopulmonary dysfunction. J Biol Chem. 288:520–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang MC, Li CL, Cui J, Jiao M, Wu T, Jing
LI and Nan KJ: BMI-1, a promising therapeutic target for human
cancer. Oncol Lett. 10:583–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang L, Li J and Song L: Bmi-1, stem
cells and cancer. Acta Biochim Biophys Sin (Shanghai). 41:527–534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koning NJ, de Lange F, Vonk AB, Ahmed Y,
van den Brom CE, Bogaards S, van Meurs M, Jongman RM, Schalkwijk
CG, Begieneman MP, et al: Impaired microcirculatory perfusion in a
rat model of cardiopulmonary bypass: The role of hemodilution. Am J
Physiol Heart Circ Physiol. 310:H550–H558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Link MS, Myerburg RJ and Estes NA III:
American Heart Association Electrocardiography and Arrhythmias
Committee of Council on Clinical Cardiology, Council on
Cardiovascular Disease in Young, Council on Cardiovascular and
Stroke Nursing, Council on Functional Genomics and Translational
Biology, and American College of Cardiology: Eligibility and
disqualification recommendations for competitive athletes with
cardiovascular abnormalities: Task force 12: Emergency action
plans, resuscitation, cardiopulmonary resuscitation, and automated
external defibrillators: A scientific statement from the American
heart association and American college of cardiology. Circulation.
132:e334–e338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Huang C, Liu S, Bai J, Fan X, Guo
J, Jia Y, Zhang Z, Chen X, Jia Y, et al: Effects of quercetin on
intracavernous pressure and expression of nitrogen synthase
isoforms in arterial erectile dysfunction rat model. Int J Clin Exp
Med. 8:7599–7605. 2015.PubMed/NCBI
|
|
16
|
Barteková M, Šimončíková P, Fogarassyová
M, Ivanová M, Okruhlicová L', Tribulová N, Dovinová I and Barančík
M: Quercetin improves postischemic recovery of heart function in
doxorubicin-treated rats and prevents doxorubicin-induced matrix
metalloproteinase-2 activation and apoptosis induction. Int J Mol
Sci. 16:8168–8185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen XC, Yang YP, Xiao TT, Peng J and Liu
XD: Protective effect of oxymatrine on myocardial fibrosis induced
by acute myocardial infarction in rats involved in
TGF-β1-smads signal pathway. J Asian Nat Prod Res.
13:215–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uraoka M, Nakajima Y, Kurita T, Suzuki A,
Takata K and Sato S: Landiolol, an ultra short acting
beta1-blocker, improves pulmonary edema after cardiopulmonary
resuscitation with epinephrine in rats. J Anesth. 24:67–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye S, Weng Y, Sun S, Chen W, Wu X, Li Z,
Weil MH and Tang W: Comparison of the durations of mild therapeutic
hypothermia on outcome after cardiopulmonary resuscitation in the
rat. Circulation. 125:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duppen N, Etnel JR, Spaans L, Takken T,
van den Berg-Emons RJ, Boersma E, Schokking M, Dulfer K, Utens EM,
Helbing W and Hopman MT: Does exercise training improve
cardiopulmonary fitness and daily physical activity in children and
young adults with corrected tetralogy of Fallot or Fontan
circulation? A randomized controlled trial. Am Heart J.
170:606–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durukan AB, Gurbuz HA, Salman N, Unal EU,
Ucar HI and Yorgancioglu CE: Ventilation during cardiopulmonary
bypass did not attenuate inflammatory response or affect
postoperative outcomes. Cardiovasc J Afr. 24:224–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guizilini S, Alves DF, Bolzan DW, Cancio
AS, Regenga MM, Moreira RS, Trimer R and Gomes WJ: Sub-xyphoid
pleural drain as a determinant of functional capacity and clinical
results after off-pump coronary artery bypass surgery: A randomized
clinical trial. Interact Cardiovasc Thorac Surg. 19:382–387. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sayed S, Idriss NK, Sayyedf HG, Ashry AA,
Rafatt DM, Mohamed AO and Blann AD: Effects of propofol and
isoflurane on haemodynamics and the inflammatory response in
cardiopulmonary bypass surgery. Br J Biomed Sci. 72:93–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu K, Mei F, Wang Y, Xiao N, Yang L, Wang
Y, Li J, Huang F, Kou J, Liu B and Qi LW: Quercetin oppositely
regulates insulin-mediated glucose disposal in skeletal muscle
under normal and inflammatory conditions: The dual roles of AMPK
activation. Mol Nutr Food Res. 60:551–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CT, Zhang L, Wu HW, Wei L, Xu B and
Li DM: Doxycycline attenuates acute lung injury following
cardiopulmonary bypass: Involvement of matrix metalloproteinases.
Int J Clin Exp Pathol. 7:7460–7468. 2014.PubMed/NCBI
|
|
26
|
He ZJ, Huang ZT, Chen XT and Zou ZJ:
Effects of matrix metalloproteinase 9 inhibition on the blood brain
barrier and inflammation in rats following cardiopulmonary
resuscitation. Chin Med J (Engl). 122:2346–2351. 2009.PubMed/NCBI
|
|
27
|
Liu K, Shen L, Wang J, Dong G, Wu H, Shao
H and Jing H: The preventative role of curcumin on the lung
inflammatory response induced by cardiopulmonary bypass in rats. J
Surg Res. 174:73–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minamishima S, Bougaki M, Sips PY, Yu JD,
Minamishima YA, Elrod JW, Lefer DJ, Bloch KD and Ichinose F:
Hydrogen sulfide improves survival after cardiac arrest and
cardiopulmonary resuscitation via a nitric oxide synthase
3-dependent mechanism in mice. Circulation. 120:888–896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mendoza-Paredes A, Liu H, Schears G, Yu Z,
Markowitz SD, Schultz S, Pastuszko P, Greeley WJ, Nadkarni V, Kubin
J, et al: Resuscitation with 100%, compared with 21%, oxygen
following brief, repeated periods of apnea can protect vulnerable
neonatal brain regions from apoptotic injury. Resuscitation.
76:261–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shuja F, Tabbara M, Li Y, Liu B, Butt MU,
Velmahos GC, DeMoya M and Alam HB: Profound hypothermia decreases
cardiac apoptosis through Akt survival pathway. J Am Coll Surg.
209:89–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wiedemann D, Schachner T, Bonaros N,
Weidinger F, Kolbitsch C, Friedrich G, Laufer G and Bonatti J: Does
obesity affect operative times and perioperative outcome of
patients undergoing totally endoscopic coronary artery bypass
surgery? Interact Cardiovasc Thorac Surg. 9:214–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Q, Chen L, Lu Q, Sharma S, Li L,
Morimoto S and Wang G: Quercetin attenuates doxorubicin
cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol.
171:4440–4454. 2014. View Article : Google Scholar : PubMed/NCBI
|