Introduction
Gastric cancer is the fourth most prevalent cancer
worldwide (1,2) and the second leading cause of
cancer-associated mortality (3).
Despite the important advances in cancer therapy, gastric cancer
remains a major malignancy and a serious threat to human health
(4). Surgical, radiotherapeutic
and chemotherapeutic strategies have recently become available for
the treatment of gastric cancer (5); however, radiotherapy and chemotherapy
exert severe adverse effects, and the development of drug
resistance is common. Therefore, it is necessary to develop novel,
effective and safe agents for the treatment of gastric cancer.
Recently, natural products have attracted attention. Studies have
reported that compounds from natural resources are suitable
alternatives for controlling cancer with minimal toxicity and high
efficacy (6–8).
Isoliquiritigenin (ISL) is a flavonoid with a
chalcone structure that is derived from licorice compounds
(9,10). It is found ubiquitously in foods
and beverages, and tobacco. ISL has been reported to exhibit a wide
range of distinct biological properties and pharmacological
activities, including anti-inflammatory, anti-oxidation,
antiplatelet aggregation, cardioprotective effects against
ischemia-reperfusion, and estrogenic properties (11–13).
ISL has also been reported to suppress the proliferation and to
induce the apoptosis of numerous cancers in vitro and in
vivo (14), including colon
and breast cancer (15–18).
At present, the anticancer effects and underlying
mechanisms of ISL on gastric cancer have not been fully elucidated.
The present study conducted a series of preliminary experiments to
suggest that ISL may inhibit the proliferation, migration and
invasion of MKN28 gastric cancer cells, which may be associated
with phosphoinositide 3-kinase (PI3K)/protein kinase B
(AKT)/mammalian target of rapamycin (mTOR) signaling
pathway-mediated apoptosis and autophagy.
Materials and methods
Cell culture and treatment
The MKN28 human gastric cancer cell line and the
GES-1 human gastric normal epithelial mucosa cell line were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), penicillin (100 U/ml)
and streptomycin (0.1 mg/ml; both Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and incubated at 37°C in a humidified
atmosphere containing 5% CO2. When the cells entered the
logarithmic growth phase, they were washed with PBS three times,
and were then digested by trypsin (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). Once the cells became
rounded, the digestion was terminated and the cells were placed in
culture medium, after which they were pipetted into 6-well plates
for subsequent experiments. When cell confluence in the wells
reached ~80%, the cells were treated with ISL (MedChemExpress,
Monmouth Junction, NJ, USA) or 0.1% dimethyl sulfoxide (DMSO;
Amresco, LLC, Solon, OH, USA) as a negative control (NC).
Cell Counting Kit-8 (CCK8)
proliferation detection
CCK-8 (Beijing Solarbio Science & Technology
Co., Ltd. Beijing, China) was used to assess the effects of ISL on
MKN28 and GES-1 cell proliferation. MKN28 or GES-1 cells (100 µl)
were seeded into 96-well plates at a cellular density of 1,000
cells/well. To the NC group, 0.1% DMSO was added, whereas 20 µM ISL
was added to the experimental group. Cells were cultured for 0, 24,
48 and 72 h time intervals at 37°C, and the cell viabilities at
each time interval were subsequently detected. Prior to detection,
10 µl CCK8 solution was added to each well for 1.5 h at 37°C. The
optical density (OD) value was measured at 450 nm using a
microplate reader to obtain the proliferation curve.
Transwell migration and invasion
assays
For the invasion assay, the inner layer of a 24-well
Transwell system (EMD Millipore, Billerica, MA, USA) was pre-coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at a 1:6
dilution. Cells treated with 20 µM ISL or 0.1% DMSO for 24 h were
suspended in serum-free medium and 100-µl cell suspension
(~1×104 cells) was added to the upper chambers. The
lower chambers were filled with culture medium. Following overnight
incubation, cells on the surface of the upper chamber were removed
with a cotton swab. Cells on the bottom were fixed in 4%
paraformaldehyde for 0.5 h at room temperature, stained with 0.1%
crystal violet dye for 20 min at room temperature and washed with
PBS at room temperature. Subsequently, images were captured and the
cells in five random fields were counted manually using light
microscopy.
The migration assay was similar to the invasion
assay, with the exception that the Transwell system was not coated
with Matrigel, and the number of cells tested was 5,000.
Apoptosis analysis
Following treatment with 20 µM ISL or 0.1% DMSO
(control group) for 24 h, cells were collected and digested with
trypsin (Beijing Solarbio Science & Technology Co., Ltd.).
Cells were then washed using precooled PBS (4°C). The cell density
was adjusted to 1–5×106/ml by adding 1X binding buffer.
Subsequently, 100-µl cell suspension was placed in a 5 ml flow
tube, and 5 µl Annexin V/fluorescein isothiocyanate (FITC; BD
Biosciences, Franklin Lakes, NJ, USA) was added and mixed by
flicking the tube for 5 min in the dark at room temperature,
followed by incubation with 10 µl propidium iodide for 10–15 min in
the dark room at room temperature. Following this, 400 µl PBS was
added and mixed, and the tubes were subsequently placed on ice. The
results were detected using a flow cytometer (BD Biosciences) in 1
h and analyzed using FlowJo v10.0 software (FlowJo LLC, Ashland,
OR, USA).
Western blot analysis
To extract proteins, cells in the experimental and
NC groups were added to 6-well plates and treated with
radioimmunoprecipitation assay lysis buffer (CW Biotech, Beijing,
China) plus protease inhibitor. Protein concentration was measured
using a Bicinchoninic Acid Protein Assay kit (CW Biotech), after
which the proteins were heated for 5 min at 95°C. Vertical
electrophoresis was performed with ~20 µg protein in each lane. The
proteins were isolated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% skim milk powder at room temperature for 1 h and were incubated
with primary antibodies overnight at 4°C. The following rabbit
anti-human primary antibodies were used: AKT (1:1,000; cat. no.
9272; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p-)AKT (1:1,000; cat. no. 4060; Cell Signaling
Technology, Inc.), mTOR (1:1,000; cat. no. 2972; Cell Signaling
Technology, Inc.), p-mTOR (1:1,000; cat. no. 2971; Cell Signaling
Technology, Inc.), B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no.
4223; Cell Signaling Technology, Inc.), caspase-3 (1:1,000; cat.
no. 9664; Cell Signaling Technology, Inc.), Bcl-2-associated X
protein (Bax; 1:1,000; cat. no. 5023; Cell Signaling Technology,
Inc.), microtubule-associated proteins 1A/1B light chain 3B (LC3;
1:1,000; cat. no. 14600-1-AP; ProteinTech Group, Inc., Chicago, IL,
USA), Beclin 1 (1:1,000; cat. no. 11306-1-AP; ProteinTech Group,
Inc.), p62 (1:1,000; cat. no. 5114; Cell Signaling Technology,
Inc.) and GAPDH (1:5,000; cat. no. 10494-1-AP; ProteinTech Group,
Inc.). The membranes were washed three times (5 min/wash) with
Tris-buffered saline containing 0.05% Tween-20. The membranes were
then incubated with goat anti-rabbit immunoglobulin G (H+L),
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat.
no. SA00001-2; ProteinTech Group, Inc.) at 37°C for 1 h. After
washing of the membranes, enhanced chemiluminescence (ProteinTech
Group, Inc.) was used to detect the signals. Quantity One v4.6.9
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
to scan the gray value of the blots, and GAPDH was used as an
internal control. The relative expression levels were calculated by
normalizing the target protein expression to the expression of the
internal control.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, IL, USA). Data are
expressed as the means ± standard deviation. Statistical comparison
between two groups was carried out with Student's t-test and
one-way analysis of variance followed by a Dunnett's post hoc test
was used to compare >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
ISL inhibits proliferation of MKN28
cells
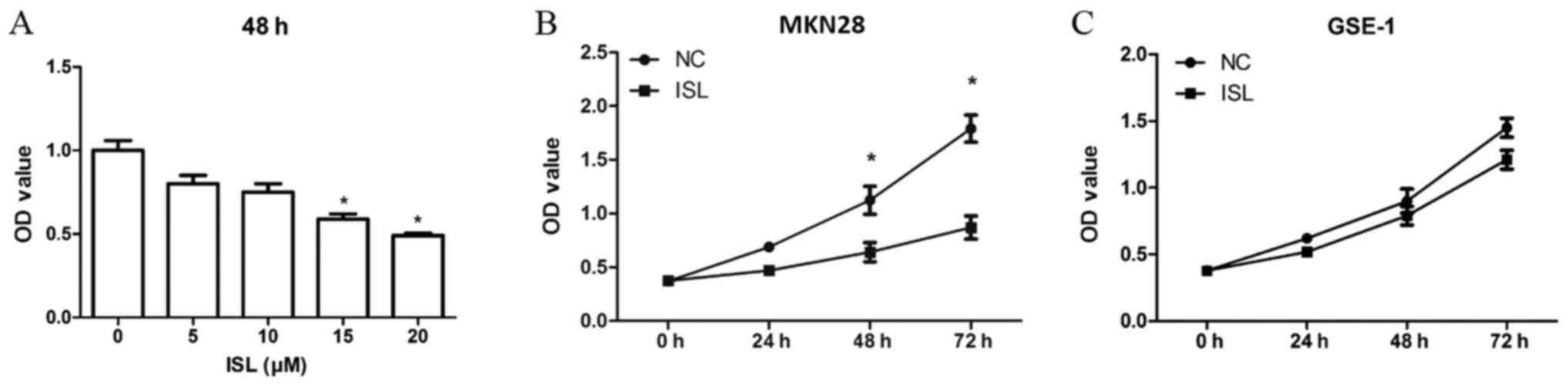
A CCK8 assay was performed to detect the potential
effects of ISL on the proliferation of gastric cancer cells. ISL
treatment decreased the proliferation of MKN28 cells compared with
in the NC group in a concentration-dependent manner (Fig. 1A). In addition, MKN28 cells were
treated with ISL (20 µM) for 0, 24, 48 and 72 h. The OD values at
48 and 72 h were significantly different between the ISL and NC
groups (P<0.05; Fig. 1B), thus
suggesting that ISL could effectively reduce MKN28 cell
proliferation at 48 h. In addition, the effects of ISL on GES-1
cell proliferation were investigated. The results indicated that
ISL exerted little inhibitory effect on GES-1 cell proliferation
(Fig. 1C). Therefore, subsequent
experiments were performed only using MKN28 cells. In summary, ISL
clearly inhibited MKN28 cell proliferation but exhibited little
effect on GES-1 cell proliferation.
ISL inhibits migration and invasion of
MKN28 cells
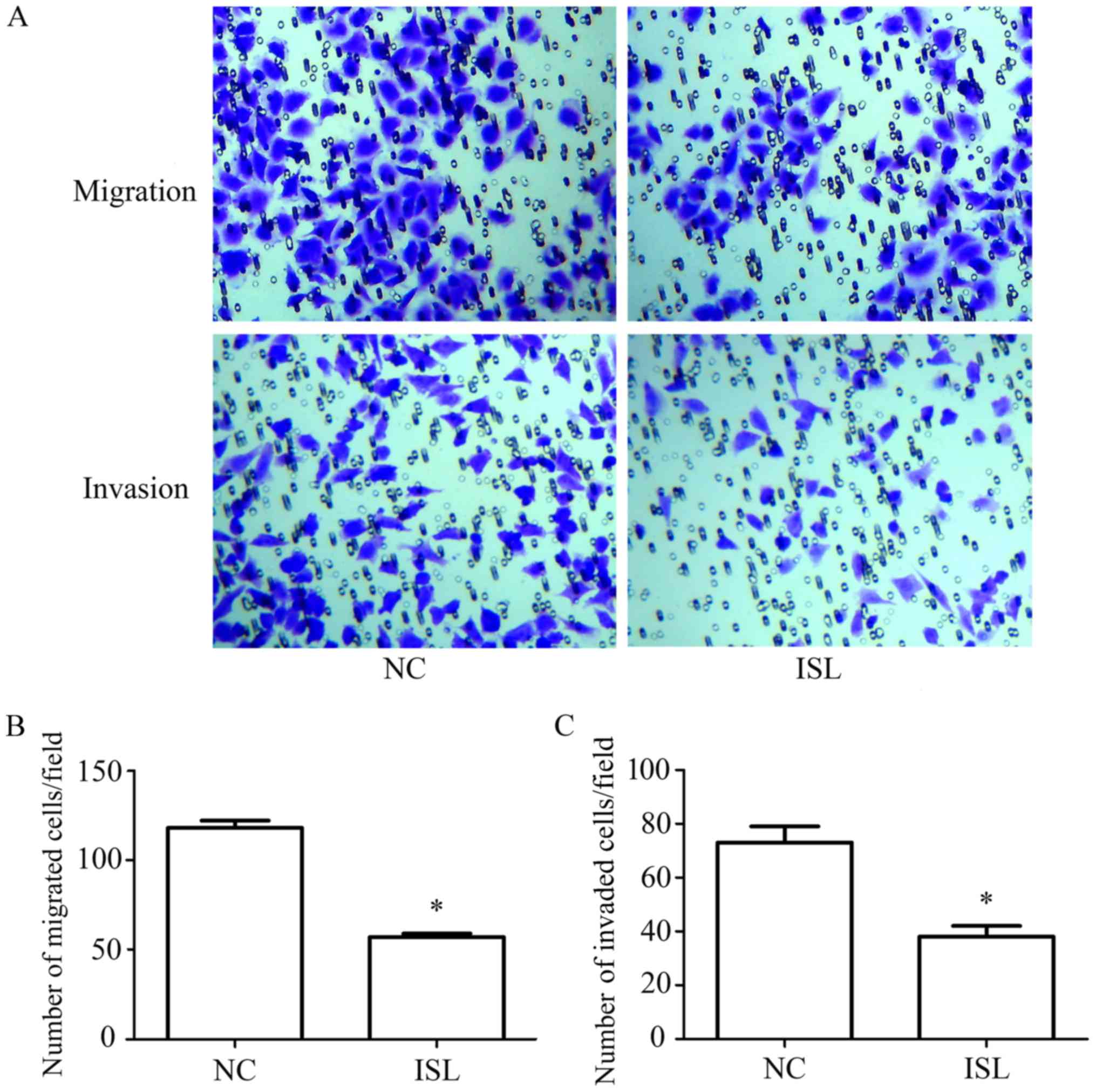
A Transwell assay was used to determine the effects
of ISL on the migration and invasion of MKN28 cells (Fig. 2A). The Transwell migration assay
revealed that the number of migrated cells in the ISL group was
significantly decreased compared with in the NC group (P<0.05;
Fig. 2B). In addition, the
Transwell invasion assay demonstrated that the number of invaded
cells was reduced in the ISL group compared with in the NC group
(Fig. 2C; P<0.05). These
results indicated that ISL markedly reduced the invasion and
migration of MKN28 cells.
ISL promotes apoptosis of MKN28
cells
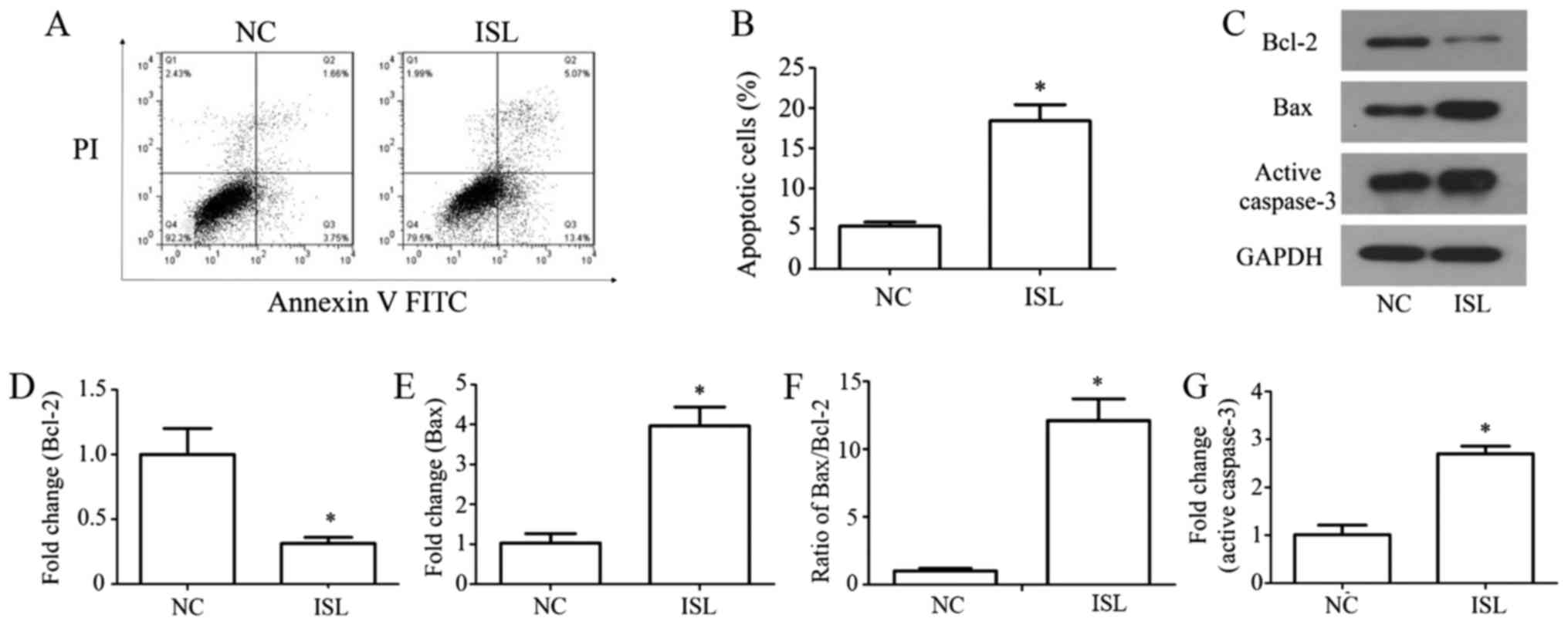
The apoptosis of MKN28 cells was evaluated using the
FITC Annexin V Apoptosis assay and flow cytometry, and
apoptosis-associated proteins were detected by western blotting.
The results demonstrated that treatment with ISL significantly
increased cell apoptosis compared with in the NC group (Fig. 3A and B; P<0.05). Western
blotting (Fig. 3C) indicated that
the protein expression levels of Bcl-2 were reduced (P<0.05;
Fig. 3D), whereas those of Bax
were increased compared with in the NC group (P<0.05; Fig. 3E). Furthermore, the ratio of
Bax/Bcl-2 was significantly increased following ISL treatment
compared with in the NC group (Fig.
3F; P<0.05), and active caspase-3 expression was increased
following ISL administration (Fig. 3C
and G; P<0.05). These results suggested that ISL may promote
the apoptosis of MKN28 cells.
ISL triggers autophagy in MKN28
cells
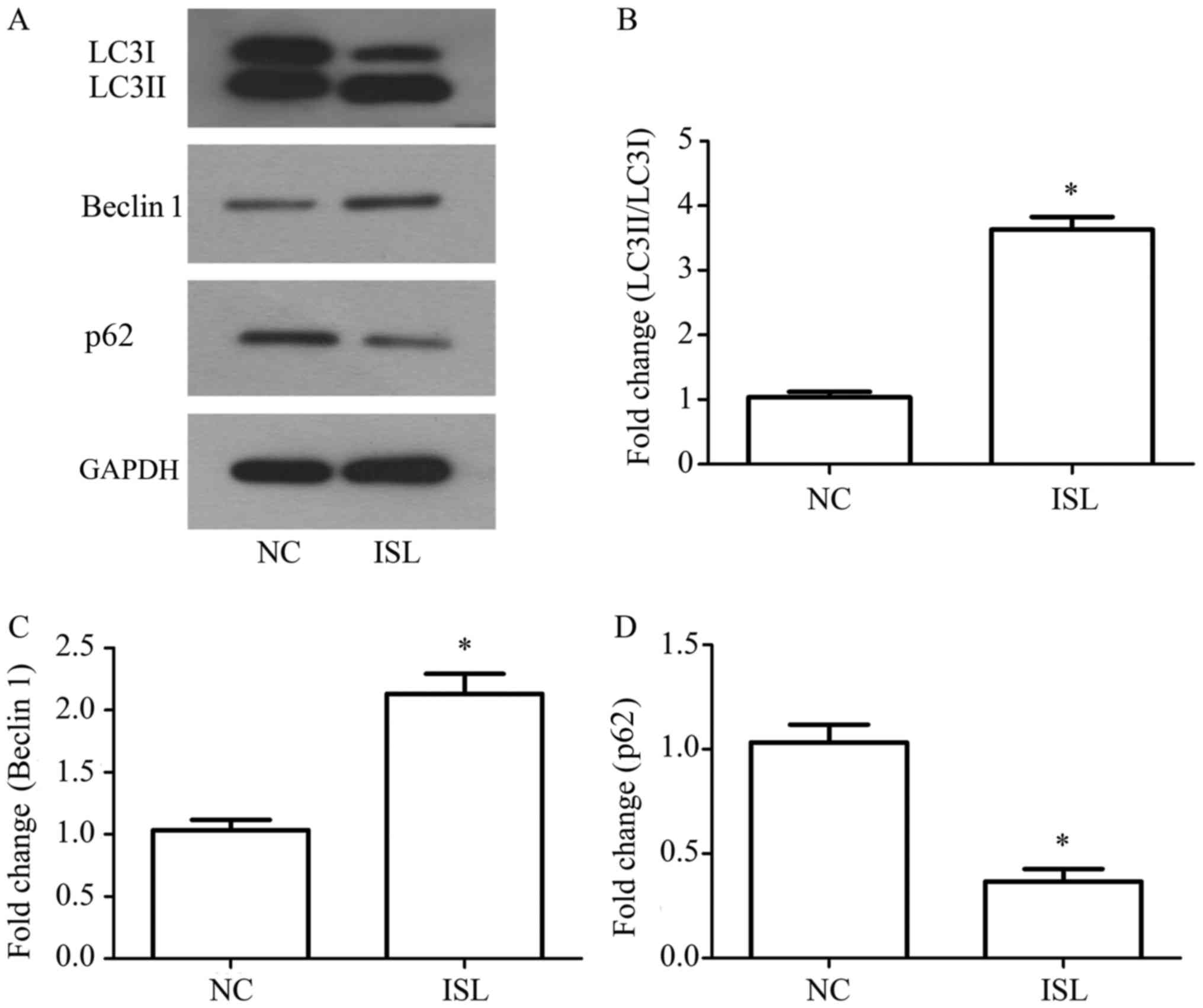
Autophagy in MKN28 cells was detected by western
blot analysis, which measured alterations in the protein expression
levels of LC3I, LC3II, Beclin 1 and p62 (Fig. 4A). Compared with in the NC group,
the ratio of LC3II/LC3I and the expression levels of Beclin 1 were
clearly upregulated in response to ISL treatment, whereas p62 was
downregulated (P<0.05; Fig.
4B-D). These results indicated that ISL may promote autophagy
in MKN28 cells.
ISL downregulates the PI3K/AKT/mTOR
signaling pathway in MKN28 cells
The effects of ISL on the expression levels of
PI3K/AKT/mTOR signaling pathway-related proteins in MKN28 cells
were investigated by western blot analysis, which was performed to
analyze AKT, p-AKT, mTOR and p-mTOR protein expression (Fig. 5A). The protein expression levels of
p-AKT were significantly decreased in ISL-treated MKN28 cells, with
no significant differences in the total expression levels of AKT
(P<0.05; Fig. 5B and C).
Furthermore, p-mTOR was significantly decreased following ISL
treatment, with no significant differences in the total mTOR levels
(P<0.05; Fig. 5D and E). These
results suggested that the effects of ISL on MKN28 may be
associated with downregulation of the PI3K/AKT/mTOR signaling
pathway.
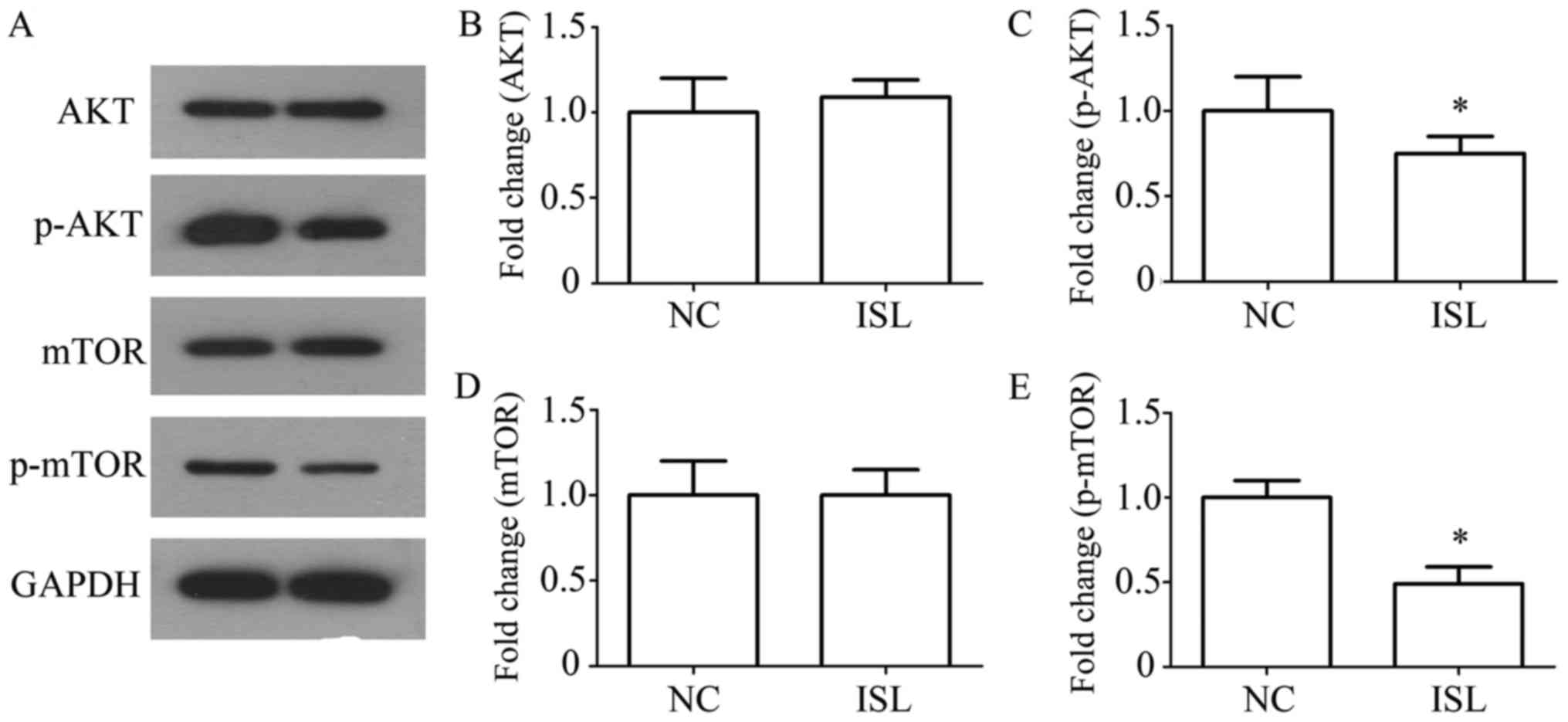 | Figure 5.ISL downregulates the PI3K/AKT/mTOR
signaling pathway in MKN28 cells. (A) AKT, p-AKT, mTOR and p-mTOR
protein expression levels were determined by western blotting.
GAPDH was used as a loading control. Statistical analysis of the
protein expression levels of (B) AKT, (C) p-AKT, (D) mTOR and (E)
p-mTOR following ISL treatment. Results are presented as the means
± standard deviation, n=5. *P<0.05 vs. NC. AKT, protein kinase
B; ISL, isoliquiritigenin; mTOR, mammalian target of rapamycin; NC,
negative control; p-, phosphorylated. |
Discussion
The findings of the present study demonstrated that
ISL inhibited the proliferation, migration and invasion of MKN28
cells. ISL also promoted apoptosis and autophagy in MKN28 cells.
These effects may be associated with inhibition of the
PI3K/AKT/mTOR signaling pathway.
ISL is a chalcone-type dietary flavonoid with
relatively low toxicity; numerous studies have reported that ISL
demonstrates potential antitumor activity. It has previously been
reported that ISL inhibits cancer proliferation, including in
prostate, oral and cervical cancer (19–21).
In 2001, Ma et al (22)
revealed that ISL may induce apoptosis of MGC-803 human gastric
cancer cells through calcium- and Deltapsi (m)-dependent pathways.
Lin et al (23) identified
that ISL promotes MKN45 cell apoptosis, and enhances
chemosensitivity in combination with 5-fluorouracil by targeting
glucose-regulated protein 78. Previous studies have identified the
marked antitumor activities of ISL, including migration inhibition,
cell cycle arrest, apoptosis induction, initiation of oxidative
stress and autophagy (24,25); however, the effects of ISL on MKN28
cell proliferation, migration, invasion and autophagy have not been
reported previously, to the best of our knowledge. The present
study demonstrated that ISL may possess the ability to inhibit
MKN28 cell proliferation, migration and invasion, and to induce
apoptosis and trigger autophagy in MKN28 cells. These results are
in line with previous findings in other cancers and imply that ISL
may be a novel agent for gastric cancer treatment.
Cancer cells are characteristically aggressive, with
the capabilities of immediate development and early metastasis.
Extensive studies (10,18,20,21,24)
have demonstrated that agents that prevent tumorigenesis do so by
disorganizing tumor initiation, proliferation, migration and
invasion via cell death pathways, including apoptosis and
autophagy. As important regulatory factors in the process of cell
apoptosis, Bcl-2 family members are divided into two groups:
Anti-apoptotic proteins (Bcl-2) and pro-apoptotic proteins (Bax)
(26,27). Caspase-3 is downstream of the
activator caspases. Therefore, activation of Bax and caspase-3
causes cells apoptosis (28). In
the present study, western blot analysis indicated that Bax and
caspase-3 expression levels were significantly increased, whereas
Bcl-2 expression levels were significantly decreased. The results
of the present study indicated that ISL may effectively induce
MKN28 cell apoptosis, which is consistent with findings in T24
human bladder cancer cells (10).
LC3 is a specific autophagy marker and LC3I is converted to LC3II
during autophagy; therefore, the levels of LC3II/LC3I may indicate
the occurrence of autophagy (29).
The present study detected the ratio of LC3II/LC3I as an autophagy
indicator. In general, autophagy is associated with decreased
levels of p62, suggesting that steady state levels of this protein
reflect autophagy status (30). It
is generally accepted that Beclin 1 is integral to the formation of
autophagosomes in autophagy; therefore, the expression levels of
the Beclin 1 were also investigated (31). The present study indicated that ISL
significantly increased LC3II/LC3I expression levels, whereas p62
expression levels were markedly decreased in ISL-treated cells.
Furthermore, the expression levels of Beclin 1 were markedly
increased following ISL treatment.
Previous studies have concluded that ISL can affect
cancer cell proliferation, migration, invasion, apoptosis and
autophagy via various signaling pathway, including c-Jun N-terminal
kinase/activator protein-1, vascular endothelial growth factor
(VEGF)/VEGF receptor 2 and PI3K/AKT (19,32,33).
The findings of the present study demonstrated that ISL may inhibit
cancer via the PI3K/AKT/mTOR signaling pathway. The PI3K/AKT/mTOR
signaling pathway serves an important role in cell proliferation
and tumorigenesis (34–37). It is generally accepted that the
PI3K/AKT/mTOR signaling pathway contributes to the proliferation of
cancer cells. AKT participates in cell proliferation (38) and this pathway has also been
reported to modulate cell apoptosis and growth (39), whereas mTOR regulates cell growth,
metabolism and autophagy (40).
Anticancer agents that target the PI3K/AKT/mTOR signaling pathway
can induce apoptosis, autophagy and inhibit growth. For example, a
marine sponge alkaloid derivative 4-chloro fascaplysin inhibits
tumor growth by disrupting the PI3K/AKT/mTOR signaling cascade
(41), and sinulariolide
suppresses cell migration and invasion through the PI3K/AKT/mTOR
signaling pathway in human bladder cancer cells (42).
ISL is a familiar dietary flavonoid that possesses
antitumor properties. A previous study demonstrated that ISL serves
as an inhibitor of the PI3K/AKT/mTOR signaling pathway in A375
melanoma cells (14). The present
study investigated whether the PI3K/AKT/mTOR signaling pathway was
involved in ISL-induced effects on MKN28 cells. It was identified
that ISL indeed inhibited the PI3K/AKT/mTOR signaling pathway by
downregulating the expression of p-AKT and p-mTOR. Therefore, it
was hypothesized that, in vitro, ISL may inhibit
proliferation, and induce autophagy and apoptosis, of MKN28 cells
by regulating the PI3K/AKT/mTOR signaling pathway. However, the
MKN-28 cell line has been reported to be a
problematic/misidentified cell line, which is actually a MKN74
derivative (43). Fortunately,
since they are both gastric cancer cell lines this has little
effect on the results of the present study; however, caution must
be exercised when analyzing the results and drawing
conclusions.
In conclusion, the results of the present study
indicated that ISL exerts antiproliferative, pro-apoptotic and
autophagy effects on MKN28 cells, making it a promising candidate
for gastric cancer treatment. Furthermore, suppression of the
PI3K/AKT/mTOR signaling pathway and the subsequent expression of
apoptosis and autophagy proteins may be a molecular mechanism
underlying the antitumor effects of ISL. However, since this is a
preliminary study, only one human gastric cancer cell line was
used. The effects of ISL on more gastric cancer cell lines and
animal models require further investigation in the near future. In
addition, the effects of ISL on gastric cancer chemoprevention and
the corresponding molecular mechanisms remain largely unknown and
warrant further exploration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data of the current study are available from the
corresponding author on reasonable request.
Authors' contributions
XZ and WS designed the present study. XZ and SW
performed the experiments. CW helped to analyze the results and
performed the statistical analysis. SW prepared the figures. XZ
wrote the manuscript. WS and CW carefully revised the manuscript.
All the authors have approved this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin X, Zhao Y, Song WM and Zhang B:
Molecular classification and prediction in gastric cancer. Comput
Struct Biotechnol J. 13:448–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu W, Yang Q, Liu B and Zhu Z: Serum
proteomics for gastric cancer. Clin Chim Acta. 431:179–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asghari MH, Moloudizargari M, Ghobadi E,
Fallah M and Abdollahi M: Melatonin as a multifunctional
anti-cancer molecule: Implications in gastric cancer. Life Sci.
185:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng R, Deng Q, Liu Y and Zhao P:
Curcumin inhibits gastric carcinoma cell growth and induces
apoptosis by suppressing the Wnt/beta-catenin signaling pathway.
Med Sci Monit. 23:163–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen H, Zhao S, Xu Z, Zhu L, Han Y and Ye
J: Evodiamine inhibits proliferation and induces apoptosis in
gastric cancer cells. Oncol Lett. 10:367–371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh Feng C, Wang KC, Chiang LC, Shieh DE,
Yen MH and Chang San J: Water extract of licorice had anti-viral
activity against human respiratory syncytial virus in human
respiratory tract cell lines. J Ethnopharmacol. 148:466–473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Si L, Yang X, Yan X, Wang Y and Zheng Q:
Isoliquiritigenin induces apoptosis of human bladder cancer T24
cells via a cyclin-dependent kinase-independent mechanism. Oncol
Lett. 14:241–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng F, Du Q, Peng C, Wang N, Tang H, Xie
X, Shen J and Chen J: A review: The pharmacology of
isoliquiritigenin. Phytother Res. 29:969–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Z, Park SM, Guan L, Wu Y, Lee JR, Kim
SC, Kim YW and Zhao R: Isoliquiritigenin attenuates oxidative
hepatic damage induced by carbon tetrachloride with or without
buthionine sulfoximine. Chem Biol Interact. 225:13–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yadav VR, Prasad S, Sung B and Aggarwal
BB: The role of chalcones in suppression of NF-κB-mediated
inflammation and cancer. Int Immunopharmacol. 11:295–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen XY, Li DF, Han JC, Wang B, Dong ZP,
Yu LN, Pan ZH, Qu CJ, Chen Y, Sun SG and Zheng QS: Reprogramming
induced by isoliquiritigenin diminishes melanoma cachexia through
mTORC2-AKT-GSK3β signaling. Oncotarget. 8:34565–34575.
2017.PubMed/NCBI
|
|
15
|
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie
T, Zhang J, Peng C, Lin Y and Chen J: MicroRNA-25 regulates
chemoresistance-associated autophagy in breast cancer cells, a
process modulated by the natural autophagy inducer
isoliquiritigenin. Oncotarget. 5:7013–7026. 2014.PubMed/NCBI
|
|
16
|
Zhao H, Yuan X, Li D, Chen H, Jiang J,
Wang Z, Sun X and Zheng Q: Isoliquiritigen enhances the antitumour
activity and decreases the genotoxic effect of cyclophosphamide.
Molecules. 18:8786–8798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DH, Park JE, Chae IG, Park G, Lee S
and Chun KS: Isoliquiritigenin inhibits the proliferation of human
renal carcinoma Caki cells through the ROS-mediated regulation of
the Jak2/STAT3 pathway. Oncol Rep. 38:575–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida T, Horinaka M, Takara M,
Tsuchihashi M, Mukai N, Wakada M and Sakai T: Combination of
isoliquiritigenin and tumor necrosis factor-related
apoptosis-inducing ligand induces apoptosis in colon cancer HT29
cells. Environ Health Prev Med. 13:281–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon GT, Cho HJ, Chung WY, Park KK, Moon A
and Park JH: Isoliquiritigenin inhibits migration and invasion of
prostate cancer cells: Possible mediation by decreased JNK/AP-1
signaling. J Nutr Biochem. 20:663–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsia SM, Yu CC, Shih YH, Chen Yuanchien M,
Wang TH, Huang YT and Shieh TM: Isoliquiritigenin as a cause of DNA
damage and inhibitor of ataxia-telangiectasia mutated expression
leading to G2/M phase arrest and apoptosis in oral squamous cell
carcinoma. Head Neck. 38 Suppl 1:E360–E371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu YL, Chia CC, Chen PJ, Huang SE, Huang
SC and Kuo PL: Shallot and licorice constituent isoliquiritigenin
arrests cell cycle progression and induces apoptosis through the
induction of ATM/p53 and initiation of the mitochondrial system in
human cervical carcinoma HeLa cells. Mol Nutr Food Res. 53:826–835.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Fu NY, Pang DB, Wu WY and Xu AL:
Apoptosis induced by isoliquiritigenin in human gastric cancer
MGC-803 cells. Planta Med. 67:754–757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin M, Wu D and Huang Y: Natural compounds
ursolic acid and isoliquiritigenin target GRP78 to enhance human
gastric cancer cell chemosensitivity by 5-fluorouracil. FASEB J.
30:1193–1194. 2016.
|
|
24
|
Wu CH, Chen HY, Wang CW, Shieh TM, Huang
TC, Lin LC, Wang KL and Hsia SM: Isoliquiritigenin induces
apoptosis and autophagy and inhibits endometrial cancer growth in
mice. Oncotarget. 7:73432–73447. 2016.PubMed/NCBI
|
|
25
|
Chen G, Zhu L, Liu Y, Zhou Q, Chen H and
Yang J: Isoliquiritigenin, a flavonoid from licorice, plays a dual
role in regulating gastrointestinal motility in vitro and in vivo.
Phytother Res. 23:498–506. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jergens A, Young J, Moore D, Wang C,
Hostetter J, Augustine L, Allenspach K, Schmitz S and Mosher C:
Bcl-2/caspase 3 mucosal imbalance favors T cell resistance to
apoptosis in dogs with inflammatory bowel disease. Vet Immunol
Immunopathol. 158:167–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Renault TT, Floros KV, Elkholi R, Corrigan
KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla
JJ, Buettner C, et al: Mitochondrial shape governs BAX-induced
membrane permeabilization and apoptosis. Mol Cell. 57:69–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang H, Wu Y, Guo J, Rong J, Ma L, Zhao Z,
Zuo D and Peng S: T-2 toxin induces apoptosis in differentiated
murine embryonic stem cells through reactive oxygen
species-mediated mitochondrial pathway. Apoptosis. 17:895–907.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Criollo A, Tasdemir E, Vicencio
JM, Tajeddine N, Hickman JA, Geneste O and Kroemer G: BH3-only
proteins and BH3 mimetics induce autophagy by competitively
disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L).
Autophagy. 3:374–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shvets E, Abada A, Weidberg H and Elazar
Z: Dissecting the involvement of LC3B and GATE-16 in p62
recruitment into autophagosomes. Autophagy. 7:683–688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei Y, An Z, Zou Z, Sumpter R, Su M, Zang
X, Sinha S, Gaestel M and Levine B: The stress-responsive kinases
MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through
Beclin 1 phosphorylation. elife. 4:e052892015. View Article : Google Scholar :
|
|
32
|
Wang Z, Wang N, Han S, Wang D, Mo S, Yu L,
Huang H, Tsui K, Shen J and Chen J: Dietary compound
isoliquiritigenin inhibits breast cancer neoangiogenesis via
VEGF/VEGFR-2 signaling pathway. PLoS One. 8:e685662013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen T, Deng S and Lin R: The inhibitory
effect of Isoliquiritigenin on the proliferation of human arterial
smooth muscle cell. BMC Pharmacol Toxicol. 18:572017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Hu X, Zhang W, Xu N, Wang FQ, Jia
J, Zhang WF, Sun ZJ and Zhao YF: Mammalian target of rapamycin
regulates isoliquiritigenin-induced autophagic and apoptotic cell
death in adenoid cystic carcinoma cells. Apoptosis. 17:90–101.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Xue J, Yang Y, Zhu H, Chen F, Wang
J, Lou G, Liu Y, Shi Y, Yu Y, et al: Isoliquiritigenin inhibits
interferon-γ-inducible genes expression in hepatocytes through
down-regulating activation of JAK1/STAT1, IRF3/MyD88, ERK/MAPK,
JNK/MAPK and PI3K/Akt signaling pathways. Cell Physiol Biochem.
37:501–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Safdari Y, Khalili M, Ebrahimzadeh MA,
Yazdani Y and Farajnia S: Natural inhibitors of PI3K/AKT signaling
in breast cancer: emphasis on newly-discovered molecular mechanisms
of action. Pharmacol Res. 93:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dillon RL, White DE and Muller WJ: The
phosphatidyl inositol 3-kinase signaling network: Implications for
human breast cancer. Oncogene. 26:1338–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen L, Wang J, Wang B, Yang J, Gong Z,
Zhao X, Zhang C and Du K: MiR-126 inhibits vascular endothelial
cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol.
95:365–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arsham AM, Plas DR, Thompson CB and Simon
MC: Phosphatidylinositol 3-kinase/Akt signaling is neither required
for hypoxic stabilization of HIF-1 alpha nor sufficient for
HIF-1-dependent target gene transcription. J Biol Chem.
277:15162–15170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma S, Guru SK, Manda S, Kumar A,
Mintoo MJ, Prasad VD, Sharma PR, Mondhe DM, Bharate SB and Bhushan
S: A marine sponge alkaloid derivative 4-chloro fascaplysin
inhibits tumor growth and VEGF mediated angiogenesis by disrupting
PI3K/Akt/mTOR signaling cascade. Chem Biol Interact. 275:47–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng TC, Din ZH, Su JH, Wu YJ and Liu CI:
sinulariolide suppresses cell migration and invasion by inhibiting
matrix metalloproteinase-2/-9 and urokinase through the
PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar
Drugs. 15:pii: E238. 2017. View Article : Google Scholar
|
|
43
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR and Freshney RI: Check your cultures! A list of
cross-contaminated or misidentified cell lines. Int J Cancer.
127:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|