Introduction
During the past decades, adult stem cells (ASCs)
have been separated from different tissues. As one type of ASCs,
human dental pulp stem cells (HDPSCs) isolated from human dental
pulp in adult permanent and exfoliated deciduous teeth (SHED)
(1,2) have the capability of differentiation
into osteoblasts, odontoblasts, adipocytes and neuronal-like cells
(3). Strikingly, HDPSCs can
regenerate a dentin-pulp-like complex in normal human teeth
(4), showing that HDPSCs represent
a novel ASC population (2).
In spite of the great potential in tissue
engineering (5,6), the molecular mechanisms underlying
cell apoptosis in HDPSCs remain unclear. Interestingly, there are
very few data on the occurrence of apoptosis in the cells of the
dental pulp (7). A disintegrin and
metalloproteinase 28 (ADAM28) was reported to be involved in the
proliferation, differentiation, and apoptosis of HDPSCs (8), but a prior study by Muthna et
al reported that the irradiation of adult HDPSCs provokes
activation p53, cell cycle arrest and senescence instead of
apoptosis (3). So far, few
evidences showed the occurrence of apoptosis in HDPSCs.
As reviewed previously (9–11),
three typical pathways, including extrinsic pathway, intrinsic
pathway and a perforin/granzyme pathway, were involved in cell
apoptosis. Each of three pathways requires activation of caspase-8,
caspase-9 and caspase-10, which in turn activate caspase-3.
However, whether HDPSCs contain the same pathways remains
unknown.
Here, we checked the existences of the extrinsic and
intrinsic pathways of apoptosis in HDPSCs isolated from adult
permanent teeth and deciduous teeth by RNA interference (RNAi),
RT-qPCR and other experiments. RNAi has been widely accepted as an
excellent system for the targeted silencing of gene expression, in
which activation of small-interfering RNA [(siRNA), ~21
nucleotides] pathway results in the degradation of a specific
targeted mRNA (12,13). The specificity and potency of siRNA
in cell culture and in animal studies has suggested that it can be
a powerful therapeutic agent. In present study, we knocked down the
expressions of caspase-8 and caspase-9 by RNAi experiments,
respectively, and observed significant reduction of HDPSCs
apoptosis in the caspase-9 RNAi group instead of the caspase-8 RNAi
group, showing that differences of functional consequences of
caspase-8 and caspase-9. The caspase-3 expression and activity
assays revealed that caspase-3 activated by caspase-9 regulated
apoptosis in HDPSCs.
Materials and methods
Ethics statement
All procedures used in this study conformed to the
tenets of the Declaration of Helsinki. The Ethics Committee
guidelines of Stomatological Hospital, Southern Medical University
approved the protocols used. Informed consent was obtained from all
participants.
Subjects and cell culture
Six normal deciduous teeth and 6 adult permanent
teeth were collected from 6- to 13-year-old children and normal
adults, respectively. All the teeth were extracted according to the
agreements of patients and regular medical processes. The intact
teeth were separated, immersed in a solution of 0.25%
Chloramphenicol solution (30 min), and then kept in PBS solution
with Penicillin (60 mg/l) and Streptomycin (100 mg/l). The dental
pulp was isolated from the teeth, and washed by PBS solutions with
Penicillin and Streptomycin, then digested in a solution of 1%
collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h
at 37°C. The cells were filtered by 150 mesh nylon, centrifuged
1,000 × g for 5 min, and countered by blood counting chamber. After
these processes, the cells were then mixed in DMEM solution (20%
FBS, 100 µM ascorbic acid, 2 mM L-glutamine, 60 mg/l Penicillin and
100 mg/l Streptomycin). Anti-immunoglobulin M Micro Beads
(Miltenyi-Biotec, Bergisch Gladbach, Germany) were used to sort the
separated cells, according to the manufacturer's instructions. We
then obtained the cell populations that were termed as stem cells
from deciduous teeth and adult permanent teeth. These HDPSCs were
cultured for further use.
Hematoxylin and Eosin (H&E)
staining
To determine the apoptosis stages of dental pulp
cells, H&E staining was employed to observe the apoptotic
processes and stages of stem cells from deciduous teeth and adult
permanent teeth. We also used H&E staining to observe the HDPSC
apoptosis processes of RNAi groups and control groups from
deciduous teeth.
Apoptosis assay
For Annexin V-FITC apoptosis assay, separated stem
cells from deciduous teeth and adult permanent teeth were cultured,
trypsinized, washed, and stained with Annexin V/PI apoptosis kit
(Multi Sciences Biotech, Dalian, China) in the dark for 15 min at
room temperature. Then, the stained cells were analyzed by flow
cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mitochondria were extracted by Cell Mitochondria
Isolation kit (Beyotime Institute of Biotechnology, Shanghai,
China). Using TRIzol (Takara Biotechnology Co., Ltd., Dalian,
China), poly-A+ RNA were extracted from the mitochondria,
cytoplasm, and whole cells of the HDPSCs from deciduous teeth and
adult permanent teeth. RT-qPCR was performed to check the
expression of Cytochrome c in these samples. The Cytochrome
c primers consisted of the following sequences: (Forward)
5′-CCAATGAAGATGGGGAGATG-3′ and (reverse) 5′-CCGTGAGCAGGGAGAAGAC-3′.
The primer pair for the β-actin housekeeping gene was made up of
the following sequences: (Forward) 5′-ATCGTGCGTGACATTAAGGAGAAG-3′
and (reverse) 5′-AGGAAGGAAGGCTGGAAGAGTG-3′.
Furthermore, we examined the expression of
caspase-9, caspase-3 and caspase-8 by RT-qPCR in dental pulp from
deciduous teeth and adult permanent teeth. The primers for these
three genes were made up of the following sequences: (caspace-9
forward) 5′-AACCCTAGAAAACCTTACCCC-3′, (caspace-9 reverse)
5′-CATCACCAAATCCTCCAGAAC-3′, (caspase-3 forward)
5′-AGCAAACCTCAGGGAAACATT-3′, (caspase-3 reverse)
5′-CTCAGAAGCACACAAACAAAACT-3′, (caspase-8 forward)
5′-GGGAGGAGTTGTGTGGGGTA-3′, (caspase-8 reverse)
5′-CAGTCATCGTGGGGCTTGA-3′.
Each sample of cDNA templates (1 µl) was added to
Bestar® SYBR Green qPCR Master Mix (DBI Bioscience,
Shanghai, China). The amplification protocol consisted of a
pre-denaturation step at 94°C for 2 min, and 40 cycles of the
following: A denaturation step at 94°C for 20 sec, annealing at
58°C for 20 sec and extension at 72°C for 20 sec. Melting curve
data were collected to verify PCR specificity. Each mRNA sample was
analyzed in triplicate. In addition, the expression was calculated
by relative quantification using β-actin as reference control. Fold
expression changes were determined by the 2−ΔΔCq method
(14).
RT-qPCR was performed to check the expression of the
RNAi samples of caspase-8 and caspase-9 in the HDPSCs from
deciduous teeth. The primer sequences of caspase-8, caspase-9 and
β-actin were the same as above.
Western blot
Using lysis buffer (cat. no. P0013) (Beyotime
Institute of Biotechnology), total protein was extracted from the
mitochondria, cytoplasm, and whole cells of the HDPSCs from
deciduous teeth and adult permanent teeth. The protein
concentration was determined by BCA assay kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). 20 µg protein was separated by
SDS-PAGE (10% stacking gel and 5% separation gel) and transferred
to a nitrocellulose membrane. The membranes were blocked in 5%
skimmed milk for 1 h at room temperature, respectively incubated
with rabbit anti-human monoclonal antibody against Cytochrome
c (dilution, 1:800; cat. no. 4280), mouse anti-human
monoclonal antibody against caspase-9 (dilution, 1:2,000; cat. no.
9508), rabbit anti-human monoclonal antibody against caspase-3
(dilution, 1:1,000; cat. no. 14220) and rabbit anti-human
monoclonal antibody against caspase-8 (dilution, 1:1,500; cat. no.
4790) (Cell Signaling Technology, Inc., Danvers, MA, USA) at room
temperature for 1 h. The membranes were washed with 25 ml TBS
containing 0.1% Tween-20 (TBST) followed by an incubation of
secondary antibody (HRP goat anti-rabbit and goat anti-mouse IgG)
(dilution, 1:20,000; cat. nos. BA1054 and BA1051; Boster
Bioengineering Co., Ltd., Wuhan, China) at room temperature for 40
min. After final washing with 25 ml TBST, the membranes were
developed using chemiluminescence and exposed to X-ray films. GAPDH
(dilution, 1:10,000; cat. no. RC-5G5; KangChen Bio-tech, Shanghai,
China) was used as positive control.
Similarly, we also used western blotting to check
the expression of the RNAi samples of caspase-8 and caspase-9 in
the HDPSCs from deciduous teeth, using GAPDH (RC-5G5) (KangChen
Bio-tech) as positive control.
RNAi
RNAi was employed to knock down the expression of
caspase-8 and caspase-9 in the HDPSCs from deciduous teeth. We
designed and chemically synthesized four DNA oligos for each gene
and then chose the best one for RT-qPCR assays. The siRNA oligo
sequences for caspase-8 and caspase-9 were as follows: (caspase-8)
siRNA1, 5′-CUACCAGAAAGGUAUACCUTT-3′, siRNA2:
5′-GAGGGUCGAUCAUCUAUUATT-3′, siRNA3: 5′-GGGUCGAUCAUCUAUUAAUTT-3′,
and siRNA4: 5′-GAGCUGCUCUUCCGAAUUATT-3′; (caspase-9) siRNA1:
5′-GAUGCCUGGUUGCUUUAAUTT-3′, siRNA2: 5′-CGGUGAAAGGGAUUUAUAATT-3′,
siRNA3: 5′-CACCCAGUGACAUCUUUGUTT-3′, and siRNA4:
5′-GCCACUGCCUCAUUAUCAATT-3′. The empty vector, LV3-shRNAs was
transfected into HDPSCs, serving as a negative control. All siRNA
sequences were examined for specificity using a BLAST search
(15) and failed to show homology
to any other genes in Homo sapiens.
Both RT-qPCR and western blotting were performed to
assay the expression the transfected and untransfected HDPSCs from
deciduous teeth. β-actin and GAPDH were used as internal and
positive control in RT-qPCR and Western blot assays, respectively.
After investigating the expression, we finally chose the first
(caspase-8 siRNA1) and second (caspase-9 siRNA2) primer of
caspase-8 and caspase-9 for Lentivirus construct (LV3) and
following RNAi and RT-qPCR assays, respectively.
Furthermore, the untransfected HDPSCs, HDPSCs
transfected with empty vector, caspase-8 and caspase-9 siRNAs
(caspase-8-siRNA and caspase-9-siRNA), were cultured, trypsinized,
washed, and stained with Annexin V-APC/7-AAD apoptosis kit (Multi
Sciences Biotech) in the dark for 15 min at room temperature. Then,
the stained cells were analyzed by flow cytometry.
Caspase activity assay
Cultured HDPSCs, separated from deciduous teeth,
were harvested and lysed with lysis buffer (cat. no. P0013;
Beyotime Institute of Biotechnology). After 16,000–20,000 × g
centrifugation at 4°C for 10–15 min, the protein concentration was
measured using Bradford method. The caspase-3 activity of
untransfected HDPSCs, HDPSCs transfected with empty vector, HDPSCs
transfected with caspase-8 and caspase-9 siRNAs (caspase-8-siRNA
and caspase-9-siRNA) were assayed with Caspase-3 Activity Assay kit
(Beyotime Institute of Biotechnology) according to manufacturing
instruction. In order to check the correlation of caspase-3
expression and activity, RT-qPCR assays were also performed to
measure the expression of caspase-3 in all these four groups of
cells.
Statistical analysis
An unpaired Student's t-test and an one-way analysis
of variance (ANOVA) in IBM SPSS platform were used to perform
statistical analysis. For two groups, unpaired two-tailed Student's
t tests were used; for more than two group comparisons, one-way
ANOVAs were used followed by the post hoc Tukey's HSD (honest
significant difference) test. Statistical significance was
determined with unpaired Student's t-test or One-way ANOVA test.
P<0.05 was regarded as statistically significant (*P<0.05,
**P<0.01). Additionally, Pearson correlation analysis was
conducted in Excel to calculate possible correlation between
caspase-3 expression and activity.
Results
Characterization of HDPSCs
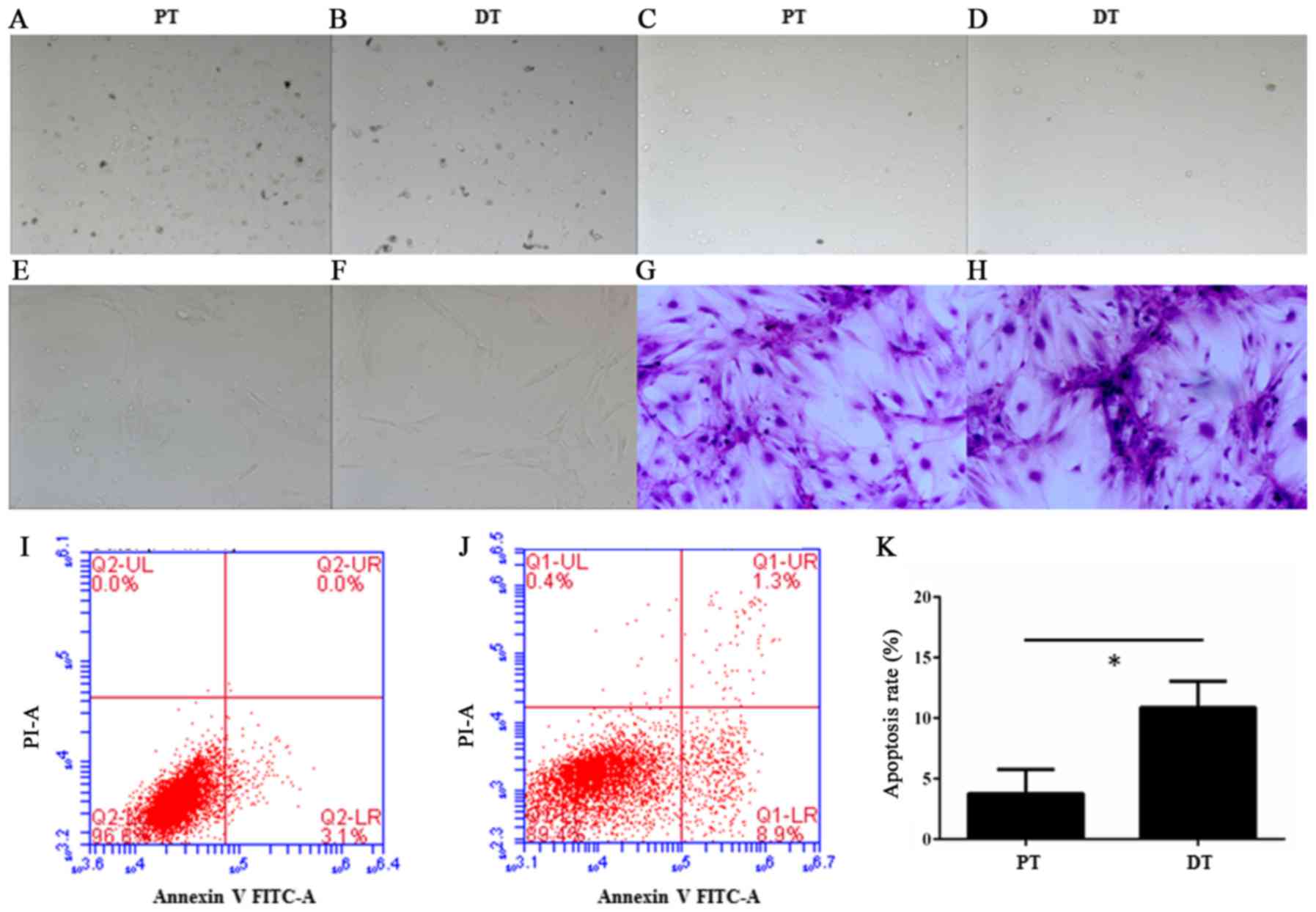
The separated cells from deciduous teeth and adult
permanent teeth were round or irregular shape (Fig. 1A-D) after primary separation and
enrichment by Anti-immunoglobulin M Micro Beads (Miltenyi-Biotec).
However, the HDPSCs were fusiform after culture for 2 weeks
(Fig. 1E-H). This observation is
similar to previous report (16),
in which SHED and DPSCs displayed a fibroblastic morphology.
Moreover, in the Annexin V-FITC apoptosis assay, a
significant increase of apoptosis was observed in the deciduous
teeth than in adult permanent teeth (P=0.014<0.05, unpaired
Student's t-test) (Fig. 1I-K).
Expression of Cytochrome c, caspase-9,
caspase-8 and caspase-3
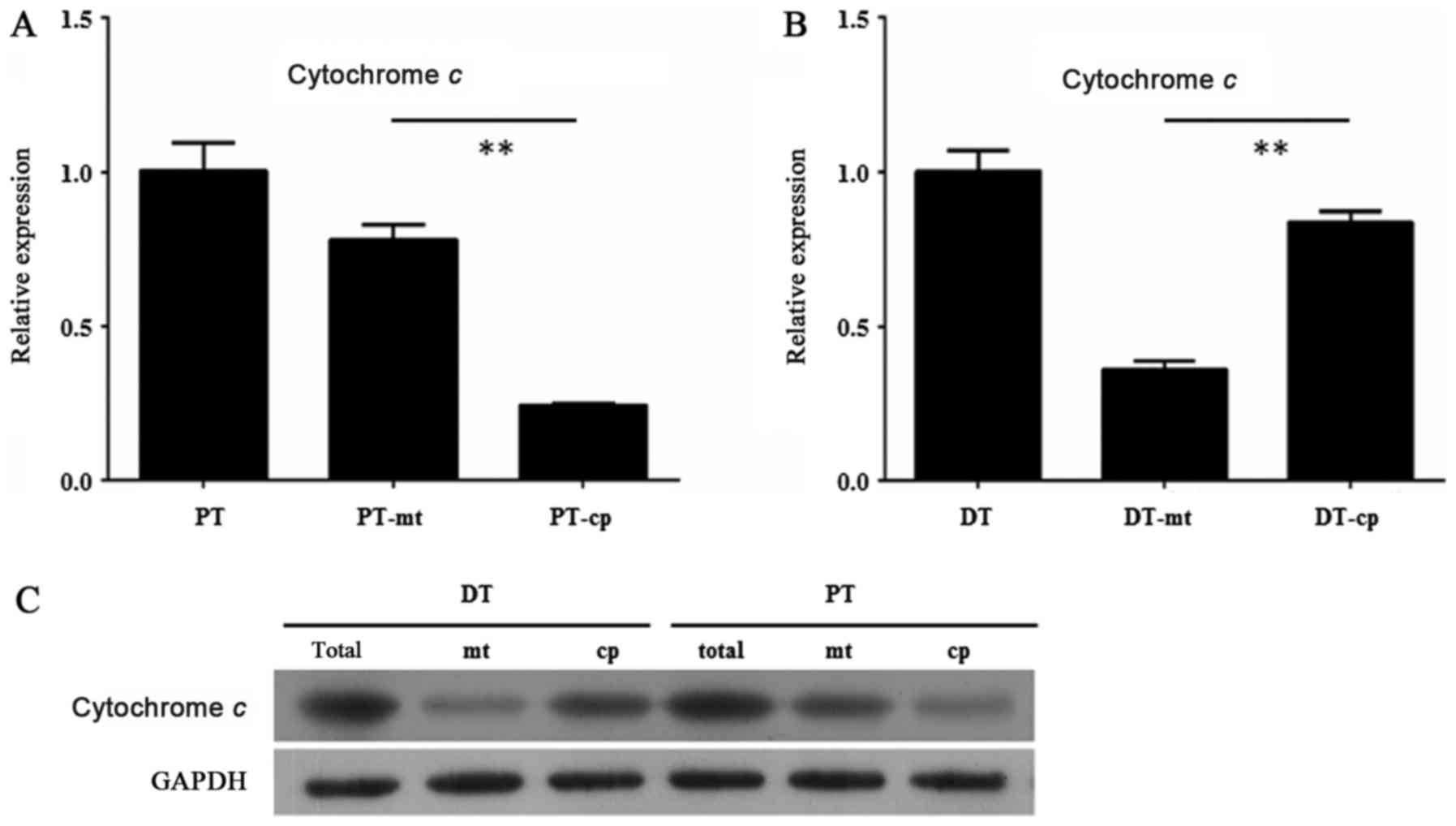
In order to find the mechanisms of the different
apoptosis level between the HDPSCs from deciduous teeth and adult
permanent teeth, we examined the expression of Cytochrome c
in the mitochondria of the HDPSCs from deciduous teeth and adult
permanent teeth by RT-qPCR and Western blot assay. The results
showed that Cytochrome c exhibited a relative higher
expression in mitochondria than in cytoplasm of the HDPSCs from
adult permanent teeth (P<0.01, unpaired Student's t-test)
(Fig. 2A). By contrast, the
expression level of Cytochrome c was significantly lower in
mitochondria than in cytoplasm of the HDPSCs from deciduous teeth
(P<0.01, unpaired Student's t-test) (Fig. 2B). These results were confirmed by
Western blot assay, in which the extent of Cytochrome c
protein level in mitochondria and cytoplasm of the HDPSCs from
adult permanent teeth and deciduous teeth was consistent with that
of its transcript level (Fig.
2C).
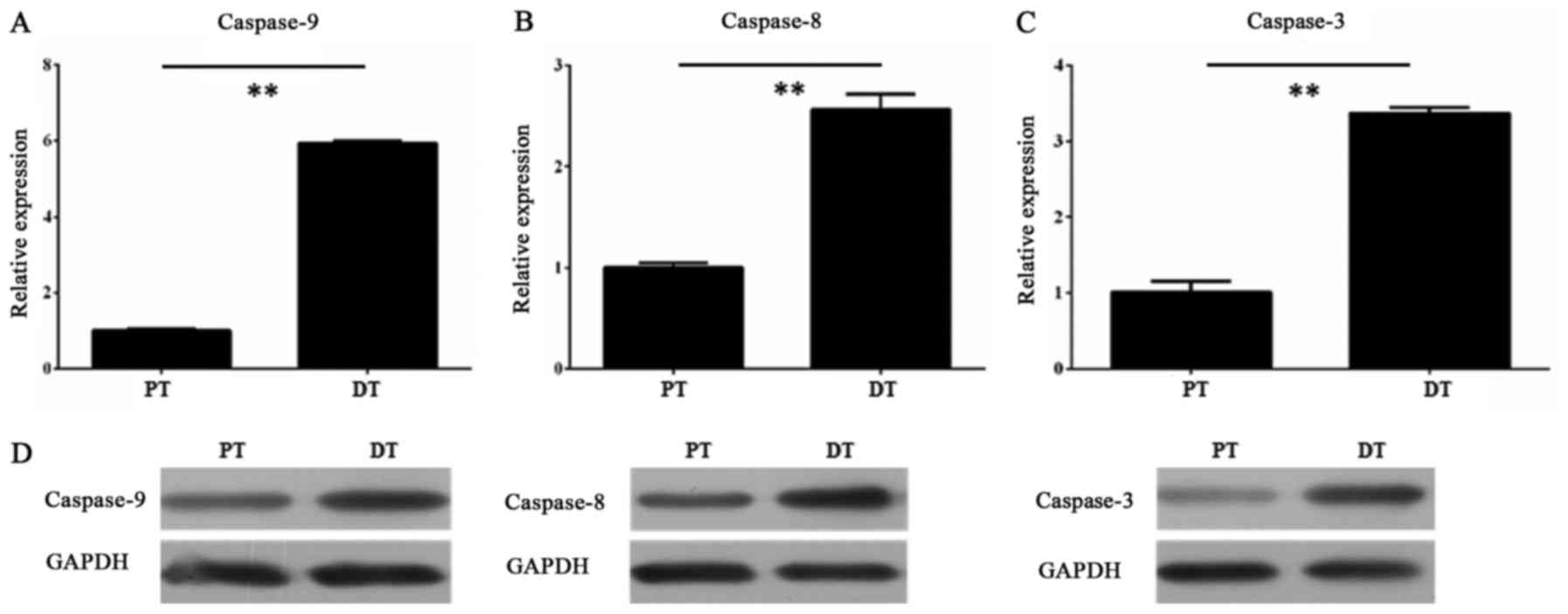
Furthermore, we measured the expression levels of
caspase-9, caspase-8 and caspase-3 in the HDPSCs from adult
permanent teeth and deciduous teeth, respectively. The RT-qPCR
results showed that all three genes had higher transcript level in
the HDPSCs from deciduous teeth than in that from adult permanent
teeth (P<0.01, unpaired Student's t-test) (Fig. 3A-C). The Western blot assay
confirmed the RT-qPCR results, revealing that the protein levels of
all three genes were also consistent with their transcript levels
(Fig. 3D).
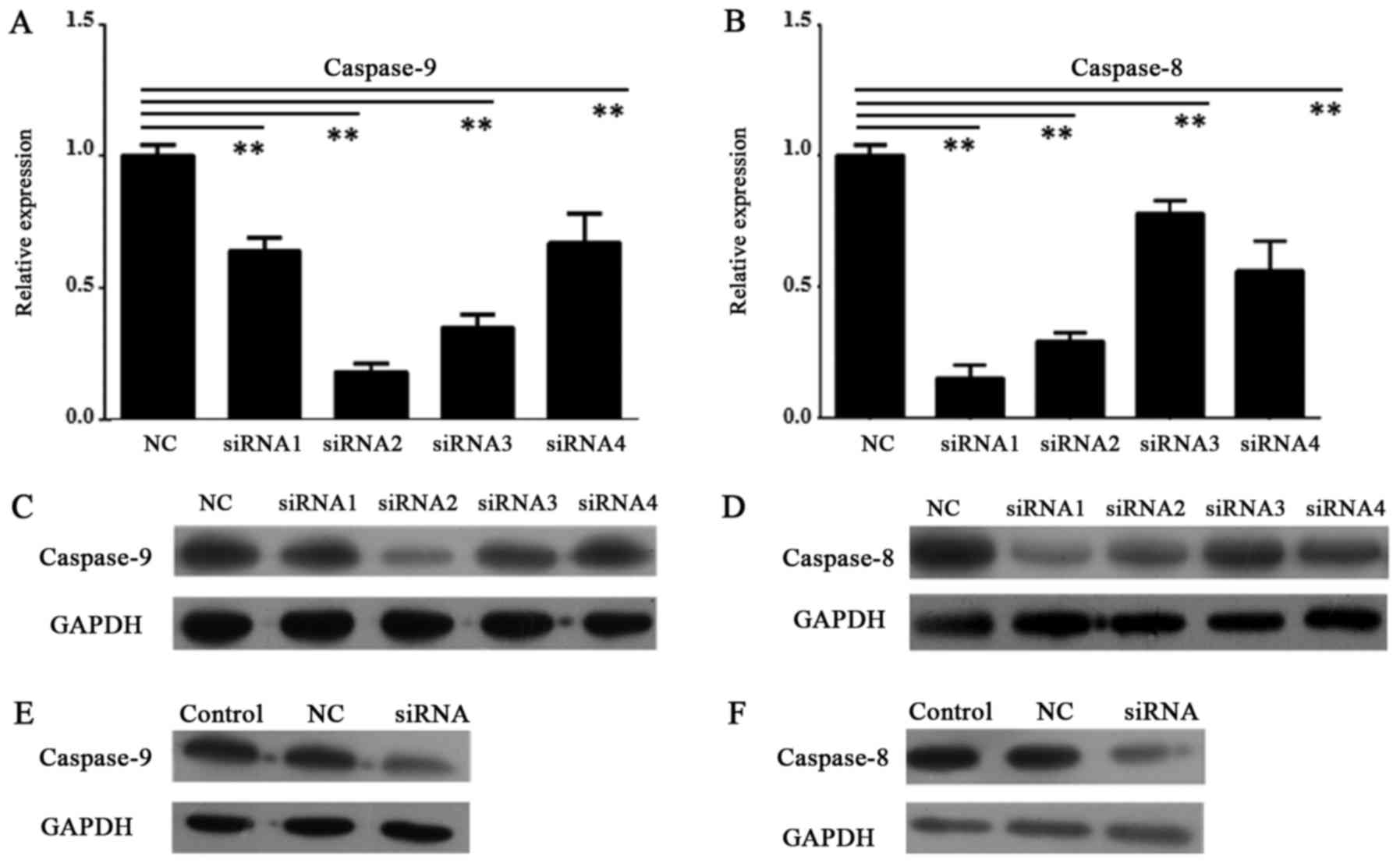
Knock down of caspase-9 induced
reduction of apoptosis
In order to knock down the expression of caspase-8
and caspase-9 in HDPSCs from deciduous teeth, we designed four
primers for each gene. After tests by RT-qPCR and Western blot, the
first primer of caspase-8 (caspase-8 siRNA1) and the second one of
caspase-9 (caspase-9 siRNA2) had greatest reduction of gene
expression in both transcript and protein levels (P<0.01,
one-way ANOVAs and Tukey's post hoc tests) (Fig. 4A-D). Then, caspase-8 siRNA1 and
caspase-9 siRNA2 were selected to conduct RNAi experiments. These
data revealed efficient knocking down of both genes in HDPSCs
(Fig. 4A-D).
After knocking down of caspase-8 and caspase-9 by
caspase-8 siRNA1 and caspase-9 siRNA2, respectively, the protein
expression of them were greatly reduced in Western blot assays
(Fig. 4E and F).
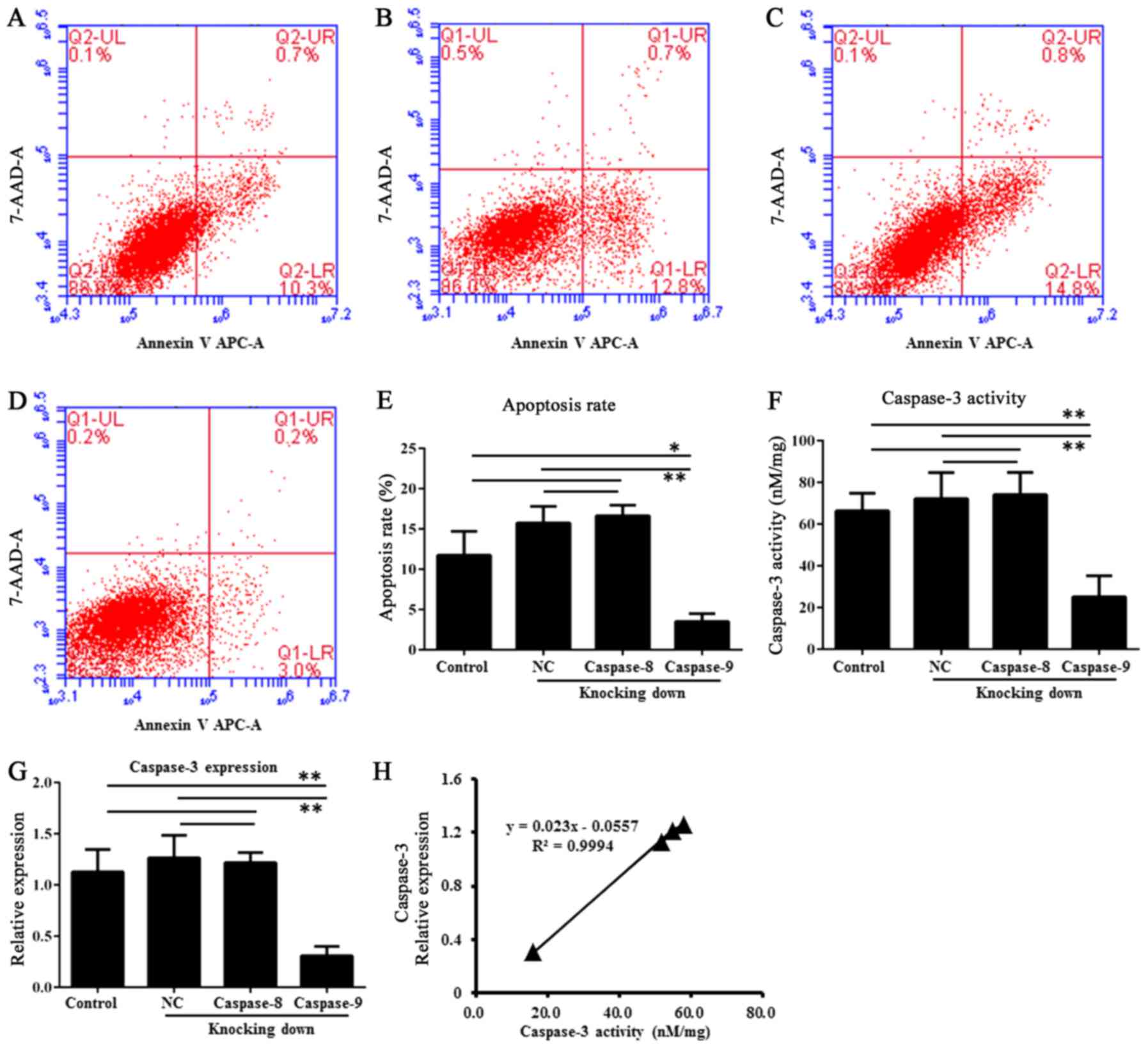
The Annexin V-FITC apoptosis assay showed caspase-9
had a reduction of apoptosis in the RNAi group (transfected HDPSCs)
than in the untransfected HDPSCs (P<0.01, one-way ANOVAs and
Tukey's post hoc tests) and in the HDPSCs transfected with empty
vector (P<0.01, one-way ANOVAs and Tukey's post hoc tests)
(Fig. 5A-E). This observation
indicated that caspase-9 was involved in apoptosis of HDPSCs. By
contrast, caspase-8 showed no significant difference of apoptosis
among these groups (P>0.05, one-way ANOVAs and Tukey's post hoc
tests) (Fig. 5A-E).
In previous reviews (9–11),
caspase-9 was required to activate caspase-3 and result in cell
apoptosis. Whether the expression changes of the upstream genes of
caspase-9 in this pathway contribute to cell apoptosis of HDPSCs
remains unclear. Here, we employed RT-qPCR approach to check the
expression of four upstream genes (Bax, Bak, Apaf-1 and
cyt C) of caspase-9. The results showed no significant
difference of them among the RNAi group, the untransfected HDPSCs
and the HDPSCs transfected with empty vector (data not shown here).
Therefore, cell apoptosis of HDPSCs ought to be caused by
expression changes of caspase-9 instead of its upstream genes.
Caspase-3 activity of HDPSCs
The caspase-3 activity assays showed that HDPSCs
transfected with caspase-9 siRNA2 have lower average caspase-3
activity (15.79±6.33 nM/mg) than untransfected HDPSCs (51.57±6.55
nM/mg) and HDPSCs transfected with empty vector (57.81±10.04 nM/mg)
(Fig. 5F). After RNAi of
caspase-9, the caspase-3 activity was significantly reduced
compared to that in untransfected HDPSCs (P<0.01, one-way ANOVAs
and Tukey's post hoc tests) and that in HDPSCs transfected with
empty vector (P<0.01, one-way ANOVAs and Tukey's post hoc
tests). By contrast, a different pattern was observed after
caspase-8 RNAi treatment. There were no significant differences of
caspase-3 activity among the RNAi group, untransfected HDPSCs
(P>0.05, one-way ANOVAs and Tukey's post hoc tests) and the
HDPSCs transfected with empty vector (P>0.05, one-way ANOVAs and
Tukey's post hoc tests) (Fig. 5F).
Consistent with caspase-3 activity, the expression of caspase-3
also exhibited significant differences between the caspase-9 RNAi
group and untransfected HDPSCs (P<0.01, one-way ANOVAs and
Tukey's post hoc tests) or HDPSCs transfected with empty vector
(P<0.01, one-way ANOVAs and Tukey's post hoc tests) (Fig. 5G). There were also no significant
differences of caspase-3 expression among the caspase-8 RNAi group,
untransfected HDPSCs (P>0.05, one-way ANOVAs and Tukey's post
hoc tests) and the HDPSCs transfected with empty vector (P>0.05,
one-way ANOVAs and Tukey's post hoc tests) (Fig. 5G). Therefore, caspase-3 activity
was positively correlated to its expression levels (Fig. 5H). There was a significantly
positive correlation between caspase-3 expression and activity with
a Pearson value of 0.9994 (P<0.05).
Discussion
Up to date, there are only a few studies about
HDPSCs and apoptosis (7).
According to a previous review (10), mitochondrial Cytochrome c
played dual roles in controlling cellular energetic metabolism and
apoptosis. In mammalian cells, a major caspase-activated apoptosis
pathway is initiated by Cytochrome c releasing from
mitochondria, which can induce caspase activation and subsequent
cell death (17). The relationship
of mitochondria and apoptosis have been well studied in various
species, such as yeast, nematode, fly, mouse and human (18) showing that it is a common mechanism
in all these species. The apoptosis of odontoclasts during
physiological root resorption of human deciduous teeth was
discovered, showing the presence of apoptosis in human deciduous
teeth (19). In present study, the
expression of Cytochrome c confirmed the possibility of
Cytochrome c-mediated apoptosis pathway in HDPSCs from both
deciduous teeth and adult permanent teeth.
The mitochondrial mechanisms of apoptosis were
summarized in prior reviews (9,11,20).
The extrinsic and intrinsic pathways require activation of
caspase-8 and caspase-9, respectively, which then activate
caspase-3, forming two major pathways of mitochondrial apoptosis
(9–11). The expression of caspase-9,
caspase-3 and caspase-8 in the HDPSCs from deciduous teeth and
adult permanent teeth supported the existence of apoptosis pathway.
Moreover, higher expression level of all three genes in the HDPSCs
from deciduous teeth might indicate higher possibility of apoptosis
pathways comparing with the HDPSCs from adult permanent teeth,
though it needs further confirmation. The higher expression of
caspase-3 in HDPSCs from deciduous teeth was consistent with
previous study, while caspase-8 expression show some differences
(21). The expression difference
of caspase-8 might be due to the examined materials we used in this
study instead of deciduous teeth and adult permanent teeth.
HDPSCs were thought to originate from migrating
neural crest cells during development, because they reside
predominantly within the perivascular niche of dental pulp
(22). According to Zhao et
al, ADAM28 was involved in the proliferation, differentiation,
and apoptosis of HDPSCs (8),
however, whether other pathways were involved in apoptosis of
HDPSCs remains unknown. As mentioned above, the extrinsic and
intrinsic pathways were typical in apoptosis, which needs
activation of caspase-9, caspase-8 and caspase-3 (9–11).
The knock down of caspase-9 induced reduction of apoptosis in the
HDPSCs from deciduous teeth, while that of caspase-8 did not. This
result suggested that the caspase-9-mediated pathway may be more
important in apoptosis than caspase-8-mediated pathway in
HDPSCs.
In previous reviews (9–11),
caspase-3 was activated by caspase-8 or caspase-9, though whether
the expression of caspase-3 was regulated by caspase-8 or caspase-9
remians unclear. The HDPSCs transfected with caspase-8 siRNA1 and
caspase-9 siRNA2 showed that knocking down of caspase-9 instead of
caspase-8 induced significant reduction of caspase-3 expression and
activity. Therefore, caspase-3 expression and activity should be
regulated by caspase-9 in HDPSCs. This line of evidence also
supported that caspase-9 followed by activated caspase-3 were
involved in HDPSCs cell apoptosis. Additionally, caspase-8 did not
regulate caspase-3 expression in HDPSCs from deciduous teeth.
Whether caspase-8 regulate caspase-3 expression in other HDPSCs or
other types of cells need further study.
As we known, HDPSCs were capable to form dentin-pulp
complex-like structures or a woven bone-like structure in
vivo, therefore, they had great potential for tissue
engineering (4,23,24).
Our study here uncovered the apoptosis pathway in HDPSCs, which may
provide theoretical framework for further application.
Acknowledgements
The authors would like to thank Guangzhou Genedenovo
Biotechnology Co., Ltd. for their technical support and revision of
manuscript.
Funding
This study was supported by a grant from Guangzhou
City Science and Technology Plan (grant no. 1563000340).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQ conceived and designed the study, and wrote the
paper. QH, YXC, MWY, JXF and QL collected the clinical samples,
performed the experiments, analyzed the data, interpreted the
results, and prepared figures for the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures used in this study conformed to the
tenets of the Declaration of Helsinki. The Ethics Committee
guidelines of Stomatological Hospital, Southern Medical University
approved the protocols used. Informed consent was obtained from all
participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: Stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gronthos S, Brahim J, Li W, Fisher LW,
Cherman N, Boyde A, DenBesten P, Robey PG and Shi S: Stem cell
properties of human dental pulp stem cells. J Dent Res. 81:531–535.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muthna D, Soukup T, Vavrova J, Mokry J,
Cmielova J, Visek B, Jiroutova A, Havelek R, Suchanek J, Filip S,
et al: Irradiation of adult human dental pulp stem cells provokes
activation of p53, cell cycle arrest, and senescence but not
apoptosis. Stem Cells Dev. 19:1855–1862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakopoulou A and About I: Stem cells of
dental origin: Current research trends and key milestones towards
clinical application. Stem Cells Int. 2016:42098912016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Shin SJ, Song Y and Kim E: In vivo
experiments with dental pulp stem cells for pulp-dentin complex
regeneration. Mediators Inflamm. 2015:4093472015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi C, Yaegaki K, Calenic B,
Ishkitiev N, Imai T, Ii H, Aoyama I, Kobayashi H, Izumi Y and
Haapasalo M: Hydrogen sulfide causes apoptosis in human pulp stem
cells. J Endod. 37:479–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z, Liu H and Wang D: ADAM28
manipulates proliferation, differentiation, and apoptosis of human
dental pulp stem cells. J Endod. 37:332–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Yang J and Jones DP: Mitochondrial
control of apoptosis: The role of cytochrome c. Biochim Biophys
Acta. 1366:139–149. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sui G, Soohoo C, Affar el B, Gay F and Shi
Y, Forrester WC and Shi Y: A DNA vector-based RNAi technology to
suppress gene expression in mammalian cells. Proc Natl Acad Sci
USA. 99:5515–5520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altschul SF, Madden TL, Schäffer AA, Zhang
J, Zhang Z, Miller W and Lipman DJ: Gapped BLAST and PSI-BLAST: A
new generation of protein database search programs. Nucleic Acids
Res. 25:3389–3402. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura S, Yamada Y, Katagiri W, Sugito
T, Ito K and Ueda M: Stem cell proliferation pathways comparison
between human exfoliated deciduous teeth and dental pulp stem cells
by gene expression profile from promising dental pulp. J Endod.
35:1536–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karbowski M and Youle RJ: Dynamics of
mitochondrial morphology in healthy cells and during apoptosis.
Cell Death Differ. 10:870–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domon T, Taniguchi Y, Inoue K, Ushijima N,
Taishi Y, Hiramatsu A, Wakita M and Yoshida S: Apoptosis of
odontoclasts under physiological root resorption of human deciduous
teeth. Cell Tissue Res. 331:423–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polster BM and Fiskum G: Mitochondrial
mechanisms of neural cell apoptosis. J Neurochem. 90:1281–1289.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodrigues LV, Del Puerto HL, Brant JM,
Leite RC and Vasconcelos AC: Caspase-3/caspase-8, bax and bcl2 in
pulps of human primary teeth with physiological root resorption.
Int J Paediatr Dent. 22:52–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stokowski A, Shi S, Sun T, Bartold PM,
Koblar SA and Gronthos S: EphB/ephrin-B interaction mediates adult
stem cell attachment, spreading, and migration: Implications for
dental tissue repair. Stem Cells. 25:156–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
About I, Bottero MJ, de Denato P, Camps J,
Franquin JC and Mitsiadis TA: Human dentin production in vitro. Exp
Cell Res. 258:33–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
d'Aquino R, De Rosa A, Laino G, Caruso F,
Guida L, Rullo R, Checchi V, Laino L, Tirino V and Papaccio G:
Human dental pulp stem cells: From biology to clinical
applications. J Exp Zool B Mol Dev Evol. 312B:408–415. 2009.
View Article : Google Scholar : PubMed/NCBI
|