Introduction
Pancreatic cancer is a common form of cancer of the
highly malignant digestive system. The incidence of pancreatic
cancer is high in western developed countries. According to
statistics in 2012 in Europe and America, 137,286 cases of new
pancreatic cancer were diagnosed, and pancreatic cancer mortality
ranked fourth in all malignant tumors (1–3).
Like most other countries, the health burden of pancreatic cancer
in our country is also on the rise, with an annual mortality rate
almost equal to the incidence (4).
Because the clinical symptoms of pancreatic cancer are relatively
vague and often diagnosed late, most patients with pancreatic
cancer have obvious regional infiltration or distant metastasis at
the time of diagnosis (5–7). Despite the joint efforts of
clinicians and scientists for decades, pancreatic cancer remains a
devastating disease with adverse consequences (8). Therefore, if we can find a way to
inhibit the invasion and metastasis of pancreatic cancer, it has
important clinical significance for the overall survival rate of
pancreatic cancer patients and inhibition of the recurrence of
pancreatic cancer.
In recent years, many studies found that microRNAs
(miRNAs) play an important regulatory role in tumorigenesis and
progression. A large number of oncogenes and tumor suppressor genes
are regulated by miRNAs (9). miRNA
is a single, non-coding 19–25 nucleotide RNA that regulates about
30% of the protein-coding mRNA in the human genome (10). miRNA can inhibit the translation of
the target mRNA or degrade the target mRNA by binding with the 3′
non-coding region (3′-UTR) of the target mRNA to exert
post-transcriptional regulation (11). There are a wide range of abnormal
miRNA expression in tumor tissues compared to normal tissues, some
of which have oncogenic activity while others have tumor suppressor
activity (12). In malignant
cells, the majority of tumor suppressor miRNAs are down-regulated,
while oncogenic miRNAs are up-regulated and involved in tumor
pathology through multiple mechanisms (13,14).
miRNA-661 was found to be over-expressed in the Snail-1-induced
breast cancer EMT and its targets inhibits the intercellular
adhesion protein Nectin-1 and the lipid transfer enzyme StarD 10
and leads to the down-regulation of epithelial markers (15). Another study found that
miRNA-219-5p is an important part of the Twist-EMT regulatory
pathway (16).
Among the miRNA families, miR-1271 is a newly
discovered member (17). Recent
studies show that the impact of miR-1271 on the tumor is getting
more and more attention. Many studies have found that miR-1271 has
a wide range of tumor suppressor effects (18–20).
MiR-1271 is down-regulated in many human tumors and is involved in
the inhibition of tumor epithelial-mesenchymal transition (EMT),
induction of apoptosis, and reversal of chemo-resistance (18–22).
EMT refers to the cell under the physiological or pathological
conditions into mesenchymal features similar to mesenchymal-like
phenomenon. Cells lose their own cell polarity and cell-cell
connections are the most important mechanism of induce tumor
invasion and metastasis. The EMT of the tumor cells processed by a
number of transcription factors, including ZEB1and Twist1, and
these transcription factors can inhibit the expression of
E-cadherin (23). Twist is an
inhibitor of apoptosis protein found in recent ten years. It is an
important regulatory transcription factor in the process of
embryonic development. Twist is involved in the growth and
development of normal organs, and it is also an important regulator
of EMT in malignant tumor cells (24). E-cadherin, a member of the family
of cadherin proteins in cell adhesion molecules, is an important
intercellular adhesion molecule and epithelial phenotype that has
been recognized as a suppressor of cancer metastasis. Tumors with
reduced expression of E-cadherin are more likely to metastasize
(25).
Based on the regulation mechanism of miRNA on EMT in
pancreatic cancer, the present study aimed to investigate the
inhibitory effect of miR-1271 and emodin against invasion and
metastasis of pancreatic carcinoma, and further explored the
relationship between miR-1271 and emodin. We provide theoretical
and experimental evidence for the further development of
miRNA-based targeted therapy of pancreatic cancer.
Materials and methods
Clinical specimens
A total of 58 paired pancreatic cancer and adjacent
normal tissues were identified and collected during biopsies from
58 patients with pancreatic cancer who were diagnosed by clinical
symptoms and imaging examination at the General Hospital of Tianjin
Medical University (Tianjin, China). All tissue samples were
immediately flash-frozen in liquid nitrogen and stored at −80°C.
The present study was approved by the Human Ethics Committee Review
Board at the General Hospital of Tianjin Medical University
(Tianjin, China). Informed consent was provided by every
patient.
Cell culture and treatment
Human pancreatic cancer cell lines SW1990, BXPC3,
PANC-1 and ASPC-1 as well as the normal human pancreatic ductal
epithelial cell line HPDE6c7 were originally acquired from American
Type Culture Collection ATCC (Manassas, VA, USA) and cultured in
our institute. Cells were incubated in RPMI 1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum, and 1% penicillin-streptomycin solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C with 5%
CO2. Cells were passaged every 2–3 days.
SW1990 cells (3×l04 cells/well) were
treated with various concentrations (0, 20, 40 µmol/l) of emodin
for 24, 48 h at 37°C, 5% CO2.
Cell transfection
For cell transfection, SW1990 cells were seeded in
6-well plates (4×105 per well). Then, miRNA-1271 mimics, miRNA-1271
inhibitor or the negative control was transfected into SW1990 cells
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.) following with the manufacturer's protocol. 48 h after
transfection, the cells were subjected to following experimental
analysis. Transfection efficiency was detected using qRT-PCR.
Western blot analysis
After specific treatment, total cellular proteins
from SW1990 cells were extracted by using RIPA Buffer (Auragene,
Changsha, China). BCA protein quantitative kit (Thermo Fisher
Scientific, Inc.) was carried out to measure the concentration of
protein samples. Equal amount of protein samples were resolved by
12% SDS-PAGE and then transferred onto PVDF membranes. The
membranes were blocked with 5% non-fat milk at room temperature for
1 h, followed by incubated with primary antibodies
(anti-E-cadherin, cat no. 3195; anti-ZEB1, cat no. 3396;
anti-TWIST1, cat no. 46702; dilution for all, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
Subsequently, membranes were incubated with a HRP-conjugated
secondary antibody (anti-rabbit IgG, HRP-linked antibody, cat no.
7074; 1:5,000) at room temperature for 2 h. To visualize the
protein blots, an ECL kit (Applygen Technologies, Inc., Beijing,
China) was used to visualize the protein blots according the
manufacturer's protocol.
MTT assay
The cells were evenly inoculated into 96-well plates
(Corning Incorporated, Corning, NY, USA) with 2.0×103
cells per well. 20 µl MTT solution was added in per well, incubated
at 37°C for 4 h. After removing the supernatant, 200 ul dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) was added in per well.
A microplate reader was used to determine the cell proliferation
ability by detecting the OD value at 490 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIZOL reagent (Takara, Japan) was used to extract
the total RNA from the SW1990 cells. β-actin or U6 were acted as an
internal control. cDNAs were generated by using the PrimeScript™ RT
reagent kit (Takara, Japan) in line with the manufacturer's
instructions. SYBR Premix Ex Taq (Takara, Japan) was carried out to
analyze the synthesized cDNAs according to the manufacturer's
instructions. Primer sequences used for real time PCR were listed
as following: miRNA-1271 Forward: 5′-CAGCACTTGGCACCTAGCA-3′.
miRNA-1271 Reverse: 5′-TATGGTTGTTCTCCTCTCTGTCTC-3′. E-cadherin
Forward: 5′-AAGTGCTGCAGCCAAAGACAGA-3′. E-cadherin Reverse:
5′-AGGTAGACCCACCTCAATCATCCTC-3′. ZEB1 Forward:
5′-GCACAACCAAGTGCAGAAGA-3′. ZEB1 Reverse:
5′-CATTTGCAGATTGAGGCTG-3′. Twist1 Forward:
5′-AGCTGAGCAAGATTCAGACCCTCA-3′. Twist1 Reverse:
5′-CTGCAGCTTGCCATCTTGGAGT-3′. U6 Forward: 5′-CTCGCTTCGGCAGCACA-3′.
U6 Reverse: 5′-AACGCTTCACGAATTTGCGT-3′. β-actin Forward:
5′-CAGGGCGTGATGGTGGGCA-3′. β-actin Reverse:
5′-CAAACATCATCTGGGTCATCTTCTC-3′. Relative gene quantification was
assessed using the 2−ΔΔCq method (26).
Cell invasion assay
In vitro invasion assay was performed using
transwell plates (BD Biosciences, Franklin Lakes, NJ, USA) with 8
µm pores. The cells (1×104 cells) in contains (0, 20, 40
µmol/l) emodin-DMEM medium were added to the upper chamber
pre-coated with Matrigel (BD Biosciences) of the Transwell plates.
Then emodin-DMEM medium containing 20% FBS as a chemo-attractant
was added to the lower chamber. After 48 h incubation, cells were
removed using cotton wool which on the upper surface and the cells
were fixed with methanol and stained with 0.5% crystal violet.
Images were captured and the cells were counted using a
photomicroscope (Olympus Corporation, Tokyo, Japan).
Animal models of pancreatic cancer
cell metastasis
SW1990 cells were injected into the spleens of 45
nude mice to establish an animal model of pancreatic liver
metastasis. Mice were divided into 3 groups: High dose emodin group
(gavage administration; emodin, 50 mg/kg body weight/day; day 8 to
day 35 after model establishment); low dose emodin group (gavage
administration; emodin, 20 mg/kg body weight/day; day 8 to day 35
after model establishment), and the control group (gavage
administration; 2 ml normal saline), each group of 15 mice. Six
weeks later, the nude mice were sacrificed and the liver metastasis
of pancreatic cancer in nude mice was observed. The number of tumor
nodules, the proliferation inhibition rate and the liver metastasis
inhibition rate were calculated in each group. The animal
experiments performed in the present study were approved by the
Animal Ethics Committee Review Board at Tianjin Medical University
(Tianjin, China).
Immunohistochemistry
The paraffin-embedded tissue blocks were cut into 4
µm sections using a microtome. The sections were incubated for 1 h
in 10% normal goat serum/PBS solution, then incubated overnight
with the primary antibodies in 0.1% BSA/PBS solution in humid
chambers at 4°C. Primary antibodies used were TWIST1 and ZEB-1.
Secondary antibodies were applied followed by Vectastain ABC
complex according to manufacturer protocol. Immunostaining was
visualized by 1×DAB/H2O2 solution,
subsequently counter-stained with hematoxylin, and mounted with
Permount (Sigma-Aldrich; Merck KGaA). Immunostaining without
primary antibody or with the primary antiserum preabsorbed with its
respective antigen was carried out as negative control. Alizarin
Red S and Masson's Trichrome staining protocols were used for
calcium and collagen detection, respectively.
Statistical Analysis
Results were expressed as mean values ± standard
error (mean ± S.E.). Data were analyzed by one-way analysis of
variance followed by a post hoc Tukey's test or a Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MiR-1271 is down-regulated in
pancreatic cancer
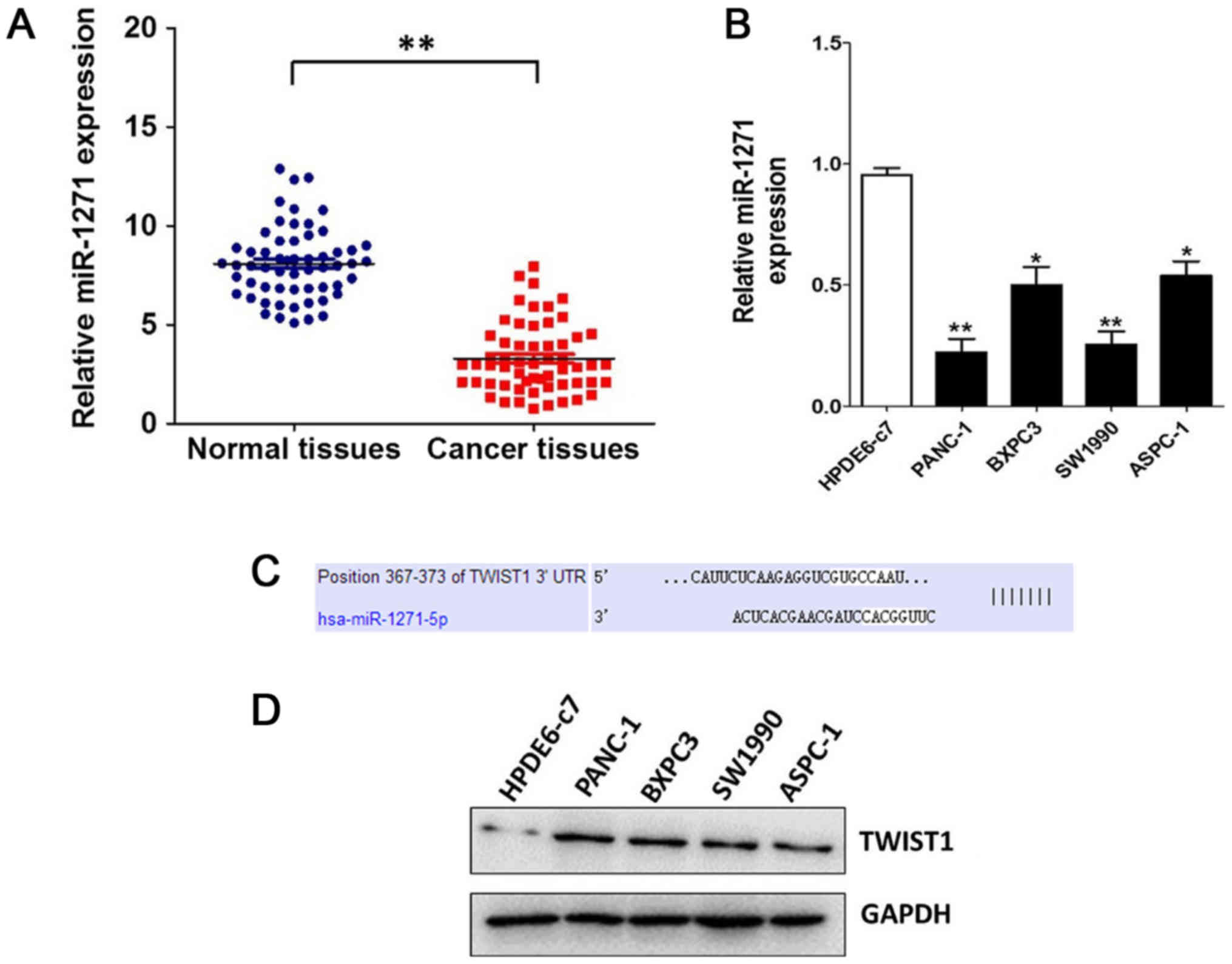
The level of miR-1271 in pancreatic cancer tissues
and cells were detected by qRT-PCR. As shown in Fig. 1, the level of miR-1271
significantly down-regulated in both pancreatic cancer tissues and
cell lines. According to the results of TargetScan, we found that
Twist1 may be a target gene of miR-1271. Compared with the human
pancreatic ductal epithelial cell line HPDE6c7, Twist1 was
up-regulated in human pancreatic cancer cell lines SW1990, BXPC3,
PANC-1 and ASPC-1.
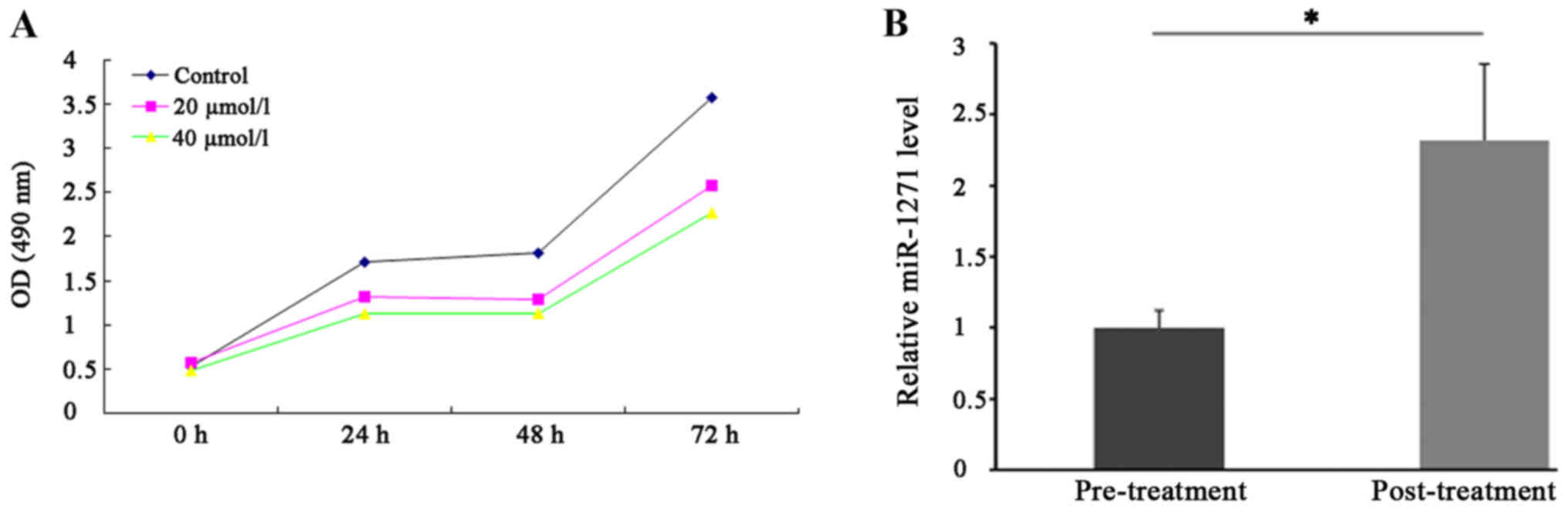
Emodin inhibits SW1990 cell
proliferation ability
As shown in Fig.
2A, we found that emodin significantly inhibited SW1990 cell
proliferation ability in a dose- and time-dependent manner. In
addition, our findings suggested that emodin treatment markedly
enhanced the expression level of miR-1271 in SW1990 cells (Fig. 2B).
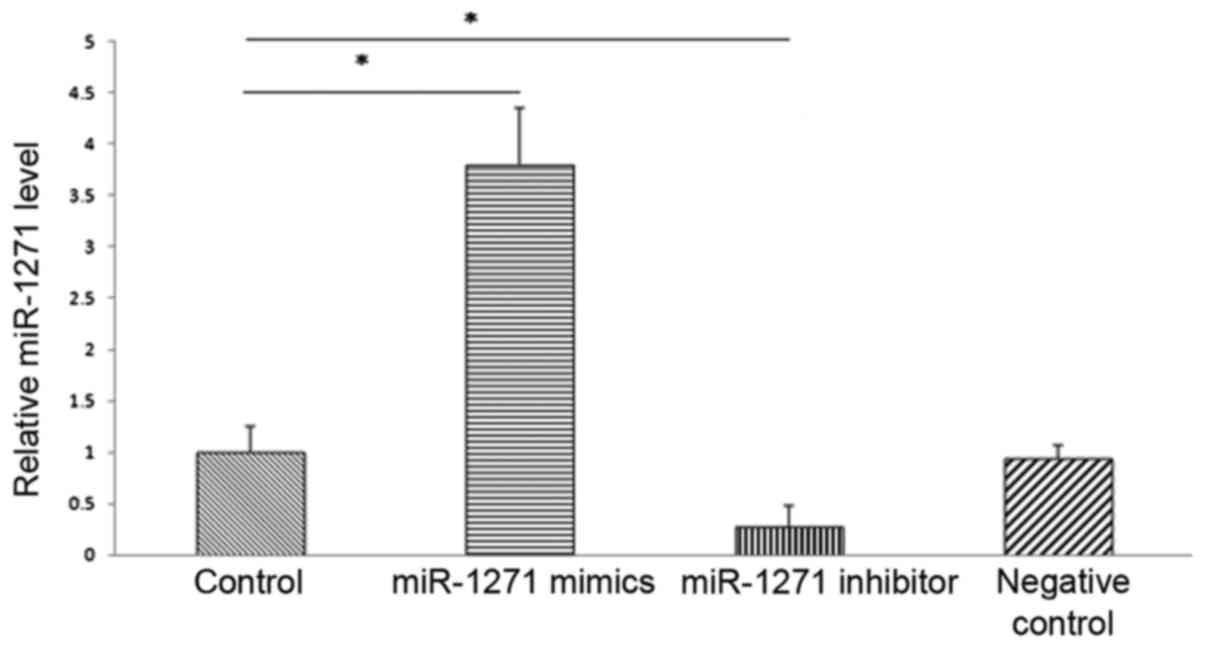
Effect of miRNA-1271 on EMT in SW1990
cells
SW1990 cells were transiently transfected with
miR-1271 mimics, miR-1271 inhibitor and empty plasmid as control.
48 h after transfection, transfection efficiency was detected using
qRT-PCR, and the results indicated that compared with the control
group, miRNA-1271 mimics significantly enhanced miR-1271
expression, while miR-1271 inhibitor significantly inhibited
miR-1271 expression (Fig. 3).
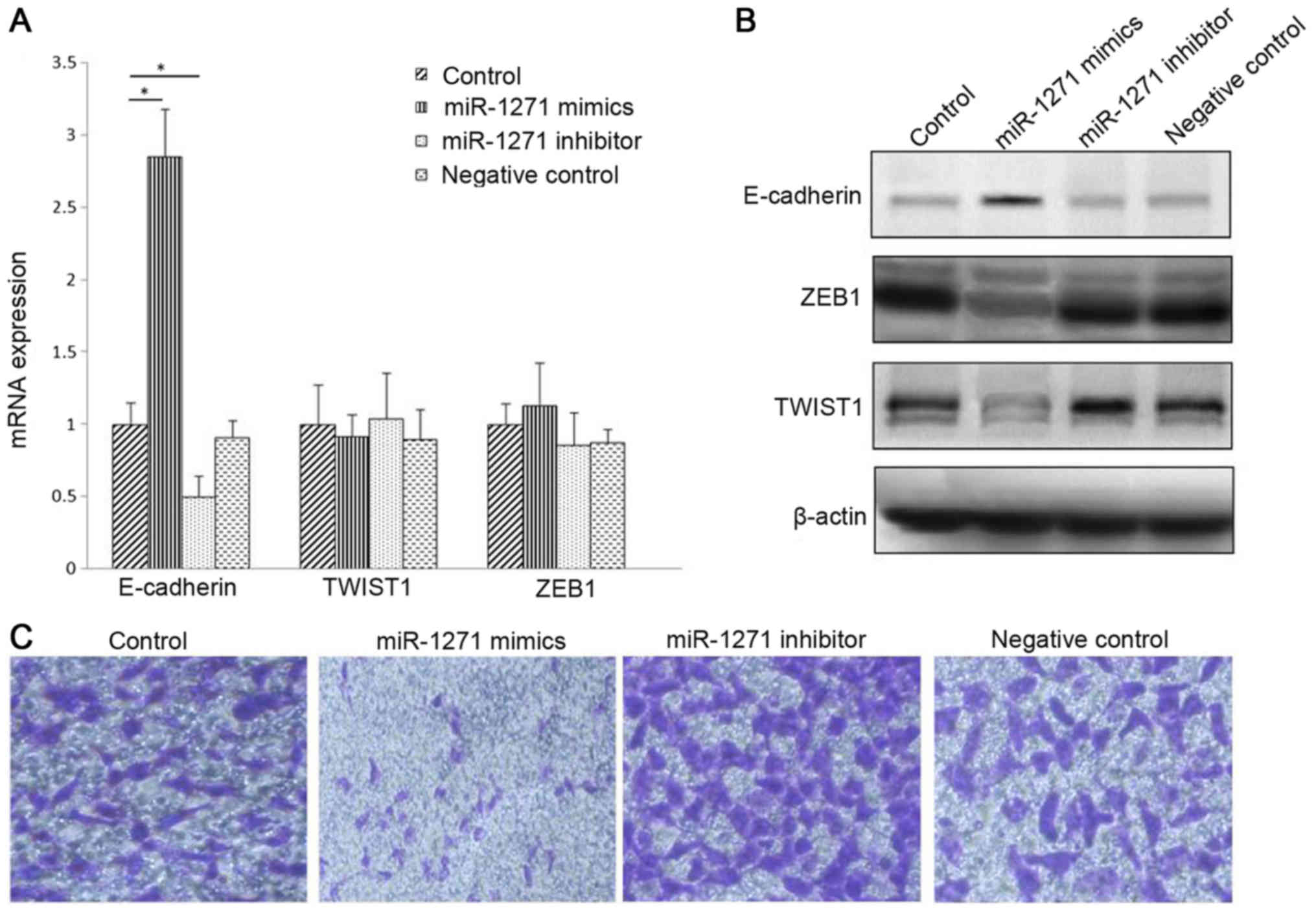
qRT-PCR also showed that compared with other experimental groups,
miR-1271 and E-cadherin mRNA expression levels were increased in
transfected miR-1271 mimics group, and mRNA levels of ZEB1 and
TWIST1 in each experimental group had no significant difference
(Fig. 4A). Western blot assay
showed that the expression of E-cadherin protein was increased
while the expression of ZEB1 and TWIST1 protein was decreased in
the miR-1271 mimics group. In the miR-1271 inhibitor group, the
opposite result was found, the E-cadherin protein level was the
lowest, while the protein expression levels of ZEB1 and TWIST1 were
significantly higher than the other groups (Fig. 4B). Transwell chamber model
experiments showed that the invasion ability of SW1990 cells in
miR-1271 mimic group was significantly lower than the other groups
(Fig. 4C). The results indicated
that miR-1271 can significantly inhibit pancreatic cancer EMT
process. MiR-1271 may act on the post-transcriptional phase of ZEB1
and TWIST1 gene, but not the mRNA expression level.
Effect of emodin on the level of
miR-1271 and EMT marker in SW1990 cells
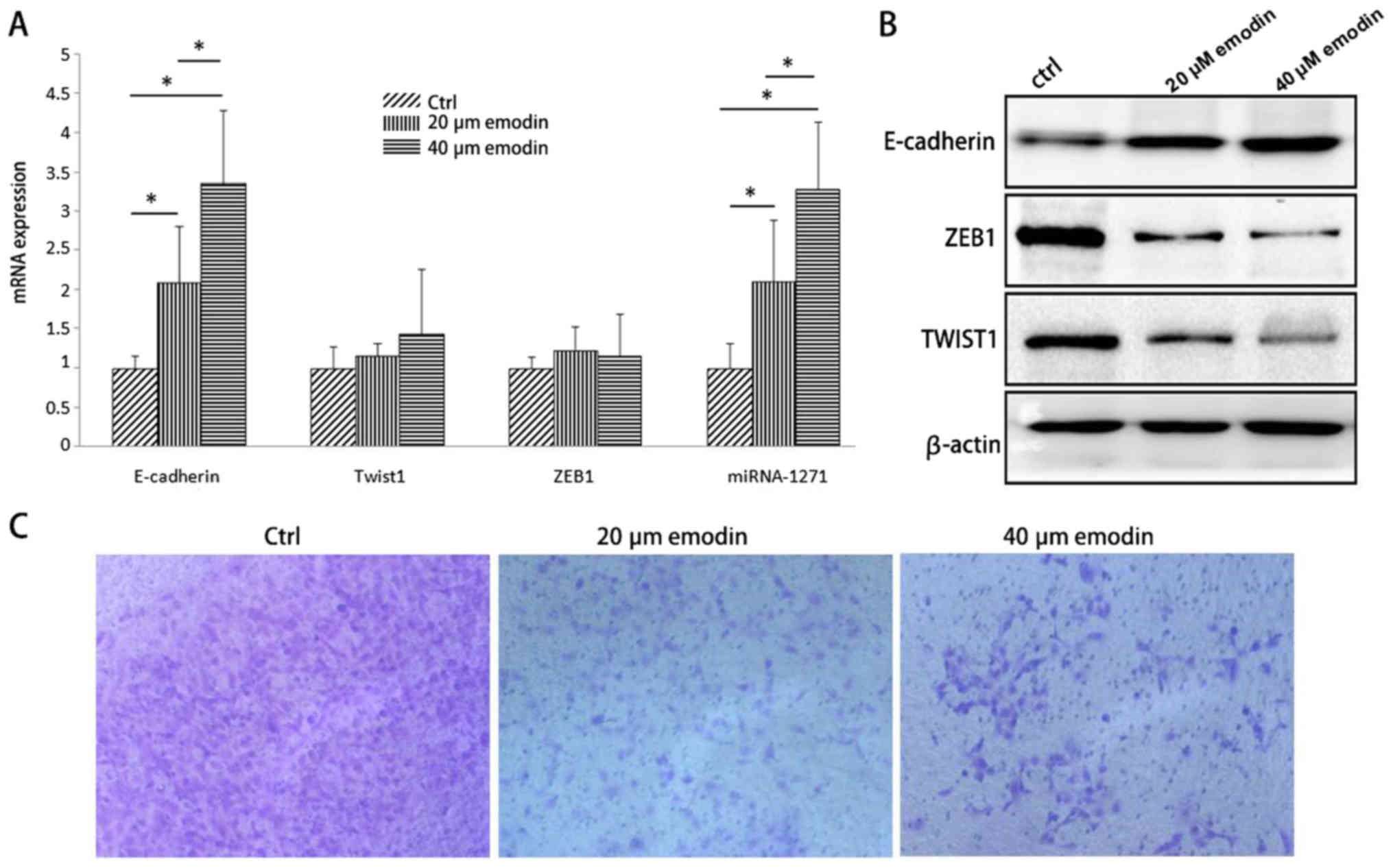
SW1990 cells were treated with various
concentrations (0, 20, 40 µmol/l) of emodin for 48 h. Then we found
that the mRNA expression levels of miRNA-1271 and E-cadherin in
SW1990 cells were increased at 20 and 40 µmol of emodin treatment,
while the mRNA levels of ZEB1 and TWIST1 were not significantly
different (Fig. 5A). When emodin
was added, the expression of E-cadherin protein increased, while
the expression of ZEB1 and TWIST1 protein decreased (Fig. 5B). Transwell chamber model
experiments showed that after emodin treatment, cell invasion of
SW1990 cells significantly decreased compared with the control
group, while the EMT inhibition effect is more significant in 40
µmol/l emodin treatment group (Fig.
5C). The experimental results indicated that emodin can inhibit
the EMT of pancreatic cancer cells by increasing the level of
miR-1271 in pancreatic cancer cells.
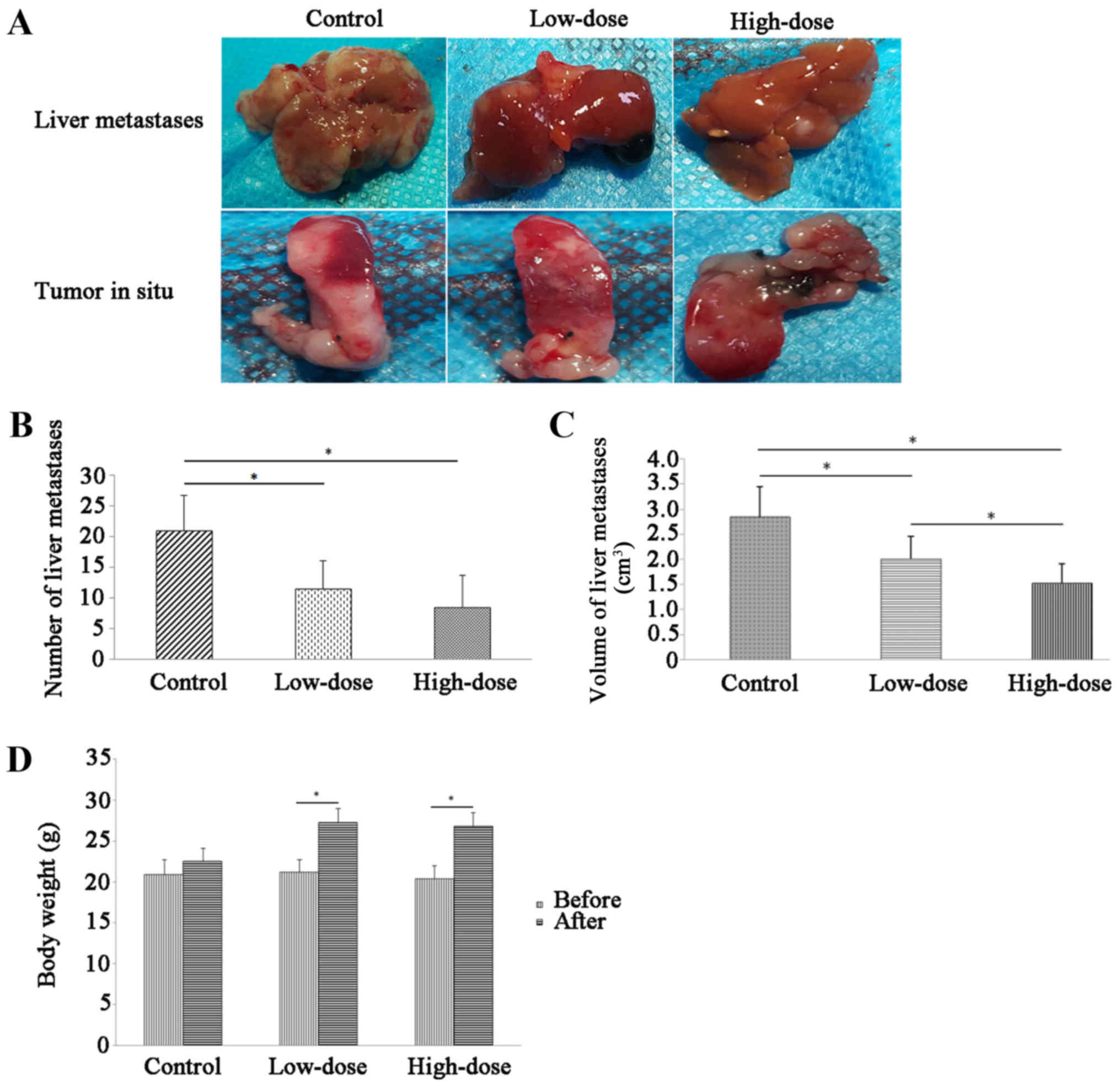
Inhibitory effect of emodin on hepatic
metastasis of pancreatic cancer in vivo
In 20 and 50 mg/kg emodin administration group, the
number of metastatic nodules, the proliferation inhibition rate and
the inhibition rate of hepatic metastasis in nude mice were better
than those in the control group (Fig.
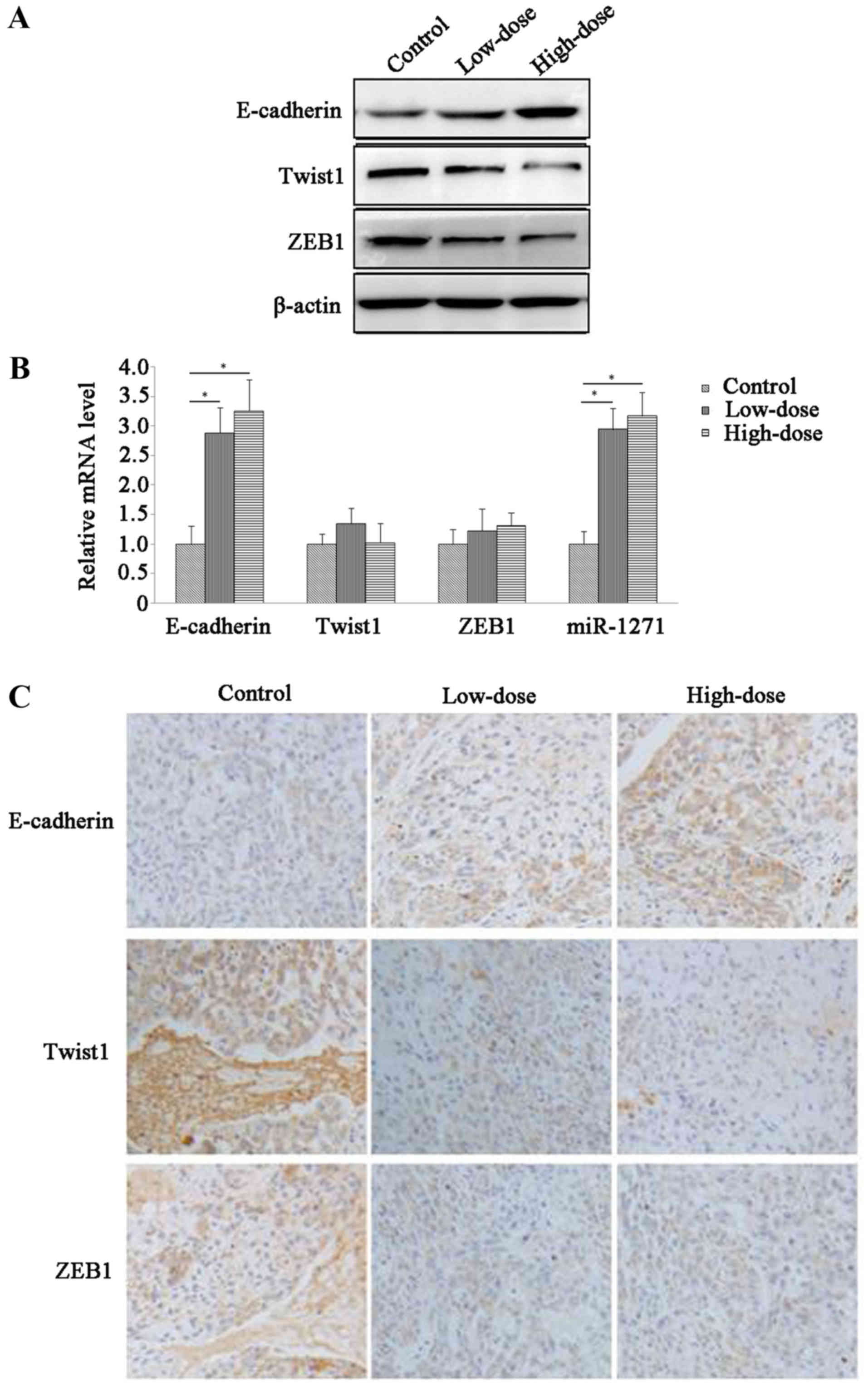
6). Emodin administration significantly increased the level of
miR-1271 and mRNA level of E-cadherin in pancreatic cancer hepatic
metastasis tissues (Fig. 7B). The
results of western blot analysis (Fig.
7A) and immunohistochemistry (Fig.
7C) showed that emodin administration significantly increased
E-cadherin protein level, however, the expression of ZEB1 and
Twist1 protein significantly decreased. In vivo experiments
showed that emodin can inhibit the EMT of pancreatic cancer cells
by increasing the content of miR-1271 in pancreatic cancer, and
then exert the therapeutic and preventive effects on the metastasis
of pancreatic cancer.
Discussion
Pancreatic cancer is a malignant digestive system
tumor with extremely high mortality rate. Currently, therapies such
as surgery, radiotherapy and chemotherapy for pancreatic cancer are
not effective (27). Therefore,
methods that can effectively treat pancreatic cancer and inhibit
its metastasis are the focus of current pancreatic cancer related
research. A large number of studies have indicated that a variety
of miRNAs are closely related to tumor development. MiR-1271, a
kind of miRNA that has tumor suppressor effect, is involved in the
inhibition of EMT, and induces the apoptosis of tumor cells
(18–22). However, there are few researches on
the effect of miR-1271 on pancreatic cancer cells and its
regulation mechanism. The present study found that miR-1271 was
significantly down-regulated in pancreatic cancer, and it can
inhibit EMT in pancreatic cancer cells.
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is a
naturally occurring pupae present in various plants, especially in
roots and barks (28). Emodin has
many pharmacological effects, such as antibacterial, antiviral
effects, anti-inflammatory effects as well as anticancer effects
(29–31). The anti-tumor drug emodin has been
shown to affect many different tumor cell lines and inhibit the
growth of leukemia, breast cancer, colon cancer and lung cancer
cells, etc (31–34). Moreover, although studies have
indicated the anti-cancer effect of emodin on pancreatic carcinoma
(35,36), the mechanism of action of emodin in
inhibiting the development of pancreatic cancer remains
incompletely elucidated. Therefore, we conducted the current study.
And we found that emodin could inhibit the proliferation ability of
pancreatic cancer cells and increased miR-1271 expression level in
pancreatic cancer cells. Moreover, we found that emodin inhibited
pancreatic cancer cell EMT by raising the level of miR-1271.
E-cadherin, a member of the cadherin family of cell
adhesion molecules, is an important intercellular adhesion molecule
and is now recognized as a cancer metastasis suppressor molecule
(when E-cadherin expression is reduced, tumors are more likely to
metastasize) (25). Twist1, a
apoptosis inhibitor protein found in recent ten years, is an
important regulatory factor in the development of malignant tumor
cells and EMT (24). ZEB (zinc
finger E-boxbinding homeobox) is an important transcription factor
whose family members include ZEB1 and ZEB2, which are important
condition factors for EMT (37).
In our study, we showed that miR-1271 may act on ZEB1 and TWIST1 at
the post-transcriptional phase, but not the mRNA level. In
addition, we found that emodin can inhibit the EMT of pancreatic
cancer cells by increasing the level of miR-1271 in pancreatic
cancer cells.
In vivo experiments showed that emodin can
inhibit the EMT of pancreatic cancer cells by increasing the
content of miR-1271 in pancreatic cancer, and then exert the
therapeutic and preventive effects on the metastasis of pancreatic
cancer.
The data of our research suggested that miR-1271
significantly decreased in pancreatic cancer cells and tissues.
Twist1 may be a target gene of miR-1271. MiR-1271 significantly
inhibited pancreatic cancer cell SW1990 EMT and invasive ability,
and emodin could inhibit SW1990 cell EMT by raising the level of
miR-1271. In addition, we found that emodin inhibited the liver
metastasis of pancreatic cancer by inhibiting EMT in vivo.
We provide theoretical and experimental evidence for the further
development of miRNA-based targeted therapy of pancreatic
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NL designed the study. CW was responsible for data
access and analysis. PZ performed literature analysis. NL, CW, PZ
and SY interpreted the results and developed the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee Review Board at The General Hospital of Tianjin Medical
University (Tianjin, China). Written informed consent was provided
by every patient. The animal experiments performed in the present
study were approved by the Animal Ethics Committee Review Board at
Tianjin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klein AP: Identifying people at a high
risk of developing pancreatic cancer. Nat Rev Cancer. 13:66–74.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heinemann V, Reni M, Ychou M, Richel DJ,
Macarulla T and Ducreux M: Tumour-stroma interactions in pancreatic
ductal adenocarcinoma: Rationale and current evidence for new
therapeutic strategies. Cancer Treat Rev. 40:118–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yachida S and Iacobuzio-Donahue CA: The
pathology and genetics of metastatic pancreatic cancer. Arch Pathol
Lab Med. 133:413–422. 2009.PubMed/NCBI
|
|
7
|
Simard EP, Ward EM, Siegel R and Jemal A:
Cancers with increasing incidence trends in the United States: 1999
through 2008. CA Cancer J Clin. 62:118–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
11
|
Kalla R, Ventham NT, Kennedy NA, Quintana
JF, Nimmo ER, Buck AH and Satsangi J: MicroRNAs: New players in
IBD. Gut. 64:504–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Dahlberg JE and Tam W: MicroRNAs
in tumorigenesis. Am J Pathol. 171:728–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou N and Mo YY: Roles of microRNAs in
cancer stem cells. Front biosci (School Ed). 4:810–818. 2012.
|
|
14
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vetter G, Saumet A, Moes M, Vallar L, Le
Béchec A, Laurini C, Sabbah M, Arar K, Theillet C, Lecellier CH and
Friederich E: miR-661 expression in SNAI1-induced epithelial to
mesenchymal transition contributes to breast cancer cell invasion
by targeting Nectin-1 and StarD10 messengers. Oncogene.
29:4436–4448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Duan P, Wang J, Lu X and Cheng J:
miR-320 inhibited ovarian cancer oncogenicity via targeting TWIST1
expression. Am J Transl Res. 9:3705–3713. 2017.PubMed/NCBI
|
|
17
|
Jensen KP and Covault J: Human miR-1271 is
a miR-96 paralog with distinct non-conserved brain expression
pattern. Nucleic Acids Res. 39:701–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maurel M, Jalvy S, Ladeiro Y, Combe C,
Vachet L, Sagliocco F, Bioulac-Sage P, Pitard V, Jacquemin-Sablon
H, Zucman-Rossi J, et al: A functional screening identifies five
microRNAs controlling glypican-3: Role of miR-1271 down-regulation
in hepatocellular carcinoma. Hepatology. 57:195–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Z, Niu X, Li C, Sheng S and Lu S:
Inhibition of the growth of non-small cell lung cancer by
miRNA-1271. Am J Transl Res. 7:1917–1924. 2015.PubMed/NCBI
|
|
20
|
Yu T, Yu HR, Sun JY, Zhao Z, Li S, Zhang
XF, Liao ZX, Cui MK, Li J, Li C and Zhang Q: miR-1271 inhibits ERα
expression and confers letrozole resistance in breast cancer.
Oncotarget. 8:107134–107148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Wang H, Liu X and Yu T: miR-1271
inhibits migration, invasion and epithelial-mesenchymal transition
by targeting ZEB1 and TWIST1 in pancreatic cancer cells. Biochem
Biophys Res Commun. 472:346–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding
Y, Shu Y and Liu P: miR-1271 regulates cisplatin resistance of
human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR and
BCL2. Anticancer Agents Med Chem. 14:884–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galván JA, Zlobec I, Wartenberg M, Lugli
A, Gloor B, Perren A and Karamitopoulou E: Expression of E-cadherin
repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells
influences tumour-budding phenotype and suggests heterogeneity of
stromal cells in pancreatic cancer. Br J Cancer. 112:1944–1950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Peng H, Xiao J, Guan A, Xie B, He
B and Chen Q: Benzo(a)pyrene enhances the EMT-associated migration
of lung adenocarcinoma A549 cells by upregulating Twist1. Oncol
Rep. 38:2141–2147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma L, Liu L, Ma Y, Xie H, Yu X, Wang X,
Fan A, Ge D, Xu Y, Zhang Q and Song C: The role of
E-Cadherin/β-catenin in hydroxysafflor yellow a inhibiting
adhesion, invasion, migration and lung metastasis of hepatoma
cells. Biol Pharm Bull. 40:1706–1715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du L and Wang-Gillam A: Trends in
neoadjuvant approaches in pancreatic cancer. J Natl Compr Canc
Netw. 15:1070–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Srinivas G, Babykutty S, Sathiadevan PP
and Srinivas P: Molecular mechanism of emodin action: Transition
from laxative ingredient to an antitumor agent. Med Res Rev.
27:591–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dey D, Ray R and Hazra B: Antitubercular
and antibacterial activity of quinonoid natural products against
multi-drug resistant clinical isolates. Phytother Res.
28:1014–1021. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu J, Cui CF, Yang L, Wang L and Jiang XH:
Emodin inhibits colon cancer cell invasion and migration by
suppressing epithelialmesenchymal transition via the Wnt/β-catenin
pathway. Oncol Res. 2018.doi: 10.3727/096504018X15150662230295.
View Article : Google Scholar
|
|
32
|
Min H, Niu M, Zhang W, Yan J, Li J, Tan X,
Li B, Su M, Di B and Yan F: Emodin reverses leukemia multidrug
resistance by competitive inhibition and downregulation of
P-glycoprotein. PLoS One. 12:e01879712017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tseng HS, Wang YF, Tzeng YM, Chen DR, Liao
YF, Chiu HY and Hsieh WT: Aloe-emodin enhances tamoxifen
cytotoxicity by suppressing ras/ERK and PI3K/mTOR in breast cancer
cells. Am J Chin Med. 45:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Li L, Guan R, Zhu D, Song N and
Shen L: Emodin inhibits ATP-induced proliferation and migration by
suppressing P2Y receptors in human lung adenocarcinoma cells. Cell
Physiol Biochem. 44:1337–1351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu L, Cui R, Liu H and Wang F: Emodin and
rhein decrease levels of hypoxia-inducible factor-1α in human
pancreatic cancer cells and attenuate cancer cachexia in athymic
mice carrying these cells. Oncotarget. 8:88008–88020. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan FP, Zhou HK, Bu HQ, Chen ZQ, Zhang H,
Xu LP, Tang J, Yu QJ, Chu YQ, Pan J, et al: Emodin enhances the
demethylation by 5-Aza-CdR of pancreatic cancer cell
tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep.
35:1941–1949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mooney SM, Talebian V, Jolly MK, Jia D,
Gromala M, Levine H and McConkey BJ: The GRHL2/ZEB feedback loop-a
key axis in the regulation of emt in breast cancer. J Cell Biochem.
118:2559–2570. 2017. View Article : Google Scholar : PubMed/NCBI
|