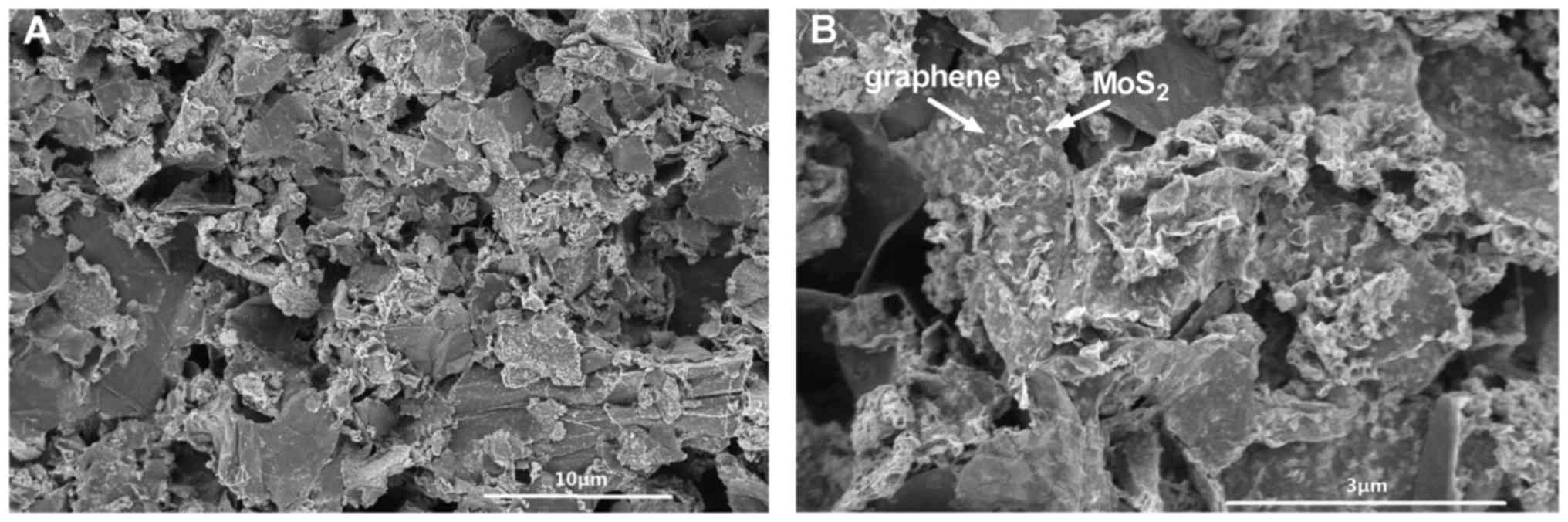
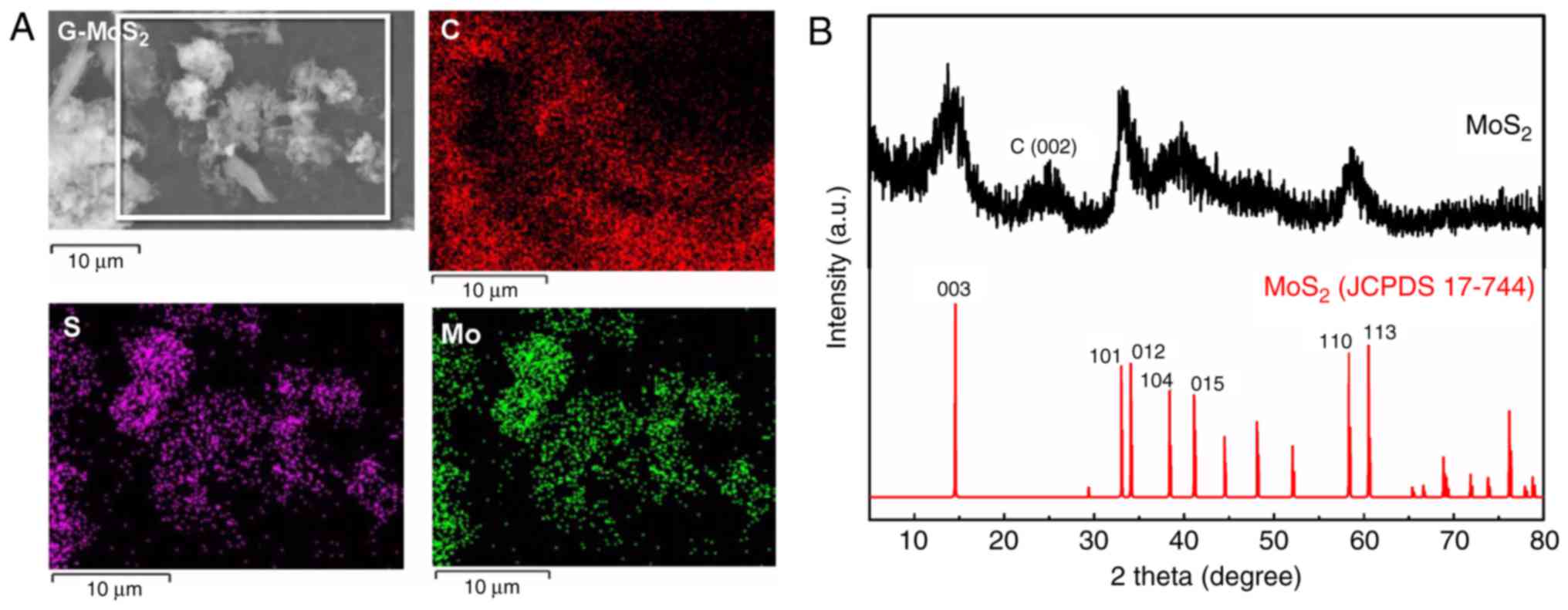
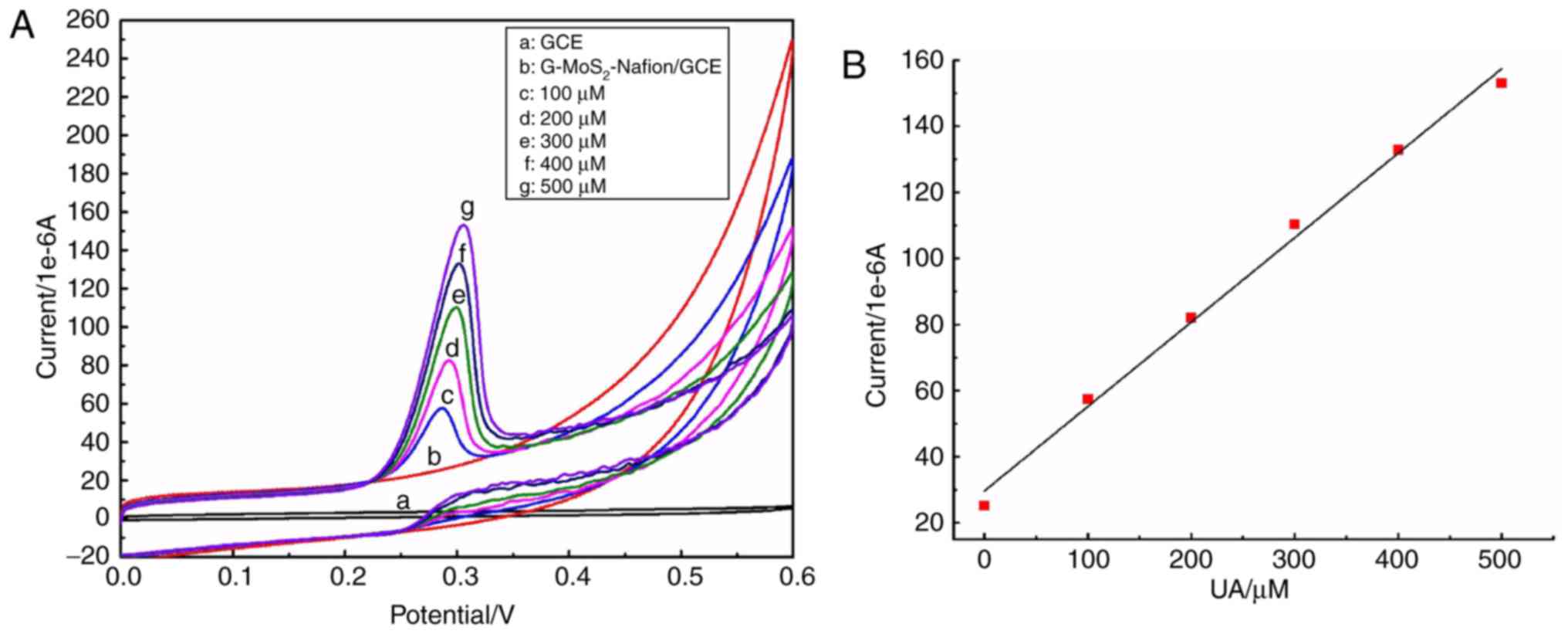
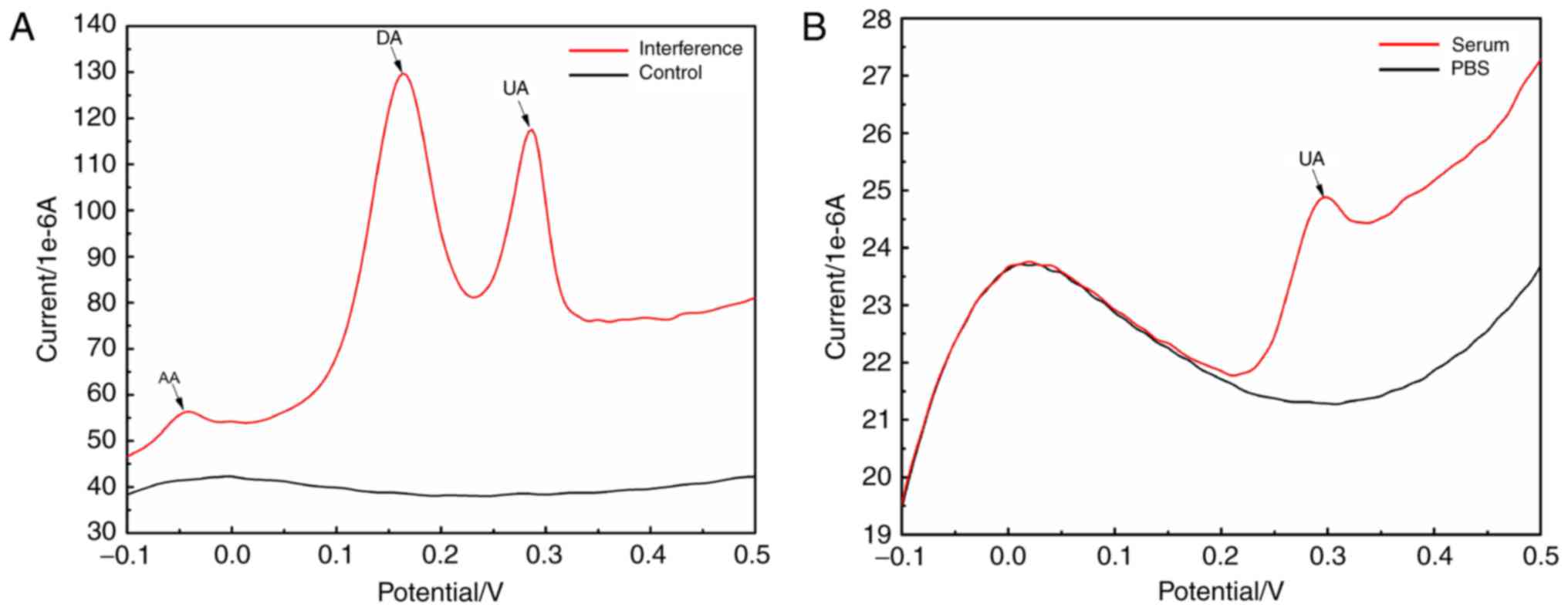
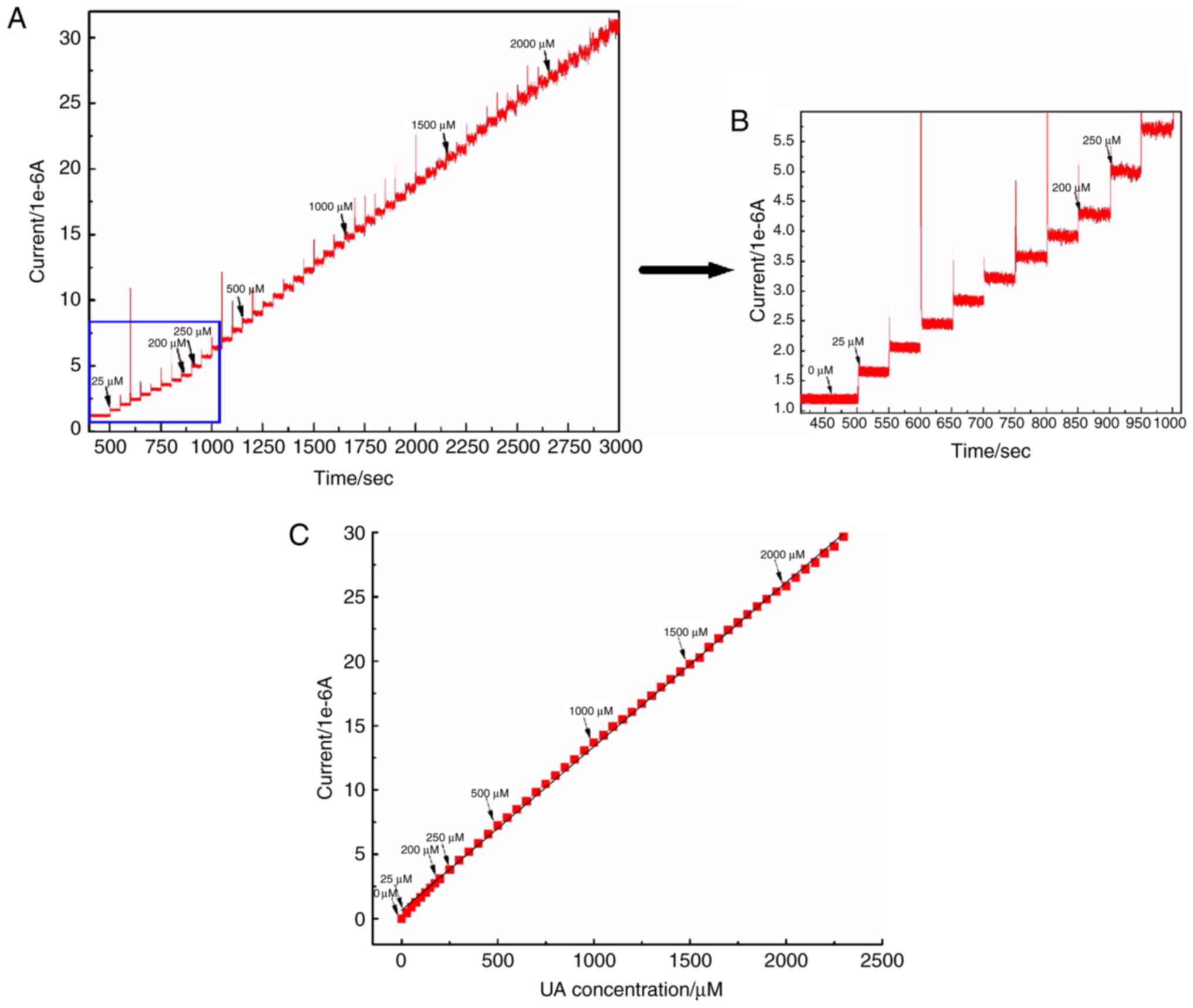
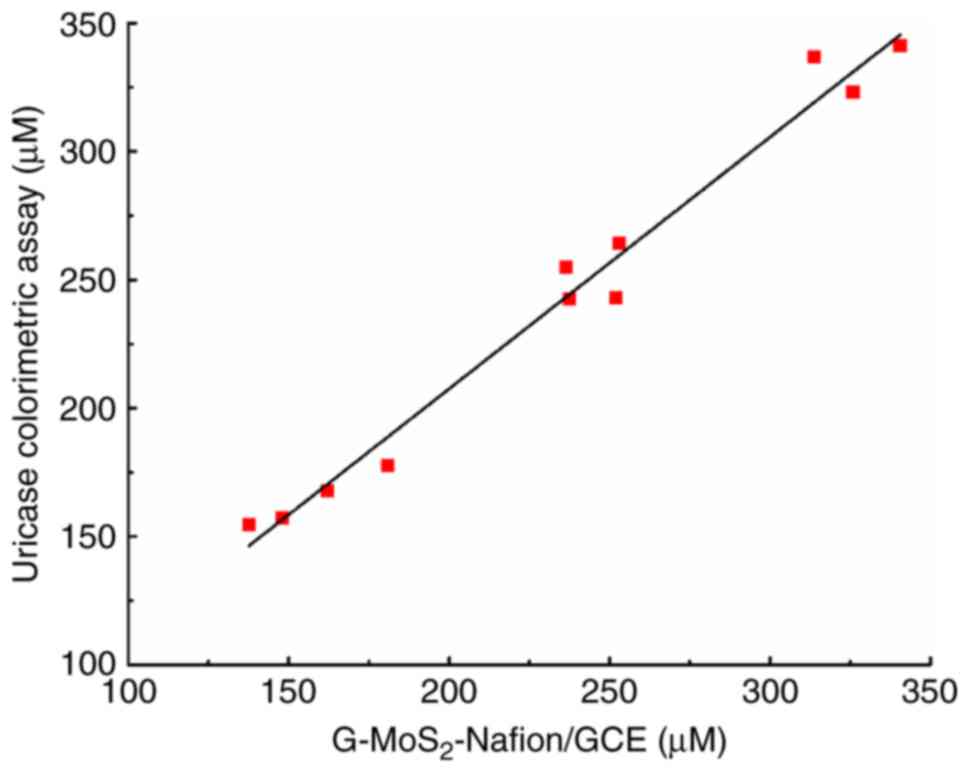
|
1
|
Popa E, Kubota Y, Tryk DA and Fujishima A:
Selective voltammetric and amperometric detection of uric acid with
oxidized diamond film electrodes. Anal Chem. 72:1724–1727. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardoso AS, Gonzaga NC, Medeiros CC and
Carvalho DF: Association of uric acid levels with components of
metabolic syndrome and non-alcoholic fatty liver disease in
overweight or obese children and adolescents. Pediatr. 89:412–418.
2013. View Article : Google Scholar
|
|
3
|
Gabison L, Chiadmi M, Colloc'h N, Castro
B, El Hajji M and Prangé T: Recapture of [S]-allantoin, the product
of the two-step degradation of uric acid, by urate oxidase. FEBS
Lett. 580:2087–2091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Filisetti-Cozzi TM and Carpita NC:
Measurement of uric acids without interference from neutral sugars.
Anal Biochem. 197:157–162. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue K, Namiki T, Iwasaki Y, Yoshimura Y
and Nakazawa H: Determination of uric acid in human saliva by
high-performance liquid chromatography with amperometric
electrochemical detection. J Chromatogr B Analyt Technol Biomed
Life Sci. 785:57–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novoselov KS, Geim AK, Morozov SV, Jiang
D, Zhang Y, Dubonos SV, Grigorieva IV and Firsov AA: Electric field
effect in atomically thin carbon films. Science. 306:666–669. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun CC, Luo FF, Wei L, Lei M, Li GF, Liu
ZL, LE WD and Xu PY: Association of serum uric acid levels with the
progression of Parkinson's disease in chinese patients. Chin Med J
(Engl). 125:583–587. 2012.PubMed/NCBI
|
|
8
|
Li S, Qian T, Wu S and Shen J:
Controllable fabrication of polystyrene/graphene core-shell
microspheres and its application in high-performance
electrocatalysis. Chem Commun (Camb). 48:7997–7999. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zen JM and Chen PJ: A selective
voltammetric method for uric acid and dopamine detection using
clay-modified electrodes. Anal. Chem. 69:5087–5093. 1997.
|
|
10
|
Geim AK and Novoselov KS: The rise of
grapheme. Nat Mater. 6:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Service RF: Carbon sheets an atom thick
give rise to graphene dreams. Science. 324:875–877. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KS, Zhao Y, Jang H, Lee SY, Kim JM,
Kim KS, Ahn JH, Kim P, Choi JY and Hong BH: Large-scale pattern
growth of graphene films for stretchable transparent electrodes.
Nature. 457:706–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee C, Wei X, Kysar JW and Hone J:
Measurement of the elastic properties and intrinsic strength of
monolayer graphene. Science. 321:385–388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JH, Jang C, Xiao S, Ishigami M and
Fuhrer MS: Intrinsic and extrinsic performance limits of graphene
devices on SiO2. Nat Nanotechnol. 3:206–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balandin AA, Ghosh S, Bao W, Calizo I,
Teweldebrhan D, Miao F and Lau CN: Superior thermal conductivity of
single-layer graphene. Nano Lett. 8:902–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Huang Y, Song Y, Zhang X, Ma Y,
Liang J and Chen Y: Room-temperature ferromagnetism of graphene.
Nano Lett. 9:220–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stepnowski P, Müller A, Behrend P, Ranke
J, Hoffmann J and Jastorff B: Reversed-phase liquid chromatographic
method for the determination of selected room-temperature ionic
liquid cations. J Chromatogr A. 993:173–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruiz-Angel MJ and Berthod A: Reversed
phase liquid chromatography of alkyl-imidazolium ionic liquids. J
Chromatogr A. 1113:101–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Zhang Q, Shan C, Li F, Han D and
Niu L: Stable, conduetive supramolecular composite of graphene
sheets with conjugated polyelectrolyte. Langmuir. 26:6708–6712.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niyogi S, Bekyarova E, Itkis ME,
McWilliams JL, Hamon MA and Haddon RC: Solution properties of
graphite and graphene. Am Chem Soc. 128:7720–7721. 2006. View Article : Google Scholar
|
|
21
|
Liu WW and Wang JN: Direct exfoliation of
graphene in organic solvents with addition of NaOH. Chem Commun.
47:6888–6890. 2011. View Article : Google Scholar
|
|
22
|
Balendhran S, Ou JZ, Bhaskaran M, Sriram
S, Ippolito S, Vasic Z, Kats E, Bhargava S, Zhuiykov S and
Kalantar-Zadeh K: Atomically thin layers of MoS2 via a two step
thermal evaporation-exfoliation method. Nanoscale. 4:461–466. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stephenson T, Li Z, Olsen B and Mitlin D:
Lithium ion battery applications of molybdenum disulfide (MoS2)
nanocomposites. Energ Environ Sci. 7:209–231. 2014. View Article : Google Scholar
|
|
24
|
Viemeusel B, Schneider T, Tremmel S,
Wartzack S and Gradt T: Humidity resistant MoS2 coatings deposited
by unbalanced magnetron sputtering. Surf Coat Tech. 235:97–107.
2013. View Article : Google Scholar
|
|
25
|
Viet Hung, pHam, Kwang-Hyun Kim, Dong-won
Jung, Pham VH, Kim KH, Jung DW, Singh K, Oh ES and Chung JS: Liquid
phase co-exfoliated MoS2-graphene composites as anode materials for
lithium ion batteries. J Power Sources. 244:280–286. 2013.
View Article : Google Scholar
|
|
26
|
Sun MY, Adjaye J and Nelson AE:
Theoretical investigations of the structures and properties of
molybdenum-based sulfide catalysts. Applied Catalysis A.
263:131–143. 2004. View Article : Google Scholar
|
|
27
|
Chen J, Kuriyama N, Yuan H, Takeshita HT
and Sakai T: Electrochemical hydrogen storage in MoS2 nanotubes. J
Am Chem Soc. 123:11813–11814. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua KH, Hua XG and Sun XJ: MorpHological
effect of MoS2 nanoparticles on catalytic oxidation and vacuum
lubrication. Applied Sur Sci. 256:2517–2523. 2010. View Article : Google Scholar
|
|
29
|
Xiao J, Choi DW, Cosimbescu L, Koech P,
Liu J and Lemmon JP: Exfoliated MoS2 nanocomposite as an anode
material for lithium ion batteries. Chem. Mater. 22:4522–4524.
2010.
|
|
30
|
Du G, Guo Z, Wang S, Zeng R, Chen Z and
Liu H: Superior stability and high capacity of restacked molybdenum
disulfide as anode material for lithium ion batteries. Chem Commun
(Camb). 46:1106–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen CM, Yang QH, Yang YG, Lv W, Wen Y,
Hou PX, Wang M and Cheng HM: Self-assembled free-standing graphite
oxide membrane. Adv Mater. 21:3007–3011. 2009. View Article : Google Scholar
|
|
32
|
Li Y, Wang H, Xie L, Liang Y, Hong G and
Dai H: MoS2 nanoparticles grown on graphene: an advanced catalyst
for the hydrogen evolution reaction. J Am Chem Soc. 133:7296–7299.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Zhang Z and Chen Y:
Morphology-controlled synthesis of MoS2 nanostructures with
different lithium storage properties. J All Comp. 600:84–89. 2014.
View Article : Google Scholar
|
|
34
|
Li XY, Ai LH and Jiang J: Nanoscale
zerovalent iron decorated on graphene nanosheets for Cr(VI) removal
from aqueous solution: Surface corrosion retard induced the
enhanced performance. Chem Eng J. 288:789–797. 2015. View Article : Google Scholar
|
|
35
|
Szentimay MN and Martin CR: Ion-exchange
selectivity of nafion films on electrode surfaces. Anal Chem.
56:1898–1902. 1984. View Article : Google Scholar
|
|
36
|
Hummers WS and Offeman RE: Preparation of
graphitic oxide. Am Chem Soc. 80:13391958. View Article : Google Scholar
|
|
37
|
Brodie BC: Bibiographic notices. Doublic J
Mecical Sci. 22:351–379. 1855.
|
|
38
|
Staudenmaier L: Verfahren zur darstellung
der Graphitsaure. Berichte der deutschen chemischen Gesellschaft.
31:1481–1487. 1898. View Article : Google Scholar
|
|
39
|
Yu L, Lee YH, Ling X, Santos EJ, Shin YC,
Lin Y, Dubey M, Kaxiras E, Kong J, Wang H and Palacios T:
Graphene/MoS2 hybrid technology for large-scale two-dimensional
electronics. Nano Lett. 14:3055–3063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang JW and Park HS: Mechanical
properties of MoS2/graphene heterostructures. App Phy Lett.
105:033–108. 2014. View Article : Google Scholar
|
|
41
|
Nengjie H, Kang J, Wei Z, Li SS, Li j and
Wei SH: Novel and Enhanced Optoelectronic Performances of
multilayer MoS2-WS2heterostructure transistors. Adv Fun Mat.
24:7025–7031. 2014. View Article : Google Scholar
|
|
42
|
Meng F, Li J and Cushing SK: Solar
hydrogen generation by nanoscale p-n junction of p-type molybdenum
disulfide/n-type nitrogen-doped reduced graphene oxide. J Am Chem
Soc. 135:10286–10289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang K and Chen W: In situ synthesis of
MoS2/graphene nanosheet composites with extraordinarily high
electrochemical performance for lithium ion batteries. Chem Commun.
47:4252–4254. 2011. View Article : Google Scholar
|
|
44
|
Wang Z, Chen T, Chen W, Chang K, Ma L,
Huang G and Chen D JM: CTAB-assisted synthesis of single-layer
MoS2-graphene composites as anode materials of Li-ion batteries. J
Mat Chem A. 1:2202–2210. 2013. View Article : Google Scholar
|