Introduction
Lung cancer is the leading cause of cancer death
worldwide (1), including China
(2). Non-small cell lung cancer
(NSCLC) accounts for >80% of lung cancer cases. Despite advances
in diagnosis and treatment, outcomes remain poor, with the 5-year
survival rate generally being less than 15% (3). In order to improve the survival rate,
numerous studies have aimed to identify novel antineoplastic
therapies that have a wide range of biological activities, fewer
side effects and low toxicity (2–4).
Oxidative stress is defined as an imbalance between
the antioxidant defenses and the production of reactive oxygen
species (ROS). It occurs when excessive production of ROS
overwhelms their elimination by protective antioxidants or when
there is a prominent decrease or lack of antioxidant defense
activity (5). ROS are involved in
a number of types of cancer (6).
Additionally, oxidative stress may affect cancer initiation and
progression through increasing DNA mutations or DNA damage, genomic
instability and cell proliferation (6,7).
The lungs are directly exposed to higher
concentrations of oxygen than other tissues and organs (8). Certain studies have demonstrated that
the levels of oxidative stress increase in advanced stages of lung
cancer, while the levels of antioxidant molecules decrease
(9–11). In particular, patients with
squamous cell carcinoma exhibit much higher oxidative stress and
ROS, which may be linked to the fact that squamous cell carcinoma
is associated with tobacco use (9,12).
Enhanced ROS/RNS in the lung may increase the risk to lung cancer
through recurring DNA damage, inhibition of apoptosis, and
activation of proto-oncogenes by initiating signal transduction
pathways (13). The
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway is
one of most important survival signaling pathways. It has been
considered as the predominant growth-factor-activated pathway that
may be activated downstream of a variety of extracellular signals
and that impacts various aspects of cellular processes, including
cell proliferation, apoptosis and survival (14). Therefore, numerous types of cancer
have been linked to PI3K activity, including lung cancer (15), breast cancer (16), leukemia (17) and melanoma (18), among others (19,20).
Therefore, this pathway presents a challenge and an opportunity for
cancer therapy. A recent study demonstrated that the PI3K/Akt
pathway may be a novel target in antineoplastic therapy (19). However, in a number of cases,
activation of the PI3K/Akt pathway alone is not responsible for
oncogenic transformation and therefore, the antineoplastic effect
of PI3K/mechanistic target of rapamycin (mTOR) inhibitor is not
satisfied. In addition, cancer cells resistant to PI3K inhibition
remain a problem.
Hydrogen is an efficient antioxidant and has been
identified as a novel therapeutic antioxidant recently, as it was
revealed to selectively reduce cytotoxic ROS in tissues (21–23).
In in vivo and in vitro studies, H2 has exhibited
protective antioxidant, anti-apoptotic and anti-inflammatory
properties. Hydrogen is safe and effective for distribution into
the cytoplasm without the need for specific receptors to overcome
hydrophilicity (24,25). In rat models, inhaled hydrogen gas
is able to reduce myocardial ischemia reperfusion injury and the
infarct size of focal cerebral (26). Due to the fact that hydrogen gas is
flammable and inconvenient for clinical use, hydrogen-rich saline
with a therapeutic concentration of hydrogen is an alternative
model of molecular hydrogen. The protective effect of hydrogen-rich
saline is largely due to its ability to reduce the ROS-associated
pathologies.
Clinically, combination therapy is most widely used
in the treatment of cancer, including lung cancer. The main aim is
to achieve synergistic therapeutic effect, to reduce dose and
cytotoxicity, and to delay or minimize the induction of drug
resistance (27,28).
In the present study, we hypothesized that the
combination of hydrogen-rich saline and a highly potent PI3K
inhibitor, LY294002, would decrease the proliferation of cancer
cells more than single-agent therapy through anti-oxidation and
regulation of apoptosis. The present study aimed to demonstrate the
effect of the release of inflammatory and apoptosis cytokines.
Furthermore, it focused on the influence of combination therapy on
the PI3K/Akt signaling pathway and the mechanism underpinning this,
which may be applied to clinical tumor therapy.
Materials and methods
Reagents
Assay kits for superoxide dismutase (SOD) and
malondialdehyde (MDA) were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). A propidium iodide (PI)
double staining kit and Annexin V-fluorescein isothiocyanate (FITC)
were purchased from Nanjing KeyGene Biotech Co., Ltd. (Nanjing,
China). PI was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). LY-294002 was purchased from Sigma-Aldrich (Merck KGaA),
Stock solution (50 mM) of LY-294002 was dissolved in DMSO and
further diluted to a final concentration of 20 µM. Antibodies were
obtained from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA).
Other reagents were obtained locally.
Cell lines and cell culture
The human NSCLC A549 cell line was obtained from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The A549 cell line was maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum and
penicillin/streptomycin at 37°C in an atmosphere of 5%
CO2 and 95% air.
Hydrogen saline
Molecular hydrogen (H2) was dissolved in saline
under high pressure (0.6 MPa) to a supersaturated level for 2 h.
Hydrogen-rich saline was freshly prepared each week and was
sterilized by g radiation. It was ensured that hydrogen-rich saline
was maintained at a concentration of more than 0.6 mmol/l. The
hydrogen concentration was detected by gas chromatography using the
method previously described by Ohsawa et al (22).
Cell proliferation inhibition
assay
The inhibition rate of cell proliferation was tested
using an MTT assay. A549 cells were grown in 96-well plates at a
density of 5×103 cells per well for 24 h, prior to being
treated with different concentrations of LY294002 (primary
concentration, 20 µM, the concentration of each well is 1/3 of the
previous well), hydrogen-rich saline (with a hydrogen concentration
of 0.6 mmol/l) or a combination of hydrogen-rich saline and
LY294002. After 48 h, 20 µl MTT solution [2 mg/ml in
phosphate-buffered saline (PBS)] was added into each well and the
cells were incubated for an additional 4 h at 37°C. The medium was
completely removed and then 150 µl DMSO was added to solubilize the
MTT formazan crystals. Finally, the optical density was read at 570
nm (OD570) using a Microplate reader (Model 550; Bio-Rad
Laboratories, Inc., Hercules, MA, USA). Results were presented as
the mean of 3 independent experiments each conducted in duplicate.
The half-maximal inhibitory concentration (IC50) values were
calculated from dose-response curves utilizing GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA).
SOD and MDA measurement
A549 cells were grown in 6-well plates at a density
of 1×106 cells per well for 24 h, prior to being treated
with hydrogen-rich saline (0.6 mmol/l), LY294002 (20 µM) or a
combination of hydrogen-rich saline and LY294002 for 24 h. The
cells were subsequently harvested, disrupted ultrasonically on ice
and centrifuged at 2,500 × g for 10 min at 4°C. The supernatants
were collected and stored at −20°C for subsequent analysis. The
concentrations of MDA and SOD were evaluated using an MDA and SOD
detection kit according to the manufacturer's protocols.
Annexin V/PI flow cytometry
analysis
Cell apoptosis was measured using an Annexin V-FITC
Apoptosis kit, according to the manufacturer's protocols. A549
cells were grown in 6-well plates at a density of 1×106
cells per well for 24 h, prior to being treated with hydrogen-rich
saline (0.6 mmol/l), LY294002 (20 µM) or a combination of
hydrogen-rich saline and LY294002 for 24 h. Following staining,
flow cytometry was performed using a FACScan flow cytometer. Cells
stained as Annexin V-positive and PI-negative were considered
apoptotic, while cells that double stained as Annexin V-positive
and PI-positive were considered late apoptotic or necrotic. Data
were analyzed using the Cell Quest Software Program
(FACSCalibur™.; BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis
The cells were washed twice with ice-cold PBS
following treatment for 24 h. Total protein was extracted using
cell lysis buffer (1 M Tris.HCL pH 7.5, 8M Urea, 150 mM β-ME and 1%
protease inhibitor cocktail). Protein concentration was determined
by bicinchoninic acid (BCA) protein assay. Equal amounts of protein
(50 µg) from each sample were separated by SDS-PAGE. Proteins were
subsequently transferred onto polyvinylidene difluoride membranes.
Next, the membranes were blocked with 5% skimmed milk for half an
hour in room temperature, prior to being incubated with primary
antibodies against HO-1, p65 and p-Akt separately overnight at 4°C,
followed by being washed three times with PBS. The membranes were
subsequently incubated with secondary antibodies for 2 h in room
temperature. Following washing, the membranes were visualized with
an ECL kit and quantified using ImageJ Software (National
Institutes of Health, Bethesda, MD, USA). Experiments were repeated
at least three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using an mirVana
miRNA Isolation kit (Qiagen GmbH, Hilden, Germany). Equal amounts
of RNA samples were reverse transcribed into cDNA using a
high-capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). RT-qPCR was
performed using first-strand cDNA with TaqMan probes and TaqMan
Universal PCR Master mix (Roche Diagnostics, Basel, Switzerland)
with the following conditions: Denaturation at 95°C for 10 sec,
annealing at 58°C for 20 sec and elongation at 72°C for 20 sec, and
GAPDH served as an internal control. PCR was performed using the
following primers: HO-1 forward, 5′-GATAGAGCGCAACAAGCAGAA-3′ and
reverse, 5′-CAGTGAGGCCCATACCAGAAG-3′; P65 forward,
5′-GGGAAGGAACGCTGTCAGAG-3′ and reverse,
5′-TAGCCTCAGGGTACTCCATCA-3′; and GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse, 5′-CTCCACGACGTACTCAGCG-3′.
Experiments were repeated at least three times. The relative mRNA
expression was measured using the ∆∆Cq method (29).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6 (GraphPad Software, Inc.). Data are presented as the mean ±
standard error of the mean. The statistical significance of
differences between groups in vitro experiments was
determined by the Student t-test. The statistical significance of
differences between combinations with theoretical combination in
vitro experiments was determined by one-way analysis of
variance followed by a Turkey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
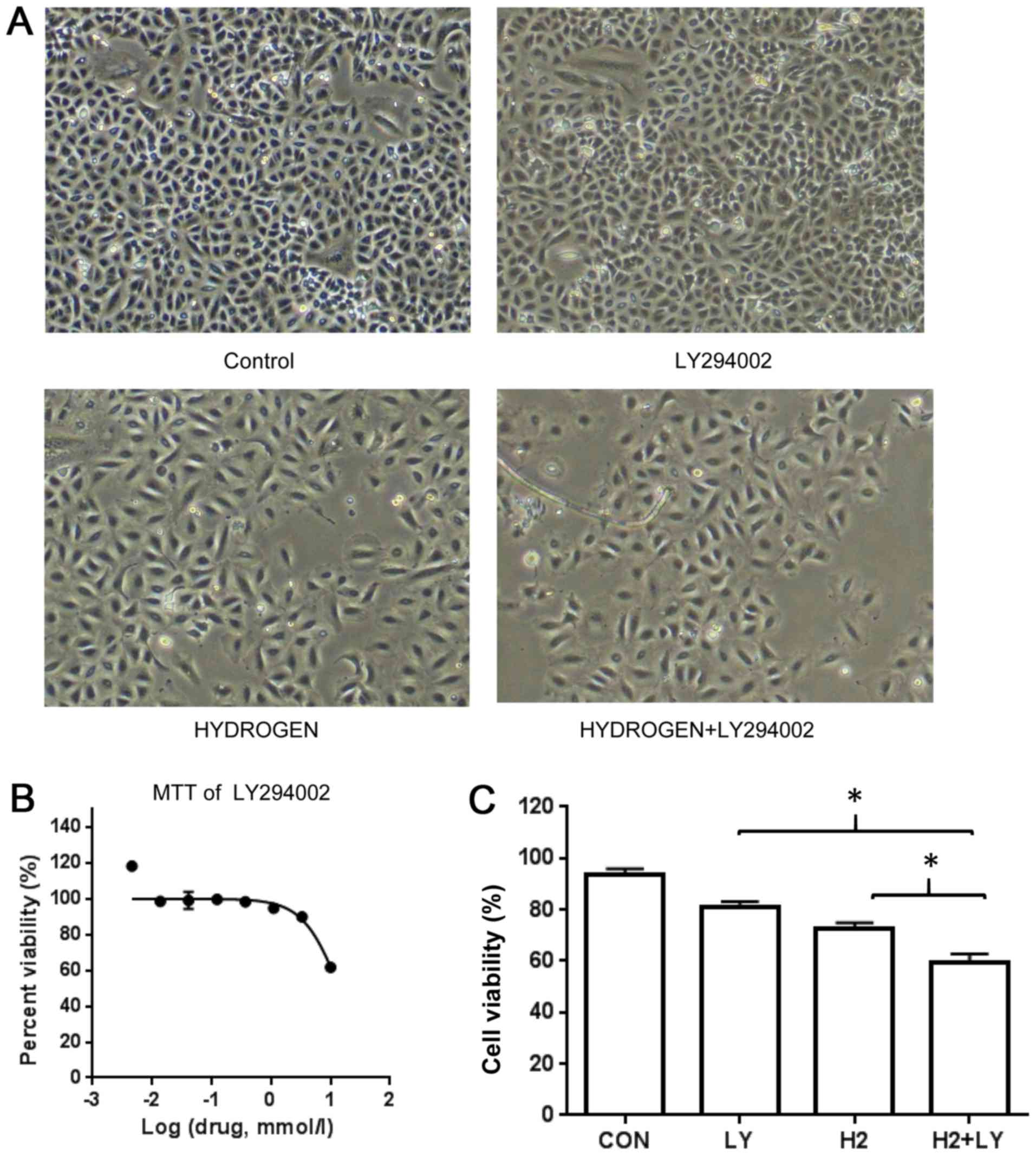
Inhibition of cell proliferation by
hydrogen-rich saline and the PI3K inhibitor, LY294002, in A549
cells
To investigate the proliferation inhibition effects
of hydrogen-rich saline alone and in combination with LY-294002 in
A549 cells, the cells were treated with hydrogen-rich saline,
LY-294002 or a combination of the two for 48 h, and the inhibition
of cell proliferation rate was measured using an MTT assay. We
observed that growth of A549 cells was suppressed in treatment
group (Fig. 1A). Cell
proliferation was suppressed following treatment with either
hydrogen-rich saline or LY-294002 alone (Fig. 1B). However, compared with treatment
with hydrogen-rich saline or LY-294002 alone (Fig. 1C), the combination of the two
resulted in increased inhibition.
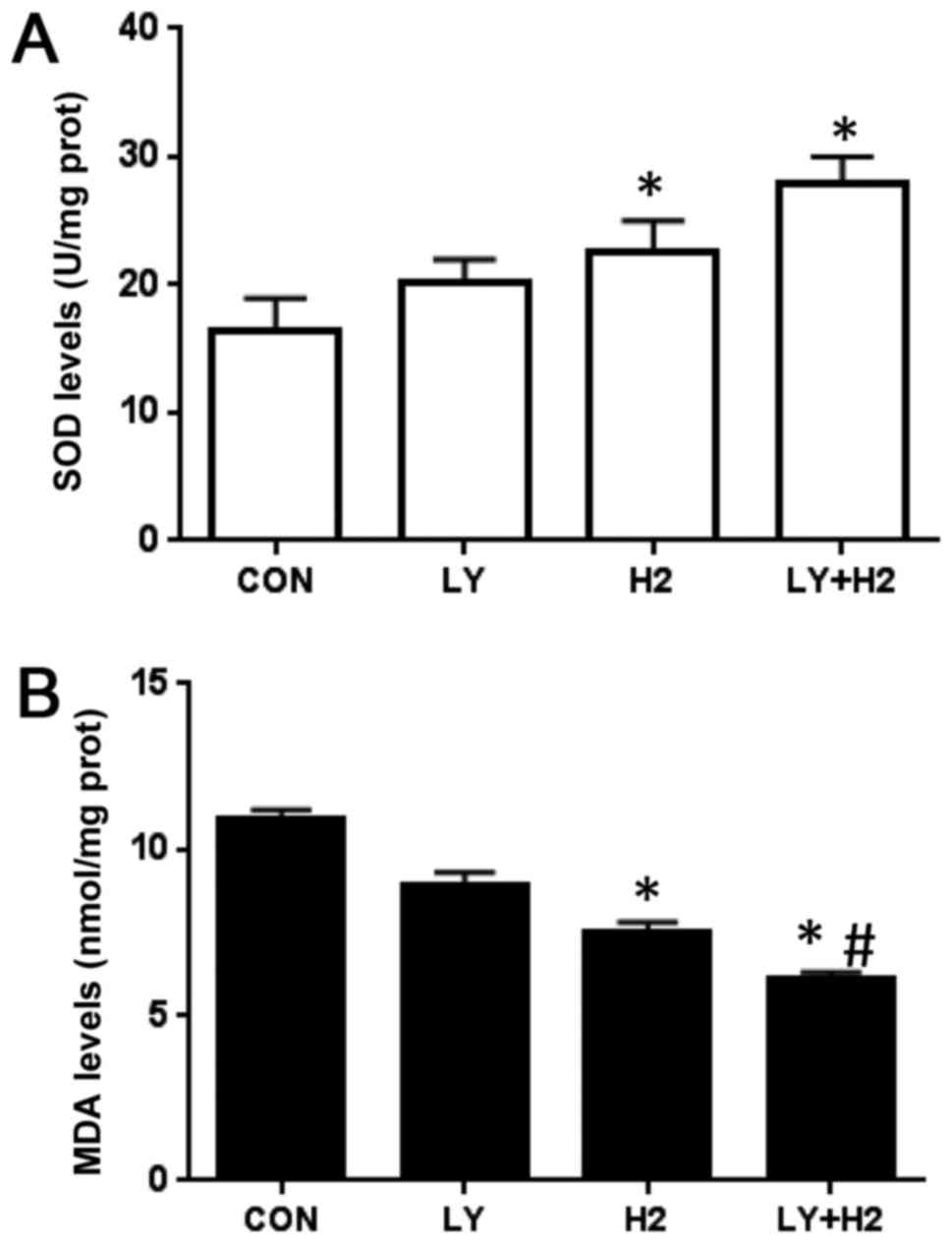
Effect of hydrogen-rich saline and the
PI3K inhibitor, LY294002, on the concentrations of ROS and MDA in
A549 cells
To further investigate the effect of a combination
of hydrogen-rich saline and the PI3K inhibitor, LY294002, on
intracellular oxidant generation in A549 cells, the concentrations
of ROS and MDA were examined. The results indicated that the
combination of hydrogen-rich saline and LY294002 increased the
concentrations of SOD (Fig. 2A),
and markedly decreased the levels of MDA, compared with the control
group and the hydrogen-rich saline monotherapy group (Fig. 2B). These results demonstrated that
the combination of the two treatments suppressed intracellular
oxidant generation in A549 cells.
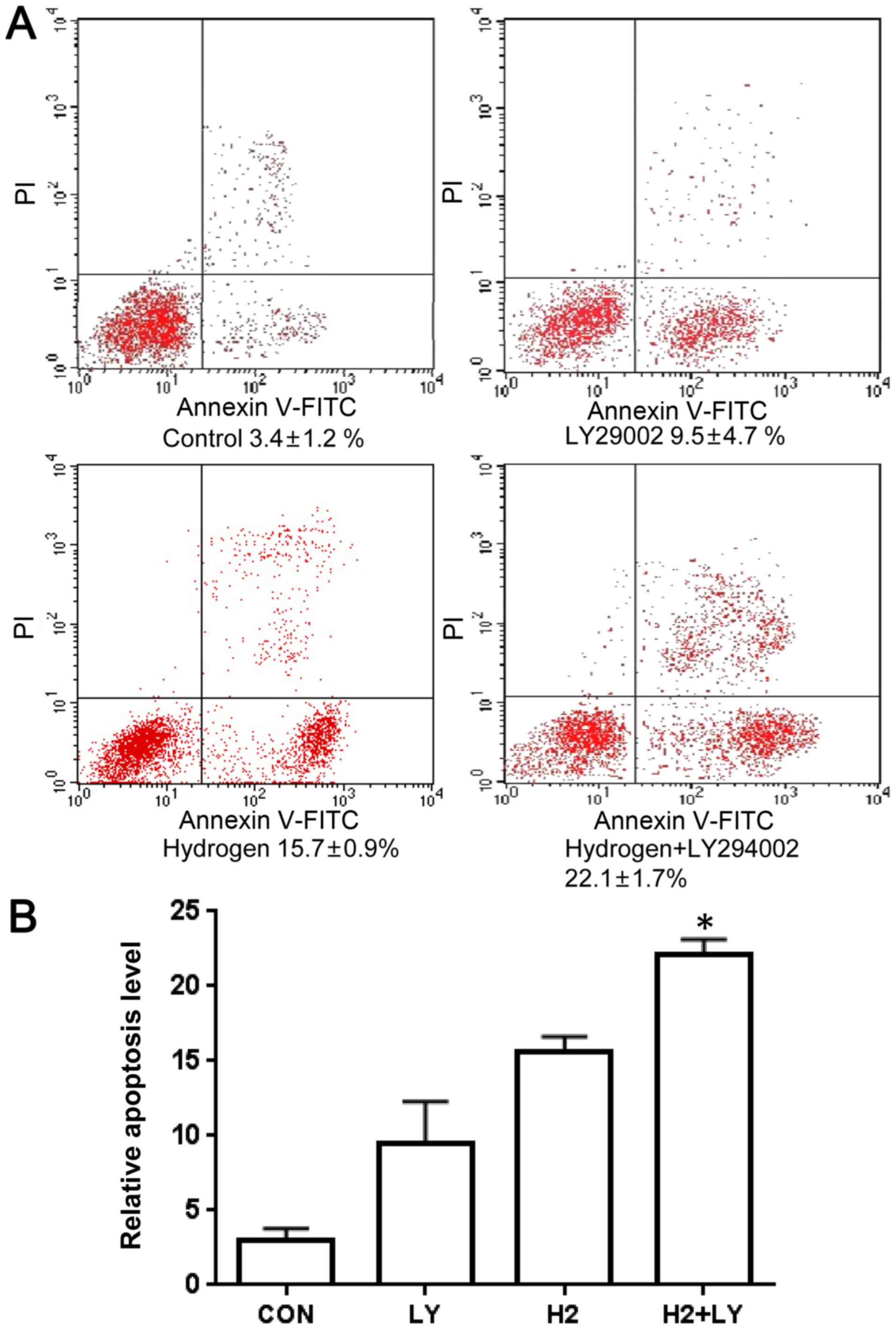
Induction of apoptosis by
hydrogen-rich saline and the PI3K inhibitor, LY294002, in A549
cells
A549 cells were treated with hydrogen saline, the
PI3K inhibitor, LY294002, or the combination of hydrogen saline
with LY294002 for 24 h, prior to being analyzed by flow cytometry.
In comparison with the control group, the numbers of early and late
apoptotic cells were markedly increased (Fig. 3A and B). The proportion of early
and late apoptotic cells in the combination treatment group was
markedly increased, compared with the control group. The proportion
of apoptotic cells was increased in the three groups, but the
combination of the two treatments induced a more marked increase
than treatment with hydrogen-rich saline or LY-294002 alone.
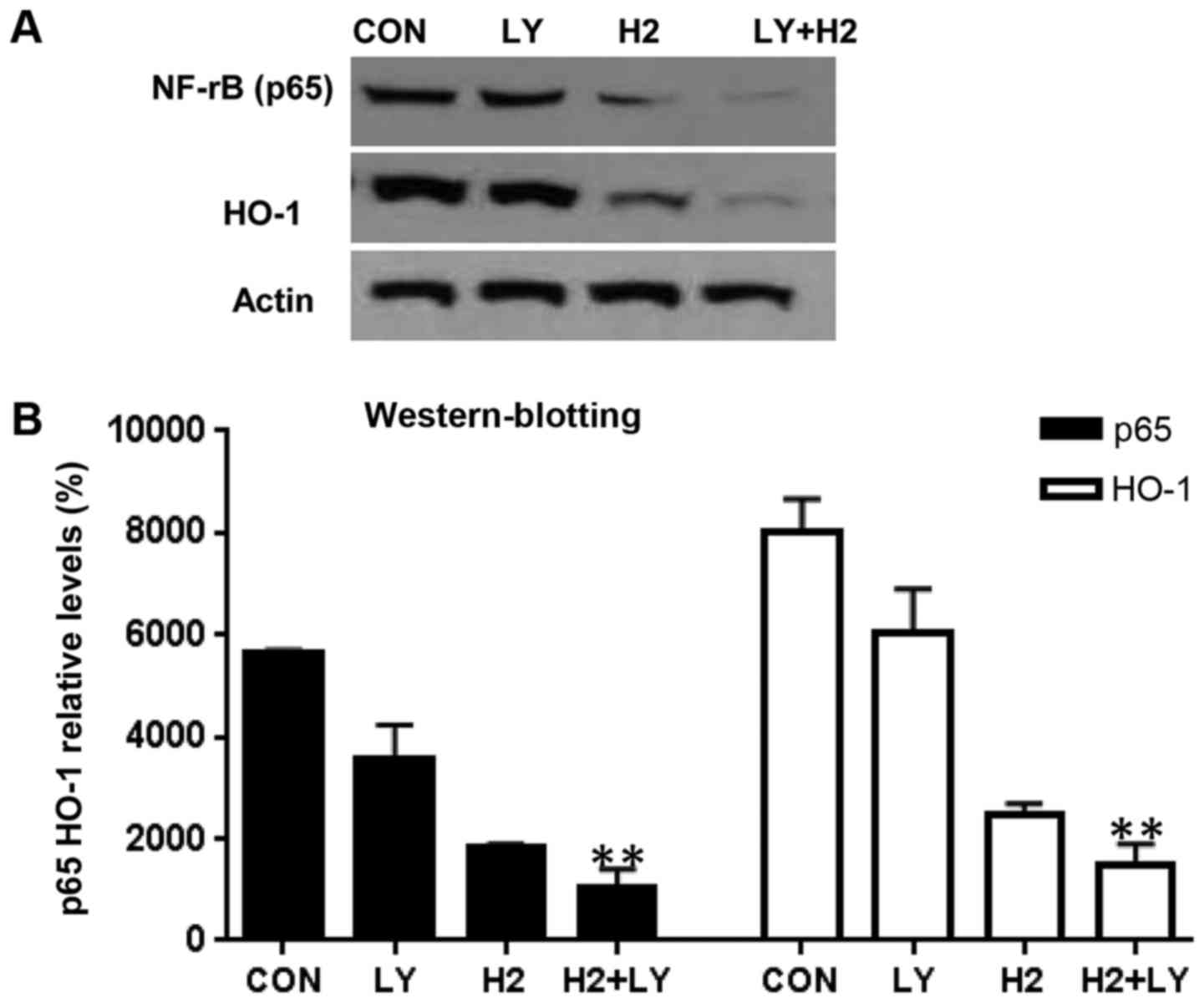
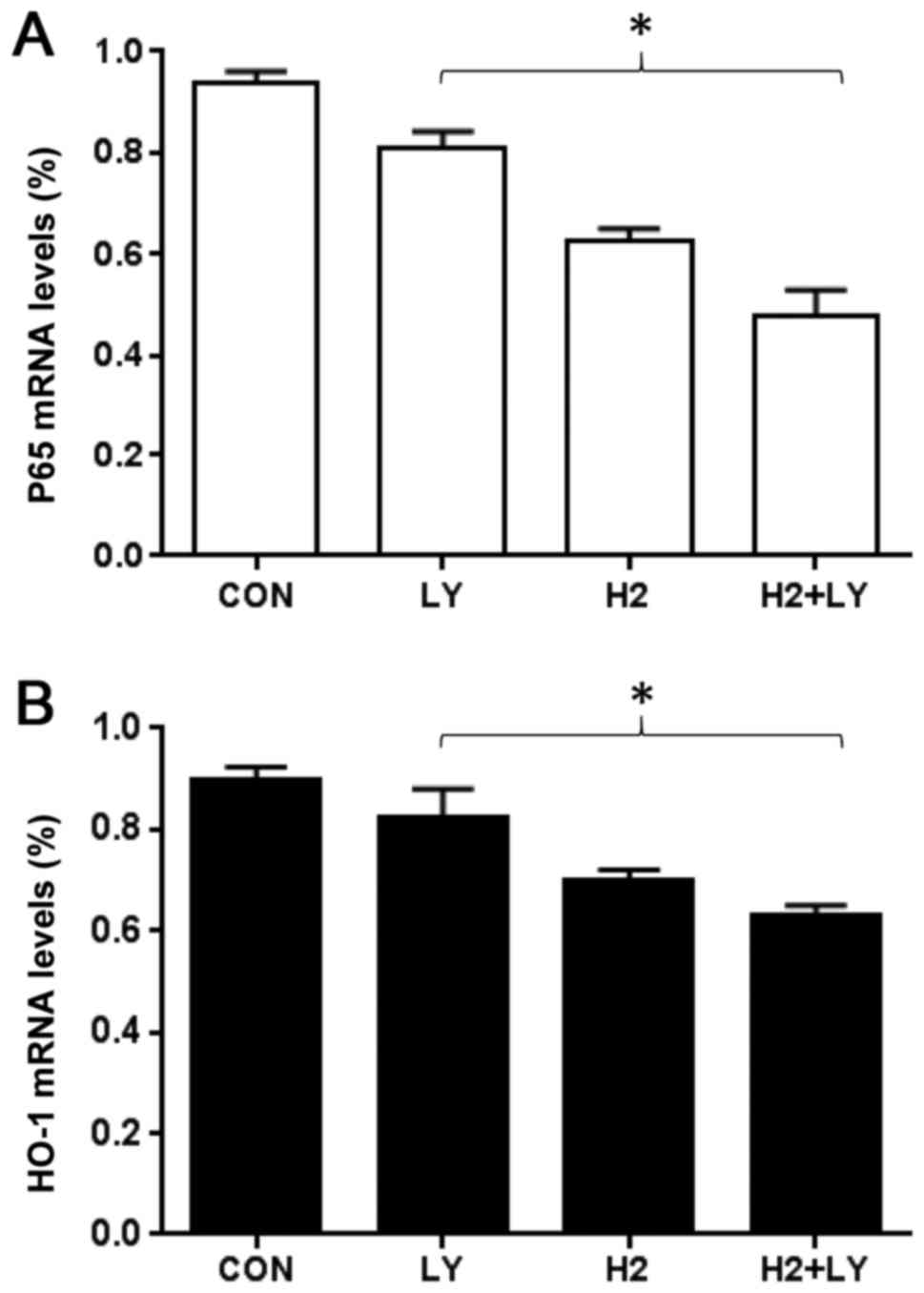
Inhibition of HO-1 and NF-κB p65
protein and mRNA expression in A549 cells by combination of
hydrogen-rich saline and the PI3K inhibitor, LY294002
To investigate the mechanism of anti-oxidation
induced by hydrogen-rich saline alone, LY294002 alone and the
combination of hydrogen-rich saline and LY294002, the present study
tested the effect of three treatment groups on HO-1 and NF-κB p65
protein and mRNA expression levels. Western blot analysis revealed
that hydrogen-rich saline monotherapy led to a decrease in HO-1 and
p65 levels as compared with the control cells (Fig. 4A and B). RT-qPCR analysis revealed
that hydrogen-rich saline monotherapy led to a decrease in HO-1 and
p65 mRNA expression levels, compared with the control cells
(Fig. 5A and B). The protein and
mRNA expression levels were higher following combined treatment
with hydrogen-rich saline and LY294002, compared with treatment
with either of the agents alone.
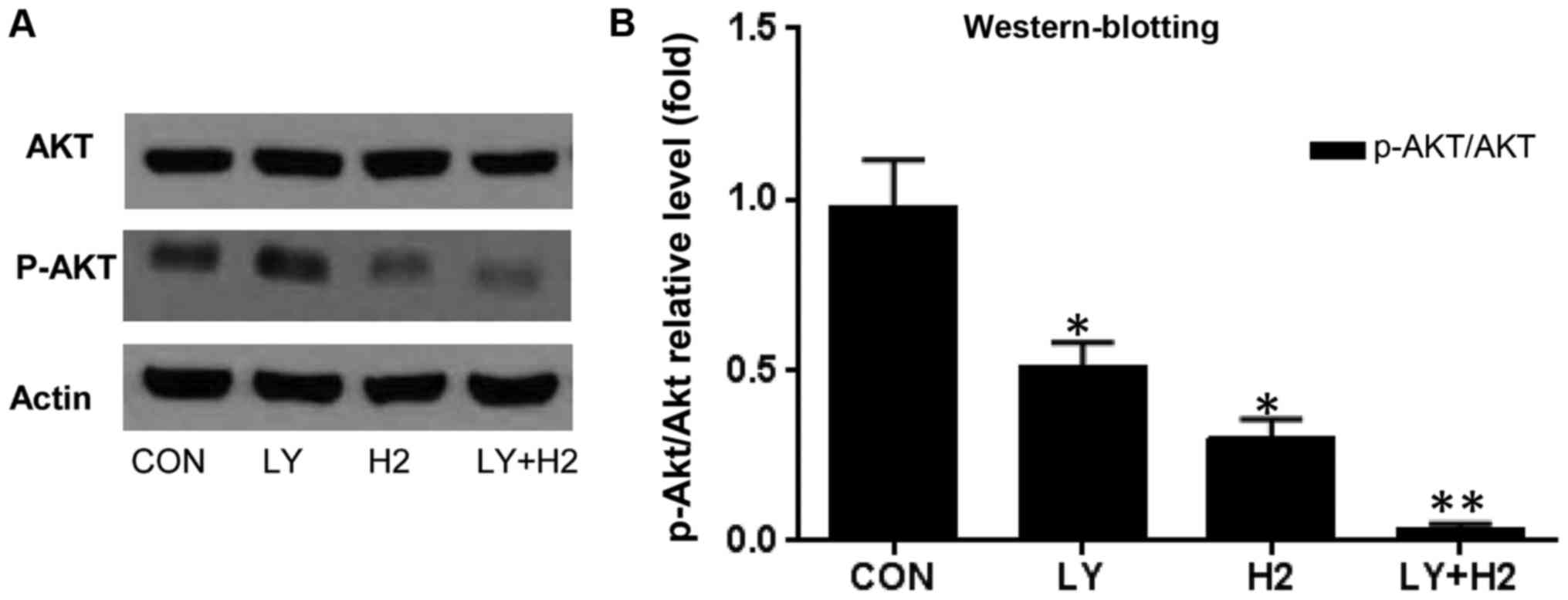
Inhibition of the PI3K/Akt pathway by
hydrogen-rich saline alone and in combination with LY294002
In order to better understand the molecular basis of
the anti-proliferation and apoptosis induced by hydrogen-rich
saline alone and in combination with LY294002, the present study
examined the expression of p-Akt, an effector of the PI3K/Akt/mTOR
signaling pathway, following treatment with hydrogen-rich saline
alone and in combination with LY294002 for 24 h. The protein
expression of p-Akt was decreased in response to hydrogen-rich
saline alone, LY294002 alone and a combination of the two, while
the p-Akt protein expression level remained lower in the combined
treatment group than following drug treatment alone (Fig. 6A and B). These results indicated
that the combination therapy has a synergistic effect on
anti-proliferation and the induction of apoptosis possibly through
inhibiting the PI3K/Akt pathway.
Discussion
To the best of our knowledge, oxidative stress play
a key role in tumorigenesis (6,30–32).
Oxidative stress may affect the initiation and progression of
cancer by leading to DNA mutations, DNA damage, inducing genomic
instability and cell proliferation (33–36).
In particular, oxidative damage serves a pivotal
role in pulmonary disease as the lungs are exposed daily to
oxidants, which are generated either endogenously or exogenously
(e.g., air pollutants and cigarette smoke) (10); tobacco smoke and particulate matter
in air pollution may generate ROS and therefore increase the
incidence of lung cancer and respiratory diseases (37). Furthermore, synergistic effects in
the generation of ROS contribute toward lung cancer through
inducing oxidative stress and inflammation with a high DNA damage
potential (10). Since oxidative
stress is a crucial event in cancer cells, this may also provide an
opportunity to kill malignant cells.
Hydrogen has been generally recognized as a
therapeutic anti-oxidative and anti-apoptotic tool (22,38).
Numerous studies have demonstrated that hydrogen may selectively
react with exclusively detrimental ROS, while hydrogen does not
disturb physiological metabolic oxidation-reduction reactions or
disrupt the ROS involved in cell signaling (22,31,39).
Hydrogen-rich saline, which is easily administered and is safe for
clinical application, is a viable approach with the same properties
of anti-oxidation, anti-inflammation and anti-apoptosis, and has
been used in several recent animal disease studies (40–42).
However, a study has demonstrated that hydrogen therapy may inhibit
the proliferation of tongue carcinoma cells (43) and reduce the size of skin tumors.
Further studies will elucidate the molecular mechanism of
hydrogen-mediated inhibition of cancer cell proliferation.
The present study initially applied hydrogen-rich
saline alone to lung cancer cells. Subsequently, the effect of
hydrogen-rich saline on apoptosis and inflammatory cytokines, and
the pathway involved in this process, was investigated. The results
demonstrated the following: i) Hydrogen-rich saline treatment alone
inhibited A549 cell proliferation; ii) hydrogen-rich saline
treatment alone decreased MDA expression and increased SOD
activity; iii) hydrogen-rich saline treatment alone induced A549
cell apoptosis; iv) in in vitro experiment, treatment with
hydrogen significantly suppressed the effect on protein and mRNA
expression of HO-1 and NF-κB p65 in A549 cell and v) hydrogen-rich
saline suppressed the expression of p-Akt and the expression levels
of HO-1 and p65.
The results of the present study demonstrated that
hydrogen-rich saline alone may downregulate the expression of NF-κB
p65, which is a redox-regulated transcription factor. The
activation of NF-κB serves an important role in regulating the
expression of a number of early stress response genes, which are
associated with oxidative stress-induced cell death in a wide range
of tumor types (44). Therefore,
it is suggested that hydrogen-rich saline may attenuate oxidative
stress by inhibiting the activation of NF-κB. The present study
demonstrated that hydrogen-rich saline alone downregulated the
expression of HO-1, which is a stress response protein (45). As HO-1 expression and activity are
markedly increased by stressful conditions, the major role of HO-1
is involved in antioxidant and anti-inflammatory responses
(46,47). However, the induction of HO-1,
which may provide cells with a growth advantage, is not always
adequate to protect the cells. HO-1 may be overexpressed in tumor
cells (45,48,49).
In the present study, HO-1 was downregulated in vitro,
oxidative stress was markedly decreased by hydrogen-rich saline and
the expression of p-Akt was suppressed. These results indicated
that it is possible that the anti-proliferation and apoptosis
inducing functions of hydrogen-rich saline are primarily achieved
by downregulating transcriptional factors (NF-κB and p-Akt) via the
PI3K/Akt pathway.
The PI3K/Akt pathway is involved crucially in the
development and progression of several types of cancer by aiding in
promoting cell proliferation and allowing cells to evade apoptosis.
PI3K/Akt pathway activation is associated with poor outcomes in
certain types of cancer (50). In
addition, numerous novel ‘targeted agents’ have been specifically
exploited to act on PI3K/Akt and downstream component-related
targets due to the ubiquitous nature of the activation of this
pathway in cancer. Furthermore, multiple PI3K/Akt pathway
inhibitors are now under active clinical development (20).
In NSCLC cells, these small molecule inhibitors have
been demonstrated to shift the apoptotic threshold in cancer cells
following treatment with other molecule inhibitors or standard
chemotherapy (51).
The present study focused on the efficacy of a PI3K
inhibitor (LY294002) in combination with anti-oxidative treatment
with hydrogen-rich saline. Regarding the administration of
hydrogen-rich saline or LY294002 separately as single agents, the
results of the present study demonstrated that the combination
treatment of A549 cells may be more effective than single
treatment. Combination treatment enhanced the efficacy of
anti-proliferation and apoptosis induction. Similarly, these data
demonstrated that usage of the two agents in combination may
decrease MDA expression levels, inhibit p-Akt activity, and reduce
expression of HO-1 and NF-κB. The results of the present study
supported the hypothesis that the combination therapy has the
effect of anti-proliferation and apoptosis induction through
inhibiting the activation of Akt phosphorylation and reducing the
downstream expression of HO-1 and NF-κB. Therefore, combining PI3K
inhibitor (LY294002) and hydrogen-rich saline may negatively
regulate A549 cell proliferation through inhibition of the PI3K/Akt
pathway. The present study has suggested that this combination is
able to serve as dual PI3K pathway inhibitors, which may be
attractive targets for the treatment of lung cancer, with a low
risk of side effects. However, due to the fact that the present
study using combination are unable to assay clear mechanisms of
PI3K in the cells, clarification of the intracellular signaling
network of cells should be verified in future experiments.
In conclusion, the present study demonstrated that
hydrogen-rich saline in combination with LY294002 treatment in A549
cancer cells leads to constitutive inhibition of the PI3K/Akt
signaling pathway and acts as a negative regulator for cell
proliferation and expression of HO-1. Furthermore, the drug
combination approach used in the present study may be useful for
the study of novel cancer therapeutics and may directly aid future
drug development and patient stratification in clinical trials.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grant from
the Medical Research Projects of Chongqing Municipal Health and
Family Planning Commission (grant no. 2016MSXM057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ and SLG planned the experiments. GL, SC and CL
performed the experiments. LZ and YL analyzed the data. YJ prepared
the figures. YJ and LZ drafted the manuscript. YJ and LZ proof read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Z, Dai Y, Fu H, Zheng Y, Bao D, Yin Y,
Chen Q, Nie X, Hao Q, Hou D and Cui Y: Curcumin exerts a protective
effect against premature ovarian failure in mice. J Mol Endocrinol.
60:261–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duracková Z: Some current insights into
oxidative stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI
|
|
6
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Visconti R and Grieco D: New insights on
oxidative stress in cancer. Curr Opin Drug Discov Devel.
12:240–245. 2009.PubMed/NCBI
|
|
8
|
Kinnula VL and Crapo JD: Superoxide
dismutases in the lung and human lung diseases. Am J Respir Crit
Care Med. 167:1600–1619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esme H, Cemek M, Sezer M, Saglam H, Demir
A, Melek H and Unlu M: High levels of oxidative stress in patients
with advanced lung cancer. Respirology. 13:112–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valavanidis A, Vlachogianni T, Fiotakis K
and Loridas S: Pulmonary oxidative stress, inflammation and cancer:
Respirable particulate matter, fibrous dusts and ozone as major
causes of lung carcinogenesis through reactive oxygen species
mechanisms. Int J Environ Res Public Health. 10:3886–3907. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Møller P, Folkmann JK, Forchhammer L,
Bräuner EV, Danielsen PH, Risom L and Loft S: Air pollution,
oxidative damage to DNA, and carcinogenesis. Cancer Lett.
266:84–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hawthorn L, Stein L, Panzarella J, Loewen
GM and Baumann H: Characterization of cell-type specific profiles
in tissues and isolated cells from squamous cell carcinomas of the
lung. Lung Cancer. 53:129–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azad N, Rojanasakul Y and Vallyathan V:
Inflammation and lung cancer: Roles of reactive oxygen/nitrogen
species. J Toxicol Environ Health B Crit Rev. 11:1–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dillon RL, White DE and Muller WJ: The
phosphatidyl inositol 3-kinase signaling network: Implications for
human breast cancer. Oncogene. 26:1338–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gustafson AM, Soldi R, Anderlind C,
Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu
G, et al: Airway PI3K pathway activation is an early and reversible
event in lung cancer development. Sci Transl Med. 2:26ra252010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fry MJ: Phosphoinositide 3-kinase
signalling in breast cancer: How big a role might it play? Breast
Cancer Res. 3:304–312. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martelli AM, Evangelisti C, Chappell W,
Abrams SL, Bäsecke J, Stivala F, Donia M, Fagone P, Nicoletti F,
Libra M, et al: Targeting the translational apparatus to improve
leukemia therapy: Roles of the PI3K/PTEN/Akt/mTOR pathway.
Leukemia. 25:1064–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakao A, Toyoda Y, Sharma P, Evans M and
Guthrie N: Effectiveness of hydrogen rich water on antioxidant
status of subjects with potential metabolic syndrome-an open label
pilot study. J Clin Biochem Nutr. 46:140–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchholz BM, Kaczorowski DJ, Sugimoto R,
Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ and Nakao A:
Hydrogen inhalation ameliorates oxidative stress in transplantation
induced intestinal graft injury. Am J Transplant. 8:2015–2024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohta S: Molecular hydrogen is a novel
antioxidant to efficiently reduce oxidative stress with potential
for the improvement of mitochondrial diseases. Biochim Biophys
Acta. 1820:586–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takaki A, Kawai D and Yamamoto K: Multiple
hits, including oxidative stress, as pathogenesis and treatment
target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci.
14:20704–20728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desai UN, Shah KP, Mirza SH, Panchal DK,
Parikh SK and Rawal RM: Enhancement of the cytotoxic effects of
Cytarabine in synergism with Hesperidine and Silibinin in Acute
Myeloid Leukemia: An in-vitro approach. J Cancer Res Ther.
11:352–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barreiro E, Fermoselle C, Mateu-Jimenez M,
Sánchez-Font A, Pijuan L, Gea J and Curull V: Oxidative stress and
inflammation in the normal airways and blood of patients with lung
cancer and COPD. Free Radic Biol Med. 65:859–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khansari N, Shakiba Y and Mahmoudi M:
Chronic inflammation and oxidative stress as a major cause of
age-related diseases and cancer. Recent Pat Inflamm Allergy Drug
Discov. 3:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glasauer A and Chandel NS: Targeting
antioxidants for cancer therapy. Biochem Pharmacol. 92:90–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ames BN and Gold LS: Animal cancer tests
and cancer prevention. J Natl Cancer Inst Monogr. 125–132.
1992.PubMed/NCBI
|
|
34
|
Guyton KZ and Kensler TW: Oxidative
mechanisms in carcinogenesis. Br Med Bull. 49:523–544. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schulte-Hermann R, Timmermann-Trosiener I,
Barthel G and Bursch W: DNA synthesis, apoptosis, and phenotypic
expression as determinants of growth of altered foci in rat liver
during phenobarbital promotion. Cancer Res. 50:5127–5135.
1990.PubMed/NCBI
|
|
36
|
Klaunig JE, Xu Y, Isenberg JS, Bachowski
S, Kolaja KL, Jiang J, Stevenson DE and Walborg EF Jr: The role of
oxidative stress in chemical carcinogenesis. Environ Health
Perspect. 106 Suppl 1:S289–S295. 1998. View Article : Google Scholar
|
|
37
|
Strak M, Janssen NA, Godri KJ, Gosens I,
Mudway IS, Cassee FR, Lebret E, Kelly FJ, Harrison RM, Brunekreef
B, et al: Respiratory health effects of airborne particulate
matter: The role of particle size, composition, and oxidative
potential-the RAPTES project. Environ Health Perspect.
120:1183–1189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wood KC and Gladwin MT: The hydrogen
highway to reperfusion therapy. Nat Med. 13:673–674. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen H, Sun YP, Li Y, Liu WW, Xiang HG,
Fan LY, Sun Q, Xu XY, Cai JM, Ruan CP, et al: Hydrogen-rich saline
ameliorates the severity of l-arginine-induced acute pancreatitis
in rats. Biochem Biophys Res Commun. 393:308–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen HG, Xie KL, Han HZ, Wang WN, Liu DQ,
Wang GL and Yu YH: Heme oxygenase-1 mediates the anti-inflammatory
effect of molecular hydrogen in LPS-stimulated RAW 264.7
macrophages. Int J Surg. 11:1060–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen
H, Han H, Hou L, Gong G and Wang G: Molecular hydrogen ameliorates
lipopolysaccharide-induced acute lung injury in mice through
reducing inflammation and apoptosis. Shock. 37:548–555.
2012.PubMed/NCBI
|
|
42
|
Kajiyama S, Hasegawa G, Asano M, Hosoda H,
Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M, et al:
Supplementation of hydrogen-rich water improves lipid and glucose
metabolism in patients with type 2 diabetes or impaired glucose
tolerance. Nutr Res. 28:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang CS, Kawamura T, Toyoda Y and Nakao
A: Recent advances in hydrogen research as a therapeutic medical
gas. Free Radic Res. 44:971–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao
YP and Sun XJ: Hydrogen-rich saline reduces oxidative stress and
inflammation by inhibit of JNK and NF-κB activation in a rat model
of amyloid-beta-induced Alzheimer's disease. Neurosci Lett.
491:127–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sass G, Leukel P, Schmitz V, Raskopf E,
Ocker M, Neureiter D, Meissnitzer M, Tasika E, Tannapfel A and
Tiegs G: Inhibition of heme oxygenase 1 expression by small
interfering RNA decreases orthotopic tumor growth in livers of
mice. Int J Cancer. 123:1269–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nitti M, Piras S, Marinari UM, Moretta L,
Pronzato MA and Furfaro AL: HO-1 induction in cancer progression: A
matter of cell adaptation. Antioxidants (Basel). 6:pii: E29.
2017.PubMed/NCBI
|
|
47
|
Lien GS, Wu MS, Bien MY, Chen CH, Lin CH
and Chen BC: Epidermal growth factor stimulates nuclear factor-κB
activation and heme oxygenase-1 expression via c-Src, NADPH
oxidase, PI3K, and Akt in human colon cancer cells. PLoS One.
9:e1048912014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Was H, Dulak J and Jozkowicz A: Heme
oxygenase-1 in tumor biology and therapy. Curr Drug Targets.
11:1551–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goodman AI, Choudhury M, da Silva JL,
Schwartzman ML and Abraham NG: Overexpression of the heme oxygenase
gene in renal cell carcinoma. Proc Soc Exp Biol Med. 214:54–61.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schmidt M, Hövelmann S and Beckers TL: A
novel form of constitutively active farnesylated Akt1 prevents
mammary epithelial cells from anoikis and suppresses
chemotherapy-induced apoptosis. Br J Cancer. 87:924–932. 2002.
View Article : Google Scholar : PubMed/NCBI
|