Introduction
Melanoma is a malignant tumor caused by excessive
proliferation of abnormal melanocytes and most commonly occurs in
the skin, but also occurs in the mucosa and eye choroid. In
addition, melanoma accounts for a large proportion of cases of skin
tumour-associated mortality (1).
There are several factors that can induce the onset of melanoma,
including sunlight exposure, family history, occurrence of nevus,
giant congenital melanocytic nevi and dysplastic nevi syndrome
(2,3). In addition, the morbidity of melanoma
varies with race and geographical region; for example, the
incidence in Caucasians is much higher compared with in people of
African descent. Although the survival rate for patients with
melanoma has been greatly improved due to advances in research and
treatment, the overall morbidity of melanoma is still increasing
with an annual increase of 3 to 5% (4,5).
Since melanoma cells possess a high degree of malignancy, rapid
growth rate and early metastatic characteristics, finding effective
treatments to suppress proliferation of these cells is particularly
important.
Naphthalimides, a class of compounds that binds DNA
by intercalation, are known as potential anti-cancer agents
(6). Numerous clinical trials have
been performed using naphthalimides, including mitonafide, DMP840,
amonafide and elinafide (7).
However, dose-limiting bone marrow toxicity, leading to
thrombocytopenia, anaemia and leukopenia, has meant that
naphthalimides in general and amonafide in particular have failed
to pass Phase III clinical trials (8). Amonafide is metabolized by N-acetyl
transferase-2 to form N-acetyl amonafide, which is a toxic
metabolite (9,10). To prevent toxicity and improve
therapeutic outcome, a class of bis-intercalating agents were
derived from naphthalimides (11).
UNBS3157, a naphthalimide derivative, was designed to overcome the
metabolism that induces the clinical haematotoxicity of amonafide.
Several studies have reported that UNBS3157 possesses a three- to
four-fold higher maximum tolerated dose regardless of route of
administration and does not induce blood toxicity at therapeutic
doses (12–14).
UNBS5162,
N-{2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]
isoquinolin-5-yl}urea, is a novel naphthalimide derivative that can
be generated by UNBS3157 hydrolysis in physiological saline
(15). The anti-cancer activity of
UNBS5162 was investigated by Mijatovic et al (16) using experimental models of
refractory human prostate cancer and the results revealed that
expression of proangiogenic (C-X-C motif) ligand (CXCL) chemokines
was almost completely eliminated following UNBS5162 treatment.
However, the anti-tumor effects of UNBS5162 in other diseases
remain unknown. The aim of the present study was to investigate the
effects of UNBS5162 on melanoma and its overall mechanism of
action.
Materials and methods
Cell culture
The M14 human melanoma cell line was obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were maintained in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 100 U/ml
penicillin, 0.1 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were cultured in a humidified
atmosphere containing 5% CO2. Once cell confluence
reached ~80%, they were treated with 10 µM UNBS5162
(MedChemExpress, Princeton, NJ, USA) or 0.1% dimethyl sulfoxide
(DMSO, Amresco, LLC, Solon, OH, USA) for 24 h at room temperature.
DMSO-treated cells were used as the negative control (NC)
group.
Western blotting
Total protein was extracted from UNBS5162- and
DMSO-treated cells using radioimmunoprecipitation assay buffer
(Beijing ComWin Biotech Co., Ltd., Beijing, China), followed by
centrifugation at 12,000 × g and 4°C for 10 min. Protein
concentration was determined using bicinchoninic acid assay and
equal amounts of proteins (20 µg/lane) were separated by 8–15%
SDS-PAGE. Following electrophoresis, the proteins on the gel were
transferred onto polyvinylidene difluoride membranes. Membranes
were blocked with 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature and incubated
overnight at 4°C with appropriate primary antibodies purchased from
Wuhan Sanying Biotechnology (Wuhan, China), including rabbit
anti-human Akt (cat. no. 60203-2-Ig; 1:1,000), phosphorylated
(p)-Akt (cat. no. 66444-1-Ig; 1:1,000), mTOR (cat. no. 20657-1-AP;
1:1,000), p-mTOR (cat. no. 20984-1-AP; 1:1,000), rabbit anti-human
ribosomal protein S6 kinase (p70S6K; cat. no. 66638-1-Ig; 1:1,000),
B-cell lymphoma 2 (cat. no. 60178-1-Ig; Bcl-2; 1:1,000),
Bcl-2-associated X protein (Bax; cat. no. 60267-1-Ig; 1:1,000),
active caspase-3 (cat. no. 66470-2-Ig; 1:1,000) and GAPDH (cat. no.
60004-1-Ig; 1:5,000). The membranes were subsequently washed using
Tris-buffered saline with 0.1% Tween-20 and incubated with goat
anti-mouse horseradish peroxidase (HRP)-conjugated igG secondary
antibodies (cat. no. SA00001-1; 1:5,000; Wuhan Sanying
Biotechnology, Wuhan, China) or goat anti-rabbit HRP-conjugated IgG
secondary antibodies (cat. no. SA00001-2; 1:5,000; Wuhan Sanying
Biotechnology) at room temperature for 1 h. Finally, the
immunoreactive bands were visualized by the ECL™ Prime Western
Blotting system (APG Bio Ltd., Shanghai, China). Semi-quantitative
analysis was conducted using Quantity-One software 4.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) to measure densitometric
values for each band, with GAPDH selected as the internal control.
The relative protein expression between target protein and internal
control was calculated.
Cell proliferation assay
Cell proliferation was measured using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) assay. M14 cells were seeded into 96-well plates
at 1,000 cells/well and treated with 10 µM UNBS5162 or 0.1% DMSO
for 1.5 h at 37°C. CCK-8 reagent was added every 24 h and incubated
for 1.5 h at 37°C. The optical density (OD) values of each well
were measured by a microplate reader set at 450 nm.
Transwell migration and invasion
assays
A total of 100 µl Matrigel (diluted 1:6 in
serum-free medium) was added to evenly cover 24-well transwell
inserts, and incubated at 37°C for 4–6 h until set. The transwell
chamber was washed with serum-free medium. UNBS5162- or
DMSO-treated cells (1×105, 100 µl) were suspended in
serum-free medium and added to the upper chambers. RPMI-1640 medium
containing 10% fetal bovine serum (500 µl) was added to the lower
chambers, and the cells were incubated overnight at 37°C. Residual
cells in the upper chamber were removed with a cotton swab. Cells
that passed through the Matrigel-coated membrane were fixed with 4%
paraformaldehyde for 30 min and then stained with 0.1% crystal
violet for 20 min at room temperature. Cell invasion was quantified
using an inverted light microscope (magnification, ×100; CKX41;
Olympus Corporation, Tokyo, Japan) and by randomly selecting five
regions for cell counting. The migration assay was conducted
following the same protocol as the invasion assay, but without
application of Matrigel. A total of 5,000 cells were added to the
upper chambers.
Detecting cell apoptosis by flow
cytometry
After 24 h of UNBS5162 or DMSO treatment, cells were
cultured in serum-free medium for 24 h at 37°C. Subsequently, cells
were dissociated using a trypsin solution without EDTA and
centrifuged at 2,500 × g for 5 min at 4°C. Cells were resuspended
in 100 µl Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI) solution (BestBio, Shanghai, China), and incubated at
25°C in the dark for 15 min. After staining, the incubation buffer
was removed by centrifugation at 2,500 × g for 5 min at 4°C and
cells were further cultured at 4°C for 20 min in the dark with
occasional agitation. Flow cytometry was conducted with an
excitation wavelength of 488 nm and the data were analyzed using a
FlowJo software 7.2 (Tree Star, Inc., Ashland, OR, USA). In the
bivariate scatter plots, necrotic, advanced apoptotic, early
apoptotic and live cells are represented in the upper left (Q1),
upper right (Q2), lower right (Q3) and lower left (Q4) quadrants,
respectively.
Statistical analysis
SPSS 18.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA) was used to analyze the experimental data. All
data are expressed as the means ± standard deviation of three
independent experiments. The Student's t-test was used to compare
data derived from two independent groups and P<0.05 was
considered to indicate a statistically significant difference.
Results
UNBS5162 inhibits proliferation of M14
cells
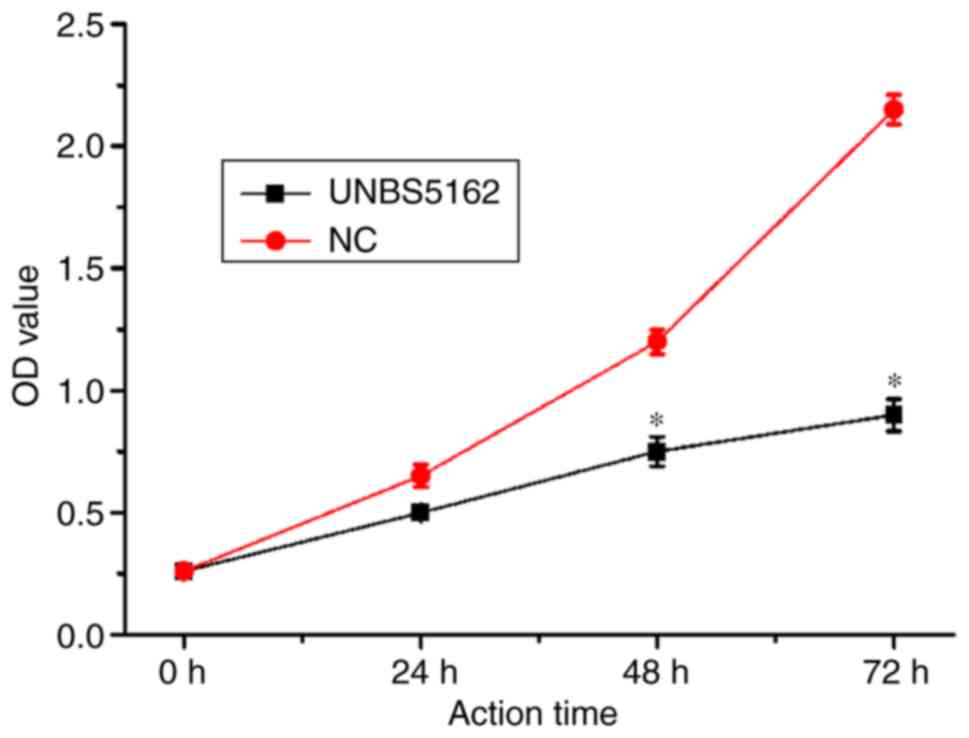
CCK-8 reagent was used to measure the effects of
UNBS5162 on M14 cell proliferation, and the results of the CCK-8
proliferation assay are shown in Fig.
1. UNBS5162 treatment inhibited proliferation of M14 cells in a
time-dependent manner. A statistically significant difference
(P<0.05) in proliferation between the UNBS5162- and DMSO-treated
groups was observed at 48 and 72 h post-treatment. These results
suggested that proliferation of M14 cells may be markedly decreased
following prolonged exposure to UNBS5162.
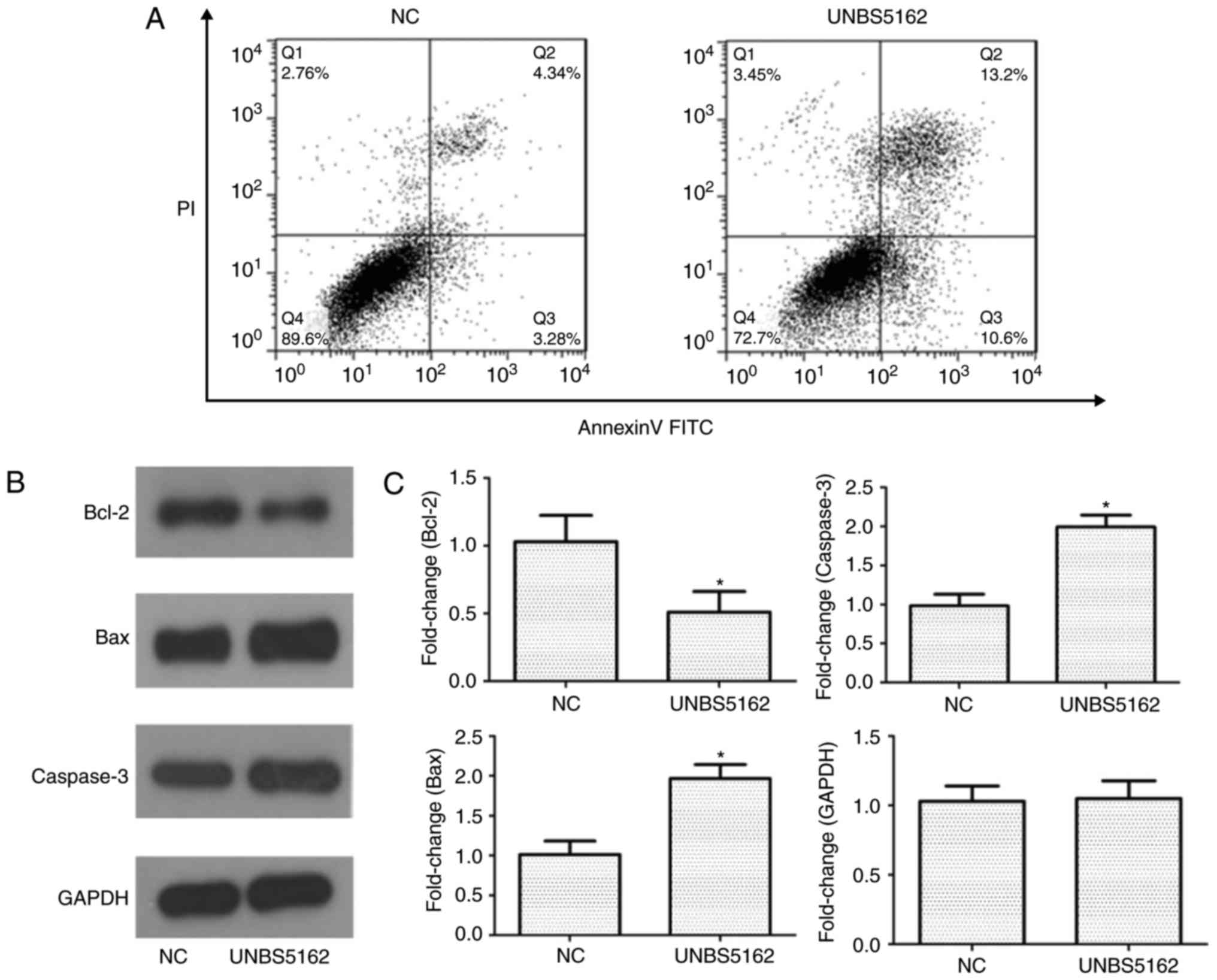
UNBS5162 induces apoptosis of M14
melanoma cells
To investigate whether UNBS5162 causes cell death
via apoptosis, Annexin V-FITC/PI staining and flow cytometric
analysis were performed. Q2 and Q3 were used to compare the
apoptotic rate of M14 cells in the experimental and control group.
As shown in Fig. 2A, the apoptotic
rate in the Q2 and Q3 quadrants of the NC group was ~4 and 3%,
whereas a higher apoptotic rate of ~13 and 11% was observed for
cells treated with UNBS5162. These findings demonstrated that total
apoptotic rate of UNBS5162-treated cells was increased (23.8±0.4%)
compared with DMSO-treated cells (7.62±0.5%). In addition, western
blotting was utilized to analyse the effects of UNBS5162 on
expression of melanoma-associated apoptosis regulators in M14
cells. Western blot analysis of apoptosis regulators following
UNBS5162 and DMSO treatment is shown in Fig. 2B. The results revealed a decrease
in the expression levels of the anti-apoptotic protein B-cell
lymphoma 2 (Bcl-2) and an increase in the expression of
proapoptotic proteins, Caspase-3 and Bcl-2-associated X protein
(Bax). The expression levels of apoptosis-associated proteins
relative to GAPDH are shown in Fig.
2C. Compared with in the NC group, expression of the
anti-apoptotic protein Bcl-2 was significantly downregulated,
whereas proapoptotic proteins Caspase-3 and Bax were significantly
upregulated in the experimental group.
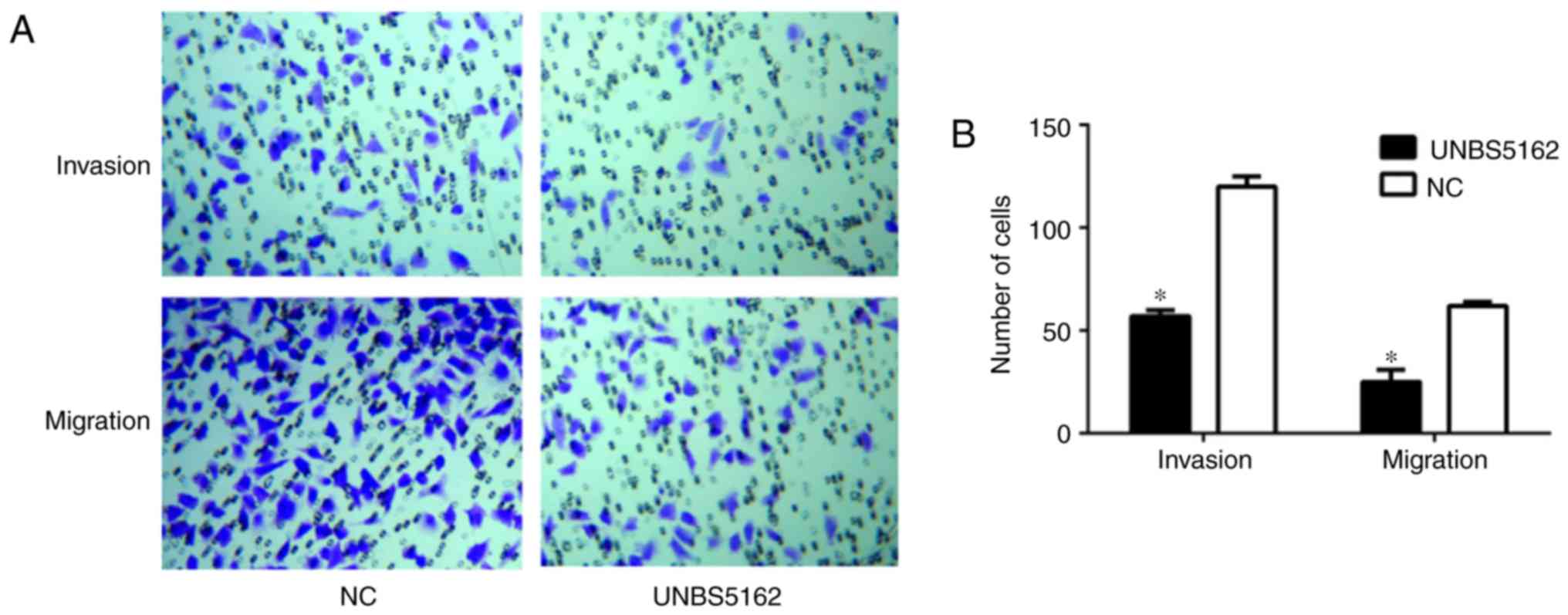
UNBS5162 inhibits migration and
invasion of M14 cells
The migration and invasion of UNBS5162- and
DMSO-treated cells were observed by microscopy, and the results are
shown in Fig. 3. The number of
cells counted in five random fields per chamber was reduced in the
experimental group compared with in the NC group (Fig. 3A), indicating that the number of
migrated (25±6 vs. 57±3 cells) and invasive (62±2 vs. 120±5 cells)
M14 cells was significantly reduced following UNBS5162 treatment
(P<0.05; Fig. 3B). Conversely,
a large number of cells treated with DMSO was observed in both the
migration and invasion assays, as shown in Fig. 3A and B. The results revealed that
the invasion and migration of UNBS5162-treated M14 cells was
inhibited.
UNBS5162 suppresses activation of the
PI3K signaling pathway in M14 cells
The PI3K signaling pathway is considered to be an
important signaling pathway in tumours, and the associated proteins
Akt and mTOR have a key role in promoting proliferation and
metastasis of tumour cells. In addition, p70S6K, a downstream
molecule of the PI3K signaling pathway, has been associated with
proliferation of M14 cells (17).
Western blot analysis was used to evaluate the protein expression
levels of Akt, mTOR and p70S6K in M14 cells following UNBS5162
treatment, and the results are shown in Fig. 4A and B. The results revealed a
significant downregulation of phosphorylated (p)-Akt and p-mTOR. In
addition, the expression levels of p70S6K, a protein associated
with cellular proliferation, were significantly downregulated in
the experimental grouped compared with in the NC group. These
results suggested that UNBS5162 treatment may suppress
PI3K/Akt/mTOR signaling in M14 melanoma cells.
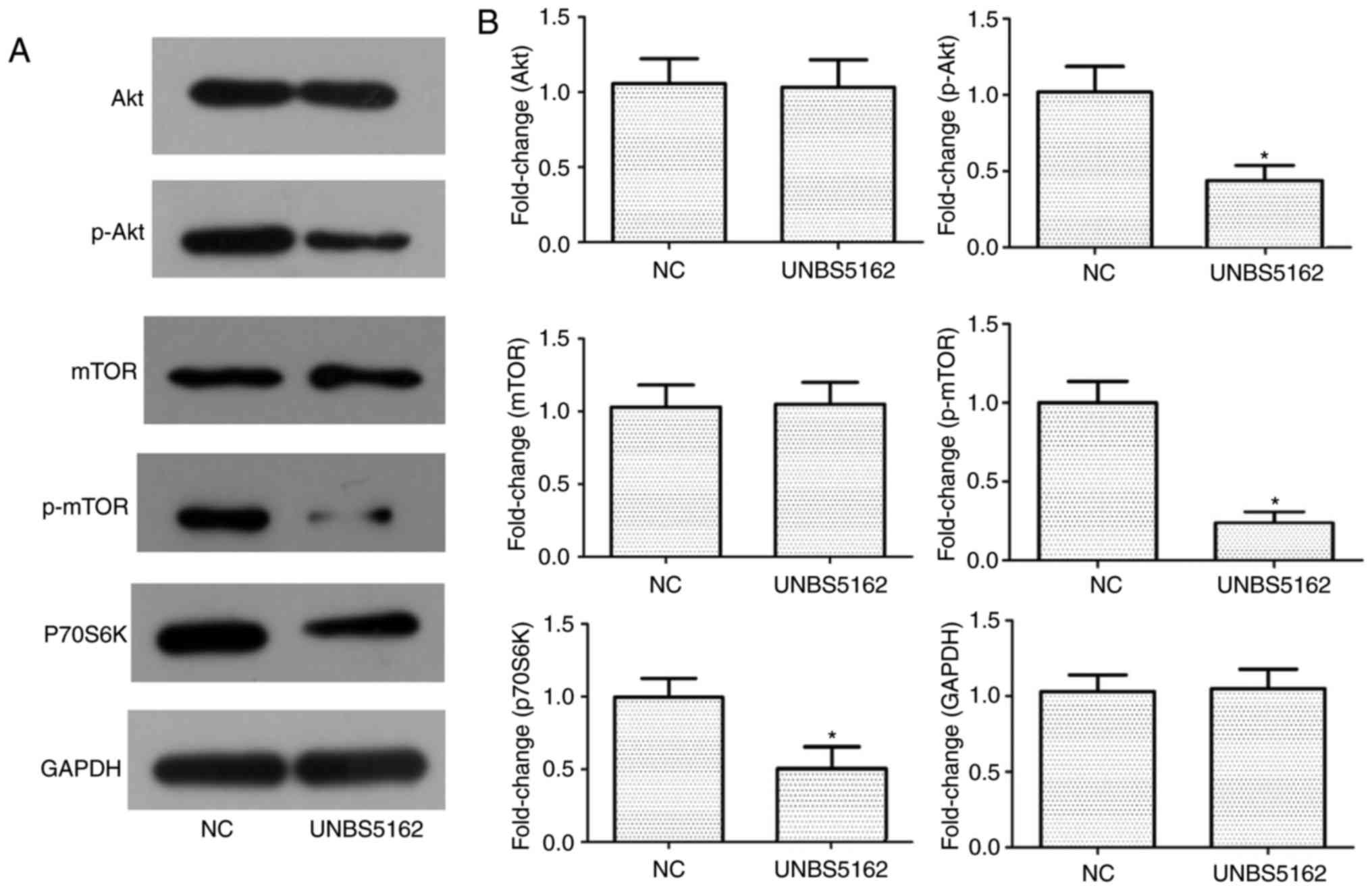 | Figure 4.Activation of the PI3K/Akt signaling
pathway is inhibited in UNBS5162-treated M14 cells. (A) Expression
levels of Akt, p-Akt, mTOR, p-mTOR, p70S6K and internal control
GAPDH in M14 cells were determined by western blot analysis. (B)
Semi-quantitative analyses of protein expression levels are shown.
Results are presented as the means ± standard deviation from three
independent experiments. *P<0.05 vs. the NC group. Akt, protein
kinase B; mTOR, mammalian target of rapamycin; NC, negative
control; p70S6K, p70 ribosomal protein S6 kinase; p-,
phosphorylated; PI3K, phosphatidylinositol-4,5-bisphosphate
3-kinase. |
Discussion
The novel naphthalimide, UNBS5162, has been
demonstrated to be a DNA-targeting compound that is synthesized by
UNBS3157 hydrolysis in physiological saline. UNBS3157 was initially
designed to avoid the metabolism that induces haematotoxicity of
amonafide (12). It has previously
been demonstrated that UNBS3157, a non-haematotoxic naphthalimide,
possesses significant anti-cancer activity in vivo (13). Therefore, a better understanding of
the efficacy and mechanism of action of UNBS5162, a UNBS3157
derivative, is required. Mijatovic et al (16) reported that repeated administration
of UNBS5162 in vivo can markedly improve survival in
orthotopic human prostate cancer models by reducing the expression
of CXCL chemokines. However, research on the anti-cancer activity
of UNBS5162 for other diseases is still lacking; therefore, the aim
of this study was to investigate the effect of UNBS5162 on
melanoma.
In the present study, a CCK-8 assay was used to
measure the proliferation of M14 cells treated with UNBS5162, and
the results demonstrated that proliferation was significantly
inhibited at 48 and 72 h post-UNBS5162 treatment compared with in
the NC group. Furthermore, the results from the transwell assays
revealed that the number of invasive and migrated M14 cells was
reduced following UNBS5162 treatment, suggesting that UNBS5162
inhibits invasion and migration of M14 cells. Notably, the
apoptotic rate of UNBS5162-treated cells was significantly
increased, which may have led to reduced invasive and migratory
ability. Similar inhibitory effects in human retinoblastoma cells
have been previously reported (18).
It has been reported that certain intracellular
proteins can regulate apoptosis, including Bcl-2, Bax and Caspase-3
(19,20). Bcl-2, an anti-apoptotic factor, is
closely associated with tumour occurrence and resistance (21). Bax, a member of the proapoptotic
protein family, when overexpressed not only allows various cell
types to undergo spontaneous apoptosis, but also promotes apoptosis
induced by other factors (22,23).
Notably, the proteins Bcl-2 and Bax are antagonistic, and have a
role in apoptosis via formation of a heterodimer (24,25).
Furthermore, Caspase-3 is required for Bax-induced apoptosis, and
is located downstream of the Bcl-2 protein family. Therefore,
activation of Caspase-3 and its role in apoptosis is directly
affected by the ratio of Bcl-2 and Bax expression (26). In the present study, the western
blotting results demonstrated that expression of the anti-apoptotic
protein Bcl-2 was reduced in M14 cells treated with UNBS5162,
whereas the proapoptotic protein Bax was highly expressed. In
addition, Caspase-3 was highly expressed in UNBS5162-treated cells.
These results suggested that UNBS5162 may contribute to enhancement
of apoptosis in M14 cells.
The molecular mechanisms underlying the effects of
UNBS5162 on proliferation and apoptosis were also investigated
using M14 cells. Apoptosis is a programmed cell death process,
which can be triggered by alterations in the intracellular and
extracellular environment or by a death signal, and involves
activation, expression and regulation of a series of genes.
Activation of the PI3K/Akt/mTOR signaling pathway can inhibit
stimulation-induced apoptosis and enhance cell cycle progression,
and sequentially improve survival and increase proliferation of
tumour cells (27,28). PI3K in the PI3K/Akt/mTOR signaling
pathway can increase activation of Akt by stimulating a signaling
cascade that produces phosphatidylinositol triphosphate (29,30).
Akt is generally located at important intersections of multiple
signaling pathways and can respond to various of intra- or
extracellular stimuli by regulating survival signals (31,32).
Furthermore, the serine/threonine-protein kinase mTOR is a
downstream effector of the PI3K/Akt signaling pathway, which acts
as a key regulator of metastasis, invasion, survival and
proliferation of tumour cells by activating p70S6K (33,34).
It has previously been suggested that the expression of Bcl-2 and
Bax proteins may be regulated by kinases and that their activation
could be affected by mTOR (35).
In the present study, p-Akt and p-mTOR expression
was downregulated following treatment of M14 cells with UNBS5162.
Similarly, the expression levels of p70S6K, a downstream molecule
in the PI3K/Akt/mTOR signaling pathway, were significantly reduced
in the experimental group compared with in the NC group.
Downregulation of proteins in the PI3K/Akt/mTOR signaling pathway
indicated that UNBS5162 treatment may inhibit PI3K/Akt/mTOR
signaling in M14 melanoma cells. Additionally, the migratory and
invasive abilities of UNBS5162-treated cells were significantly
suppressed. Wang et al (18) reported that the functional roles of
UNBS5162 in human retinoblastoma cells may be regulated by the
activity of the Akt-mTOR pathway in vitro. Additionally, the
preclinical development of UNBS5162 in prostate cancer has been
studied by Mahieu et al (15); the results indicated that UNBS5162
has anti-cancer effects in prostate cancer. Therefore, UNBS5162 may
be a potential therapeutic agent for melanoma.
In conclusion, the results of the present study
demonstrated that UNBS5162 significantly inhibits proliferation,
invasion and migration of M14 cells. In addition, it may be
hypothesized that UNBS5162 induces apoptosis through regulation of
the PI3K/Akt/mTOR signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XLS performed the experiments and analyzed the data,
and was also a major contributor in writing the manuscript. LY, JX,
YMZ and JZC made substantial contribution to the study conception
and design. ZPL, HYL, XZC and JHF performed the statistical
analysis. JHF was involved in the drafting of the manuscript and
gave the final approval for publication. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trinh VA: Current management of metastatic
melanoma. Am J Health Syst Pharm. 65 Suppl 9:S3–S8. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mete M, Sirakov NM, Griffin J and Menter
A: A novel classification system for dysplastic nevus and malignant
melanomaProceedings of the IEEE International Conference on Image
Processing. Phoenix, AZ: pp. 3414–3418. 2016
|
|
3
|
Cho E, Rosner BA and Colditz GA: Risk
factors for melanoma by body site. Cancer Epidemiol Biomarkers
Prev. 14:1241–1244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trautmann F, Meier F, Seidler A and
Schmitt J: Effects of the German skin cancer screening programme on
melanoma incidence and indicators of disease severity. Br J
Dermatol. 175:912–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whiteman DC, Green AC and Olsen CM: The
Growing Burden of Invasive Melanoma: Projections of Incidence Rates
and Numbers of New Cases in Six Susceptible Populations through
2031. J Invest Dermatol. 136:1161–1171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingrassia L, Lefranc F, Kiss R and
Mijatovic T: Naphthalimides and azonafides as promising anti-cancer
agents. Curr Med Chem. 16:1192–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ralhan R and Kaur J: Alkylating agents and
cancer therapy. Expert Opin Ther Pat. 17:1061–1075. 2007.
View Article : Google Scholar
|
|
8
|
Xie L, Cui J, Qian X, Xu Y, Liu J and Xu
R: 5-Non-amino aromatic substituted naphthalimides as potential
antitumor agents: Synthesis via Suzuki reaction, antiproliferative
activity, and DNA-binding behavior. Bioorg Med Chem. 19:961–967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu H, Huang M, Yang F, Chen Y, Miao ZH,
Qian XH, Xu YF, Qin YX, Luo HB, Shen X, et al: R16, a novel
amonafide analogue, induces apoptosis and G2-M arrest via poisoning
topoisomerase II. Mol Cancer Ther. 6:484–495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonini I, Volpini R, Dal Ben D,
Lambertucci C and Cristalli G: Design, synthesis, and biological
evaluation of new mitonafide derivatives as potential antitumor
drugs. Bioorg Med Chem. 16:8440–8446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Braña MF, Cacho M, García MA, de
Pascual-Teresa B, Ramos A, Domínguez MT, Pozuelo JM, Abradelo C,
Rey-Stolle MF, Yuste M, et al: New analogues of amonafide and
elinafide, containing aromatic heterocycles: Synthesis, antitumor
activity, molecular modeling, and DNA binding properties. J Med
Chem. 47:1391–1399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dumont P, Ribaucour F, Quaquebeke EV,
Darro F and Kiss R: UNBS3157, a new amonafide derivative with
improved in vivo efficacy and decreased toxicity. Cancer Res.
66:11052006.PubMed/NCBI
|
|
13
|
Van Quaquebeke E, Mahieu T, Dumont P,
Dewelle J, Ribaucour F, Simon G, Sauvage S, Gaussin JF, Tuti J, El
Yazidi M, et al:
2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl}carbamoyl)acetamide
(UNBS3157), a novel nonhematotoxic naphthalimide derivative with
potent antitumor activity. J Med Chem. 50:4122–4134. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seliga R, Pilatova M, Sarissky M, Viglasky
V, Walko M and Mojzis J: Novel naphthalimide polyamine derivatives
as potential antitumor agents. Mol Biol Rep. 40:4129–4137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahieu T, Mijatovic T, Quaquebeke EV,
Lefranc F, Vynckt FV, Darro F and Kiss R: UNBS5162 is a novel
naphthalimide derivative that induces autophagy and senescence in
human prostate cancer cells. Mol Cancer Ther. 6:3373S2007.
|
|
16
|
Mijatovic T, Mahieu T, Bruyère C, De Nève
N, Dewelle J, Simon G, Dehoux MJM, van der Aar E, Haibe-Kains B,
Bontempi G, et al: UNBS5162, a novel naphthalimide that decreases
CXCL chemokine expression in experimental prostate cancers.
Neoplasia. 10:573–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babchia N, Calipel A, Mouriaux F, Faussat
AM and Mascarelli F: The PI3K/Akt and mTOR/P70S6K signaling
pathways in human uveal melanoma cells: Interaction with B-Raf/ERK.
Invest Ophthalmol Vis Sci. 51:421–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Shen J and Wang J: UNBS5162
inhibits proliferation of human retinoblastoma cells by promoting
cell apoptosis. OncoTargets Ther. 10:5303–5309. 2017. View Article : Google Scholar
|
|
19
|
Kiliçkan L, Gonca S, Dalçik C, Dalçik H,
Solak M, Bayindir O, Süzer K, Omay O and Calikan E: General
anesthesia with thoracic epidural anesthesia in the cardiopulmonary
bypass surgery reduces apoptosis by upregulating antiapoptotic
protein Bcl-2. J Cardiovasc Surg (Torino). 47:315–322.
2006.PubMed/NCBI
|
|
20
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wojtuszkiewicz A, Schuurhuis GJ, Kessler
FL, Piersma SR, Knol JC, Pham TV, Jansen G, Musters RJ, van Meerloo
J, Assaraf YG, et al: Exosomes Secreted by Apoptosis-Resistant
Acute Myeloid Leukemia (AML) Blasts Harbor Regulatory Network
Proteins Potentially Involved in Antagonism of Apoptosis. Mol Cell
Proteomics. 15:1281–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang L, Han J, Ben-Hail D, He L, Li B,
Chen Z, Wang Y, Yang Y, Liu L, Zhu Y, et al: A New Fungal Diterpene
Induces VDAC1-dependent Apoptosis in Bax/Bak-deficient Cells. J
Biol Chem. 290:23563–23578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh L, Pushker N, Saini N, Sen S, Sharma
A, Bakhshi S, Chawla B and Kashyap S: Expression of pro-apoptotic
Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin
Experiment Ophthalmol. 43:259–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y
and Na LX: Curcumin triggers apoptosis via upregulation of
Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes.
Mol Med Rep. 12:1151–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou S, Wang Y and Zhu JJ: Simultaneous
Detection of Tumor Cell Apoptosis Regulators Bcl-2 and Bax through
a Dual-Signal-Marked Electrochemical Immunosensor. ACS Appl Mater
Interfaces. 8:7674–7682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Liu J, Gao J, Meng M, Qin X and
Wang T: Up-regulations of Bax and caspase-3 and down-regulation of
Bcl-2 after Xinfeng capsule treatment in adjuvant-induced arthritis
rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:457–461. 2016.(In
Chinese). PubMed/NCBI
|
|
27
|
Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei
M, Wang L and Zhong M: Leptin regulates proliferation and apoptosis
of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J
Biosci. 37:91–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z, Liu Y and Wang C: Oncogenic
NanogP8 expression regulates cell proliferation and migration
through the Akt/mTOR signaling pathway in human gastric cancer -
SGC-7901cell line. OncoTargets Ther. 9:4859–4866. 2016. View Article : Google Scholar
|
|
31
|
Leleu X, Coiteux V, Corm S, Robu D, Dupire
S, Moreau AS, Gay J, Berthon C, Pascal L, Cliquenois E, et al: The
AKT signaling pathway is the key regulator of cell survival and
homing in Waldenstrom macroglobulinemia. Hématologie. 14:14–17.
2008.
|
|
32
|
Yang H, Zhou J, Mi J, Ma K, Fan Y, Ning J,
Wang C, Wei X, Zhao H and Li E: HOXD10 acts as a tumor-suppressive
factor via inhibition of the RHOC/AKT/MAPK pathway in human
cholangiocellular carcinoma. Oncol Rep. 34:1681–1691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asnaghi L, Bruno P, Priulla M and Nicolin
A: mTOR: A protein kinase switching between life and death.
Pharmacol Res. 50:545–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu YD, Zheng QX, Wu HB, Guo XD, Li JF and
Hao SF: The effects of rapamycin on expression ratio of Bax/Bcl-2
and the expression of activated caspase-3 in different types of
tumor cells. Tumor. 33(2)2013.
|