Introduction
Ethanol-induced gastric mucosa injury represents one
of the most refractory and frequently represented disease group in
clinical settings, including gastritis, gastric ulcers and gastric
mucosal stress injury (1).
Patients with gastric mucosa injury commonly suffer from hyperemia,
edema, erosion and hemorrhage (2).
Chronic alcohol consumption is one of the most important factors
contributing to the high incidence and prevalence of gastric mucosa
injury (3). Ethanol-induced
gastric mucosal injury (acute or chronic), with the imbalance of
protective and injury-inducing factors of the mucosa (4), maybe attenuated by the regulation of
anti-inflammatory and antioxidant pathways (5,6).
Reactive oxygen species (ROS) have previously been demonstrated
have important roles in disease pathogenesis and to be involved in
complex physiological processes associated with oxidative stress,
such as cell signaling and apoptosis (7–9). In
clinical and animal model studies, chronic alcohol consumption was
revealed to be associated with suppressed superoxide dismutase
(SOD) activity, reduced glutathione (GSH) levels and the oxidation
of GSH/glutathione disulfide (GSSG) redox potentials, as well as
enhanced levels of the lipid peroxide product, malondialdehyde
(MDA) (10–12).
The major bioactive polyphenols of tea (Camellia
sinensis) are epigallocatechin gallate (EGCG) (13), a member of the catechin group of
polyphenols found in tea, and theaflavins (TFs), which are a
polymerized type of catechin (14). Tea polyphenols, which are
predominantly extracted from green tea, have been extensively
investigated (15). However,
despite the beneficial properties of black tea, the polyphenolic
compound TF derivatives have not been widely studied. Previous
studies investigating black tea have revealed that it exhibits
chemopreventive and chemotherapeutic effects (16,17).
There are four major TFs in black tea: TF1, TF-3-gallate,
TF-3′-gallate and TF-3, 3′-digallate (TF3), of which TF3 is the
most abundant (18,19). Tea polyphenols serve predominantly
as antioxidants; and they have also been demonstrated to serve as
anticancer agents in numerous preclinical and clinical studies
(20,21). TFs have been reported to attenuate
inflammatory responses in human gingival fibroblasts as a result of
antibacterial properties (22),
and to suppress the secretion of inflammation-associated factors
and enhance the secretion of antimicrobial peptides in oral
epithelial cells in vitro (23). An investigation using animal models
with oxidative stress revealed that tea polyphenols functioned as
antioxidants primarily by scavenging ROS and attenuating the
suppression of the activity of antioxidant enzymes, such as SOD and
GSH (24). Furthermore, TFs have
been demonstrated to suppress hematopoietic stem cell (HSC)
senescence and reduce oxidative stress to protect mouse HSCs from
radiation injury in vivo (25).
In addition to the role of oxidative stress, studies
have indicated that the underlying molecular mechanisms of
ethanol-induced gastric diseases may involve multiple signaling
pathways, including apoptosis and mitogen-activated protein kinase
(MAPK) pathways, such as extracellular signal-regulated kinase
(ERK)1, ERK2, c-Jun N-terminal kinase (JNK) and p38 kinase (p38)
MAPK pathways (26,27). Apoptosis is induced by oxidative
stress and the subsequent increases in superoxide and hydroxyl
radicals, and MAPK pathways have important roles in cell
proliferation, differentiation and apoptosis. TFs have previously
been revealed to inhibit H2O2- and
inflammation-induced apoptosis in neural cells (28,29).
Furthermore, the phosphorylation levels of ERK1/2 and JNK have been
previously demonstrated to be suppressed by EGCG in epidermal cells
(30) and by both EGCG and green
tea polyphenols in lung carcinogenesis models (31).
The aim of the present study was to investigate
whether TFs may attenuate ethanol-induced oxidative stress in
gastric mucosa epithelial cells and to investigate the potential
associated underlying molecular mechanisms, including apoptosis and
MAPK pathways. The results of the present study indicates that TFs
may represent a novel therapeutic agent for the treatment of
ethanol-induced injury in gastric mucosa epithelial cells, which
may provide insight for future studies investigating
ethanol-induced gastric diseases.
Materials and methods
Cell culture
TF3 (>90.0%) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) and is approved by the Food and
Drug Administration (32,33). GES-1 human gastric mucosa
epithelial cells were obtained from the American Type Culture
Collection (Manassas, VA, USA) in order to investigate the effects
of TF3 on ethanol-induced injury in vitro. For the similar
reactions to ethanol with primary gastric mucosa epithelial cells,
the GES-1 cell line has been widely used in the study of the
effects of ethanol on the gastric mucosa, and so the GES-1 cell
line was determined to be appropriate for use in the present study
(34,35). Cells were cultured in RPMI 1640
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin/streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in a 5% CO2-containing
humidified incubator at 37°C. When cells reached 70% confluence,
cell morphologies were observed under a DMi8 optical microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
When cells reached 70% confluence, they were divided
into five groups: EtOH group (treated with 0.5 mol/l ethanol for 24
h at room temperature); TF-1, TF-2 and TF-3 groups (respectively
treated with 20, 40 and 80 µg/ml TF3 for 6 h, and then 0.5 mol/l
ethanol for 24 h, at room temperature) and the Control group
(without any treatment). Cell viability and proliferation abilities
were subsequently investigated to determine the degree of injury
exhibited by GES-1 cells following treatment with ethanol, and
whether administration of TF3 attenuates these effects.
Cell Counting Kit-8 (CCK-8) cell
viability assay
The cell viability of GES-1 cells treated with
varying concentrations of TF3 (20, 40 and 80 µg/ml) for 6 h prior
to ethanol (0.5 mol/l) treatment were investigated using the CCK-8
assay (Beyotime Institute of Biotechnology, Haimen, China) at
specific time intervals (0, 6, 12, 24 and 48 h), according to the
manufacturer's protocol. Briefly, cells (5×103
cells/well) were seeded with CCK-8 reagent (20 µl) in 96-well
plates and incubated for 1 h at 37°C in an atmosphere of 5%
CO2. The optical density values were analyzed under 450
nm using a microplate reader (Bio-Tek Instruments, Inc., Winooski,
VT, USA).
Carboxyfluorescein diacetate
succinimidyl ester (CFSE) cell proliferation assay
The CFSE assay was used to investigate the
proliferation ability of cells in all groups following treatment
with ethanol for 24 h, with or without pretreatment with 20, 40 and
80 µg/ml TF3 for 6 h. Proliferation was assessed based on the even
distribution of CFSE fluorescence when cell division occurs. The
CellTrace CFSE Cell Proliferation kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to investigate GES-1 cell proliferation
according to the manufacturer's protocol. Cells were mixed with
preheated PBS (1 ml) in sterile centrifuge tubes to reach a final
concentration of 1×106 cells/ml. A total of 2 µl CFSE (5
mmol/l) reagent was added to the mixture, which was subsequently
incubated for 10 min at 37°C. Following culture in 10 ml RPMI 1640
containing 10% FBS for 5 min on ice in the dark, cells were seeded
into 24-well plates (1×105 cells/well) and cultured in
an incubator containing 5% CO2 at 37°C for 4 h. Finally,
cells were investigated using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) and CellQuest™ Pro Software
version 5.1 (BD Biosciences).
ROS detection
ROS levels were measured using
dichlorofluorescein-diacetate (DCTH-DA), an oxygen-sensitive
fluorescence probe. Briefly, DCFH-DA (10 µmol/l) was added to EtOH,
TF-1, TF-2, TF-3 and Control cell groups (1×105
cells/well) in a 6-well plate. Following incubation for 30 min at
37°C in the dark, cells were washed with PBS three times to remove
excess dye, and were immediately collected and analyzed using a
FACSCalibur flow cytometer (BD Biosciences) and CellQuest™ Pro
Software version 5.1 (BD Biosciences).
Detection of oxidative
stress-associated factors
MDA levels, SOD activity and the GSH/GSSG ratio in
the EtOH, TF-1, TF-2, TF-3 and Control cell groups were determined.
MDA levels were detected by thiobarbituric acid in a Lipid
Peroxidation MDA assay kit (Beyotime Institute of Biotechnology).
SOD activities were investigated using Total Superoxide Dismutase
assay kit with WST-8 (Beyotime Institute of Biotechnology).
Additionally, GSH/GSSG redox potentials were determined using
2,2-dithio-bis-nitrobenzoic acid in the GSH and GSSG assay kit
(Beyotime Institute of Biotechnology). All assays were performed
according to the manufacturer's protocols.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection
The apoptosis rates of GES-1 cells were determined
using an Annexin V-FITC/PI double-staining assay, according to the
manufacturer's protocol (BioVision, Inc., Milpitas, CA, USA). In
the present study, cells in the EtOH, TF-1, TF-2, TF-3 and Control
cell groups, with the initial concentration of 1×106
cells/ml, were seeded in a 96-well plate. Following this, Annexin
V/FITC (40 µg/ml, 5 µl) and PI (20 µg/ml, 10 µl) were added to the
cell suspension. Following incubation for 15 min at room
temperature, cells were analyzed using a FACSCalibur flow
cytometer. A wavelength of 488 nm was used as the exciting light
wavelength, and wavelengths of 515 nm (FITC) and 560 nm (PI) were
used as detecting light wavelengths.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) from the EtOH, TF-1, TF-2, TF-3 and
Control cell groups, and cDNA was obtained using a EN-QuantiTect
Reverse Transcription (Qiagen GmbH, Hilden, Germany), according to
the manufacturer's protocols of 37°C for 10 min, and 85°C for 15
sec. The thermocycling conditions for qPCR were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing/extension at 60°C for
30 sec. qPCR was performed in an ABI 7300 Thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using a SYBR Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The 2−∆∆Cq method used to normalize gene expression to
the housekeeping gene (GAPDH) (36). The primer sequences used for PCR
are presented in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
|
| Sequence |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
CCATCTTCCAGGAGCGAGAT |
TGCTGATGATCTTGAGGCTG |
| Caspase-3 |
TGAGCCATGGTGAAGAAGGA |
TCGGCCTCCACTGGTATTTT |
| Bax |
AACATGGAGCTGCAGAGGAT |
CCAATGTCCAGCCCATGATG |
| Bcl-2 |
TTCTTTGAGTTCGGTGGGGT |
CTTCAGAGACAGCCAGGAGA |
Western blot analysis
Total proteins were extracted from cells in EtOH,
TF-1, TF-2, TF-3 and Control cell groups. Cells were lysed using
lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 0.02% NaN2,
100 µg/ml phenylmethanesulfonyl fluoride, 1 µg/ml aprotinin, and 1%
Triton X-100), and centrifuged at 12,000 × g for 30 min at 4°C.
Total protein concentration was determined via a BCA assay
(Beyotime Institute of Biotechnology). Following this, proteins (20
µg/well) were separated via 12% SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA). Following blocking using 5% non-fat dry milk for 1 h at
room temperature, the membranes were probed using the following
rabbit primary antibodies overnight at 4°C: Anti-caspase-3 (cat no.
ab13847; 1:500; Abcam, Cambridge, UK), anti-B-cell lymphoma-2
(Bcl-2;cat. no. ab59348; 1:1,000; Abcam), anti-Bcl-2-associated X
(Bax; cat. no. ab32503; 1:2,000; Abcam), anti-GAPDH (cat. no.
ab9485; 1:2,500; Abcam), anti-ERK1/2 (cat. no. 4695; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-phosphorylated
(p)-ERK1/2 (cat. no. 4370; 1:2,000; Cell Signaling Technology,
Inc.), anti-JNK (cat. no. 9252; 1:1,000; Cell Signaling Technology,
Inc.), anti-p-JNK (cat. no. 4668; 1:1,000; Cell Signaling
Technology, Inc.), anti-p38 (cat. no. 8690; 1:1,000; Cell Signaling
Technology, Inc.) and anti-p-p38 (cat. no. 4511; 1:1,000; Cell
Signaling Technology, Inc.). GAPDH was used as the loading control.
Following this, membranes were probed with horseradish
peroxidase-conjugated goat anti-rabbit IgG H&L (cat. no.
ab6721; 1:5,000; Abcam) for 2 h at room temperature. PVDF membranes
were subsequently exposed to X-ray film and visualized using an
enhanced chemiluminescence detection system, GE ECL Start (GE
Healthcare, Chicago, IL, USA). Lab Works Image Acquisition and
Analysis software PLUS 4.1 (UVP, LLC, Phoenix, AZ, USA) was used to
quantify the band intensities.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using SPSS software (version 22.0; IBM Corp., Armonk, NY,
USA). Statistical differences were determined via one-way analysis
of variance and Dunnett's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
TFs enhance the viability of GES-1
cells injured by ethanol
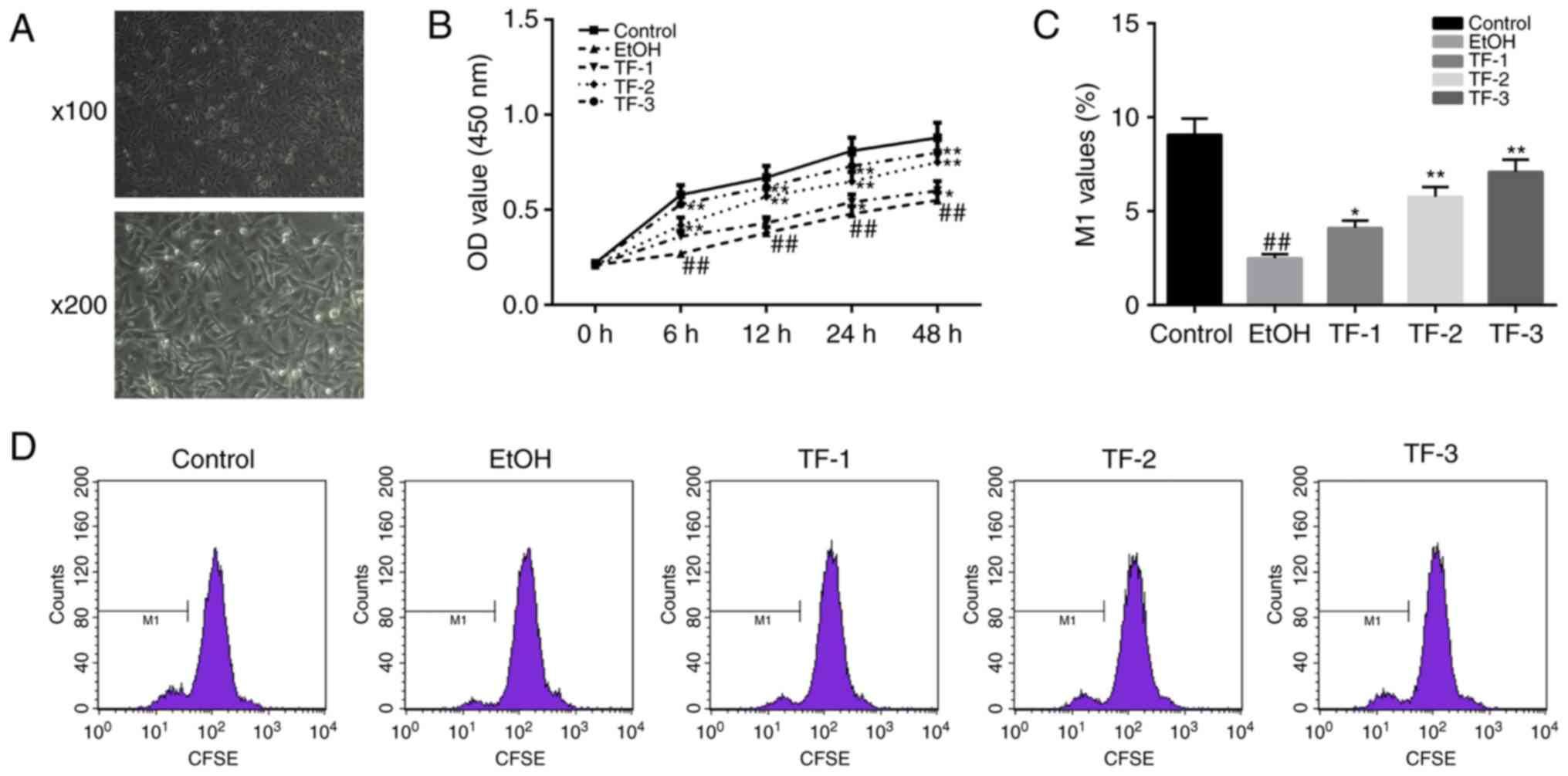
After 24 h of culture, cell morphologies of GES-1
gastric mucosa epithelial cells were observed by optical
microscopy, when cells attained 70% confluence. Cells were observed
regular polygonal or fusiform cell morphologies, and uniform
interstitials (Fig. 1A). Once the
cells had reached 70% confluence, the CCK-8 assay was performed to
determine the effect of the administration of differing
concentrations of TFs (20, 40 and 80 µg/ml) on the cell viability
of GES-1 cells treated with 0.5 mol/l ethanol for specific time
intervals (0, 6, 12, 24 and 48 h). The results revealed that the
cell viability of the EtOH group was significantly suppressed at
all time-points compared with the control group (P<0.01;
Fig. 1B). Furthermore, the results
of the CCK-8 assay revealed that TFs enhanced cell viability
following treatment with ethanol in a dose-dependent manner
compared with the EtOH group (P<0.05; Fig. 1B).
The CFSE assay was performed to further investigate
the potential TF-induced promotion of GES-1 cell proliferation in
cells treated with ethanol (0.5 mol/l) for 24 h. The results of the
flow cytometry analysis demonstrated that the cell proliferation
ability (reflected by M1 values) was significantly inhibited
(decreased M1 values) in the EtOH group compared with the control
group (P<0.01); however, this effect was significantly reversed
(increased M1 values) following treatment with TF in a
dose-dependent manner (P<0.05; Fig.
1C and D).
TFs attenuate oxidative stress induced
by ethanol in GES-1 gastric mucosa epithelial cells
As an indicator of oxidative stress, ROS levels were
detected as a marker of oxidative stress. DCTH-DA fluorescence
detection was performed and the results revealed that the EtOH
group exhibited significantly enhanced ROS levels compared with the
control group (P<0.01; Fig. 2A and
B). Furthermore, treatment with TFs reversed this effect in a
dose-dependent manner, compared with the EtOH group (P<0.05;
Fig. 2A and B).
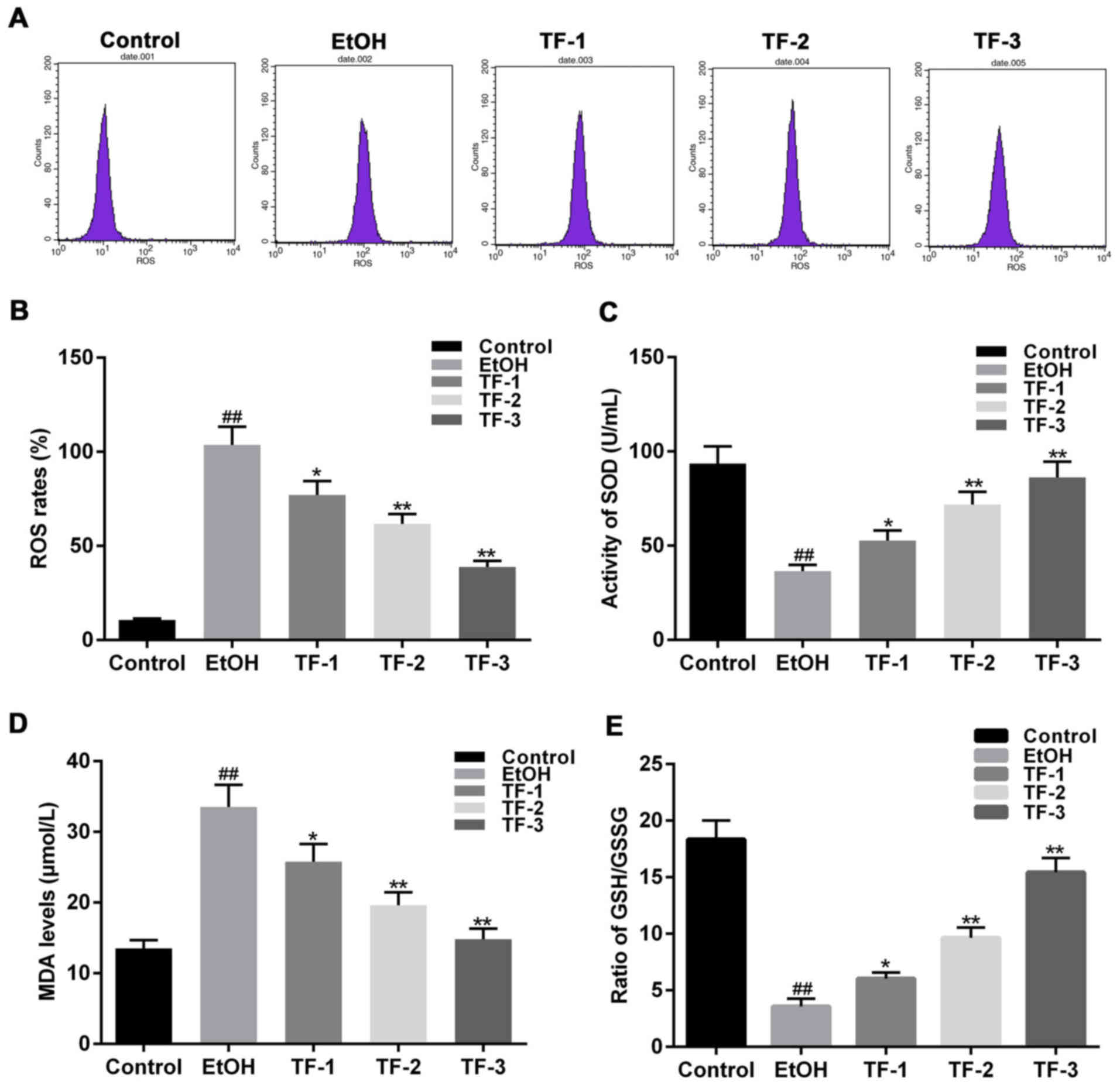 | Figure 2.TFs attenuate oxidative stress
induced by ethanol in GES-1 gastric mucosa epithelial cells. ROS
levels were investigated via administration of DCTH-DA to GES-1
cells following treatment with 0.5 mol/l ethanol. (A)
Representative flow cytometry graphs for each cell group following
the addition of DCTH-DA. (B) ROS levels were quantified by flow
cytometry and statistically compared among the groups. Levels of
(C) SOD, (D) MDA and (E) GSH/GSSG were determined using commercial
kits. ##P<0.01 vs. control group; *P<0.05 and
**P<0.01 vs. EtOH group. TFs, theaflavins; ROS, reactive oxygen
species; DCTH-DA, dichlorofluorescein-diacetate; SOD, superoxide
dismutase; MDA, malondialdehyde; GSH, glutathione; GSSG,
glutathione disulfide; EtOH group, GES-1 cells treated with 0.5
mol/l ethanol only; TF-1 group, GES-1 cells treated with 20 µg/ml
TFs prior to 0.5 mol/l ethanol treatment; TF-2, GES-1 cells treated
with 40 µg/ml TFs prior to 0.5 mol/l ethanol treatment; TF-3, GES-1
cells treated with 80 µg/ml TFs prior to 0.5 mol/l ethanol
treatment. |
In addition, the levels of other common oxidative
stress-associated factors, including SOD, GSH and MDA, were
investigated. The activity of SOD in the EtOH group was
significantly suppressed compared with the control group
(P<0.01; Fig. 2C). By contrast,
the levels of MDA in the EtOH group were significantly increased
compared with the control group (P<0.01; Fig. 2D). Furthermore, the activity of the
GSH/GSSG redox potential was significantly suppressed in the EtOH
group compared with the control group (P<0.01; Fig. 2E). However, treatment with TFs
reversed the effects of ethanol treatment on the levels of ROS,
SOD, MDA and GSH/GSSG in a dose-dependent manner (Fig. 2).
TFs inhibit cell apoptosis in GES-1
cells injured by ethanol
Annexin V-FITC/PI double-staining assay and flow
cytometry were performed to determine the apoptosis rates exhibited
by the control, EtOH, TF-1, TF-2 and TF-3 cell groups. The results
demonstrated that the apoptosis rate was significantly increased in
the EtOH group compared with the control group (P<0.01) and that
treatment with TFs significantly reversed this effect in a
dose-dependent manner (P<0.05; Fig.
3A and B).
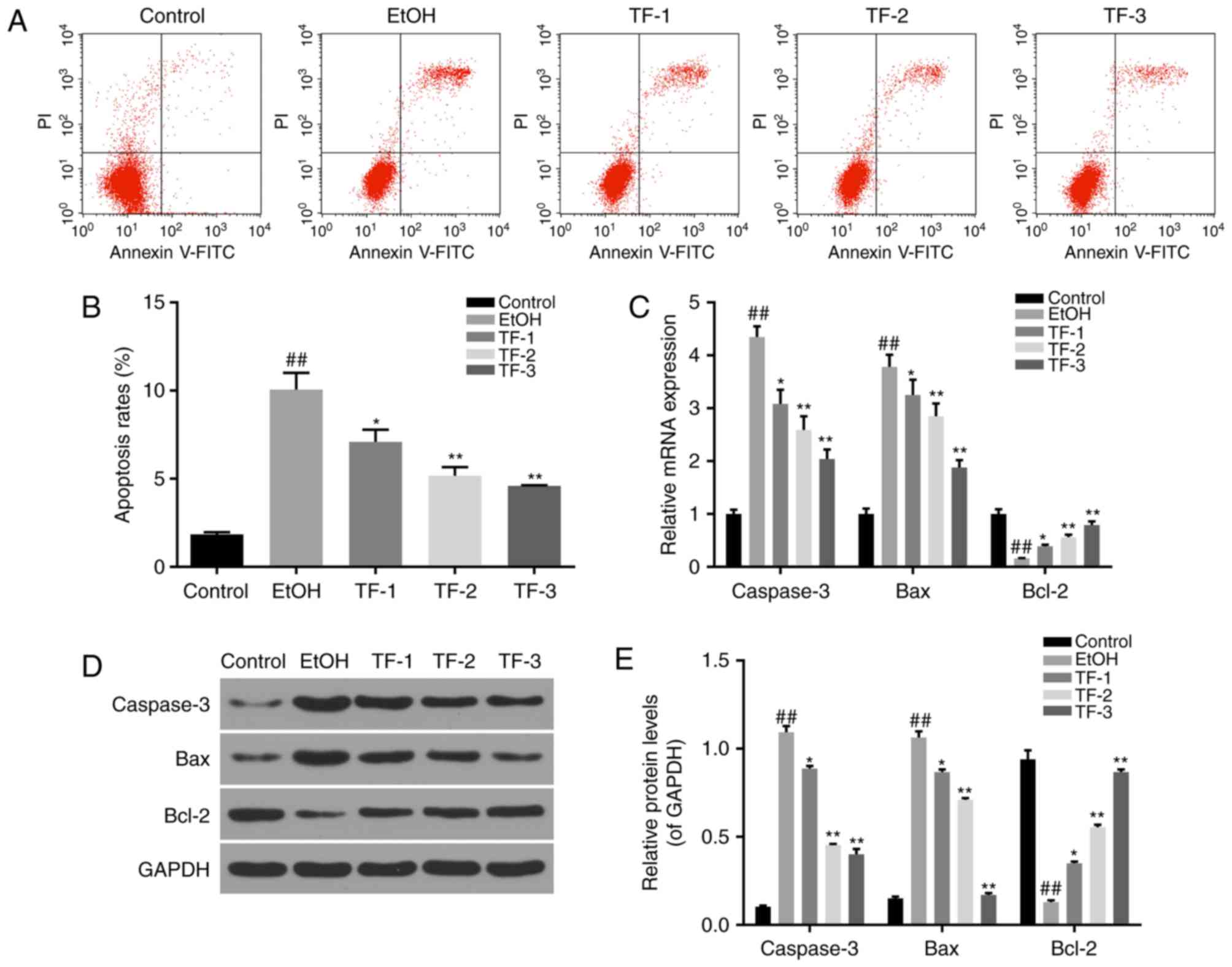 | Figure 3.TFs suppress cell apoptosis in GES-1
cells treated with ethanol. Annexin V-FITC/PI double staining
followed by flow cytometry was performed to measure apoptosis rates
in the different cell groups. (A) Representative flow cytometry
scatter plots following Annexin V-FITC/PI double staining, lower
right + upper right quadrants were considered to indicate apoptotic
cells. (B) Apoptosis rates determined by flow cytometry were
statistically compared among the groups. (C) Reverse
transcription-quantitative polymerase chain reaction was performed
to measure the mRNA levels of apoptosis-associated factors,
includingcaspase-3, Bax and Bcl-2. (D) Representative western blot
bands obtained for the protein expression of caspase-3, Bax and
Bcl-2 apoptosis-associated factors. (E) Densitometric analysis was
performed to quantify and statistically compare the protein levels
of caspase-3, Bax and Bcl-2 among the different cell groups.
##P<0.01 vs. control group; *P<0.05 and
**P<0.01 vs. EtOH group. TFs, theaflavins; FITC, fluorescein
isiothiocyanate; PI, propidium iodide; Bcl-2, B-cell lymphoma-2;
Bax, Bcl-2-associated X; EtOH group, GES-1 cells treated with 0.5
mol/l ethanol only; TF-1 group, GES-1 cells treated with 20 µg/ml
TFs prior to 0.5 mol/l ethanol treatment; TF-2, GES-1 cells treated
with 40 µg/ml TFs prior to 0.5 mol/l ethanol treatment; TF-3, GES-1
cells treated with 80 µg/ml TFs prior to 0.5 mol/l ethanol
treatment. |
Furthermore, the expression levels of
apoptosis-associated factors were investigated by RT-qPCR and
western blot analyses. The results demonstrated that the mRNA and
protein levels of proapoptotic factors (caspase-3 and Bax) were
significantly enhanced in the EtOH group compared with the control
group (P<0.01), while the mRNA and protein levels of the
anti-apoptotic protein Bcl-2 were significantly suppressed in the
EtOH group compared with control group (P<0.01; Fig. 3C-E). However, treatment with TFs
significantly reversed the effects of ethanol administration in a
dose-dependent manner (P<0.05; Fig.
3C-E).
TFs alleviate oxidative stress via
MAPK pathways in GES-1 cells treated with ethanol
The phosphorylation levels of ERK, JNK and p38 were
investigated by western blot analyses in control, EtOH, TF-1, TF-2
and TF-3 cell groups. The results demonstrated that the protein
levels of p-ERK (Fig. 4A and B),
p-JNK (Fig. 4C and D) and p-p38
(Fig. 4E and F) were significantly
increased in EtOH groups compared with the control group
(P<0.01). However, treatment with TFs significantly attenuated
this effect in a dose-dependent manner compared with the EtOH group
(P<0.05; Fig. 4). The total
levels of ERK, JNK and p38 did not exhibit any significant
differences between the control, EtOH, TF-1, TF-2 and TF-3 cell
groups (Fig. 4).
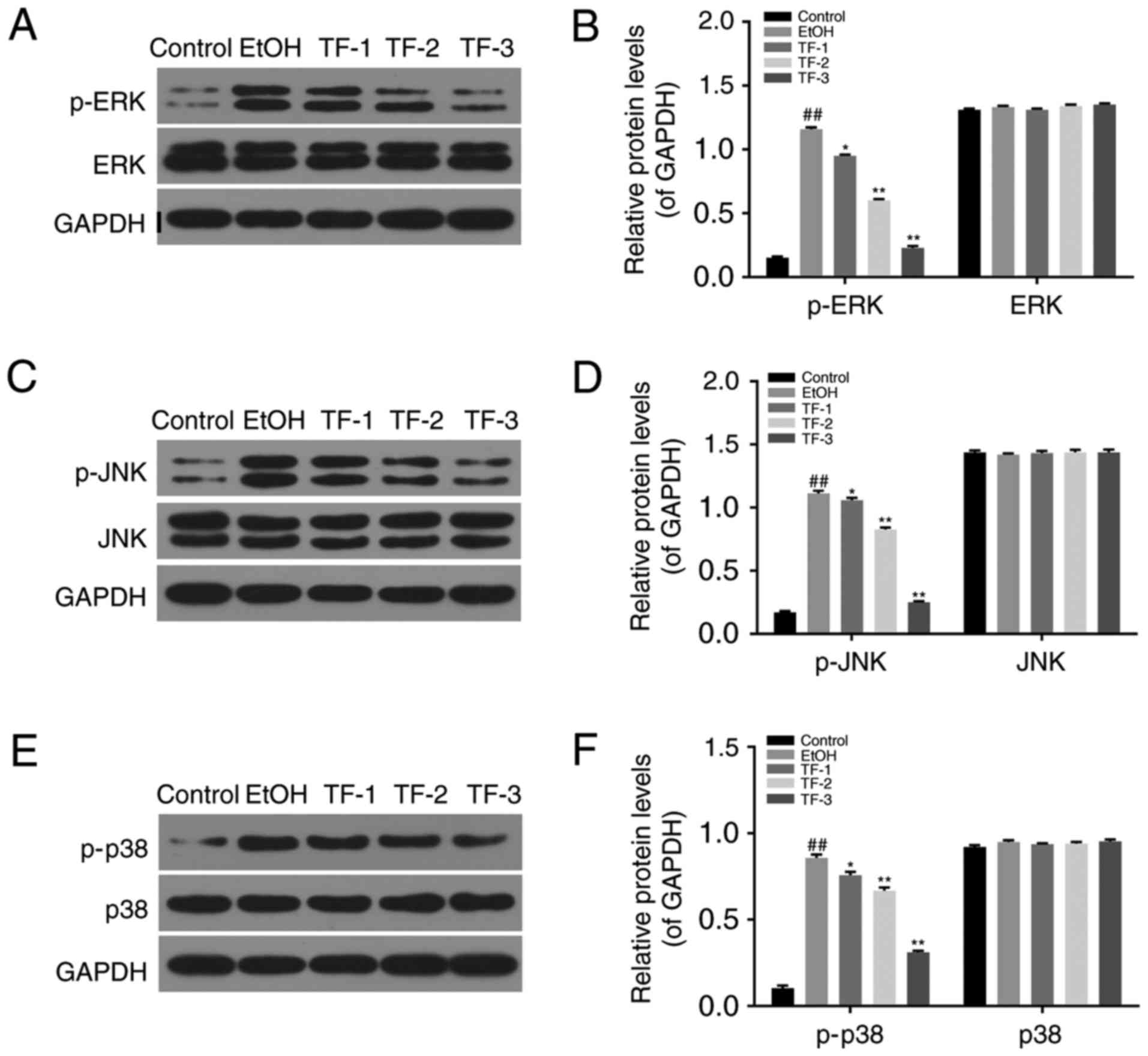 | Figure 4.TFs attenuate oxidative stress in
GES-1 cells treated with ethanol via MAPK pathways. Western blot
analyses were performed to measure the phosphorylation levels of
ERK, JNK and p38 MAPK proteins. (A) Representative western blot
bands obtained for the protein expression of p-ERK and ERK. (B)
Densitometric analysis was performed to quantify and statistically
compare the protein levels of p-ERK and ERK. (C) Representative
western blot bands obtained for the protein expression of p-JNK and
JNK. (D) Densitometric analysis was performed to quantify and
statistically compare the protein levels of p-JNK and JNK. (E)
Representative western blot bands obtained for the protein
expression of p-p38 and p38. (F) Densitometric analysis was
performed to quantify and statistically compare the protein levels
of p-p38 and p38. ##P<0.01 vs. control group;
*P<0.05 and **P<0.01 vs. EtOH group. TFs, theaflavins; MAPK,
mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; p38, p38
kinase; p-, phosphorylated-; EtOH group, GES-1 cells treated with
0.5 mol/l ethanol only; TF-1 group, GES-1 cells treated with 20
µg/ml TFs prior to 0.5 mol/l ethanol treatment; TF-2, GES-1 cells
treated with 40 µg/ml TFs prior to 0.5 mol/l ethanol treatment;
TF-3, GES-1 cells treated with 80 µg/ml TFs prior to 0.5 mol/l
ethanol treatment. |
Discussion
It is well established that chronic alcohol
consumption is one of the most important risk factors for the
induction of digestive tract diseases (37). Chronic, excessive alcohol
consumption may lead to the induction of various acute digestive
tract diseases, including gastritis, gastric ulcers and
gastrorrhagia, as a result of subsequent gastric mucosa injury,
particularly via oxidative stress and apoptosis (38,39).
Tea polyphenols, which are predominantly extracted from green tea,
have been widely investigated and have been revealed to exhibit
antioxidative properties (40,41).
TFs, polyphenolic compounds that are the major beneficial component
of black tea, have also been demonstrated to exert antioxidative
effects and thus require further investigation (42–44).
In the present study, GES-1 gastric mucosa
epithelial cells were used to construct an oxidative stress cell
model using ethanol treatment to induce injury. The viability of
GES-1 cells was significantly suppressed following treatment with
ethanol (0.5 mol/l) after 6, 12, 24 and 48 h, while the levels of
oxidative stress and apoptosis were significantly enhanced
following treatment with 0.5 mol/l ethanol. However, the results
demonstrated that treatment of GES-1 cells with TFs attenuated the
effects of ethanol administration on levels of oxidative stress,
apoptosis and cell viability in a dose-dependent manner. The
underlying molecular mechanisms of this process were investigated
further by analyzing oxidative stress, apoptosis and MAPK pathways
that are important for cell proliferation.
ROS are involved in cell oxidative stress
mechanisms, predominantly via the induction of lipid peroxidation
in the phospholipid membrane, the production of oxygen radicals and
the induction of cell apoptosis (26,27).
Antioxidant enzymes, such as SOD and GSH, eliminate ROS to maintain
the balance of ROS generation and elimination during cell normal
metabolism (45,46). Excessive accumulation of ROS leads
to the destruction of this balance and induces oxidative stress and
cell apoptosis (28). Consistent
with previous research, the results of the present study revealed
that ROS and MDA levels were significantly increased following
treatment with ethanol, whereas the activities of SOD and GSH
antioxidant enzymes were significantly decreased following
treatment with ethanol. Furthermore, the results of the present
study demonstrated that administration of TFs attenuated the
effects of ethanol treatment in GES-1 cells in a dose-dependent
manner.
Apoptosis, a form of programmed cell death, is
important for normal cell survival. Bcl-2 family members and
caspases are important regulators of mitochondria-mediated
apoptosis (47,48). Bcl-2 family members, which possess
a BH homology domain, such as Bcl-2, Bax and Bcl-2-like 1, have
been previously demonstrated to be regulators of the mitochondria
membrane potential via competitive level variations of
antiapoptotic factors (e.g. Bcl-2) and proapoptotic factors (e.g.
Bax) (29). In addition, such
factors modify the release of cytochrome c in order to
regulate the activation of downstream caspase pathways (49). Caspases are a family of
cysteine-aspartic proteases. Cell apoptosis in mammals is
predominantly induced by caspases, some of which function as
apoptosis activators and others function as apoptosis executioners
(50). Caspase-3 is the most
important executive factor in the apoptosis pathway (51). The present study demonstrated that
treatment with TFs downregulated the expression levels of Bax and
caspase-3, which were otherwise induced by ethanol injury in GES-1
cells. Furthermore, treatment with TFs upregulated the expression
levels of Bcl-2, which were suppressed following treatment with
ethanol alone. Therefore, TFs may protect GES-1 cells against
ethanol injury via the regulation of cell apoptosis.
MAPK pathways have important roles in cell
proliferation, differentiation, apoptosis and inflammation
(52,53). Studies have indicated that
extracellular signals are transferred between cells via the MAPK
pathway in order to induce various cellular responses (54,55).
ERK, JNK and p38 are important proteins in MAPK pathways (56). ERK is closely associated with cell
viability and proliferation, while JNK and p38 are involved in
apoptotic pathways and are more readily activated by stimuli in the
extracellular environment, including oxidative stress, ultraviolet
irradiation, high temperatures, ischemia reperfusion and
inflammatory factors (57,58). The results of the present study
revealed that treatment with ethanol upregulated the
phosphorylation levels of ERK, JNK and p38, indicating the
activation of associated MAPK pathways so as to induce oxidative
stress and apoptosis in GES-1 cells. Furthermore, the results
revealed that treatment with TFs protected GES-1 cells from
ethanol-induced injury via downregulation of the phosphorylation of
ERK, JNK and p38.
In conclusion, the results of the current study
indicated that TFs may attenuate ethanol-induced oxidative stress
and apoptosis in GES-1 gastric mucosa epithelial cells by
increasing the function of SOD and GSH in order to reduce ROS and
MDA levels, regulating the activity of apoptosis pathways, such as
via the upregulation of Bcl-2 expression and the downregulation of
Bax and Caspase-3 expression levels, and by suppressing the
activation of ERK, JNK and p38 MAPK pathways. The results of the
present study indicate that TFs, a type of national and
health-benefitting properties, may represent a novel therapeutic
agent for the treatment and/or prevention of diseases resulting
from ethanol-induced gastric mucosa injury. However, the effect of
TFs on gastric mucosa injury in a clinical setting requires further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
ZW and HL conducted all the experiments, HL also
produced the manuscript, and HX made substantial contributions to
the conception and design of the present study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boutemine IM, Amri M, Amir ZC, Fitting C,
Mecherara-Idjeri S, Layaida K, Sennoun N, Berkane S, Cavaillon JM
and Touil-Boukoffa C: Gastro-protective, therapeutic and
anti-inflammatory activities of Pistacia lentiscus L. Fatty oil
against ethanol-induced gastric ulcers in rats. J Ethnopharmacol.
224:273–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chi YC, Lee SL, Lee YP, Lai CL and Yin SJ:
Modeling of human hepatic and gastrointestinal ethanol metabolism
with kinetic mechanism-based full rate equations of the component
alcohol dehydrogenase isozymes and allozymes. Chem Res Toxicol.
31:556–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Araujo ERD, Guerra GCB, Araujo DFS, de
Araujo AA, Fernandes JM, de Araujo Junior RF, da Silva VC, de
Carvalho TG, Ferreira LS and Zucolotto SM: Gastroprotective and
antioxidant activity of kalanchoe brasiliensis and kalanchoe
pinnata leaf juices against indomethacin and ethanol-induced
gastric lesions in rats. Int J Mol Sci. 19(pii): E12652018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Zhou W, Gu Y, Dai J, Li X, Tai P,
Li Y, Ma X and Zhang Y: Protective effect of Pu-erh tea extracts
against ethanol-induced gastric mucosal damage in rats. Biomed Rep.
8:335–342. 2018.PubMed/NCBI
|
|
5
|
Chen H, Liao H, Liu Y, Zheng Y, Wu X and
Su Z, Zhang X, Lai Z, Lai X, Lin ZX and Su Z: Protective effects of
pogostone from Pogostemonis Herba against ethanol-induced gastric
ulcer in rats. Fitoterapia. 100:110–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong J, Zhang Z, Zhang X, Chen F, Tan Y,
Li H, Jiang J and Zhang J: Effects and possible mechanisms of
Alpinia officinarum ethanol extract on indomethacin-induced gastric
injury in rats. Pharm Biol. 56:294–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moris D, Spartalis M, Tzatzaki E,
Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI,
Tsilimigras DI and Theocharis S: The role of reactive oxygen
species in myocardial redox signaling and regulation. Ann Transl
Med. 5:3242017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slomiany A, Piotrowski E, Piotrowski J and
Slomiany BL: Impact of ethanol on innate protection of gastric
mucosal epithelial surfaces and the risk of injury. J Physiol
Pharmacol. 51:433–447. 2000.PubMed/NCBI
|
|
9
|
Shindo Y, Konagaya M, Harasawa S, Miwa T
and Osamura Y: The role of histamine in ethanol-induced gastric
mucosal injury in the rat. Tokai J Exp Clin Med. 22:59–64.
1997.PubMed/NCBI
|
|
10
|
Liu J, Wang J, Shi Y, Su W, Chen J, Zhang
Z, Wang G and Wang F: Short chain fatty acid acetate protects
against ethanol-induced acute gastric mucosal lesion in mice. Biol
Pharm Bull. 40:1439–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang HJ, Kim MJ, Kwon DY, Kang ES, Kang S
and Park S: Gastroprotective actions of Taraxacum coreanum Nakai
water extracts in ethanol-induced rat models of acute and chronic
gastritis. J Ethnopharmacol. 208:84–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Shang P, Liu T, Xu H, Ren D, Zhou
W, Wen A and Ding Y: Gastroprotective effects of chebulagic acid
against ethanol-induced gastric injury in rats. Chem Biol Interact.
278:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Yin B, Lv L, Wang Z, He J, Chen Z,
Wen X, Zhang Y, Sun W, Li Y and Zhao Y: Gastroprotective effect of
aucubin against ethanol-induced gastric mucosal injury in mice.
Life Sci. 189:44–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ilacqua AN, Shettler JA, Wernke KM, Skalla
JK and McQuade KJ: Theaflavins from black tea affect growth,
development, and motility in Dictyostelium discoideum. Biochem
Biophys Res Commun. 491:449–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pereira-Caro G, Moreno-Rojas JM, Brindani
N, Del Rio D, Lean MEJ, Hara Y and Crozier A: Bioavailability of
black tea theaflavins: Absorption, metabolism, and colonic
catabolism. J Agric Food Chem. 65:5365–5374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thakur VS, Gupta K and Gupta S: The
chemopreventive and chemotherapeutic potentials of tea polyphenols.
Curr Pharm Biotechnol. 13:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaveri NT: Green tea and its polyphenolic
catechins: Medicinal uses in cancer and noncancer applications.
Life Sci. 78:2073–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao Y, Rankin GO, Tu Y and Chen YC:
Theaflavin-3,3′-digallate decreases human ovarian carcinoma OVCAR-3
cell-induced angiogenesis via Akt and Notch-1 pathways, not via
MAPK pathways. Int J Oncol. 48:281–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan H, Wang F, Rankin GO, Rojanasakul Y,
Tu Y and Chen YC: Inhibitory effect of black tea pigments,
theaflavin3/3′-gallate against cisplatin-resistant ovarian cancer
cells by inducing apoptosis and G1 cell cycle arrest. Int J Oncol.
51:1508–1520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sur S and Panda CK: Molecular aspects of
cancer chemopreventive and therapeutic efficacies of tea and tea
polyphenols. Nutrition 43–44. 1–15. 2017.
|
|
21
|
Yang CS, Wang X, Lu G and Picinich SC:
Cancer prevention by tea: Animal studies, molecular mechanisms and
human relevance. Nat Rev Cancer. 9:429–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong L, Qi X, Huang S, Chen S, Wu Y and
Zhao L: Theaflavins inhibit pathogenic properties of P. Gingivalis
and MMPs production in P. Gingivalis-stimulated human gingival
fibroblasts. Arch Oral Biol. 60:12–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lombardo Bedran TB, Morin MP, Palomari
Spolidorio D and Grenier D: Black tea extract and its theaflavin
derivatives inhibit the growth of periodontopathogens and modulate
interleukin-8 and beta-defensin secretion in oral epithelial cells.
PLoS One. 10:e01431582015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frei B and Higdon JV: Antioxidant activity
of tea polyphenols in vivo: Evidence from animal studies. J
Nutrition. 133:3275s–3284s. 2003. View Article : Google Scholar
|
|
25
|
Han X, Zhang J, Xue X, Zhao Y, Lu L, Cui
M, Miao W and Fan S: Theaflavin ameliorates ionizing
radiation-induced hematopoietic injury via the NRF2 pathway. Free
Radic Biol Med. 113:59–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang H, Wang Y, Yin X, Liu X and Xuan H:
Ethanol extract of propolis and its constituent caffeic acid
phenethyl ester inhibit breast cancer cells proliferation in
inflammatory microenvironment by inhibiting TLR4 signal pathway and
inducing apoptosis and autophagy. BMC Complement Altern Med.
17:4712017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sims EK, Lakhter AJ, Anderson-Baucum E,
Kono T, Tong X and Evans-Molina C: MicroRNA 21 targets BCL2 mRNA to
increase apoptosis in rat and human beta cells. Diabetologia.
60:1057–1065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Cai S, Li J, Xiong L, Tian L, Liu
J, Huang J and Liu Z: Neuroprotective effects of theaflavins
against oxidative stress-induced apoptosis in PC12 cells. Neurochem
Res. 41:3364–3372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anandhan A, Essa MM and Manivasagam T:
Therapeutic attenuation of neuroinflammation and apoptosis by black
tea theaflavin in chronic MPTP/probenecid model of Parkinson's
disease. Neurotox Res. 23:166–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Z, Ma W, Huang C and Yang CS:
Inhibition of tumor promoter-induced activator protein 1 activation
and cell transformation by tea polyphenols, (−)-epigallocatechin
gallate, and theaflavins. Cancer Res. 57:4414–4419. 1997.PubMed/NCBI
|
|
31
|
Lu G, Liao J, Yang G, Reuhl KR, Hao X and
Yang CS: Inhibition of adenoma progression to adenocarcinoma in a
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung
tumorigenesis model in A/J mice by tea polyphenols and caffeine.
Cancer Res. 66:11494–11501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shao J, Meng Q and Li Y: Theaflavins
suppress tumor growth and metastasis via the blockage of the STAT3
pathway in hepatocellular carcinoma. Onco Targets Ther.
9:4265–4275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ganguly S, G TK, Mantha S and Panda K:
Simultaneous determination of black tea-derived catechins and
theaflavins in tissues of tea consuming animals using
ultra-performance liquid-chromatography tandem mass spectrometry.
PLoS One. 11:e01634982016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang M, Gao PF, Li HQ, Tian PY and Fan
XM: Ghrelin inhibition of ethanol-induced gastric epithelial cell
apoptosis is mediated by miR-21. Int J Clin Exp Pathol.
8:4662–4672. 2015.PubMed/NCBI
|
|
35
|
Chang W, Bai J, Tian S, Ma M, Li W, Yin Y,
Deng R, Cui J, Li J, Wang G, et al: Autophagy protects gastric
mucosal epithelial cells from ethanol-induced oxidative damage via
mTOR signaling pathway. Exp Biol Med (Maywood). 242:1025–1033.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olguin-Martinez M, Hernandez-Espinosa DR
and Hernandez-Munoz R: High alpha-Tocopherol dosing increases lipid
metabolism by changing redox state in damaged rat gastric mucosa
and liver after ethanol treatment. Clin Sci (Lond). 132:1257–1272.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Isbil-Buyukcoskun N, Cam B, Suyen GG and
Ozluk K: Effects of intracerebroventricularly injected
glucagon-like peptide-2 on ethanol-induced gastric mucosal damage
in rats. Endocr Res. Apr 9–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sahin HH, Cumbul A, Uslu U, Yilmaz Z,
Ercan F and Alican I: The effect of 1,25 dihydroxyvitamin D3 on
HCl/Ethanol-induced gastric injury in rats. Tissue Cell. 51:68–76.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo L, Guo J, Liu H, Zhang J, Chen X, Qiu
Y and Fu S: Tea polyphenols suppress growth and virulence-related
factors of Haemophilus parasuis. J Vet Med Sci. 80:1047–1053. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun L, Gidley MJ and Warren FJ: Tea
polyphenols enhance binding of porcine pancreatic alpha-amylase
with starch granules but reduce catalytic activity. Food Chem.
258:164–173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arent SM, Senso M, Golem DL and McKeever
KH: The effects of theaflavin-enriched black tea extract on muscle
soreness, oxidative stress, inflammation, and endocrine responses
to acute anaerobic interval training: A randomized, double-blind,
crossover study. J Int Soc Sports Nutr. 7:112010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fatima M, Kesharwani RK, Misra K and Rizvi
SI: Protective effect of theaflavin on erythrocytes subjected to in
vitro oxidative stress. Biochem Res Int. 2013:6497592013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu YY, Li W, Xu Y, Jin EH and Tu YY:
Evaluation of the antioxidant effects of four main theaflavin
derivatives through chemiluminescence and DNA damage analyses. J
Zhejiang Univ Sci B. 12:744–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
do Carmo TLL, Azevedo VC, de Siqueira PR,
Galvao TD, Dos Santos FA, Dos Reis Martinez CB, Appoloni CR and
Fernandes MN: Reactive oxygen species and other biochemical and
morphological biomarkers in the gills and kidneys of the
Neotropical freshwater fish, Prochilodus lineatus, exposed to
titanium dioxide (TiO2) nanoparticles. Environ Sci
Pollut Res Int. Jun 1–2018.(Epub ahead of print). View Article : Google Scholar
|
|
46
|
Li H, Cao F, Zhao F, Yang Y, Teng M, Wang
C and Qiu L: Developmental toxicity, oxidative stress and
immunotoxicity induced by three strobilurins (pyraclostrobin,
trifloxystrobin and picoxystrobin) in zebrafish embryos.
Chemosphere. 207:781–790. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Liu J, Ye G, Gan F, Hamid M, Liao S
and Huang K: Protective effects of zymosan on heat stress-induced
immunosuppression and apoptosis in dairy cows and peripheral blood
mononuclear cells. Cell Stress Chaperones. Jun 2–2018.(Epub ahead
of print). View Article : Google Scholar
|
|
48
|
Meng LQ, Wang Y, Luo YH, Piao XJ, Liu C,
Wang Y, Zhang Y, Wang JR, Wang H, Xu WT, et al: Quinalizarin
induces apoptosis through reactive oxygen Species (ROS)-Mediated
mitogen-activated protein kinase (MAPK) and signal transducer and
activator of transcription 3 (STAT3) signaling pathways in
colorectal cancer cells. Med Sci Monit. 24:3710–3719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochromec. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han DK, Chaudhary PM, Wright ME, Friedman
C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM and Hood L: MRIT, a
novel death-effector domain-containing protein, interacts with
caspases and BclXL and initiates cell death. Proc Natl Acad Sci
USA. 94:pp. 11333–11338. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Scatena R, Bottoni P, Botta G, Martorana
GE and Giardina B: The role of mitochondria in pharmacotoxicology:
A reevaluation of an old, newly emerging topic. Am J Physiol Cell
Physiol. 293:C12–C21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Becatti M, Barygina V, Mannucci A, Emmi G,
Prisco D, Lotti T, Fiorillo C and Taddei N: Sirt1 protects against
oxidative stress-induced apoptosis in fibroblasts from psoriatic
patients: A new insight into the pathogenetic mechanisms of
psoriasis. Int J Mol Sci. 19(pii): E15722018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Miao LS, Cai YM, He JX, Zhang ZN,
Wu G and Zheng J: TXNIP knockdown alleviates hepatocyte ischemia
reperfusion injury through preventing p38/JNK pathway activation.
Biochem Biophys Res Commun. 502:409–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Soustek MS, Balsa E, Barrow JJ,
Jedrychowski M, Vogel R, Jan S, Gygi SP and Puigserver P:
Inhibition of the ER stress IRE1alpha inflammatory pathway protects
against cell death in mitochondrial complex I mutant cells. Cell
Death Dis. 9:6582018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feng W, Li J, Liao S, Ma S, Li F, Zhong C,
Li G, Wei Y, Huang H, Wei Q, et al: Go6983 attenuates titanium
particle-induced osteolysis and RANKL mediated osteoclastogenesis
through the suppression of NFkappaB/JNK/p38 pathways. Biochem
Biophys Res Commun. Jun 5–2018.(Epub ahead of print). View Article : Google Scholar
|
|
57
|
Paudel P, Jung HA and Choi JS:
Anthraquinone and naphthopyrone glycosides from Cassia obtusifolia
seeds mediate hepatoprotection via Nrf2-mediated HO-1 activation
and MAPK modulation. Arch Pharm Res. 41:677–689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
You MM, Chen YF, Pan YM, Liu YC, Tu J,
Wang K and Hu FL: Royal Jelly attenuates LPS-induced inflammation
in BV-2 microglial cells through modulating NF-kappaB and p38/JNK
signaling pathways. Mediators Inflamm. 2018:78343812018. View Article : Google Scholar : PubMed/NCBI
|