Introduction
Abdominal aortic aneurysm (AAA) is the most common
true aneurysm, with a high tendency of rupture following
occurrence, resulting in its being classified as a serious threat
to human health (1). Since the
formation of this condition is a typically long process, for the
majority of AAA cases, there are no obvious clinical symptoms at
the early stage, and abdominal aortic aneurysm is finally diagnosed
at the early stage of arterial expansion (2). From the perspective of aneurysm
accumulation range, almost all the AAA cases are associated with
infrarenal aorta, and only 5% involve the renal aorta (2,3). A
total of 25% of the AAA cases may be the invasion of the medullary
artery, however isolated skeletal aneurysm is relatively rare, and
isolated suprarenal AAA cases are rarer, unless the invasion of the
thoracic aorta or renal abdominal aorta results in chest AAA
(4).
Interleukin (IL)-6 is a primary multifunctional
pro-inflammatory cytokine, secreted by a variety of cell types,
including T lymphocytes, B lymphocytes, monocytes, epithelial cells
and certain tumor cells, involved in inflammation and the immune
response, which affect cell survival, proliferation and apoptosis
(5). It results in neutrophil
infiltration by regulating the expression of chemokines and
adhesion molecules and recruiting mononuclear macrophage
accumulation to release a large number of cytokines and growth
factors to maintain the continuity of inflammation and to promote
the development of multiple tumors, including the growth of
neuroblastoma, cervical cancer and angiogenesis (6).
It has previously been demonstrated that the effect
of IL-6 is associated with various signaling pathways, particularly
the signal transducer and activator of transcription (STAT)
signaling pathway (6). This is the
primary pathway that results in IL-6 promoting various biological
effects, including local tumor inflammation, angiogenesis and
expression of genes that have an impact on cell cycle, and in the
family of STAT transcription factors, STAT3 is the most important
(7). STAT3 transcription factor is
an oncogene, which exhibits an important role in the regulation of
inflammation and the immune response, and it is highly expressed
and aberrantly activated in breast, colon and prostate cancers, AAA
and other tumors (8). The
persistent activation of STAT3 is closely associated with tumor
formation, and promotion of tumor cell proliferation, angiogenesis,
invasion and metastasis, therefore inhibiting the expression and
activation of STAT3 may inhibit tumor growth significantly
(9). Previous studies have
demonstrated that there is an interaction between STAT3 and nuclear
factor (NF)-κB signaling pathways, which jointly promote tumor
development (8,10). NF-κB is a key molecule in
regulating inflammation and immune responses, is a hub for the
regulation of inflammatory gene expression, and also a cancer gene
expressed highly in multiple tumors and activated abnormally to
regulate cell growth, apoptosis, angiogenesis, invasion and
metastasis (11).
Embelin is the primary component of white acid vine
fruit (12). This plant is
traditionally used as the first-line anti-inflammatory drug to
relieve rheumatism and fever. Relevant research revealed that
embelin also has antioxidant, hepato-protective, antibacterial,
anti-diabetic, and anti-inflammatory effects in other organs
(12,13). Embelin additionally blocks the
NF-ΚB signaling pathway that is the key protein associated with the
ischemia-reperfusion injury-induced inflammatory response (14). The aim of the present study was to
elucidate how embelin inhibits AAA and its underlying molecular
mechanism.
Materials and methods
Animals, animal groups and Angiotensin
II-induced AAA in mice
All animal experiment operations were conducted
according to nursing and use guidance for animal experiment
operations of National Institutes of Health in Heilongjiang
Provincial Hospital (15). Male,
C57BL/6 mice (20–22 g; 6 weeks old, n=38) were purchased from
Animal laboratory of Harbin Medical University (Harbin,
Heilongjiang, China) and were raised in a laboratory animal room
with a 12 h light/dark cycle at 24±2°C, 50–60% humidity, and free
access to food and water. All mice were randomly distributed into
sham group (n=6), AAA model group (n=8) and low embelin (25 mg/kg,
n=8), medium embelin (50 mg/kg, n=8) and high embelin (100 mg/kg,
n=8) treatment groups. AAA model mice were induced by chronic
infusion of 1,000 ng/kg/min Angiotensin II using mini-osmotic pumps
(cat. no. 1004, Alzet; Durect Corporation, Cupertino, CA, USA). AAA
model mice were gavaged with normal saline, and embelin treatment
groups were gavaged with 25, 50 and 100 mg/kg of Embelin. Following
28 days, mice were sacrificed. The experiments were approved by the
Animal Ethical and Welfare Committee of the First Hospital of
Qiqiha'er (Qiqiha'er, China).
Toluidine blue staining
Mice were anesthetized using 50 mg/kg of sodium
pentobarbital (intraperitoneally) and the aortas were immediately
separated and washed with PBS. Samples were perfused with 4%
paraformaldehyde for 30 min at room temperature. Samples were
embedded using paraffin and cut into 5–6 µm sections. Sections were
deparaffinized and rehydrated in a descending ethanol series and
Tissue-Clear® (Sakura Finetek UK Ltd, Thatcham,
England). Then, sections were stained in toluidine blue working
solution for 15 min at room temperature and dehydrated using
several degradations of ethanol. Samples were normalized to aortic
vessel wall area (mm2) and total numbers per aorta since
they were few in number.
ELISA analysis
Aortic tissues from mice treated with or without
embelin were acquired, and expression levels tumor necrosis factor
(TNF)-α, interleukin (IL)-1β, IL-6, IL-18, in addition to
superoxide dismutase (SOD), malondialdehyde (MDA) activities, and
monocyte chemoattractant protein (MCP)-2 and epithelial
neutrophil-activating peptide (CXCL5) secretions were measured.
Aortic tissues (10 mg) were homogenized with
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) and centrifuged at
14,000 × g at 4°C for 10 min to collect protein extract. TNF-α
(E-EL-M0049c), IL-1β (E-EL-M0037c), IL-6 (E-EL-M0044c), IL-18
(E-EL-M0730c), SOD (E-EL-M2398c), MDA (E-EL-0060c), GSH
(E-EL-0026c), GSH-PX (E-EL-M0950c), MCP-2 (E-EL-H1158c) and CXCL5
(E-EL-M0471c) activities were evaluated using ELISA kits (all from
Elabscience Biotechnology Co., Ltd. Wuhan, China).
Western blot analysis
Aortic tissues (50 mg) was homogenized with RIPA
lysis buffer (Beyotime Institute of Biotechnology) and centrifuged
at 14,000 × g at 4°C for 10 min to collect protein extract. Protein
was quantitated with BCA assay (Beyotime Institute of
Biotechnology) and 50 µg protein was separated on 10% SDS-PAGE gels
and blotted onto a nitrocellulose membrane. Membranes were blocked
using 5% skimmed milk powder in Tris buffered Tween-20 and
incubated with primary antibodies against matrix metallopeptidase
(MMP)-9 (13667; 1:2,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), phosphorylated (p)-STAT3 (9145; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), p-p38 (4511; 1:1,000; Cell
Signaling Technology, Inc.), NF-κB (8242; 1:2,000; Cell Signaling
Technology, Inc.) and GAPDH (AF1186; 1:5,000; Beyotime Institute of
Biotechnology) at 4°C for 12–16 h. Anti-rabbit IgG conjugated to
horseradish peroxidase secondary antibody (7074; 1:5,000; Cell
Signaling Technology, Inc.) was incubated with the membranes at
37°C for 1 h. Membranes were visualized using enhanced
chemiluminescence Prime Western blotting reagent (GE Healthcare
Life Sciences) and ImageLab version 3.0 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used for densitometry.
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Multigroup
comparisons were assessed using one-way analysis of variance and
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
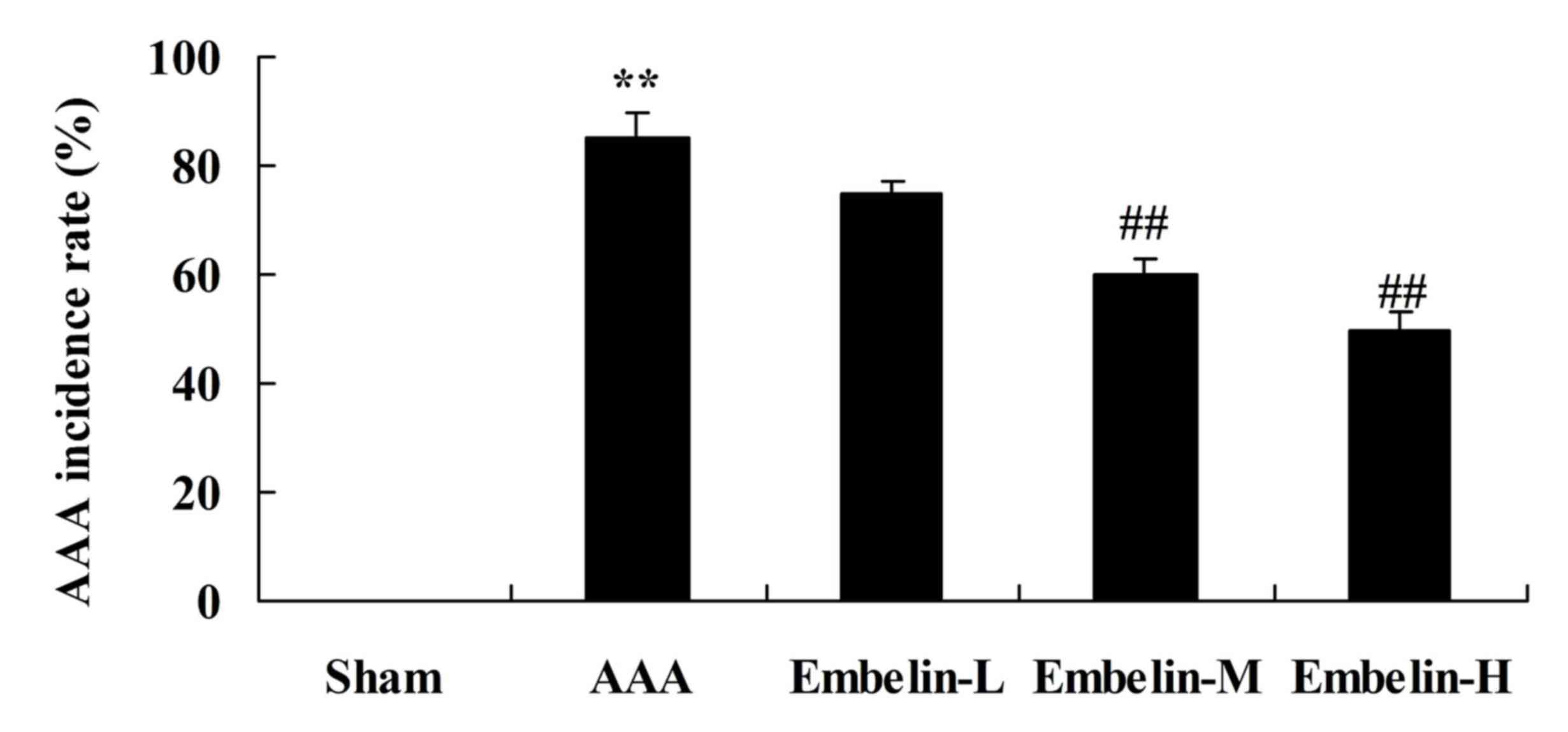
Embelin inhibits AAA incidence rate in
Angiotensin II infused mice
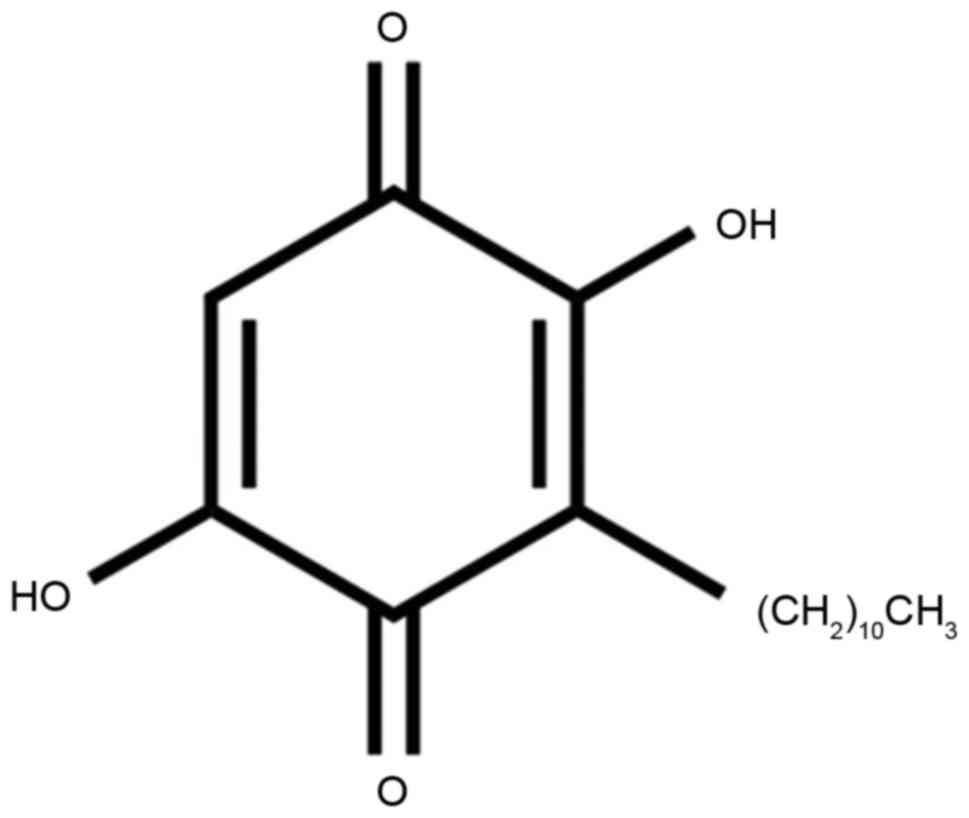
The present study first examined the effect of
embelin (Fig. 1) on AAA incidence
rate in mice. It was demonstrated that that AAA incidence rate was
markedly increased in the AAA model group, compared with sham group
(Fig. 2). Treatment with embelin
significantly inhibited the AAA incidence rate in mice compared
with the AAA model group (Fig.
2).
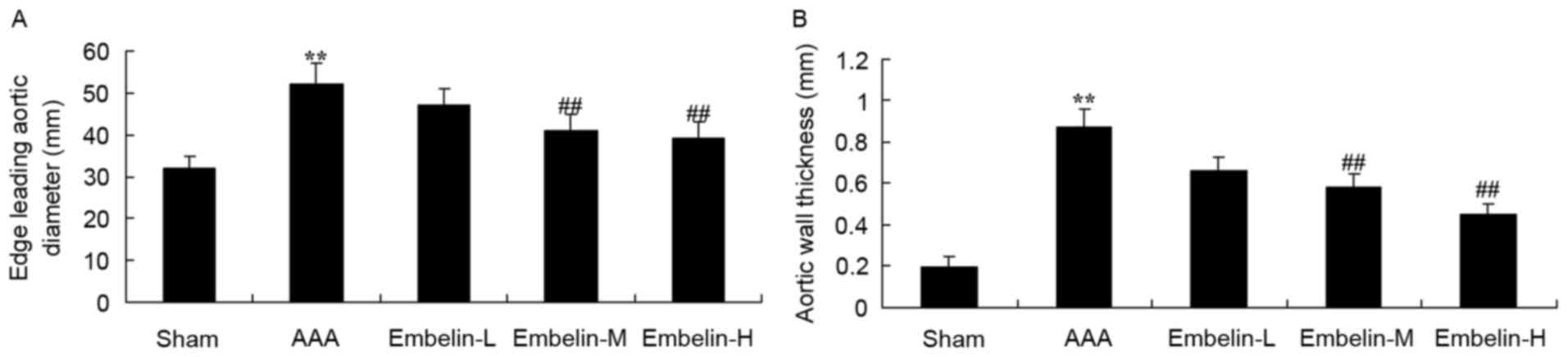
Embelin inhibits vascular remodeling
in Angiotensin II infused mice
Next, the effect of embelin was examined on
AAA-induce vascular remodeling. As presented in Fig. 3, the thickness of edge leading
aortic diameter and aortic wall thickness in the AAA model group
were significantly promoted, compared with sham group. Treatment
with embelin significantly inhibited edge leading aortic diameter
and aortic wall thickness in AAA mice, compared with AAA model
group (Fig. 3).
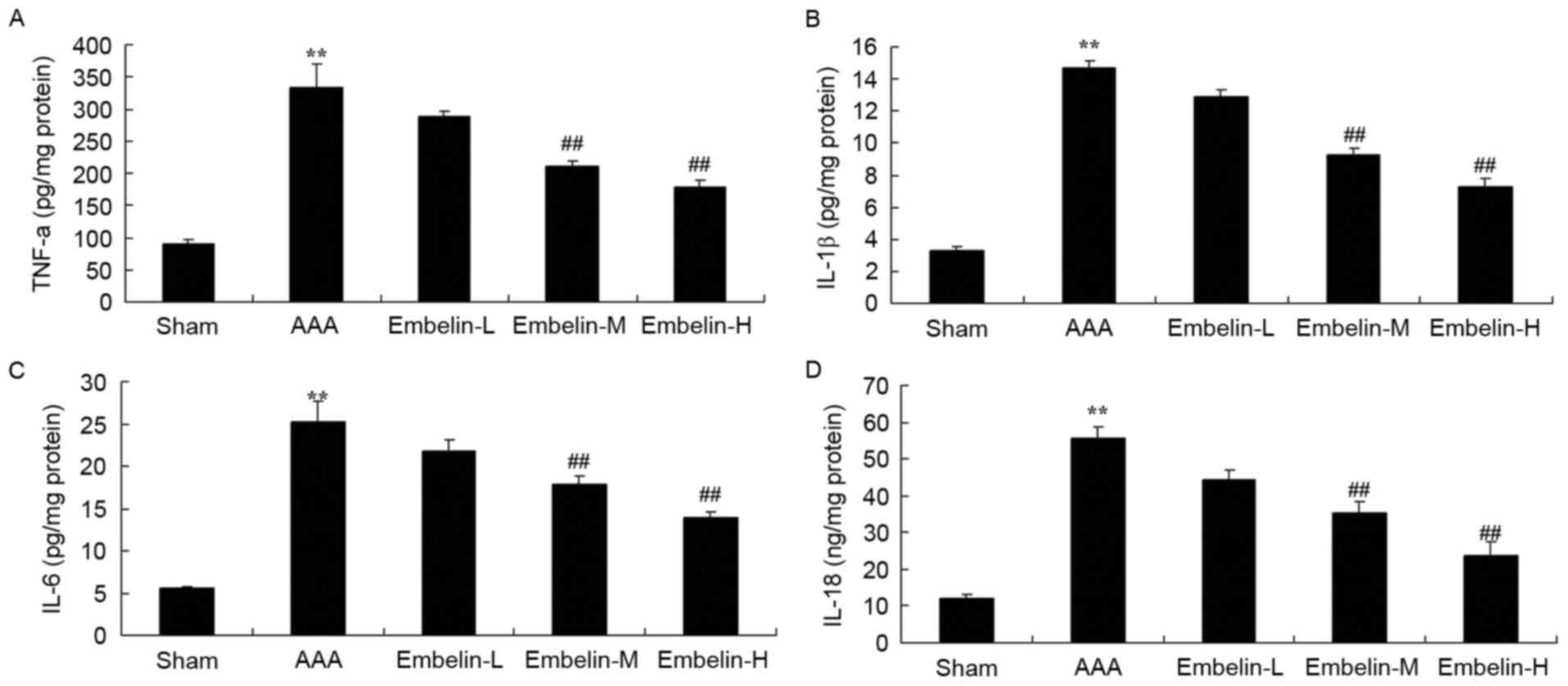
Embelin inhibits inflammatory reaction
in Angiotensin II infused mice
The present study analyzed the anti-inflammatory
effect of embelin in Angiotensin II infused mice. In Angiontensin
II-induced AAA mice, TNF-α, IL-1β, IL-6 and IL-18 expression levels
were significantly increased compared with control group (Fig. 4). Notably, treatment with embelin
suppressed TNF-α, IL-1β, IL-6 and IL-18 levels in AAA mice compared
with AAA model group (Fig. 4).
Embelin inhibits oxidative stress in
Angiotensin II infused mice
The present study next investigated the effects of
embelin on oxidative stress injury in Angiotensin II infused mice.
It was observed that SOD, GSH and GSH-Px level activities were
markedly decreased and MDA level activities in model group were
markedly increased compared with sham group (Fig. 5). Embelin treatment significantly
increased SOD, GSH and GSH-PX level activities and inhibited MDA
level activities in AAA mice compared with AAA model group
(Fig. 5).
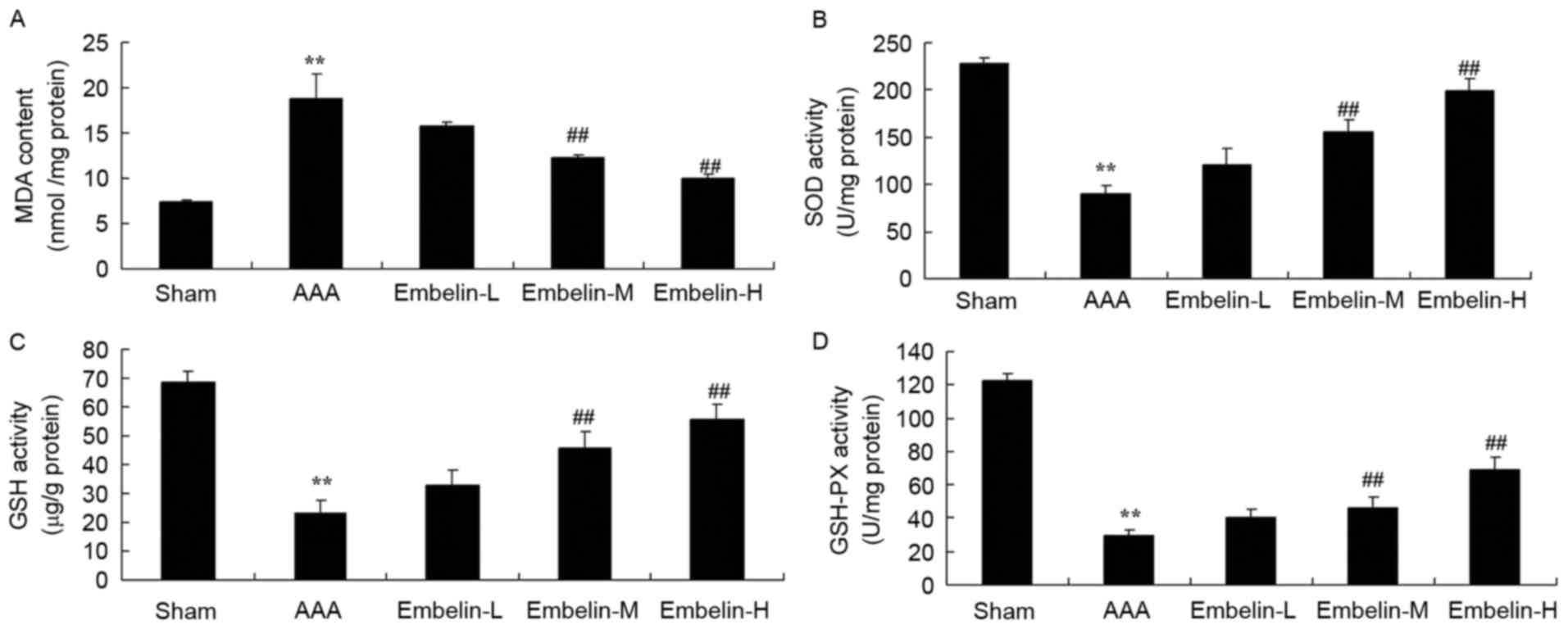 | Figure 5.Embelin inhibits oxidative stress in
Angiotensin II infused mice. Embelin inhibited (A) MDA and
increased (B) SOD, (C) GSH and (D) GSH-PX in Angiotensin II infused
mice. **P<0.01 vs. sham group; ##P<0.01 vs. AAA model group.
Sham, sham group; AAA, abdominal aortic aneurysm model group;
Embelin-L, low embelin treatment group (25 mg/kg); Embelin-M,
medium embelin treatment group (50 mg/kg); Embelin-H, high embelin
treatment group (100 mg/kg); MDA, malondialdehyde; SOD, superoxide
dismutase; GSH, glutathione; GSH-PX, glutathione peroxidase. |
Embelin inhibits MMP-9 and p-STAT3
protein expression in Angiotensin II infused mice
It has previously been demonstrated that MMP-9
regulates inflammatory factors in AAA model mice, therefore the
present study investigated the effect of embelin on MMP-9 and
p-STAT3 protein expression. As presented in Fig. 6, there was a significant increase
of MMP-9 protein expression in the AAA model group, compared with
the sham group. In AAA mice, embelin treatment significantly
suppressed the MMP-9 and p-STAT3 protein expression levels,
compared with the AAA model group (Fig. 6).
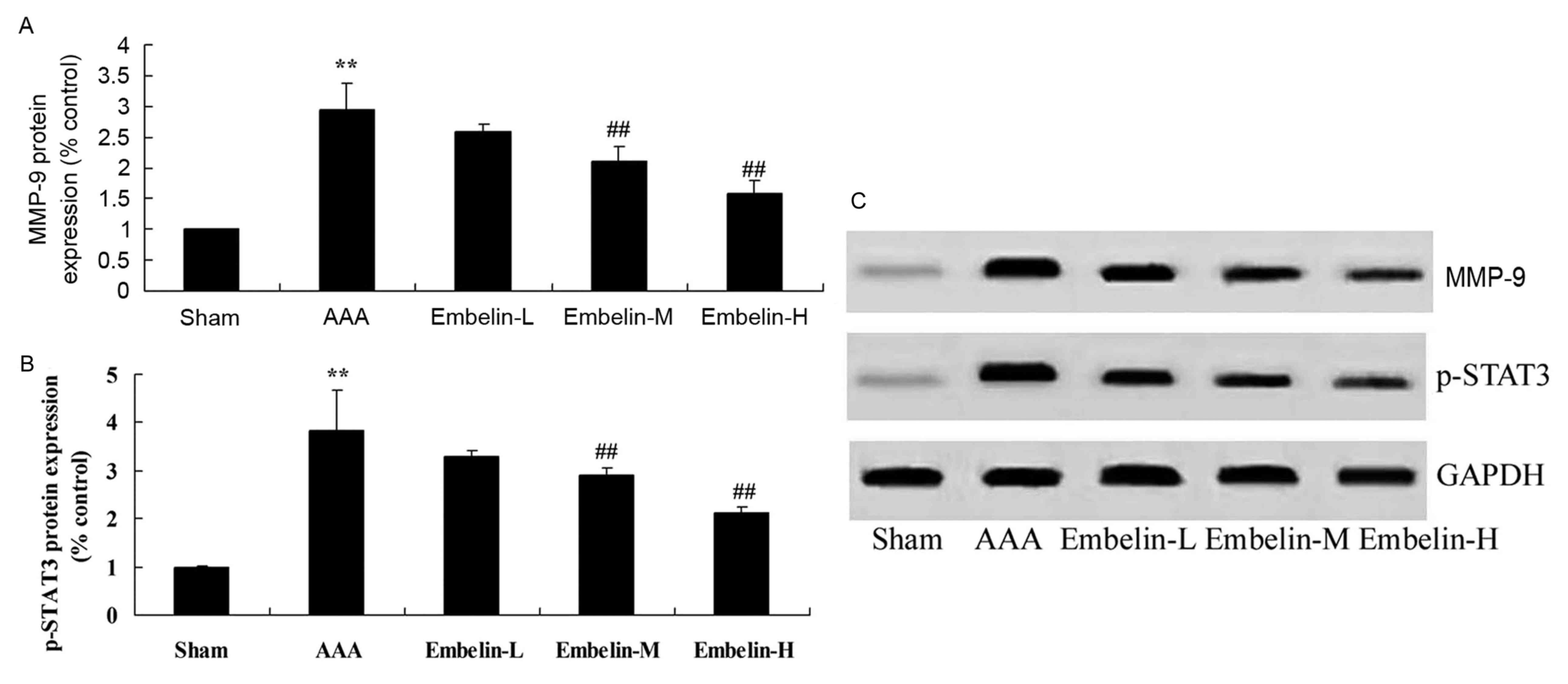 | Figure 6.Embelin inhibits MMP-9 and p-STAT3
protein expression in Angiotensin II infused mice. (A and B)
Quantitative analysis and (C) representative image of MMP-9 and
p-STAT3 protein expression levels, detected by western blotting
assay, in Angiotensin II infused mice. **P<0.01 vs. sham group;
##P<0.01 vs. AAA model group. Sham, sham group; AAA, abdominal
aortic aneurysm model group; Embelin-L, low embelin treatment group
(25 mg/kg); Embelin-M, medium embelin treatment group (50 mg/kg);
Embelin-H, high embelin treatment group (100 mg/kg); MMP-9, matrix
metallopeptidase-9; p, phosphorylated; STAT3, signal transducer and
activator of transcription 3. |
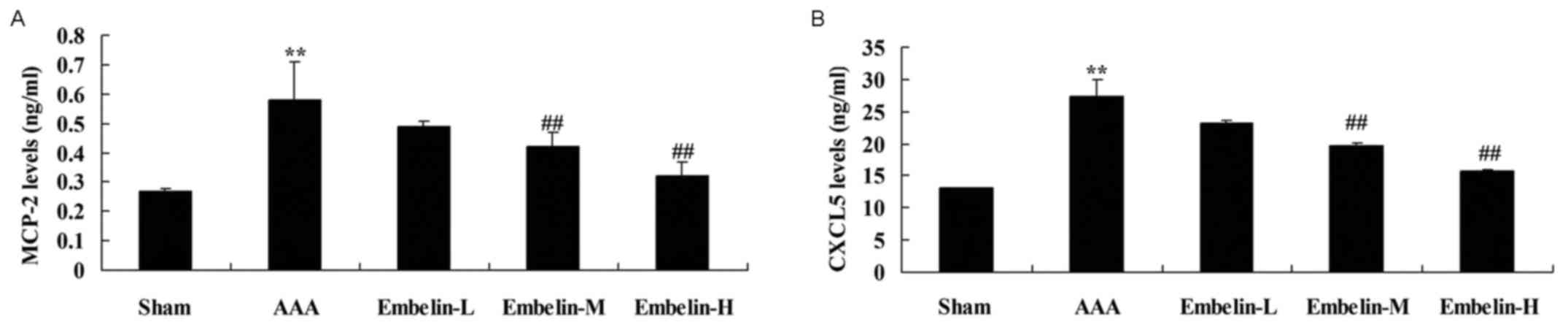
Embelin inhibits MCP-2 activity and
CXCL5 levels in Angiotensin II infused mice
In order to identify MCP-2 activity and CXCL5 levels
in Angiotensin II infused mice, the present analyzed the effect of
embelin these factors in the AAA model group. MCP-2 activity and
CXCL5 levels were significantly increased in AAA model mice,
compared with sham group (Fig. 7).
Treatment with embelin significantly inhibited MCP-2 activity and
CXCL5 levels in AAA model mice, compared with AAA model group
(Fig. 7).
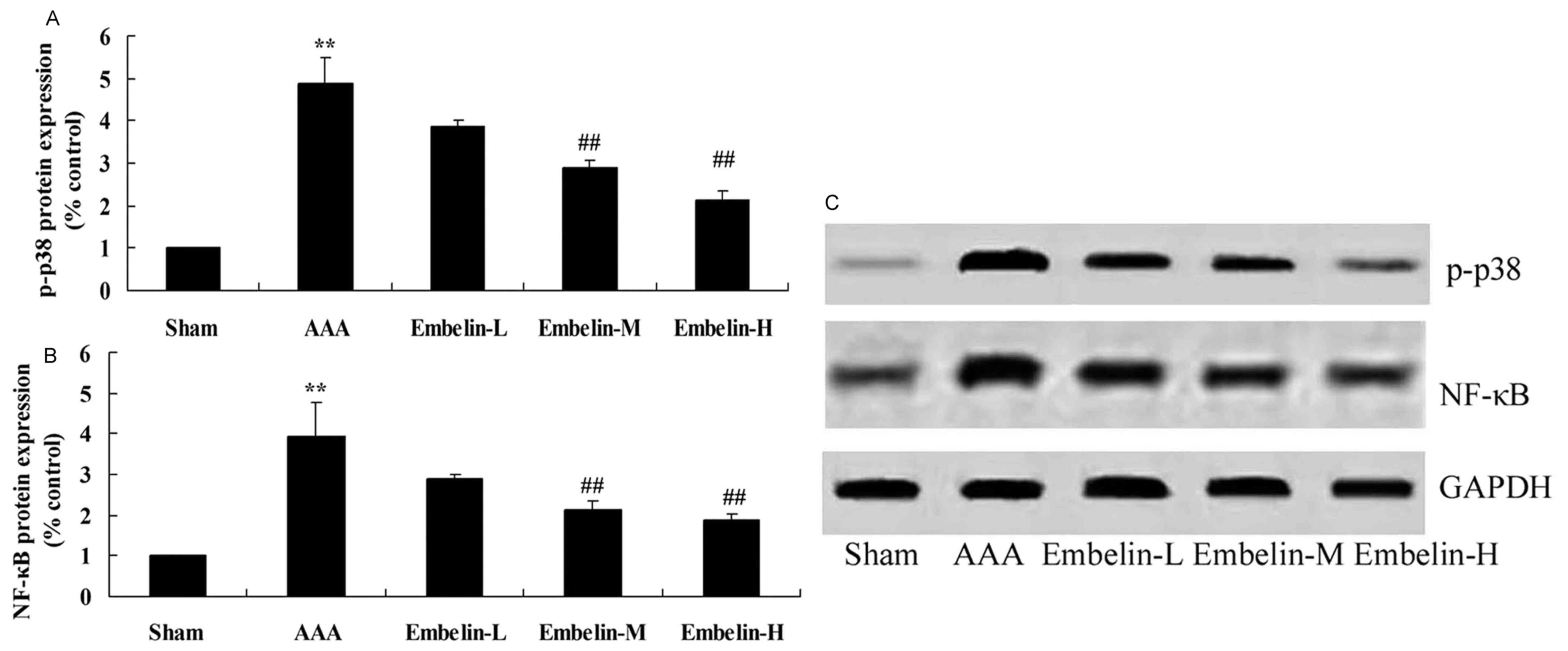
Embelin inhibits p-p38 and NF-κB
protein expression in Angiotensin II infused mice
The anti-inflammation mechanism of embelin was
investigated in AAA model mice, via p-p38 and NF-κB protein
expression quantification with western blot analysis. As presented
in Fig. 8 it was observed that
p-p38 and NF-κB protein expression in AAA model group was notably
increased compared with sham group. Compared with AAA model group,
p-p38 and NF-κB protein expression levels were significantly
suppressed in AAA mice treated with embelin (Fig. 8).
Discussion
There is an extensive difference in AAA prevalence
in different regions, with increased European and American
populations presenting with the condition compared with in Africa
and Asia (16). In 2010, Danish
research results revealed that the AAA prevalence rate was ~4.0%; a
large-scale conducted among 310 million people in the United States
in the same year revealed that the AAA prevalence rate was ~1.4%;
two large screening studies in Australia with the interval of 9
years indicated that the rates were 4.0 and 7.2%, respectively
(16). In Asian countries, a
hospital-based study that started in South Korea demonstrated that
AAA prevalence rate was 0.43% in 2009, significantly lower compared
with the European population, which was similar to the results
obtained from the screening program carried out in Japan in 2000
(17,18). In the present study, it was
demonstrated that embelin significantly inhibited the AAA incidence
rate, and decreased edge leading aortic diameter and aortic wall
thickness in AAA mice.
Lymphocytes and mononuclear cell infiltration
indicated that the autoimmune reaction is important in the
formation of AAA (19). Infection
is one of the factors for AAA formation, and it has been reported
that 55% of AAA patients suffer from mycoplasma pneumonia (20). In the formation of AAA, the active
oxygen species and antioxidants also exhibit an important role
(21). The superoxide level in AAA
tissues exhibits a 2.5-fold increase compared with the neighboring
non-aneurysmal tissue, and a 10-fold increase compared with in
normal tissue (22). A total of
two days following the injection of aggressive porcine pancreatic
elastase in the arterial wall, inducible nitric oxide synthase
exhibits a 50-fold increase. Compared with the control saline
group, 10 days following the injection of elastase in arterial
wall, the expression of the antioxidant SOD exhibits a 20-fold
decrease (22). In vitro
studies demonstrate that the active oxygen species may activate
MMP. Therefore, the imbalance of promotion of the oxidation gene
expression is important in human AAA and experimental aneurysm.
Reactive oxygen species also affect apoptosis and promote the
formation of AAA (23). In the
present study, it was demonstrated that embelin treatment
significantly increased SOD, GSH and GSH-PH and inhibited MDA
activities in AAA mice.
The incidence of AAA is quite a complex process,
which is associated with various factors. The AAA lesion structure
is characterized by the damage of elastin and collagen in the
middle wall and outer membrane. Smooth muscle cell degradation
resulted in the thinning of the middle layer, and leads to
lymphocyte and macrophage infiltration (22). The significant histological feature
of AAA is the extensive infiltration of lymphocytes and macrophages
in the vessel wall, which leads to generation of cytokines
(including interleukins, TNF, immunoreactive fibronectin) and
immunoglobulin increase in the aneurysm wall, and these cytokines
may lead to the activation of various proteases (24). Although the initiating factors of
white blood cell infiltration and transfer are not entirely clear,
the denaturation product of elastic protein in the exposed artery
walls may be used as a chemokine for macrophage infiltration
(25).
Various factors, including IL-6, IL-8 and
granulocyte chemotactic protein 2, may collect and activate various
inflammatory cells, particularly neutrophils, thereby mediating
inflammation, so as to affect infections, autoimmune diseases and
pathological process of tumors (26). STAT3 is an important nuclear
transcription factor, the gene of which is located in chromosome 12
(27). STAT3 is widely expressed
in various cells and tissues and is involved in the regulation of
cell growth and differentiation, proliferation, apoptosis and other
physiological functions (28). It
was observed that embelin suppressed TNF-α, IL-1β, IL-6 and IL-18
expression levels in AAA mice.
Currently, a large number of basic and clinical
studies have verified that MMP-9 has an important role in the
development process of AAA (29).
AAA may lead to an increase of MMP-9 content in plasma and tumor
wall tissue, however MMP-9 is a reliable indicator for judging AAA
activity, which may be associated with the size of the AAA
(29). The role of MMP-9 is not a
static, however a dynamic process. The research and study regarding
regulatory factors for the expression of MMP-9 activity and
activation process, and the exploration of specific mechanisms,
will lay the foundation for further understanding regarding AAA
mechanism, drug treatment and prevention (30,31).
The results of the present study suggested that embelin treatment
significantly suppressed the protein expression levels of MMP-9 in
AAA mice.
CXCL5, additionally termed epithelial-derived
neutrophil activating peptide 78, is a member of the CXC chemokine
family (32). CXCL5 has strong
chemotaxis for inflammatory cells (including granulocytes and
myeloid-derived suppressor cells) and a pro-angiogenesis effect,
with an important role in the formation of the tumor
microenvironment of inflammation, and is involved in tumor growth,
invasion and metastasis (33). It
has previously been demonstrated that CXCL5 is expressed in
non-tumor tissues, including stomach disease, gastric mucosa,
endometrial glands pulmonary fibrosis, inflammatory bowel disease,
liver fibrosis and cirrhosis, which are associated with
inflammatory damage; whereas in tumor tissues, including non-small
cell lung, stomach, endometrial, prostate and pancreatic cancers,
CXCL5 has a significantly increased expression compared with
non-cancerous tissue (34,35). CXCL5 may promote the development,
angiogenesis and metastasis of non-small cell lung, stomach and
endometrial cancers, in addition to AAA. CXCL5 is positively
correlated with pathological grade, malignant degree and clinical
stage of prostate cancer; high expression of CXCL5 also indicates
that the patient has a poor prognosis (36). The results of the present study
suggested that embelin significantly inhibited MCP-2 activity and
CXCL5 levels in AAA model mice.
STAT3 gene is typically associated with the
inflammatory response, and is located in the human chromosome 17
(q21) (37). Transcription factors
NF-κB and STAT3 exhibit a key role in the process of cancer
development promoted by inflammation. The stimulation and
activation of NF-κB and STAT3 via inflammation may regulate
transcription and expression of numerous genes, including those
participating in the immune response, inflammation, cell
proliferation and apoptosis (38).
Under normal physiological conditions, the activation of NF-κB and
STAT3 are strictly controlled, however in a variety of tumors, this
control is destroyed with the appearance of abnormal activation,
and regulation of the expression levels of a large number of genes
conducive to tumor growth instead occurs (39). The present study observed that
embelin significantly suppressed p-STAT3 protein expression in AAA
mice. Dai et al (40).
Demonstrated that embelin suppresses colitis-associated cancer by
limiting IL-6/STAT3 activation and the T helper cell 17 immune
response (37).
NF-κB links inflammation with cancer via the
activation of the upstream molecule IKKP, which promotes the
development of inflammation-associated tumors. Selectively
inhibiting NF-κB activation, will decrease rate of tumor
development (41). It has been
demonstrated that STAT3 and NF-κB have very important roles in the
tumor development process promoted by inflammation. Blocking the
activation of STAT3 and NF-κB signaling pathways via inflammatory
cytokines will inhibit the local inflammatory response, and will
result in the inhibition of the occurrence and development of
inflammation-associated cancers, and provide a novel strategy with
which to treat cancer (42,43).
The results of the present study demonstrated that embelin
suppressed p-p38 and NF-κB protein expression in AAA mice. Xu et
al (14) suggested that
embelin induces apoptosis through inhibition of p38 MAPK and NF-κB
signaling pathways in human gastric carcinoma.
In conclusion, these data suggest that embelin
inhibited the AAA incidence rate and decreased edge leading aortic
diameter and aortic wall thickness in AAA mice via
anti-inflammatory and anti-oxidation effects. Targeting
IL-6-induced STAT3 and inactivation of NF-κB will provide greater
therapeutic potential in the treatment of AAA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QW designed the experiment, QL and HL performed the
experiment, QL and QW analyzed the data, and QW wrote the
manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Animal Ethical
and Welfare Committee of the First Hospital of Qiqiha'er
(Qiqiha'er, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lal BK, Zhou W, Li Z, Kyriakides T,
Matsumura J, Lederle FA and Freischlag J: OVER Veterans Affairs
Cooperative Study Group: Predictors and outcomes of endoleaks in
the Veterans Affairs Open Versus Endovascular Repair (OVER) trial
of abdominal aortic aneurysms. J Vasc Surg. 62:1394–1404. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Li YS, Xu M, Wen SH, Yao X, Wu Y,
Huang CY, Huang WQ and Liu KX: Limb remote ischemic preconditioning
for intestinal and pulmonary protection during elective open
infrarenal abdominal aortic aneurysm repair: A randomized
controlled trial. Anesthesiology. 118:842–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Chen YX, Zhang B, Jiang YX, Liu
CW, Zhao RN, Wu Q and Zhang DM: Contrast-enhanced ultrasound in
detecting endoleaks with failed computed tomography angiography
diagnosis after endovascular abdominal aortic aneurysm repair. Chin
Med J (Engl). 128:2491–2497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karlsson L, Bergqvist D, Lindbäck J and
Pärsson H: Expansion of small-diameter abdominal aortic aneurysms
is not reflected by the release of inflammatory mediators IL-6,
MMP-9 and CRP in plasma. Eur J Vasc Endovasc Surg. 37:420–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones KG, Brull DJ, Brown LC, Sian M,
Greenhalgh RM, Humphries SE and Powell JT: Interleukin-6 (IL-6) and
the prognosis of abdominal aortic aneurysms. Circulation.
103:2260–2265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokje VBC, Gäbel G, Koole D, Northoff BH,
Holdt LM, Hamming JF and Lindeman JHN: IL-6: A Janus-like factor in
abdominal aortic aneurysm disease. Atherosclerosis. 251:139–146.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao M, Xu J, Clair AJ, Ehrman B, Graham
LM and Eagleton MJ: Local and systemic alterations in signal
transducers and activators of transcription (STAT) associated with
human abdominal aortic aneurysms. J Surg Res. 176:321–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YH, Yang HY, Huang SW, Ou G, Hsu YF
and Hsu MJ: Interleukin-6 induces vascular endothelial growth
factor-C expression via Src-FAK-STAT3 signaling in lymphatic
endothelial cells. PLoS One. 11:e01588392016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bode JG, Albrecht U, Häussinger D,
Heinrich PC and Schaper F: Hepatic acute phase proteins-regulation
by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk
with NF-κB-dependent signaling. Eur J Cell Biol. 91:496–505. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He G and Karin M: NF-κB and STAT3-key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dharmapatni AA, Cantley MD, Marino V,
Perilli E, Crotti TN, Smith MD and Haynes DR: The X-linked
inhibitor of apoptosis protein inhibitor embelin suppresses
inflammation and bone erosion in collagen antibody induced
arthritis mice. Mediators Inflamm. 2015:5640422015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marsh JL, Jackman CP, Tang SN, Shankar S
and Srivastava RK: Embelin suppresses pancreatic cancer growth by
modulating tumor immune microenvironment. Front Biosci (Landmark
Ed). 19:113–125. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu CL, Zheng B, Pei JH, Shen SJ and Wang
JZ: Embelin induces apoptosis of human gastric carcinoma through
inhibition of p38 MAPK and NF-κB signaling pathways. Mol Med Rep.
14:307–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CK, Luo J, Lai KP, Wang R, Pang H,
Chang E, Yan C, Sparks J, Lee SO, Cho J and Chang C: Androgen
receptor promotes abdominal aortic aneurysm development via
modulating inflammatory interleukin-1α and transforming growth
factor-β1 expression. Hypertension. 66:881–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lederle FA, Freischlag JA, Kyriakides TC,
Matsumura JS, Padberg FT Jr, Kohler TR, Kougias P, Jean-Claude JM,
Cikrit DF and Swanson KM: OVER Veterans Affairs Cooperative Study
Group: Long-term comparison of endovascular and open repair of
abdominal aortic aneurysm. N Engl J Med. 367:1988–1997. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GJ and Carpenter JP: Endologix
Investigators: The powerlink system for endovascular abdominal
aortic aneurysm repair: Six-year results. J Vasc Surg. 48:535–545.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mahmoud KM and Ammar AS: effect of
N-acetylcysteine on cardiac injury and oxidative stress after
abdominal aortic aneurysm repair: A randomized controlled trial.
Acta Anaesthesiol Scand. 55:1015–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takagi H and Umemoto T: The association
between body mass index and abdominal aortic aneurysm growth: A
systematic review. Vasa. 45:119–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruggink JL, Tielliu IF, Zeebregts CJ and
Pol RA: Mesenteric ischemia after abdominal aortic aneurysm repair:
A systemic review. J Cardiovasc Surg (Torino). 55:759–765.
2014.PubMed/NCBI
|
|
21
|
Mussa FF: Screening for abdominal aortic
aneurysm. J Vasc Surg. 62:774–778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bergqvist D, Lindeman JH, Lindholt JS and
Björck M: Antimicrobial treatment to impair expansion of abdominal
aortic aneurysm (AAA): A systematic review of the clinical
evidence. Curr Vasc Pharmacol. 11:288–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honjo H, Kumagai Y, Ishiguro T, Imaizumi
H, Ono T, Suzuki O, Ito T, Haga N, Kuwabara K, Sobajima J, et al:
Heterotopic mesenteric ossification after a ruptured abdominal
aortic aneurism: Case report with a review of literatures. Int
Surg. 99:479–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sbarzaglia P, Grattoni C, Oshoala K,
Castriota F, D'Alessandro G and Cremonesi A: AorfixTM device for
abdominal aortic aneurysm with challenging anatomy. J Cardiovasc
Surg (Torino). 55:61–70. 2014.PubMed/NCBI
|
|
25
|
Khashram M, Hider PN, Williman JA, Jones
GT and Roake JA: Does the diameter of abdominal aortic aneurysm
influence late survival following abdominal aortic aneurysm repair?
A systematic review and meta-analysis. Vascular. 24:658–667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choudhury S, Gupta P, Ghosh S, Mukherjee
S, Chakraborty P, Chatterji U and Chattopadhyay S: Arsenic-induced
dose-dependent modulation of the NF-κB/IL-6 axis in thymocytes
triggers differential immune responses. Toxicology. 357–358:85–96.
2016. View Article : Google Scholar
|
|
27
|
Lin C, Wang L, Wang H, Yang L, Guo H and
Wang X: Tanshinone IIA inhibits breast cancer stem cells growth in
vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling
pathways. J Cell Biochem. 114:2061–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma W, Sze KM, Chan LK, Lee JM, Wei LL,
Wong CM, Lee TK, Wong CC and Ng IO: RhoE/ROCK2 regulates
chemoresistance through NF-κB/IL-6/ STAT3 signaling in
hepatocellular carcinoma. Oncotarget. 7:41445–41459.
2016.PubMed/NCBI
|
|
29
|
Duellman T, Warren CL, Peissig P, Wynn M
and Yang J: Matrix metalloproteinase-9 genotype as a potential
genetic marker for abdominal aortic aneurysm. Circ Cardiovasc
Genet. 5:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adovasio R, Calvagna C, Sgorlon G, Zamolo
F, Mearelli F, Biolo G, Grassi G and Fiotti N: Growth rate of small
abdominal aortic aneurysms and genetic polymorphisms of matrix
metalloProteases-1, −3 and −9. Int J Angiol. 25:93–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tazume H, Miyata K, Tian Z, Endo M,
Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T,
Nakashima Y, et al: Macrophage-derived angiopoietin-like protein 2
accelerates development of abdominal aortic aneurysm. Arterioscler
Thromb Vasc Biol. 32:1400–1409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LY, Tu YF, Lin YC and Huang CC: CXCL5
signaling is a shared pathway of neuroinflammation and blood-brain
barrier injury contributing to white matter injury in the immature
brain. J Neuroinflammation. 13:62016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Madalli S, Beyrau M, Whiteford J, Duchene
J, Nandhra Singh I, Patel NS, Motwani MP, Gilroy DW, Thiemermann C,
Nourshargh S and Scotland RS: Sex-specific regulation of chemokine
Cxcl5/6 controls neutrophil recruitment and tissue injury in acute
inflammatory states. Biol Sex Differ. 6:272015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu X, Qiao Y, Liu W, Wang W, Shen H, Lu
Y, Hao G, Zheng J and Tian Y: CXCL5 is a potential diagnostic and
prognostic marker for bladder cancer patients. Tumour Biol.
37:4569–4577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan
J, Wang X and Li L: CXCL5/CXCR2 axis promotes bladder cancer cell
migration and invasion by activating PI3K/AKT-induced upregulation
of MMP2/MMP9. Int J Oncol. 47:690–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song J, Wu C, Zhang X and Sorokin LM: In
vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2
and MMP-9 promotes early neutrophil recruitment in IL-1β-induced
peritonitis. J Immunol. 190:401–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang DJ, Chang YY, Lin HW, Chen YC, Hsu SH
and Lin JT: Inhibitory effect of litchi (Litchi chinensis Sonn.)
flower on lipopolysaccharide-induced expression of proinflammatory
mediators in RAW264.7 cells through NF-κB, ERK, and JAK2/STAT3
inactivation. J Agric Food Chem. 62:3458–3465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hendrayani SF, Al-Harbi B, Al-Ansari MM,
Silva G and Aboussekhra A: The inflammatory/cancer-related
IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains
the active state of breast myofibroblasts. Oncotarget.
7:41974–41985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsieh YJ, Tseng SP, Kuo YH, Cheng TL,
Chiang CY, Tzeng YM and Tsai WC: Ovatodiolide of anisomeles indica
exerts the anticancer potential on pancreatic cancer cell lines
through STAT3 and NF-κB regulation. Evid Based Complement Alternat
Med. 2016:86803722016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dai Y, Jiao H, Teng G, Wang W, Zhang R,
Wang Y, Hebbard L, George J and Qiao L: Embelin reduces
colitis-associated tumorigenesis through limiting IL-6/STAT3
signaling. Mol Cancer Ther. 13:1206–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu YF, Bai YQ and Qi M: Daidzein
attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and
TGF-β1 pathways. Mol Med Rep. 14:955–962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsai SH, Huang PH, Peng YJ, Chang WC, Tsai
HY, Leu HB, Chen JW and Lin SJ: Zoledronate attenuates angiotensin
II-induced abdominal aortic aneurysm through inactivation of
Rho/ROCK-dependent JNK and NF-κB pathway. Cardiovasc Res.
100:501–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu Q, Zeng K, Ma X, Song F, Jiang Y, Tu P
and Wang X: Resokaempferol-mediated anti-inflammatory effects on
activated macrophages via the inhibition of JAK2/STAT3, NF-κB and
JNK/p38 MAPK signaling pathways. Int Immunopharmacol. 38:104–114.
2016. View Article : Google Scholar : PubMed/NCBI
|