Introduction
The development of lymphoid proliferations after
transplantation has been recognized for more than a quarter of
century as an important morbidity factor (1). The post-transplant
lymphoproliferative disorder (PTLD) refers to a heterogeneous group
of lymphoproliferative diseases, which vary from uncomplicated,
self-limiting infectious mononucleosis, to malignant lymphoma. The
histological characterization varies from reactive-appearing,
polyclonal lymphoid infiltrates or undifferentiated cells that are
morphologically indistinguishable from malignant lymphoma or plasma
cell myeloma (2–4).
PTLD is relatively rare; nevertheless, it is the
most frequent malignant disease early after transplantation, with
the majority of cases being reported in the first year after
transplantation (3,5,6).
Risk factors for PTLD development include young age and age over 50
years at transplantation, white race, unrelated or HLA-mismatched
graft, Epstein-Barr virus (EBV)-seronegative status prior to
transplant, primary EBV infection, type of organ transplant,
intensity of immunosuppression and the occurrence of concomitant
cytomegalovirus disease (3,7).
Not all PTLD cases are EBV-related, but consistent
data recognize primary EBV infection as the most important risk
factor for PTLD development (8,9).
Indeed, the immunosuppression after transplantation in an
EBV-seropositive patient reduces the activity of the patients'
EBV-specific cytotoxic T-cell surveillance, which increases the
probability of uncontrolled proliferation of EBV-infected B-cells
and subsequent progression to PTLD (10). Moreover, transplant recipients
experiencing primary EBV infection during the early post-transplant
period seem to be particularly susceptible to develop PTLD of
B-cell origin, reflecting their lack of any preexisting
EBV-specific T-cell immunity (3,10).
The overall incidence of PTLD varies from 1 to 22%
depending on the presence of risk factors, namely the transplanted
organ, patient age, EBV serostatus from recipient and donor,
aggressiveness of immunosuppression (11). The cumulative incidence of PTLD in
allogeneic hematopoietic stem cell transplantation (aHSCT)
recipients is approximately 1.0% (range 0.5–1.8%), with slightly
higher rates in the pediatric population (1,12).
Survival rates depend mainly on the type of PTLD, extent of disease
and patient age: While pediatric patients and those with localized
disease seem to have a better prognosis, monomorphic PTLDs are the
most aggressive forms (5,7,13).
The purpose of this study was to examine the
clinical and pathologic characteristics, as well as the outcome of
PTLD after aHSCT, in patients diagnosed at the Portuguese Oncology
Institute of Porto (Porto, Portugal) between 2005 and 2012.
Materials and methods
Type of study and study
participants
We retrospectively reviewed the clinic-pathological
and EBV infection data of patients that developed PTLD after aHSCT
at the Bone Marrow Transplantation Unit from Portuguese Oncology
Institute of Porto in 2005 and 2012. This retrospective study was
approved by the Ethical Committee of IPO Porto. The present study
included a total of 15 patients, 8 females (53.3%) and 7 males
(46.7%). When available, cases were histologically confirmed by an
expert pathologist and classified according the most recently
available edition of the World Health Organization (WHO)
Classification of Tumours of Haematopoietic and Lymphoid Tissues
(4th edition).
Sample processing and EBV
detection
Samples were collected in EDTA-containing tubes
(Vacutainer®; BD Biosciences, Franklin Lakes, NJ, USA)
and stored in freezing temperature prior to processing. Blood
samples were collected retrospectively from the institution
archives. DNA was extracted by MagNA Pure Compact Nucleic Acid
Isolation kit I (Roche Diagnostics GmbH, Mannheim, Germany).
DNA/RNA quality was assessed by measuring the absorbance at 260/280
nm using the NanoDrop 1000 Spectrophotometer v3.7 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA).
All patients submitted to aHSCT were monitored for
EBV infection upon request from clinicians after clinical
suspicion. EBV detection was performed at the Virology Service of
IPO Porto using the commercial Real-Time PCR kit EBV Q-PCR Alert
(Nanogen Advanced Diagnostics S.p.A., Trezzano sul Naviglio, Italy)
which targets a region from EBV nuclear antigen 1 gene (EBNA1).
Amplification was performed with the ABI PRISM 7300 Sequencer
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and results were obtained by measuring the
geometric increase of probe fluorescence during amplification and
samples were considered positive when the exponential curve
exceeded the cycle threshold line.
Regarding amplification quality, positive and
negative controls were used: as negative control we used double
distilled water in replacement of template DNA; and as positive
control we have used samples from the external quality control
panel used at the Virology Service for EBV diagnosis.
Data collection
Clinic-pathological data was extracted from
institutional databases including pre-transplant recipient age,
gender, underlying disease, HLA-donor-recipient status, EBV
serological status of the recipient, source of stem cells,
conditioning regimen and use of ATG; post-transplant information
(clinical findings, date of PTLD suspicion, date of PTLD
confirmation, PTLD type, GVHD prophylaxis, GVHD type and outcome)
and viral data (date of EBV suspicion, EBV viral load).
Statistical analysis
Statistical analysis was performed using the SPSS
version 20.0 software (IBM Corp., Armonk, NY, USA). Overall
survival was defined as the time between the date of transplant and
the date of last follow-up or mortality. The differences in
survival were calculated using the log-rank test and the
Kaplan-Meier method.
Results
The study included a total of 15 patients, 8 females
(53.3%) and 7 males (46.7%), with median age of 10 years-old (range
3–38)- Table I. Patients had a
median follow-up time of 14 months (range: 2–72). Primary diagnoses
of patients included in this study included paroxysmal nocturnal
hemoglobinuria (n=1), primary immunodeficiency (n=1), acute
lymphocytic leukemia (n=6), acute myelogenous leukemia (n=4),
chronic myelogenous leukemia (n=1),
myelodysplastic/myeloproliferative syndrome (n=1) and congenital
amegakaryocytic thrombocytopenia (n=1). Most of patients had
mismatched/unrelated donors (73.3%) and the collection of cells was
mainly performed by peripheral blood stem cells (80.0%).
Myeloablative conditioning was used in 14 patients and ATG in 12
patients. Transplant-related information for each patient is
described in Table II.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Variable | n (%) |
|---|
| Age, median (range);
years | 10 (3–38) |
| Sex |
|
| Male | 7 (46.7) |
|
Female | 8 (53.3) |
| Underlying
disease |
|
| Acute
leukemia | 10 (66.6) |
| Chronic
leukemia | 1 (6.7) |
|
Myelodysplastic/myeloproliferative
syndrome | 1 (6.7) |
|
Others | 3 (20.0) |
| HLA donor |
|
|
Match/related | 4 (26.7) |
|
Mismatched/unrelated | 11 (73.3) |
| Source of cells |
|
| PBSC | 12 (80.0) |
| BM | 2 (13.3) |
| UCB | 1 (6.7) |
| Conditioning
regimen |
|
| MAC | 14 (93.3) |
| RIC | 1 (6.7) |
| ATG |
|
| Yes | 12 (85.7) |
| No | 2 (14.5) |
 | Table II.Transplant-associated patient
information. |
Table II.
Transplant-associated patient
information.
| Patient | Age (years) | Gender | Diagnosis | Pre-conditioning | ATG | Myeloablation | Donor | Source | GVHD prophylaxis | GVHD type | Outcome |
|---|
| 1 | 25 | Male | PNH | BuCy2ATG | Yes | Yes | UMD | PB | Tacrolimus | Acute | Mortality |
| 2 | 6 | Male | PI | Bu12Cy2ATG | Yes | Yes | UMD | BM | Tacrolimus+MTX | Acute | Alive |
| 3 | 6 | Male | ALL | Bu12Cy120MelfATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Alive |
| 4 | 38 | Female | ALL |
Bu12Cy120MelfATG | Yes | Yes | UMD | PB | CSP+MTX | Chronic | Alive |
| 5 | 16 | Male | ALL |
Bu12Cy120MelfATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Mortality |
| 6 | 23 | Female | ALL | BuCyMelfATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute | Mortality |
| 7 | 8 | Male | ALL | BuCyMelfATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Mortality |
| 8 | 28 | Male | AML | BuCy2ATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Alive |
| 9 | 10 | Male | AML | Bu12Cy2ATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Chronic | Mortality |
| 10 | 36 | Female | AML | BuCy2ATG | Yes | Yes | MRD | PB | Tacrolimus+MTX | Acute/chronic | Alive |
| 11 | 6 | Female | AML | Bu16Cy4ATG | Yes | Yes | UMD | BM | Tacrolimus+MTX | Acute/chronic | Alive |
| 12 | 3 | Female | CML | BuCyATG | Yes | Yes | UMD | UCB | Tacrolimus | NA | Alive |
| 13 | 19 | Female | MDS | BuCy2ATG | Yes | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Mortality |
| 14 | 4 | Female | CAT |
AlemtuzumabFluCy | Yes | Reduced
intensity | UMD | PB | T-cell
depletion | NA | Mortality |
| 15 | 8 | Female | ALL | BuCyMelf | No | Yes | UMD | PB | Tacrolimus+MTX | Acute/chronic | Alive |
Regarding the clinical presentation of patients, 2
presented with fever, 12 had increased liver enzymes, adenomegalies
were observed in 2 patients and 12 patients had also increased
lactate dehydrogenase. EBV serological status prior to
transplantation were evaluated according to presence of IgM and IgG
titers in plasma samples. Serological status was divided in three
groups: susceptible (absence of IgM and IgG), active infection
(presence of IgM and/or IgG) and finally, past infection (absence
of IgM and presence of IgG).
The development of EBV infection was present in all
of 15 patients, with a median time of diagnosis after transplant of
68 days (range 29–464 days), with 80% (n=12) of them detected
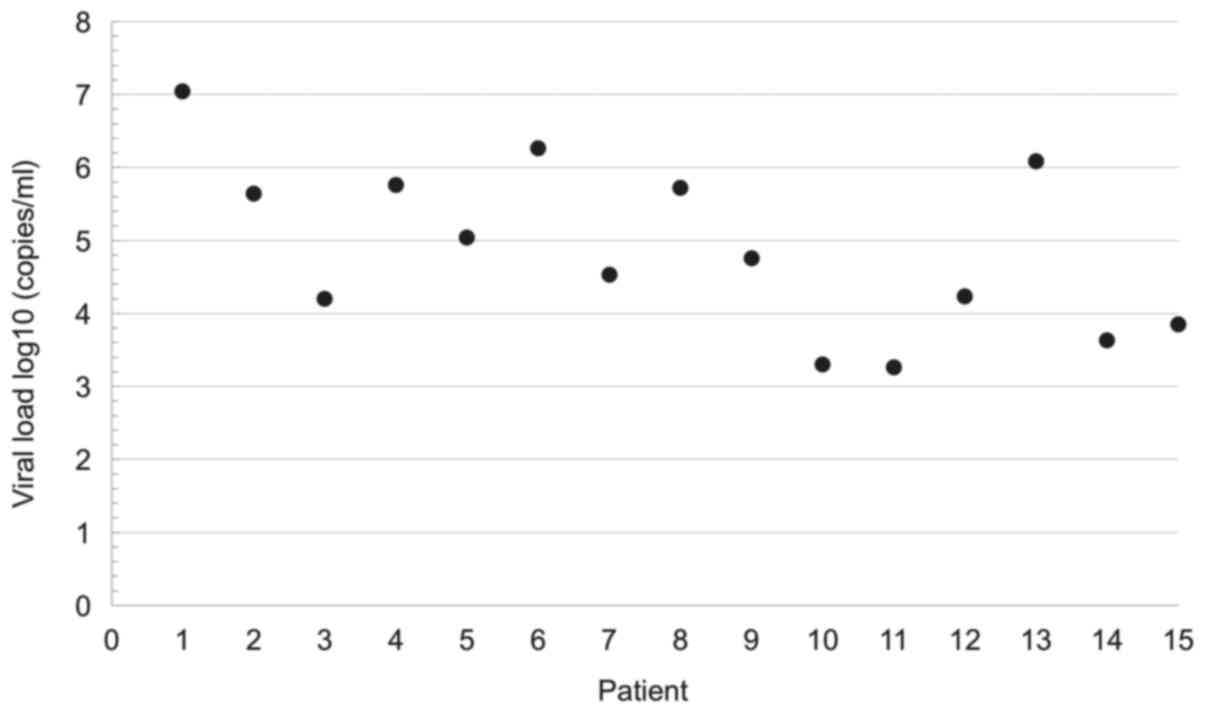
<180 days after transplant, and with a median viral load of 4.75
log10 copies/ml (range 3.30–6.26 log10
copies/ml; Fig. 1). PTLD diagnosis
occurred approximately in the same period where EBV infection
occurred (mean 135, median 75 days and range 25–485 days vs. mean
130 days, median 68 days and range 29–464 days, respectively). PTLD
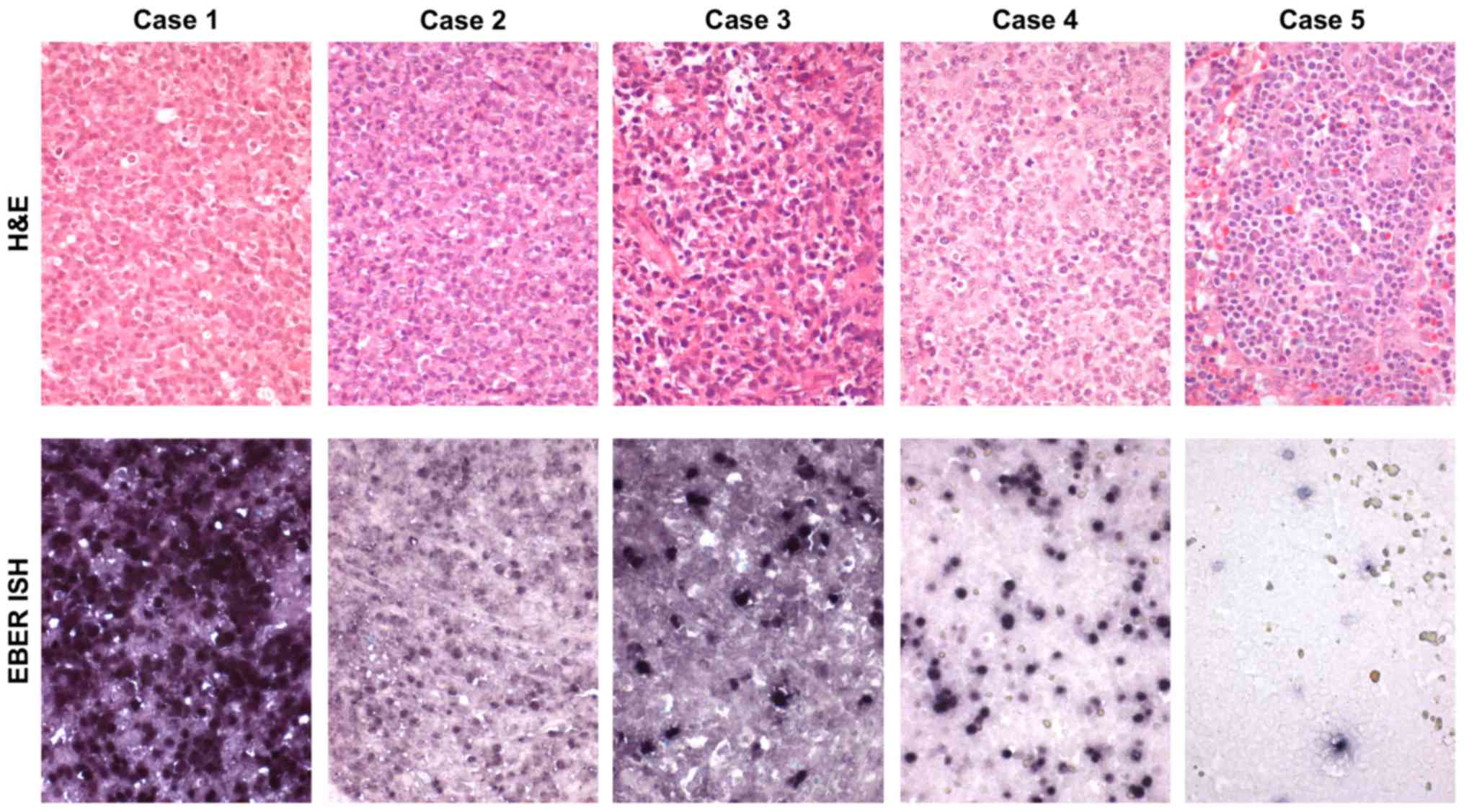
classification was available for only 5 patients and included
monomorphic-type PTLD (n=3), polymorphic PTLD (n=1) and reactive
plasmocytic hyperplasia (early lesions) (n=1) (Table III; Fig. 2). The remaining cases were not
histological confirmed, and diagnosis was established by
considering all clinical findings.
 | Table III.Characteristics of PTLD and EBV in
patients. |
Table III.
Characteristics of PTLD and EBV in
patients.
| Patient | Age (years) | Gender | Diagnosis | Clinical
findings | EBV IgM | EBV IgG | EBV serostatus | TT EBV infection
(days) | Viral load
(copies/ml) | Viral load (Log
copies/ml) | PTLD
classification | Biopsy vs. excision
(topography) |
|---|
| 1 | 25 | Male | PNH | Fever, Adenomegaly,
Hepatomegaly; ↑ liver enzymes and LDH | NR | R | Past infection | 53 |
1.10×107 | 5.64 | NA | NA |
| 2 | 6 | Male | PI | Fever; ↑ liver
enzymes | NR | R | Past infection | 29 |
4.40×105 | 4.20 | NA | NA |
| 3 | 6 | Male | ALL | ↑ liver
enzymes | NR | R | Past infection | 123 |
1.60×104 | 5.76 | NA | NA |
| 4 | 38 | Female | ALL | ↑ liver enzymes and
LDH | NR | R | Past infection | 38 |
5.70×105 | 5.04 | NA | NA |
| 5 | 16 | Male | ALL | ↑ liver enzymes and
LDH | R | R | Active
infection | 150 |
1.10×105 | 6.26 | Monomorphic (Case
2) | Biopsy
(amygdala) |
| 6 | 23 | Female | ALL | ↑ liver enzymes and
LDH | NR | R | Past infection | 68 |
1.80×106 | 4.53 | Monomorphic (Case
3) | Biopsy
(amygdala) |
| 7 | 8 | Male | ALL | ↑ liver enzymes and
LDH | NR | R | Past infection | 46 |
3.40×104 | 5.72 | NA | NA |
| 8 | 28 | Male | AML | Adenomegaly;
Pancytopenia; ↑ liver enzymes and LDH | NR | R | Past infection | 53 |
5.30×105 | 4.75 | Monomorphic (Case
1) | Excision (Cervical
lymph node) |
| 9 | 10 | Male | AML | ↑ liver
enzymes | NR | R | Past infection | 60 |
5.60×104 | 3.30 | NA | NA |
| 10 | 36 | Female | AML | ↑ LDH | NR | R | Past infection | 333 |
2.00×103 | 3.26 | Early lesions (Case
5) | Excision (Lymph
node) |
| 11 | 6 | Female | AML | ↑ liver enzymes and
LDH | NR | R | Past infection | 285 |
1.80×103 | 4.23 | NA | NA |
| 12 | 3 | Female | CML | ↑ liver enzymes and
LDH | NR | R | Past infection | 464 |
1.70×104 | 6.08 | Polymorphic (Case
4) | Excision (Cervical
lymph node) |
| 13 | 19 | Female | MDS | ↑ LDH | NR | R | Past infection | 44 |
1.20×106 | 3.63 | NA | NA |
| 14 | 4 | Female | CAT | ↑ LDH | NR | R | Past infection | 82 |
4.30×103 | 5.64 | NA | NA |
| 15 | 8 | Female | ALL | ↑ liver enzymes and
LDH | NR | NR | Susceptible | 119 |
7.00×103 | 3.85 | NA | NA |
We observed graft-vs.-host disease (GVHD) in 13
patients (93.3%): 3 with acute GVHD (20.0%), 2 with chronic GVHD
(13.3%) and 8 with both (53.3%). Considering the grade of acute
GVHD, all patients with clinical information had a grade of II or
higher. Regarding chronic GVHD, 3 patients had an evolution of
acute-to-chronic, while 7 had a de novo chronic GVHD; two
patients experienced extensive disease and 5 had only limited
disease (Table II).
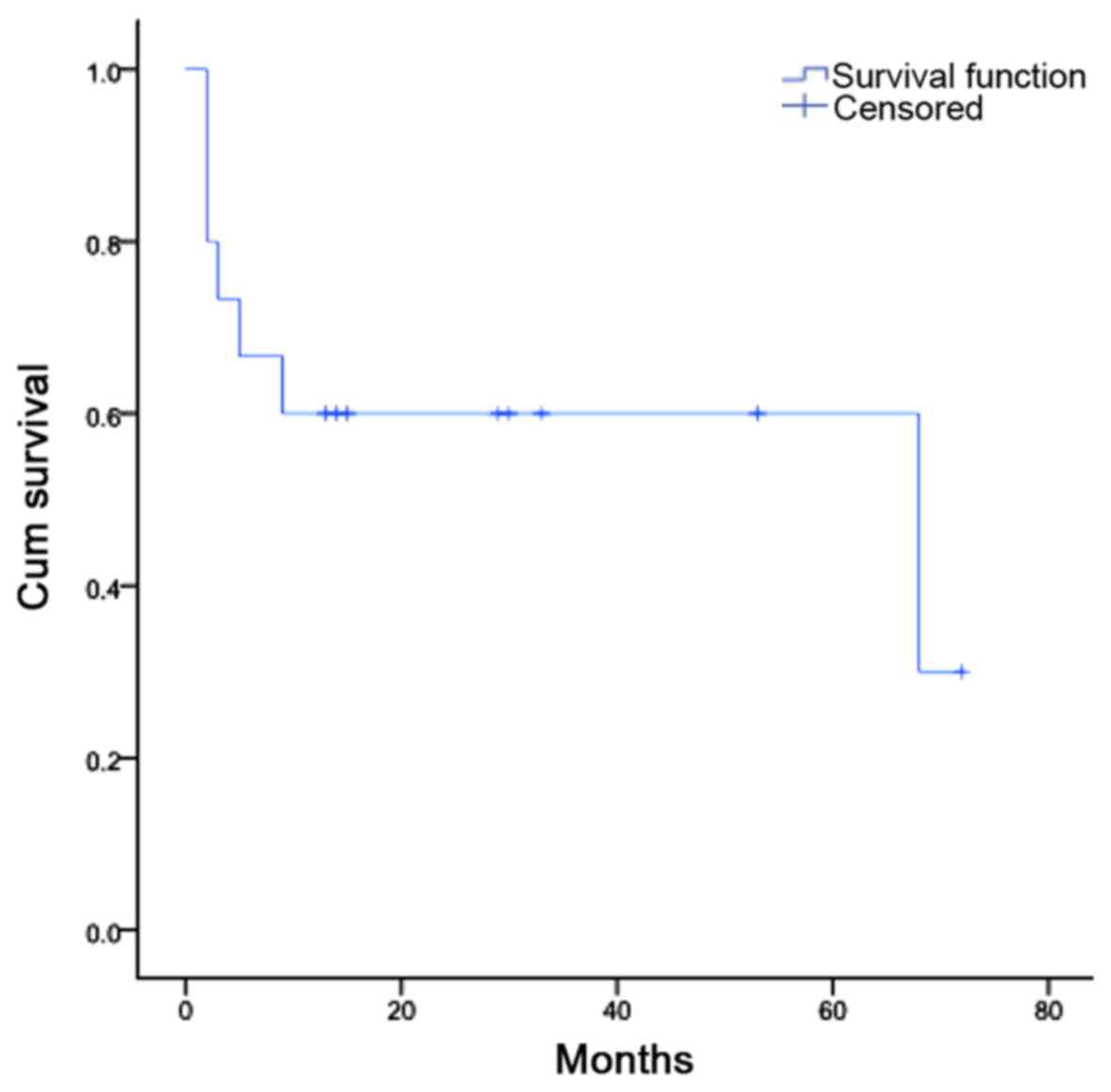
As for the overall outcome, 8 patients are still
alive (53.3%), 5 with no signs of disease (33.3%) and 3 with
evidence of disease (20.0%); and 7 patients have died (46.7%), 4
from complications associated with the transplant (26.7%) and 3
from progression of disease (20.0%) (Table II). A Kaplan-Meier plot was
obtained by evaluating the cumulative survival of these patients,
in months (Fig. 3).
Discussion
PTLD is one of the most serious complications of
immunosuppression in patients who undergo hematopoietic stem cell
transplantation, with high impact on morbidity and mortality
(14). EBV infection has been
strongly associated with the development of PTLD, and this
association is widely described in the literature (15,16).
In this retrospective analysis, we verified that
PTLD affects individuals of all age groups and with several types
of hematological malignancies, the majority having had unrelated
donors. Our patients had different types of pre-conditioning
regimens (myeloablative in 14 patients), with predominance of
busulfan and cyclophosphamide. Since types of regimens are varied,
they appear not to have a direct correlation with the development
of PTLD. ATG was used in almost all patients except for two, and
without absolute prevalence date it is difficult to confirm if its
use is directly correlated with PTLD development. GVHD prophylaxis
was performed mainly with tacrolimus, and concomitant with MTX, and
still patients have developed some type of GVHD which indicates
that altering prophylaxis regimen should be taken into
consideration.
In our case series, EBV infection was diagnosed at a
median of 68 days after transplant. EBV infection is frequently
associated with the intermediate period after aHSCT, mainly between
3 weeks to 3 months after transplant (17). Viral infection during this period
is correlated with delayed or incomplete reconstitution of specific
immunity, or patients experiencing GVHD (18). Regarding PTLD, frequently, the
median onset of development is 3 months, with a range of 2–5 months
after transplantation (13), which
is consistent with our data. Symptoms are quite nonspecific, with
patients presenting with fever, malaise, enlarged lymph nodes and
high levels of LDH, which were the factors for clinical PTLD
suspicion in our patients (2). All
patients that developed PTLD had an EBV infection at some point
after transplantation. EBV positivity is directly related to PTLD
development since its infection, or increase in viral load up to
2,000 copies/ml, occurs mainly at the same time PTLD is diagnosed.
PTLD is more frequent in EBV-seronegative patients receiving
allografts from EBV-seropositive donors and in patients with
delayed immune reconstitution due to T-cell-depletion or
HLA-mismatched donor. In a study conducted by Brunstein et
al (19), 15 of 335 patients
developed a EBV-related complication, at a median of 133 days
(range 52–407 days), which is consistent with our results.
As previously described by Bhatia et al
(20), PTLD has mortality rates
reaching up to 70–90%, which is higher than our results (46.7%).
Survival rates depend on age and stage of disease at the time of
diagnosis, with pediatric and patients with localized disease
showing the best prognosis (5). In
our study, overall patient survival was not affected by the
development of PTLD.
This is the first study to describe PTLD cases in
HSCT patients in Portugal, combining data from several years at a
reference transplantation center. This study demonstrates that EBV
infection occurs mainly between 2 and 4 months after transplant and
precedes the development of PTLD, and especially the viral load may
be important for the monitorization and early diagnosis of PTLD.
Thus, the study shows the importance of identify high-risk patients
for PTLD development and to provide them a frequent monitorization
of EBV viral load as suggested by recent guidelines (21,22).
Acknowledgements
The authors would like to acknowledge the support of
Mrs. Rute Silva from the Bone Marrow Transplant Service at IPO
Porto (Porto, Portugal) who assisted with the collection of
clinicopathological data for the present study.
Funding
Dr JMD received a grant for the development of the
project from the Portuguese League Against Cancer (Liga Portuguesa
Contra o Cancro-Núcleo Regional do Norte) between April and
September 2016.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JMD and HS designed the study, JL and RH performed
the histological analysis of cases, and CPV, LR, RB, FC and ACJr
revised the clinical information obtained from patients. IB and RM
provided the laboratory data, and JMD and HS performed data
analysis and drafted the manuscript. All authors read and revised
the manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by The Ethics
Committee of Portuguese Oncology Institute of Porto (Porto,
Portugal). The need for written informed consent was waived due to
the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Castellano-Sanchez AA, Li S, Qian J, Lagoo
A, Weir E and Brat DJ: Primary central nervous system
posttransplant lymphoproliferative disorders. Am J Clin Pathol.
121:246–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gulley ML and Tang W: Using Epstein-Barr
viral load assays to diagnose, monitor, and prevent posttransplant
lymphoproliferative disorder. Clin Microbiol Rev. 23:350–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glotz D, Chapman JR, Dharnidharka VR,
Hanto DW, Castro MC, Hirsch HH, Leblond V, Mehta AK, Moulin B,
Pagliuca A, et al: The Seville expert workshop for progress in
posttransplant lymphoproliferative disorders. Transplantation.
94:784–793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mucha K, Foroncewicz B,
Ziarkiewicz-Wróblewska B, Krawczyk M, Lerut J and Paczek L:
Post-transplant lymphoproliferative disorder in view of the new WHO
classification: a more rational approach to a protean disease?
Nephrol Dial Transplant. 25:2089–2098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalinova L, Indrakova J and Bachleda P:
Post-transplant lymphoproliferative disorder. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 153:251–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atalay A, Gökahmetoğlu S, Durmaz S,
Kandemir I, Sağlam D, Kaynar L, Eser B, Cetin M and Kılıç H:
Investigation of epstein-barr virus and parvovirus b19 DNA in
allogeneic stem cell transplant patients. Turk J Haematol.
31:155–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MJ, Kim I, Bae HM, Seo K, Park N, Yoon
SS, Park S and Kim BK: Hematopoietic stem cell transplantation
after posttransplant lymphoproliferative disorder. J Korean Med
Sci. 25:781–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caillard S, Lelong C, Pessione F and
Moulin B; French PTLD Working Group, : Post-transplant
lymphoproliferative disorders occurring after renal transplantation
in adults: Report of 230 cases from the French registry. Am J
Transplant. 6:2735–2742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Funch DP, Walker AM, Schneider G, Ziyadeh
NJ and Pescovitz MD: Ganciclovir and acyclovir reduce the risk of
post-transplant lymphoproliferative disorder in renal transplant
recipients. Am J Transplant. 5:2894–2900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen U and Preiksaitis J; AST Infectious
Diseases Community of Practice, : Epstein-barr virus and
posttransplant lymphoproliferative disorder in solid organ
transplant recipients. Am J Transplant. 9 Suppl 4:S87–S96. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bar-Natan M and Nagler A: Epstein-barr
virus-associated post-transplant lymphoproliferative disorder. Isr
Med Assoc J. 8:205–207. 2006.PubMed/NCBI
|
|
12
|
Grywalska E, Markowicz J, Grabarczyk P,
Pasiarski M and Roliński J: Epstein-barr virus-associated
lymphoproliferative disorders. Postepy Hig Med Dosw (Online).
67:481–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo L, Zhang L, Cai B, Li H, Huang W, Jing
Y, Zhu H, Zhao Y, Bo J, Wang Q, et al: Post-transplant
lymphoproliferative disease after allogeneic hematopoietic stem
cell transplantation: A single-center experience. Ann Transplant.
19:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatia S: Long-term health impacts of
hematopoietic stem cell transplantation inform recommendations for
follow-up. Expert Rev Hematol. 4:437–452; quiz 453–454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ibrahim HA and Naresh KN: Posttransplant
lymphoproliferative disorders. Adv Hematol. 2012:2301732012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JH, Park BB, Suh C, Won JH, Lee WS
and Shin HJ: Clinical characteristics of monomorphic
post-transplant lymphoproliferative disorders. J Korean Med Sci.
25:523–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burns DM, Rana S, Martin E, Nagra S, Ward
J, Osman H, Bell AI, Moss P, Russell NH, Craddock CF, et al:
Greatly reduced risk of EBV reactivation in rituximab-experienced
recipients of alemtuzumab-conditioned allogeneic HSCT. Bone Marrow
Transplant. 51:825–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Safdar A: Principles and Practice of
Cancer Infectious DiseasesCurrent Clinical Oncology. Humana Press;
New York, NY: 2011
|
|
19
|
Brunstein CG, Weisdorf DJ, DeFor T, Barker
JN, Tolar J, van Burik JA and Wagner JE: Marked increased risk of
Epstein-Barr virus-related complications with the addition of
antithymocyte globulin to a nonmyeloablative conditioning prior to
unrelated umbilical cord blood transplantation. Blood.
108:2874–2880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhatia S, Ramsay NK, Steinbuch M,
Dusenbery KE, Shapiro RS, Weisdorf DJ, Robison LL, Miller JS and
Neglia JP: Malignant neoplasms following bone marrow
transplantation. Blood. 87:3633–3639. 1996.PubMed/NCBI
|
|
21
|
Styczynski J, Einsele H, Gil L and
Ljungman P: Outcome of treatment of Epstein-Barr virus-related
post-transplant lymphoproliferative disorder in hematopoietic stem
cell recipients: a comprehensive review of reported cases. Transpl
Infect Dis. 11:383–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Styczynski J, van der Velden W, Fox CP,
Engelhard D, de la Camara R, Cordonnier C and Ljungman P; Sixth
European Conference on Infections in Leukemia, a joint venture of
the Infectious Diseases Working Party of the European Society of
Blood and Marrow Transplantation (EBMT-IDWP), the Infectious
Diseases Group of the European Organization for Research and
Treatment of Cancer (EORTC-IDG), the International
Immunocompromised Host Society (ICHS) and the European Leukemia Net
(ELN), : Management of Epstein-Barr Virus infections and
post-transplant lymphoproliferative disorders in patients after
allogeneic hematopoietic stem cell transplantation: Sixth European
Conference on Infections in Leukemia (ECIL-6) guidelines.
Haematologica. 101:803–811. 2016. View Article : Google Scholar : PubMed/NCBI
|