Introduction
Epidermal defense against the intrusion of harmful
substances from the environment, including chemicals and radiation,
acts as an important barrier to prevent skin injury (1,2).
Keratinocytes are the principal cells of the epidermis. In addition
to their barrier-serving role, these cells are involved in immune
responses within the skin; however, unperturbed keratinocytes
exhibit deficient or absent production of inflammatory mediators
(2). Under environmental or
chemical stimuli (for example, UV irradiation or ambient air
pollution), activated keratinocytes express numerous
inflammation-associated cytokines including TNF-α, interleukin
(IL)-1β, IL-8, and inducible nitric oxide synthase (iNOS), which
may result in the abnormal expression and dysregulated action of
inflammatory mediators (3,4). Activated keratinocytes may induce
strong infiltration of inflammatory cells in the epidermis, which
is associated with the underlying pathogenesis of inflammatory skin
diseases, including psoriasis, atopic dermatitis (AD) and allergic
contact dermatitis (5).
Increased expression of intercellular adhesion
molecule-1 (ICAM-1) may induce leukocyte and keratinocyte
interaction and is considered an important initiator in numerous
types of inflammatory skin diseases (6). It has been reported that tumor
necrosis factor-α (TNF-α)-induced ICAM-1 expression is a principal
mediator of increased lymphocyte infiltration into inflamed areas
in the skin. In addition, keratinocytes are activated by TNF-α,
eliciting an inflammatory response with the release of
proinflammatory cytokines and chemokines, including interleukin
(IL)-1β, IL-6, IL-8 and monocyte chemoattractant protein-1 (MCP-1),
in addition to ICAM-1 (7–9). Additionally, TNF-α-induced expression
of inflammatory mediators within keratinocytes simultaneously
activates inflammatory signaling pathways, nuclear factor-κB
(NF-κB) and mitogen-activated protein kinases (MAPKs), resulting in
aggravated inflammation (10).
Phosphorylation of inhibitor of NF-κB due to
inflammatory stimuli induces the translocation of the transcription
factor NF-κB to the nucleus and activates the transcription of
proinflammatory genes (11,12).
Extracellular signal-regulated kinase (ERK), c-Jun-N-terminal
kinase (JNK), and p38 MAPK are members of the MAPK family (12). Activation of MAPKs has also been
associated with the production of inflammatory mediators and the
regulation of numerous inflammation-associated genes (9,12).
The role of inflammation is to protect against harmful stimuli;
however, long-term and excessive inflammation may cause severe
tissue damage, which may lead to the development of
inflammation-associated diseases, including diabetes, cancer and AD
(13). Therefore, inhibiting the
activation of the NF-κB and MAPK signaling pathways may be
important for controlling and ameliorating the development of
various skin diseases. On the contrary, heme oxygenase-1 (HO-1) has
been reported to possess anti-inflammatory activity and is
regulated by cytokine and chemokine interactions in AD; HO-1 has
been suggested to alleviate inflammation (14,15).
To develop therapeutic drugs for the treatment of
dermatitis, an initial step may include the identification of
effective anti-inflammatory agents to abrogate and suppress
inflammatory mediators in keratinocytes. Spilanthol [(2E, 6Z,
8E)-N-isobutylamide-2,6,8-decatrienamide] is a bioactive compound
detected in Acmella oleracea (Spilanthes acmella) and other
Acmella species, including A. brachyglossa and A.
ciliata (16). A.
acmella exerts a variety of biological properties, including
antipyretic (17,18), anti-inflammatory (19–21),
analgesic (22–24) and antimicrobial activities
(22–26). Spilanthol inhibits
lipopolysaccharide-induced inflammatory responses in murine RAW
264.7 macrophages via inactivation of the NF-κB signaling pathway
(19). Leaf extracts of S.
acmella have exhibited immunomodulatory activity, which may be
beneficial for the treatment of rheumatism (27); spilanthol enhances immune
activities in influenza and respiratory infections (28). In addition, spilanthol is absorbed
by human skin (29); however,
whether the compound is able to modulate inflammatory responses is
unclear, and the associated mechanism underlying the effects of
spilanthol within keratinocytes requires further investigation.
The present study investigated the biological
activities and modulatory effects of spilanthol on TNF-α-induced
HaCaT cells. Spilanthol was observed to inhibit TNF-α-induced
ICAM-1 expression, which may be associated with protection against
injuries induced by cytokines, including IL-6, IL-8, MCP-1 and
ICAM-1 in the present study. In addition, the anti-inflammatory
effects of spilanthol may be mediated by enhancing HO-1 expression
and inhibiting the pJNK signaling pathway in HaCaT cells.
Materials and methods
Materials and antibodies
Spilanthol (ChromaDex, Inc., Irvine, CA, USA;
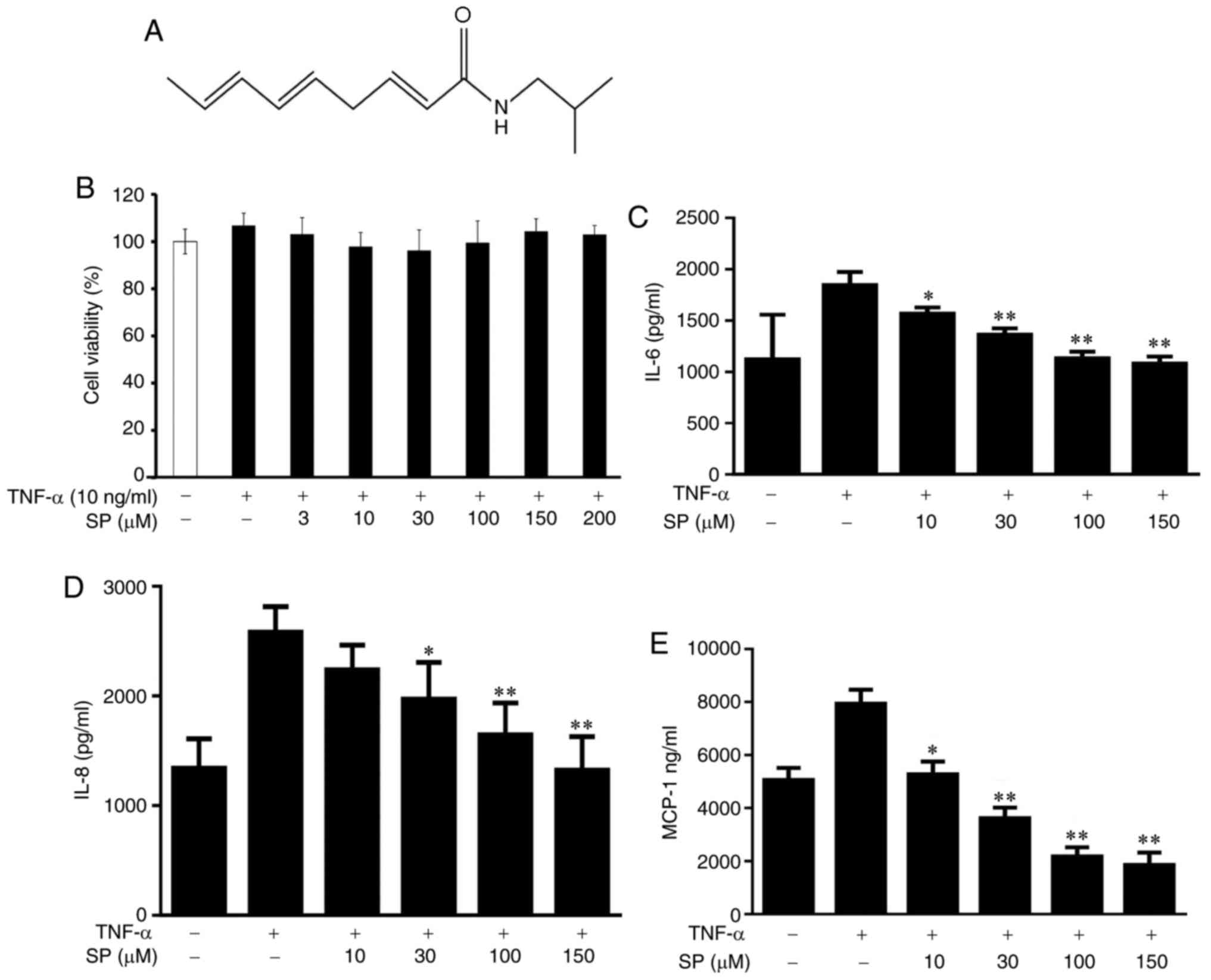
Fig. 1A) was prepared as a 100 mM
stock solution in dimethyl sulfoxide (DMSO) with a final DMSO
concentration of ≤0.1% in the culture medium. ELISA kits were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Primary antibodies against β-actin, cyclooxygenase-2 (COX-2), HO-1
and ICAM-1 were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), and JNK, ERK, p38, phosphorylated (p)-JNK,
p-ERK, and p-p38 antibodies were purchased from EMD Millipore
(Billerica, MA, USA). Calcein-AM was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). RNA was isolated using TRIzol
reagent obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Cell line and treatment
HaCaT cells (human keratinocyte cell line) were
purchased from the Bioresource Collection and Research Center
(Hsinchu, Taiwan). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Biological Industries, Ltd.,
Beit-Haemek, Israel), antibiotics (1% penicillin and streptomycin)
and 2 mM glutamine; cells were incubated in a humidified incubator
containing 5% CO2 at 37°C. THP-1 cells (a human
monocytic cell line) were purchased from the Bioresource Collection
and Research Center and incubated in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum and 2
mM L-glutamine added.
Cell viability assay
An MTT assay (Sigma-Aldrich; Merck KGaA) was
performed to assess cell viability. HaCaT cells (106
cells/well) were seeded onto 96-well plates and incubated with
spilanthol at concentrations between 3 and 200 µM for 1 h and then
TNF-α (10 ng/ml) was added and the cells co-cultured for 24 h in a
humidified incubator containing 5% CO2 at 37°C. MTT
solution (5 mg/ml) was added to each well for 2 h at 37°C.
Following removal of the MTT solution, DMSO (0.5 ml) was added to
dissolve the blue formazan crystals. Using a microplate reader
(Gene5, Synergy HT; BioTek Instruments, Inc., Winooski, VT, USA),
the absorbance was measured at 570 nm. A subsequent analysis was
conducted with 0–150 µM spilanthol. Cells without TNF-α and
spilanthol treatment served as negative control and cells treated
with TNF-α as a positive control.
ELISA for the analysis of
proinflammatory cytokines, chemokines and ICAM-1 production
HaCaT cells (106 cells/ml) were
pretreated with spilanthol (10–150 µM) for 1 h in a humidified
incubator containing 5% CO2 at 37°C, and TNF-α (10
ng/ml) was added, followed by incubation in a humidified incubator
containing 5% CO2 at 37°C for 24 h. Supernatants were
centrifuged (19,000 × g at 4°C for 5 min) and collected for
assaying the expression levels of IL-8, IL-6, MCP-1 and ICAM-1. The
present study employed specific ELISA kits (cat. nos. DY208, DY206,
DY279 and DY720, respectively; R&D Systems, Inc.) and the
optical density was spectrophotometrically measured at 450 nm with
a microplate reader (Multiskan FC; Thermo Fisher Scientific,
Inc.).
Preparation of total proteins
HaCaT cells (106 cells/ml) were seeded
onto 6-well plates and pretreated with spilanthol (10, 30, 100 and
150 µM) for 1 h in a humidified incubator containing 5%
CO2 at 37°C, and then stimulated with TNF-α (10 ng/ml)
for 24 h in a humidified incubator containing 5% CO2 at
37°C to evaluate total proteins or for 30 min to detect
phosphorylated proteins. Cell lysates were collected after
centrifuged at 19,000 × g for 15 min duration 4°C, and the proteins
were extracted in 300 µl protein lysis buffer [50 mM Tris-HCl (pH
8), 1 mM EDTA, 0.5% NP40, 150 mM NaCl and 0.1% SDS] containing a
protease inhibitor cocktail and phosphatase inhibitors
(Sigma-Aldrich; Merck KGaA). Proteins were extracted and a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) was used for quantification.
Western blot analysis
Equal amounts of protein (20–30 µg) were obtained
from HaCaT cells as mentioned above and underwent separation via
10% SDS-PAGE. The proteins were transferred to polyvinylidene
fluoride membranes (EMD Millipore). Subsequently, the membranes
were blocked with 5% BSA and incubated overnight with primary
antibodies at 4°C, including COX-2 (1:500; cat. no. sc-1746; Santa
Cruz Biotechnology, Inc.); HO-1 (1:500; cat. no. sc-10789; Santa
Cruz Biotechnology, Inc.); ERK1/2 (1:2,000; cat. no. ABS44;
Millipore), p38 (1:500; cat. no. ABS29; EMD Millipore,), JNK1/2
(1:1,000; cat. no. 06-748 EMD Millipore), phosphorylated-ERK 1/2
(1:1,000; cat. no. P27361; EMD Millipore), phosphorylated-p38
(1:1,000; cat. no. 09-272; EMD Millipore), and phosphorylated-JNK1
(1:500; cat. no. 07-175; EMD Millipore); ICAM-1 (1:1,000; cat. no.
GTX100450; Sigma-Aldrich; Merck KGaA) and β-actin (1:500; cat. no.
MAB1501; Sigma-Aldrich; Merck KGaA).
The membrane was washed with TBS with Tween-20
[TBST; 150 mM NaCl, 10 mM Tris (pH 8.0) and 0.1% Tween-20], and
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature. The bound antibodies,
including goat anti-rabbit IgG-HRP (1:10,000; cat. no. A2315; Santa
Cruz Biotechnology, Inc.) and goat anti-mouse IgG (1:10,000; cat.
no. A90-116P; Bethyl Laboratories, Inc., Montgomery, TX, USA) on
the membranes were washed with TBST and incubated in
Luminol/Enhancer Solution (EMD Millipore) according to the
manufacturer's protocols for detection of the bands; quantification
of protein expression was conducted using the BioSpectrum 600
automated system (UVP, LLC, Phoenix, AZ, USA) and its included
software.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of gene expression
ICAM-1 mRNA expression levels were determined by
RT-qPCR, with β-actin as the internal control. TRIzol solution
(Thermo Fisher Scientific, Inc.) was used to extract total RNA, and
reverse transcribed to acquire cDNA using a cDNA synthesis kit
(Thermo Fisher Scientific, Inc.). qPCR using SYBR Green Master Mix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was performed using
a spectrofluorometric thermal cycler (iCycler; Bio-Rad
Laboratories, Inc.) to quantitative the expression of specific
genes. The cycling conditions were as follows: Samples preincubated
at 95°C for 10 min. Next, the PCR was performed as 40 cycles of
95°C for 15 sec and 60°C for 1 min, followed by analysis using
TaqMan real-time quantitative PCR (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The specific primers used were as
follows: ICAM-1, forward 5′-AGACGCAGAGGACCTTAA-3′ and reverse
5′-CACACTTCACAGTTACTTGG-3′; β-actin, forward
5′-AAGACCTCTATGCCAACACAGT-3′ and reverse
5′-AGCCAGAGCAGTAATCTCCTTC-3′ (30). The specific genes were determined
by comparing the average of gene cycle quantification (Cq),
measured for each experiment and repeated three times, as described
previously (31). The comparison
Cq method was used for the specific genes determined by relative
cDNA expressions (2−∆∆Cq). 2−ΔΔCq was the
discrepancy between specific gene and housekeeping genes β-actin
for each sample.
Cell-cell adhesion assay
To perform the cell-cell adhesion assay, HaCaT cells
(106 cells/ml) were pretreated with spilanthol (10, 30,
100 and 150 µM) for 1 h in a humidified incubator containing 5%
CO2 at 37°C and subsequently incubated with TNF-α (10
ng/ml) for 24 h in a humidified incubator containing 5%
CO2 at 37°C. The control groups was treated with TNF-α
alone. THP-1 cells (106 cells/ml) labeled with
calcein-AM were co-cultured with HaCaT cells for 1 h in DMEM medium
in a humidified incubator containing 5% CO2 at 37°C.
Cells were washed with PBS and the extent of adhesion of THP-1
cells to HaCaT cells was observed under a fluorescence microscope
(3 per view; magnification, ×200; Olympus Corporation, Tokyo,
Japan) with excitation and emission wavelengths of 490 and 515 nm,
respectively. All experiments were repeated three times.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. Data were analyzed with
one-way analysis of variance and Dunnett's post hoc test using SPSS
statistical software package version 19.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition of proinflammatory cytokine
and chemokine production by pretreatment with spilanthol in
TNF-α-treated HaCaT cells
An MTT assay was used to evaluate the cytotoxicity
of HaCaT cells against spilanthol. The present study reported that
spilanthol did not significantly affect cell viability at
concentrations ≤200 µM (Fig. 1B).
Therefore, the present study conducted a subsequent analysis with
0–150 µM spilanthol. Spilanthol at 3 µM did not significantly
affect cell viability and it had no effects in pretesting and was
thus excluded from subsequent experimentation. Furthermore, the
inhibitory effects of spilanthol on TNF-α-induced proinflammatory
cytokine and chemokine production were investigated. HaCaT cells
were pretreated with various concentrations of spilanthol and
stimulated with TNF-α for 24 h. Pretreatment with spilanthol was
associated with significantly decreased expression levels of IL-6,
IL-8 and MCP-1 in TNF-α-stimulated HaCaT cells compared with TNF-α
alone (Fig. 1C-E). Spilanthol
concentrations ≥10 µM significantly inhibited IL-6 and MCP-1
expression levels; however, concentrations ≥30 µM significantly
inhibited IL-8 production. The results of the present study
suggested that spilanthol can inhibit proinflammatory cytokine
(IL-6) and chemokines (IL-8 and MCP-1) expression levels, thus
preventing inflammation.
Effects of spilanthol on TNF-α-induced
ICAM-1 protein and mRNA expression
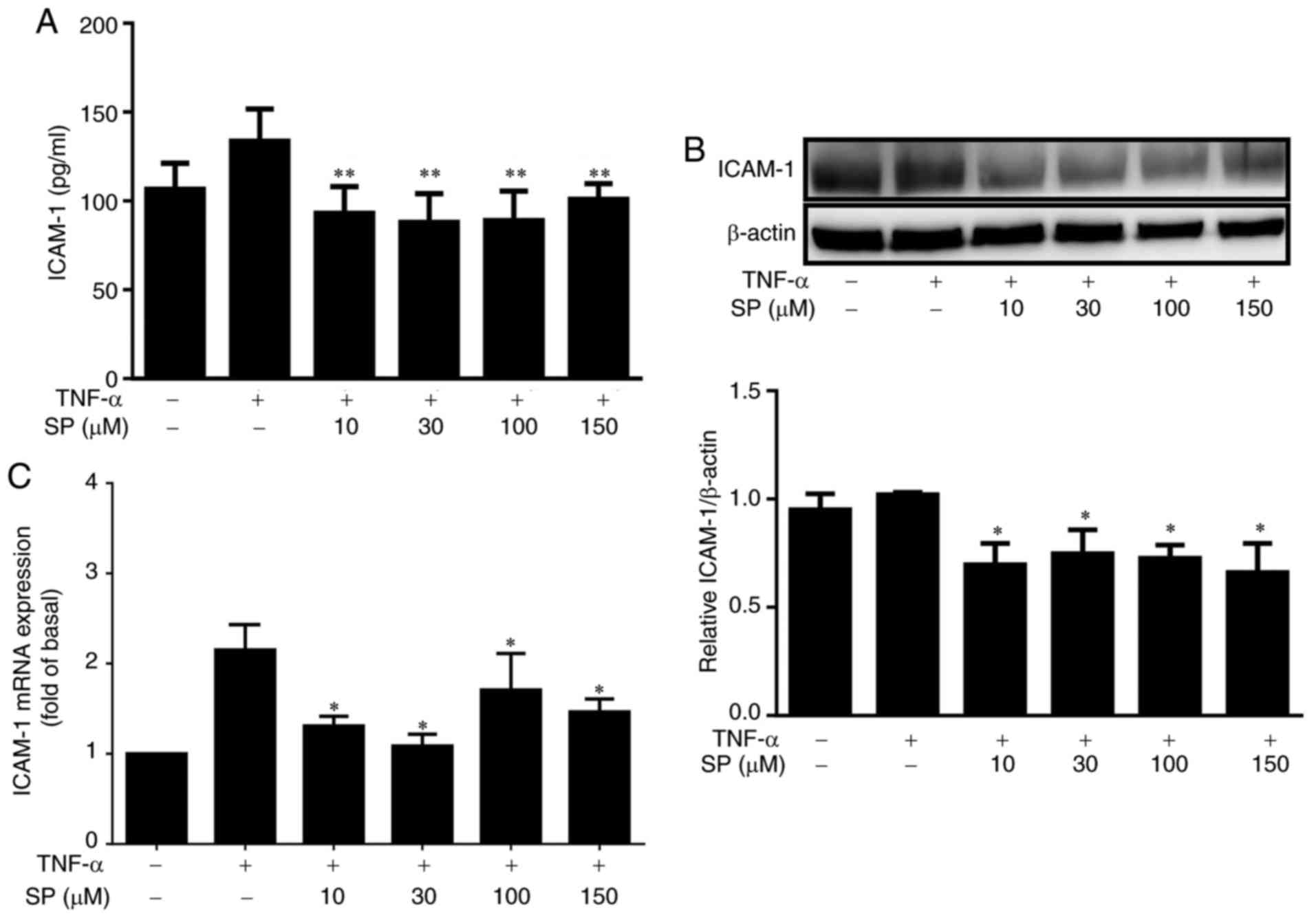
In the present study, the effects of spilanthol on
ICAM-1 expression in TNF-α-induced HaCaT cells were investigated.
Spilanthol significantly reduced ICAM-1 protein expression levels
compared with treatment with TNF-α alone, as determined by ELISA
and western blotting (Fig. 2A and
B). In addition, the mRNA expression levels of ICAM-1 were
significantly decreased (Fig. 2C)
compared with treatment with TNF-α alone, as determined by RT-qPCR.
Collectively, these results suggested that spilanthol significantly
suppressed ICAM-1 in TNF-α-induced HaCaT cells.
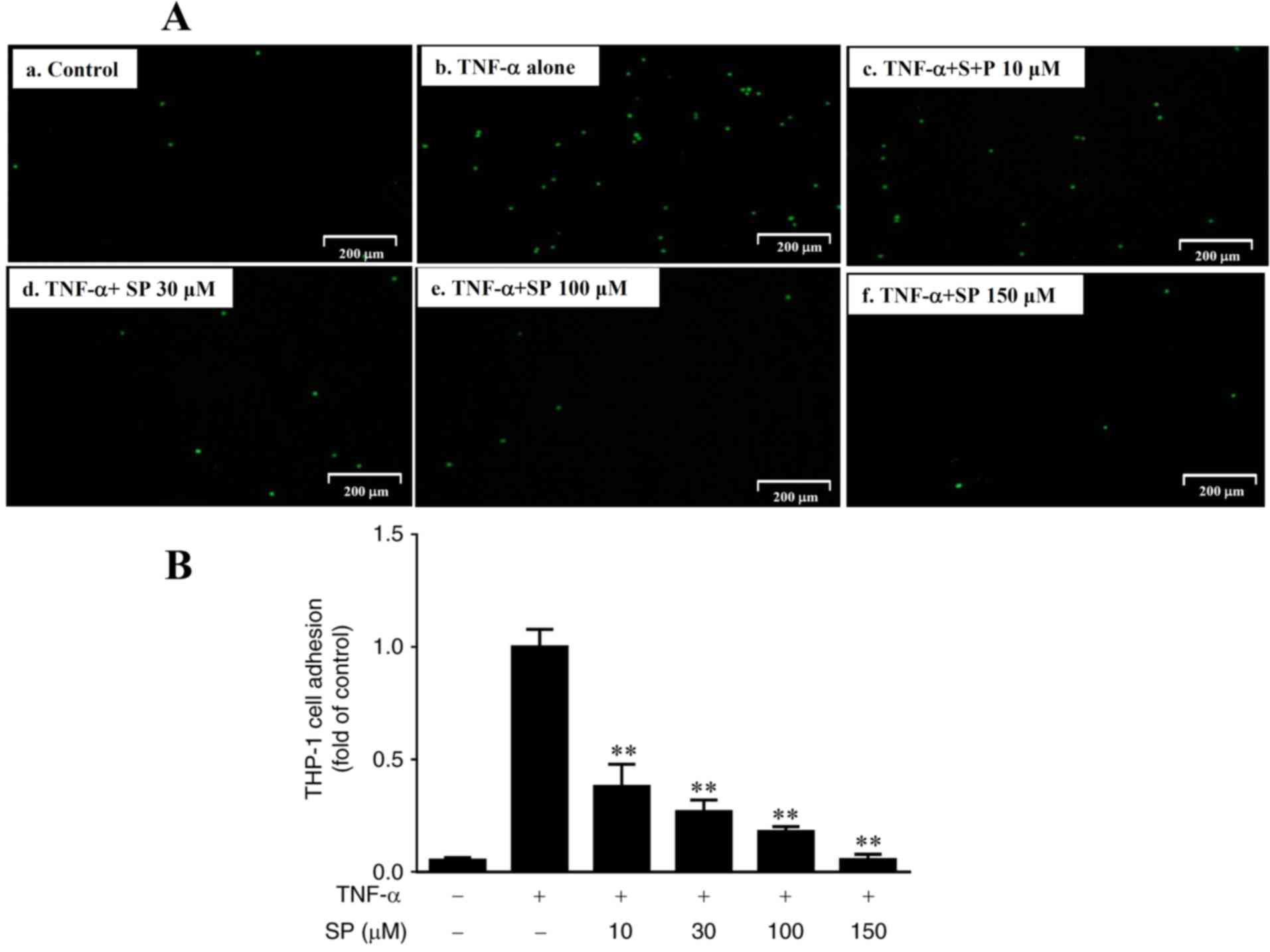
Spilanthol inhibits monocyte adhesion
to TNF-α-activated HaCaT cells
A recent study reported that increased monocyte
adhesion to human keratinocytes may be associated with increased
ICAM-1 expression (31). In the
present study, it was demonstrated that spilanthol significantly
decreased ICAM-1 expression levels in inflammatory HaCaT cells
(Fig. 2). Thus, the present study
investigated whether spilanthol may suppress TNF-α-induced monocyte
adhesion. The results revealed that THP-1 cells adhered to
TNF-α-stimulated HaCaT cells; however, the extent of monocyte
adhesion significantly decreased in a dose-dependent manner in
response to treatment with spilanthol, compared with in HaCaT cells
treated with TNF-α alone (Fig. 3).
Fluorescence density is highest in Fig. 3A-b indicating that TNF-α induced
THP-1 adhesion to HaCat cells compared with negative control
(Fig. 3A-a). SP-treatment
decreased THP-1 adhesion to HaCat cells compared with positive
control (Fig. 3A-b).
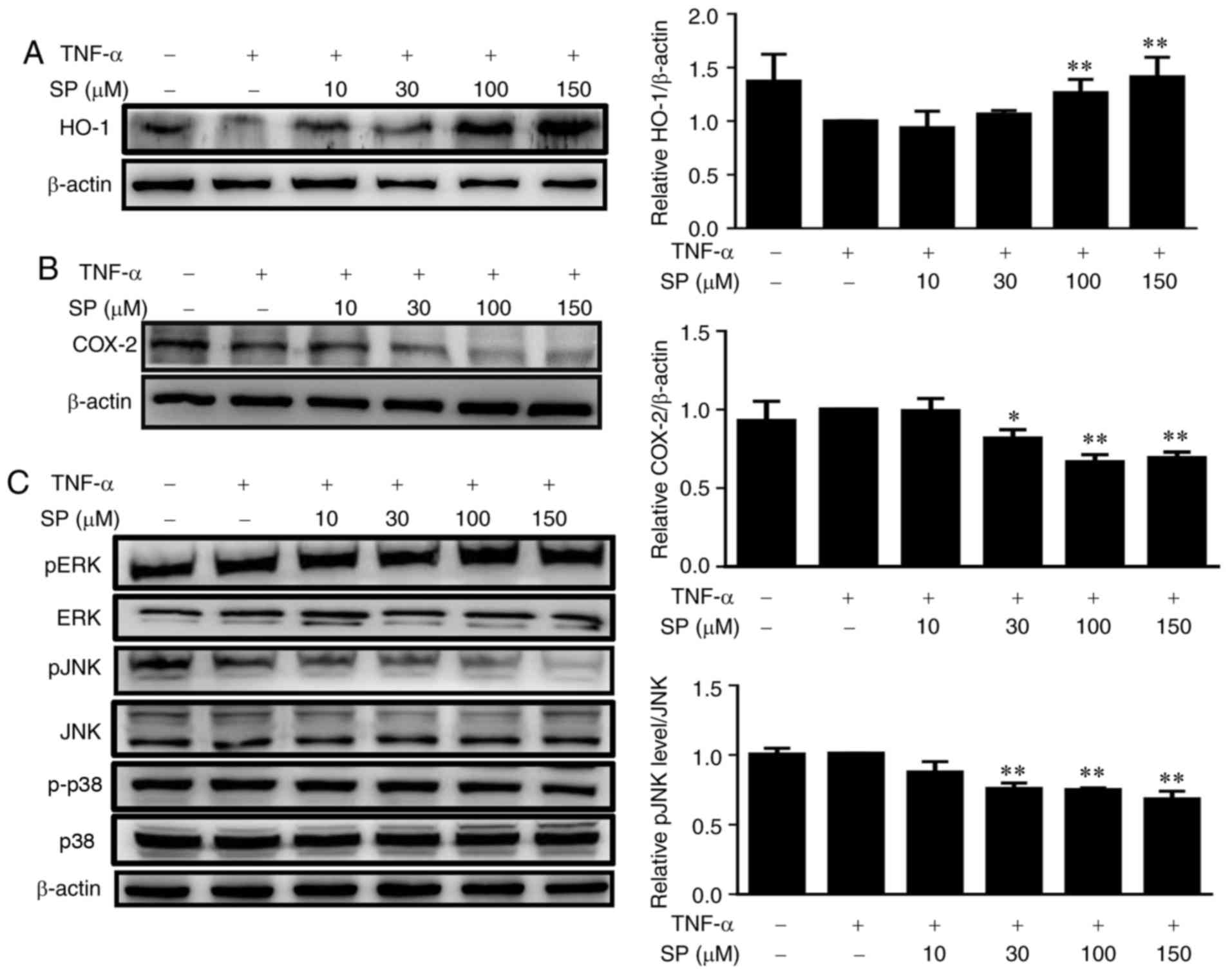
Spilanthol induces HO-1 expression and
inactivates the pJNK signaling pathway to inhibit COX-2 protein
expression in TNF-α-induced HaCaT cells
To improve understanding as to how spilanthol
reduces inflammations, the expression levels of
inflammation-associated proteins were analyzed. A recent study
suggested that the induction of HO-1 inhibits inflammation in HaCaT
cells (32). It was observed that
spilanthol concentrations ≥100 µM significantly increased the
expression levels of HO-1 expression in HaCaT cells compared with
TNF-α alone (P<0.01; Fig. 4A).
A previous study reported that COX-2 expression is associated with
inflammation in human keratinocytes (33). Thus, the present study investigated
the effects of spilanthol on TNF-α-activated HaCaT cells, and it
was indicated that pretreatment with ≥30 µM spilanthol
significantly suppressed COX-2 protein expression compared with
TNF-α-stimulated control cells (Fig.
4B). The activation of the MAPK signaling pathway is closely
associated with the expression of proinflammatory mediators in
keratinocytes (34); whether
spilanthol affects the MAPK signaling pathway in TNF-α-activated
HaCaT cells was analyzed in the present study. Treatment with ≥30
µM spilanthol significantly decreased the phosphorylation of JNK
compared with in TNF-α-activated HaCaT control cells (Fig. 4C). These results suggested that
spilanthol may induce the expression of HO-1 expression via
inactivation of pJNK to potentially inhibit the expression of COX-2
in TNF-α-activated HaCaT cells; however, spilanthol did not
suppress the phosphorylation of ERK and P38 MAPK (data not
shown).
Discussion
Epidermal keratinocytes are subjected to exposure to
harmful factors, including ultraviolet light, which may induce
keratinocyte activation and the release of inflammatory mediators
(1). ICAM-1 is expressed on
activated keratinocytes to attract more inflammatory cells to
infiltrate the inflamed skin (2–4).
Numerous reports have described the importance of ICAM-1 expression
in keratinocytes in skin inflammation-associated diseases (4,5),
which has led to a focus on the regulation of this particular
adhesion molecule in drug development for the treatment of
dermatitis. The present study reported that pretreatment with
spilanthol significantly suppressed ICAM-1 protein and mRNA
expression, as detected by ELISA and western blotting, and RT-PCR
analyses, respectively. In addition, treatment with spilanthol
reduced the extent of adhesion between HaCaT and THP-1 cells; this
may have been mediated by reductions in the secretion and
expression of ICAM-1 by HaCaT cells. Inflammatory keratinocytes
could have released proteins into culture medium and excess ICAM-1
cleaved to soluble ICAM, and moved into the supernatant. Thus,
ELISA could detect specific protein (soluble ICAM) in the
supernatant. Soluble ICAM-1 is a molecule meriting exploration in
the future. Additionally, the present study reported that
spilanthol affected the extent of monocyte adhesion to
keratinocytes, suggesting that spilanthol may attenuate the
inflammatory response via the inhibition of ICAM-1 expression in
TNF-α-induced keratinocytes.
Spilanthol possesses antioxidant and antinociceptive
properties and is of particular importance as a potential
anti-inflammatory agent (16),
which is mediated via the inactivation of NF-κB; spilanthol
negatively regulates the production of proinflammatory mediators,
including IL-1β, IL-6, TNF-α and COX-2 (19). Flavonoid extracts from S.
acmella inhibit the secretion of IL-1β and TNF-α, reducing
infection-associated inflammation, and suppress COX-2 expression,
reducing fever (16,19). These findings suggest that S.
acmella extracts have analgesic and antipyretic effects
(17,18). In addition, spilanthol exerts
anti-inflammatory effects in in vitro and in vivo
models (16–19); however, the associated
anti-inflammatory mechanisms in keratinocytes require further
investigation. In the present study, spilanthol was observed to
significantly suppress the expression of pro-inflammatory
cytokines, including IL-6, IL-8 and MCP-1 production. Additionally,
spilanthol effectively suppressed COX-2 protein expression in
keratinocytes, indicating that spilanthol may inhibit inflammatory
responses and prevent the inflammatory loop within TNF-α-induced
keratinocytes.
Of note, HO-1 expression has been reported to exert
an anti-inflammatory response in keratinocytes, and attenuates
AD-like lesions via its protective effects against inflammatory
skin diseases (7,9,15).
HO-1 expression is regulated by inflammation-associated cytokines
and chemokines associated with the development of AD (15). The present study observed that
spilanthol induced HO-1 protein expression, and decreased cytokine
and chemokine expression levels, indicating that HO-1 expression
may be associated with the underlying anti-inflammatory mechanism
of spilanthol within keratinocytes.
Furthermore, previous studies demonstrated that
flavonoid and immunosuppressive drugs (rapamycin and mycophenolic
acid) modulated the expression of ICAM-1, which is regulated by
NF-κB activation within TNF-α-induced HaCaT cells (7,9,10).
The modulatory activity of spilanthol on the signaling of NF-κB
activation was evaluated in TNF-α-induced HaCaT cells, and no
effect on the NF-κB signaling pathway detected (data not show).
Collectively, these results indicated that spilanthol may inhibit
the upregulation of proinflammatory cytokines induced by TNF-α, and
suppress ICAM-1 expression via spilanthol-induced HO-1 expression
in keratinocytes.
The p38/MAPK, ERK and JNK signaling pathways are
important in the mediation of cellular inflammation, and these MAPK
signal transduction pathways may be induced by TNF-α (2,12).
Furthermore, MAPK signaling has been associated with the expression
of pro-inflammatory mediators and skin inflammation (9). In particular, JNK signaling serves a
critical role in inflammatory responses; TNF-α may induce the
phosphorylation of JNK (35). The
present study indicated that TNF-α induced the activation of JNK
signaling via phosphorylation; however, pretreatment with
spilanthol reduced the extent of this activation. The findings of
the present study suggested that the TNF-α-induced inflammatory
response may be mediated by JNK signaling in HaCaT cells and that
spilanthol may ameliorate the inflammatory response by inhibiting
JNK phosphorylation. In addition, the JNK-MAPK signaling pathway is
also involved in ultraviolet B-induced keratinocyte inflammation
(36). Therefore, the JNK-MAPK
signaling pathway may be considered as a therapeutic target in
treatment of conditions associated with inflammation. Collectively,
these data suggested that spilanthol attenuated the production of
IL-6, IL-8 and MCP-1 induced by TNF-α, in addition to JNK
activation, in HaCaT cells.
In conclusion, to the best of our knowledge, the
present study is the first to report that spilanthol may exert an
inhibitory effect on the production of inflammation-associated
mediators, including IL-6, IL-8, MCP-1, ICAM-1 and COX-2, and may
decrease the extent of monocyte adhesion to TNF-α-induced HaCaT
cells. In addition, the anti-inflammatory activity of spilanthol
may be associated with enhanced HO-1 expression levels and the
inhibition of pJNK-MAPK signaling. The present study proposed that
spilanthol may possess therapeutic potential for the treatment of
inflammatory skin diseases.
Acknowledgements
The authors would like to thank the Graduate
Institute of Health Industry Technology of Chang Gung University of
Science and Technology and Dr Wen-Chung Huang for their support in
using bioinformatics tools.
Funding
The present study was supported by grants from the
Chang Gung Memorial Hospital (grant nos. CMRPF1F0122 and
CMRPF1G0041) and the Ministry of Science and Technology in Taiwan
(grant no. MOST 105-2320-B-255-004), and in part by a grant from
the Chang Gung University of Science and Technology (grant no.
EZRPF3FG0061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CHH, LC and SW designed and performed the
experiments. SH and CYH performed analysis and interpretation of
data. SW drafted the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coman G, Blickenstaff NR and Maibach HI:
Skin and the environment. Rev Environ Health. 29:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Subhan F, Kang HY, Lim Y, Ikram M, Baek
SY, Jin S, Jeong YH, Kwak JY and Yoon S: Fish scale collagen
peptides protect against CoCl2/TNF-α-induced cytotoxicity and
inflammation via inhibition of ROS, MAPK, and NF-κB pathways in
HaCaT cells. Oxid Med Cell Longev. 2017:97036092017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dustin ML, Singer KH, Tuck DT and Springer
TA: Adhesion of T lymphoblasts to epidermal keratinocytes is
regulated by interferon gamma and is mediated by intercellular
adhesion molecule 1 (ICAM-1). J Exp Med. 167:1323–1340. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youn GS, Kwon DJ, Ju SM, Choi SY and Park
J: Curcumin ameliorates TNF-α-induced ICAM-1 expression and
subsequent THP-1 adhesiveness via the induction of heme oxygenase-1
in the HaCaT cells. BMB Rep. 46:410–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albanesi C, Scarponi C, Giustizieri ML and
Girolomoni G: Keratinocytes in inflammatory skin diseases. Curr
Drug Targets Inflamm Allergy. 4:329–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Middleton MH and Norris DA:
Cytokine-induced ICAM-1 expression in human keratinocytes is highly
variable in keratinocyte strains from different donors. J Invest
Dermatol. 104:489–496. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H, Youn GS, An SY, Kwon HY, Choi SY
and Park J: 2,3-Dimethoxy-2′-hydroxychalcone ameliorates
TNF-α-induced ICAM-1 expression and subsequent monocyte
adhesiveness via NF-kappaB inhibition and HO-1 induction in HaCaT
cells. BMB Rep. 49:57–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nedoszytko B, Sokołowska-Wojdyło M,
Ruckemann-Dziurdzińska K, Roszkiewicz J and Nowicki RJ: Chemokines
and cytokines network in the pathogenesis of the inflammatory skin
diseases: Atopic dermatitis, psoriasis and skin mastocytosis.
Postepy Dermatol Alergol. 31:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MY, Lim YY, Kim HM, Park YM, Kang H
and Kim BJ: Synergistic inhibition of tumor necrosis
factor-alpha-stimulated pro-inflammatory cytokine expression in
HaCaT cells by a combination of rapamycin and mycophenolic acid.
Ann Dermatol. 27:32–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banno T, Gazel A and Blumenberg M: Effects
of tumor necrosis factor-alpha (TNF alpha) in epidermal
keratinocytes revealed using global transcriptional profiling. J
Biol Chem. 279:32633–32642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang CF, Liao KC and Chen CH:
2-phenylnaphthalene derivatives inhibit lipopolysaccharide-induced
pro-inflammatory mediators by downregulating of MAPK/NF-κB pathways
in RAW 264.7 macrophage cells. PLoS One. 12:e01689452017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JK, Xiong GM, Luo B, Choo CC, Yuan S,
Tan NS and Choong C: Surface modification of PVDF using
non-mammalian sources of collagen for enhancement of endothelial
cell functionality. J Mater Sci Mater Med. 27:452016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Listopad J, Asadullah K, Sievers C, Ritter
T, Meisel C, Sabat R and Döcke WD: Heme oxygenase-1 inhibits T
cell-dependent skin inflammation and differentiation and function
of antigen-presenting cells. Exp Dermatol. 16:661–670. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirino M, Kirino Y, Takeno M, Nagashima Y,
Takahashi K, Kobayashi M, Murakami S, Hirasawa T, Ueda A, Aihara M,
et al: Heme oxygenase 1 attenuates the development of atopic
dermatitis-like lesions in mice: Implications for human disease. J
Allergy Clin Immunol. 122(290–297): 297.e1–e8. 2008.
|
|
16
|
Barbosa AF, de Carvalho MG, Smith RE and
Sabaa-Srur AUO: Spilanthol: Occurrence, extraction, chemistry and
biological activities. Rev bras Farmacogn. 26:128–133. 2016.
View Article : Google Scholar
|
|
17
|
Elumalai A, Pendem N, Eswaraiah MC and
Naresh V: An updated annual review on antipyretic medicinal plants.
Int J Univers Pharm Life Sci. 2:207–215. 2012.
|
|
18
|
Chakraborty A, Devi RKB, Rita S,
Sharatchandra KH and Singh THI: Preliminary studies on
antiinflammatory and analgesic activities of Spilanthes acmella in
experimental animal models. Indian J Pharmacol. 36:148–150.
2004.
|
|
19
|
Wu LC, Fan NC, Lin MH, Chu IR, Huang SJ,
Hu CY and Han SY: Anti-inflammatory effect of spilanthol from
Spilanthes acmella on murine macrophage by down-regulating
LPS-induced inflammatory mediators. J Agric Food Chem.
56:2341–2349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hernández I, Márquez L, Martínez I,
Dieguez R, Delporte C, Prieto S, Molina-Torres J and Garrido G:
Anti-inflammatory effects of ethanolic extract and
alkamides-derived from Heliopsis longipes roots. J Ethnopharmacol.
124:649–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nair JJ, Aremu AO and Van SJ:
Anti-inflammatory effects of Leucosidea sericea (Rosaceae) and
identification of the active constituents. S Afr J Bot. 80:75–76.
2012. View Article : Google Scholar
|
|
22
|
Ratnasooriya WD and Pieris KPP:
Attenuation of persistent pain and hyperalgesia by Spilanthus
acmella flowers in rats. Pharm Biol. 43:614–619. 2005. View Article : Google Scholar
|
|
23
|
Déciga-Campos M, Rios MY and
Aguilar-Guadarrama AB: Antinociceptive effect of Heliopsis longipes
extract and affinin in mice. Planta Med. 76:665–670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arora S, Vijay S and Kumar D:
Phytochemical and antimicrobial studies on the leaves of Spilanthes
acmella. J Chem Pharm Res. 3:145–150. 2011.
|
|
25
|
Rosas-Piñón Y, Mejía A, Díaz-Ruiz G,
Aguilar MI, Sánchez-Nieto S and Rivero-Cruz JF: Ethnobotanical
survey and antibacterial activity of plants used in the Altiplane
region of Mexico for the treatment of oral cavity infections. J
Ethnopharmacol. 141:860–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prachayasittikul S, Suphapong S,
Worachartcheewan A, Lawung R, Ruchirawat S and Prachayasittikul V:
Bioactive metabolites from Spilanthes acmella Murr. Molecules.
14:850–867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Savadi R, Yadav R and Yadav N: Study on
immunomodulatory activity of ethanolic extract Spilanthes acmella
Murr. Leaves. Indian J Nat Prod Resour. 1:204–207. 2010.
|
|
28
|
Saritha KV and Naidu CV: Direct shoot
regeneration from leaf explants of Spilanthes acmella. Biol Plant.
52:334–338. 2008. View Article : Google Scholar
|
|
29
|
Boonen J, Baert B, Roche N, Burvenich C
and De Spiegeleer B: Transdermal behaviour of the N-alkylamide
spilanthol (affinin) from Spilanthes acmella (Compositae) extracts.
J Ethnopharmacol. 127:77–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang WC, Wu SJ, Tu RS, Lai YR and Liou
CJ: Phloretin inhibits interleukin-1β-induced COX-2 and ICAM-1
expression through inhibition of MAPK, Akt, and NF-κB signaling in
human lung epithelial cells. Food Funct. 6:1960–1967. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang WC, Fang LW and Liou CJ: Phloretin
attenuates allergic airway inflammation and oxidative stress in
asthmatic mice. Front Immunol. 8:1342017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kundu J, Chae IG and Chun KS: Fraxetin
induces heme oxygenase-1 expression by activation of Akt/Nrf2 or
AMP-activated protein kinase α/Nrf2 pathway in HaCaT cells. J
Cancer Prev. 21:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho JW, Park K, Kweon GR, Jang BC, Baek
WK, Suh MH, Kim CW, Lee KS and Suh SI: Curcumin inhibits the
expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT)
by inhibiting activation of AP-1: p38 MAP kinase and JNK as
potential upstream targets. Exp Mol Med. 37:186–192. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seo WY, Youn GS, Choi SY and Park J:
Butein, a tetrahydroxychalcone, suppresses pro-inflammatory
responses in HaCaT keratinocytes. BMB Rep. 48:495–500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roy PK, Rashid F, Bragg J and Ibdah JA:
Role of the JNK signal transduction pathway in inflammatory bowel
disease. World J Gastroenterol. 14:200–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhai Y, Dang Y, Gao W, Zhang Y, Xu P, Gu J
and Ye X: P38 and JNK signal pathways are involved in the
regulation of phlorizin against UVB-induced skin damage. Exp
Dermatol. 24:275–279. 2015. View Article : Google Scholar : PubMed/NCBI
|