Introduction
Taurine is a non-proteogenic and essential amino
acid in animals (1), and is known
to play a critical nutritional role in brain cell growth,
differentiation, and development (2). Huxtable (3) have reported on the functional role of
taurine in the central nervous system, as well as its functions in
cardiovascular and skeletal muscle. Rak et al (4) demonstrated that taurine plays a key
role in regeneration and neuroprotection in the injured nervous
system. Taurine is an effective antioxidant against lead-,
cadmium-, and exercise-induced oxidative stress (5), and is known to reduce the secretion
of lipids and apolipoprotein B100 in liver cancer cells (6). Taurine is also invovled in
neurotransmission, detoxification, osmoregulation, calcium
homeostasis, obesity prevention, excitotoxicity, osmotic shock
recovery, and prevention of seizures (7–13).
Traumatic brain injury (TBI) leads to cognitive
deficits, high mortality, and impaired movement (14). The most common cause of TBI is
external force to the brain, and it can be classified as closed or
penetrating head injury (15).
Ischemia, oxidative stress, apoptosis, inflammation,
excitotoxicity, and vascular and neuronal damage may also cause TBI
(16,17). Lotocki et al (18) reported that inflammation is a
well-known critical event in TBI, which may be mediated by the
secretion of cytokines and activation of glial cells. Taurine
supplementation may substantially reduce inflammatory cytokines,
such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and
IL-1β, in spinal cord injury (19). Heidari et al (20) reported a protective effect of
taurine against acute and chronic liver injuries. Recently, Wang
et al (2) investigated the
protective role of taurine against TBI. In the present study, we
investigated the therapeutic effect of taurine on levels of ROS,
malondialdehyde (MDA), reduced glutathione (GSH), glutathione
peroxidase (Gpx), superoxide dismutase (SOD), catalase,
acetylcholinesterase (AChE), TNF-α, IL-6, caspase-3, p53, bcl-2 and
bax in injured brain cells.
Materials and methods
Animals
Twenty-four male albino Wistar strain neonatal rats
were obtained from The Second Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China). The rats weighed 5–10 g and were allowed
free access to water and food with a 12-h light and dark cycle.
Rats were sacrificed by decapitation following intraperitoneal
administration of ketamine hydrochloride (80 mg/kg) and xylazine
(10 mg/kg). All experiments involving rats were monitored and
approved by the ethics committee of The Second Affiliated Hospital
of Xi'an Jiaotong University (Ref no. 2o14/2Tx1221).
Cell culture
Cortical tissues were isolated from embryonic day 15
rats and disassociated. Separated cells were cultured at a density
of 1.5×103 cells/ml on existing astrocyte cell cultures.
Co-cultures of astrocytes and neuron cells were prepared as
previously described (21). The
co-culture was supplemented with standard growth medium containing
10% fetal bovine serum and Dulbecco's modified Eagle's medium.
Experimental traumatic brain cell
injury model
Experimental traumatic brain cell injury was induced
according to Katano et al (22). Traumatic model cells were
supplemented with G5 (2%) for 12 h before the induction of injury.
The mechanical injury was induced using a standard scratch method
(23), and standard scratches were
made in 6-well plates. Cells were supplemented with standard growth
medium. After 24 h, cell survival was evaluated as lactate
dehydrogenase activity (ab102526; Abcam, Cambridge, UK).
Taurine treatment and sample
collection
Cells were treated with 100, 200, or 300 mg/l of
taurine (ab141063; Abcam) for 72 h. Following treatment, the medium
was removed carefully and the cells were washed with phosphate
buffered saline. The cells were collected, centrifuged, and stored
at −80°C.
Oxidative markers
ROS level was measured by the incubation of cells
with dichloro-dihydro-fluorescein diacetate (DCFH-DA) for 30 min,
and fluorescence was measured under a fluorescence plate reader
(24). The MDA content in the cell
supernatant was determined by measuring thiobarbituric acid
reactive species (TBARS). Briefly, the reaction tube contained 0.1
ml of cell culture supernatant, thiobarbituric acid (1.5 ml), 0.2
ml of sodium dodecyl sulfate (SDS), and acetic acid (1.5 ml). The
resultant upper layer product was measured at 534 nm (24). GSH levels were determined based on
Ellman's reaction. The absorbance was measured at 412 nm (24). Gpx activity was measured by adding
0.2 ml of Tris-HCl buffer, 0.2 ml of GSH, 0.1 ml of
H2O2, 0.2 ml of homogenate, and sodium azide
(0.1 ml) to the reaction tube. The reaction tube was centrifuged
for 10 min at 3,000 × g. Then, cell culture supernatant (0.2 ml)
and Ellman's reagent (0.1 ml) were added to the reaction tube, and
the final absorbance was measured at 340 nm (25).
SOD activity was determined by adding cell culture
supernatant (0.1 ml), nitro blue tetrazolium (0.3 ml), NADH (0.2
ml) and sodium phosphate buffer (1.2 ml). The final absorbance was
measured at 560 nm (25). Catalase
activity was determined by adding phosphate buffer (500 µl), cell
culture supernatant (500 µl) and H2O2 (500
µl). Then, TiOSO4 (500 µl) was added to the reaction
tube, and the final absorbance was measured at 420 nm (25). AChE activity was determined by the
addition of acetylcholine (0.02 ml), cell culture supernatant (0.02
ml), DTNB (0.1 ml) and phosphate buffer (3 ml) into the reaction
tube. The final absorbance was measured at 410 nm (26).
Inflammatory markers
TNF-α and IL-6 levels were determined in the cell
culture were determined by enzyme-linked immunosorbent assay
(RAB0141-1KT, Mouse ELISA kit; Sigma-Aldrich China, Inc., Shanghai,
China) (27–29).
Apoptosis markers
For the reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay, RNA was isolated from
the cells and converted into cDNA using oligo (dT) primers. Then,
qPCR was used to quantify the mRNA expression with primers specific
for caspase-3, p53, bcl-2 and bax (Table I). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as a qPCR internal control. The
2−∆∆Cq method was used to calculate the relative ratios
of expression (30). Caspase-3
protein expression was determined by immunofluorescence staining
according to Lobos et al (31) and images were taken under
fluorescence microscope (IX73 Inverted Microscope; Olympus
Corporation, Tokyo, Japan).
 | Table I.List of primers used in RT-qPCR for
mRNA expression of p53, caspase-3, bax and Bcl-2. |
Table I.
List of primers used in RT-qPCR for
mRNA expression of p53, caspase-3, bax and Bcl-2.
| Gene name | Sense primer | Anti-sense
primer |
|---|
| p53 |
5′-TAACAGTTCCTGCATGGGCGGC-3′ |
5′-AGGACAGGCACAAACACGCACC-3′ |
| Caspase-3 |
5′-TTAATAAAGGTATCCATGGAGAACACT-3′ |
5′-TTAGTGATAAAAATAGAGTTCTTTTGTGAG-3′ |
| Bax |
5′-TGGAGCTGCAGAGGATGATTG-3′ |
5′-GAAGTTGCCGTCAGAAAACATG-3′ |
| GAPDH |
5′-TCCCTCAAGATTGTCAGCAA-3′ |
5′-AGATCCACAACGGATACATT-3′ |
| Bcl-2 |
5′-CACCCCTGGCATCTTCTCCTT-3′ |
5′-AGCGTCTTCAGAGACAGCCAG-3′ |
Statistical analysis
Values are given as mean with standard deviations.
Differences between the control and taurine groups were evaluated
using the unpaired Student's t-test. One-way ANOVA was applied for
statistical analysis of data and post hoc Tukey's test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of taurine on oxidative
markers
The protective effect of taurine against
inflammation, apoptosis, and oxidative stress in TBI was
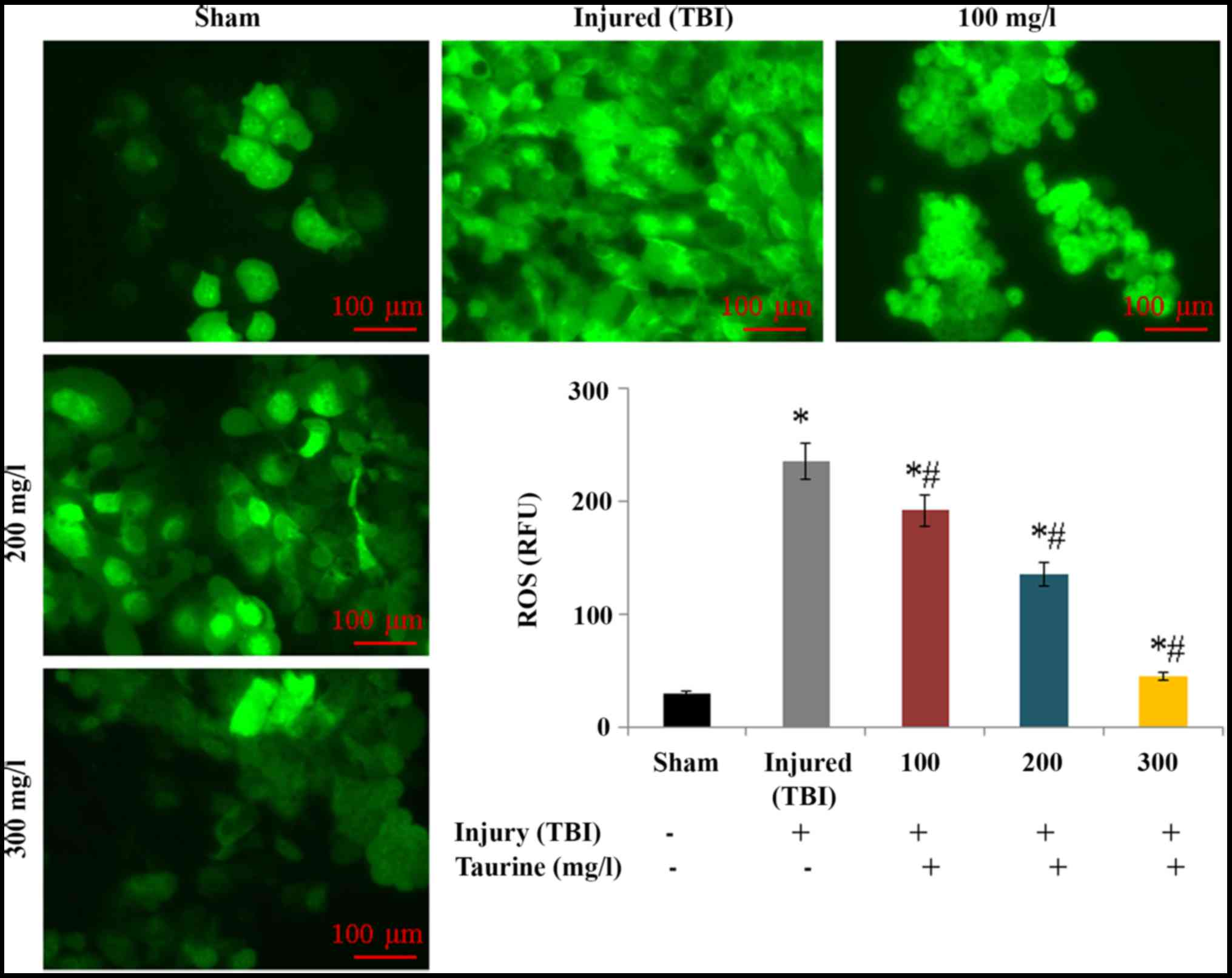
investigated in this study. Intracellular ROS levels were
substantially increased to 234.52 relative fluorescence units (RFU)
in injured brain cells. However, taurine supplementation
significantly reduced ROS levels to 191.1 (100 mg/l), 135.24 (200
mg/l), and 44.72 RFU (300 mg/l) in injured brain cells (P<0.05;
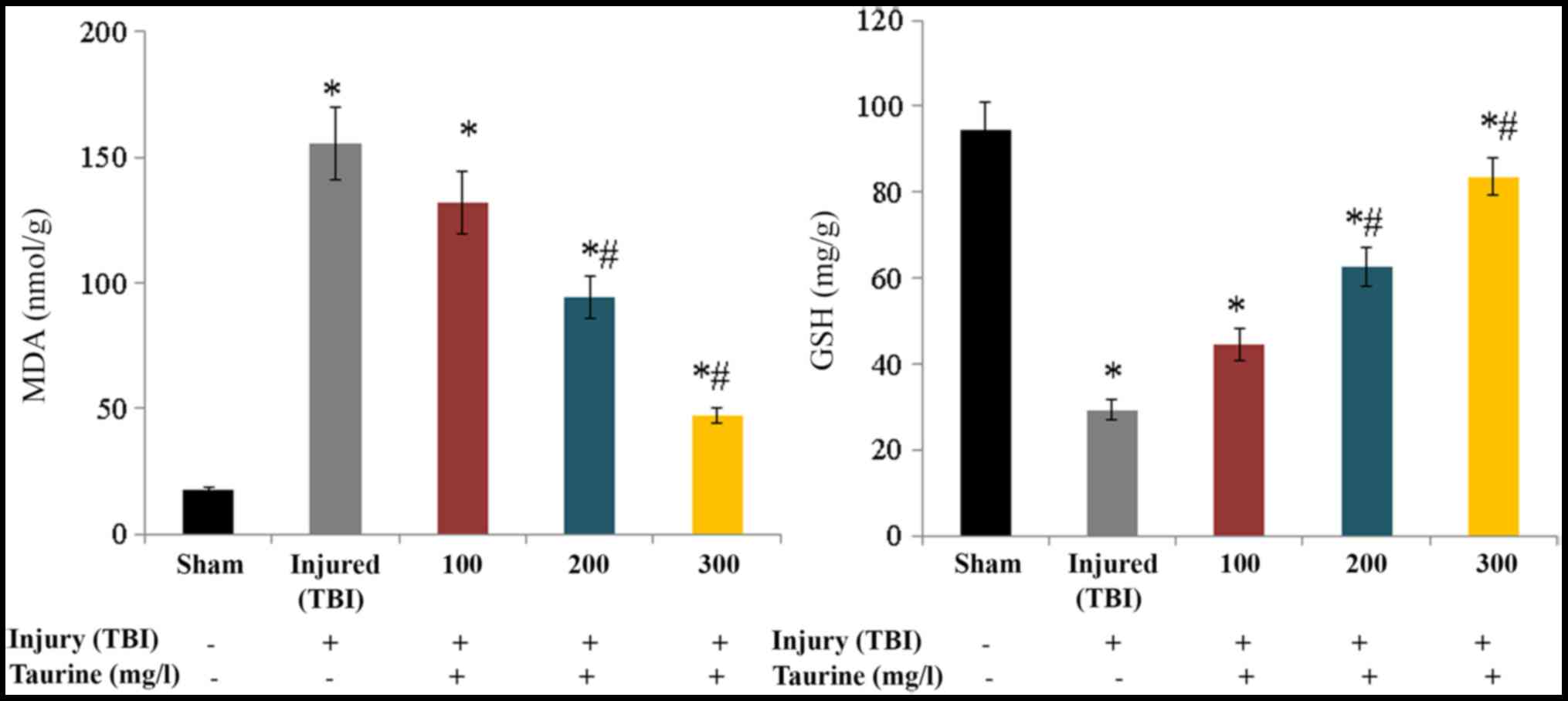
Fig. 1). Lipid peroxidation was
substantially increased to 155.32 nmol/g in injured brain cells.
Taurine supplementation significantly reduced lipid peroxidation to
131.87 (100 mg/l), 94.61 (200 mg/l), and 47.3 nmol/g (300 mg/l) in
injured brain cells (P<0.05; Fig.
2). GSH content was substantially reduced to 29.25 mg/g in
injured brain cells, while taurine supplementation significantly
increased GSH content to 44.46 (100 mg/l), 62.63 (200 mg/l), and
83.56 mg/g (300 mg/l) in injured brain cells (P<0.05; Fig. 2).
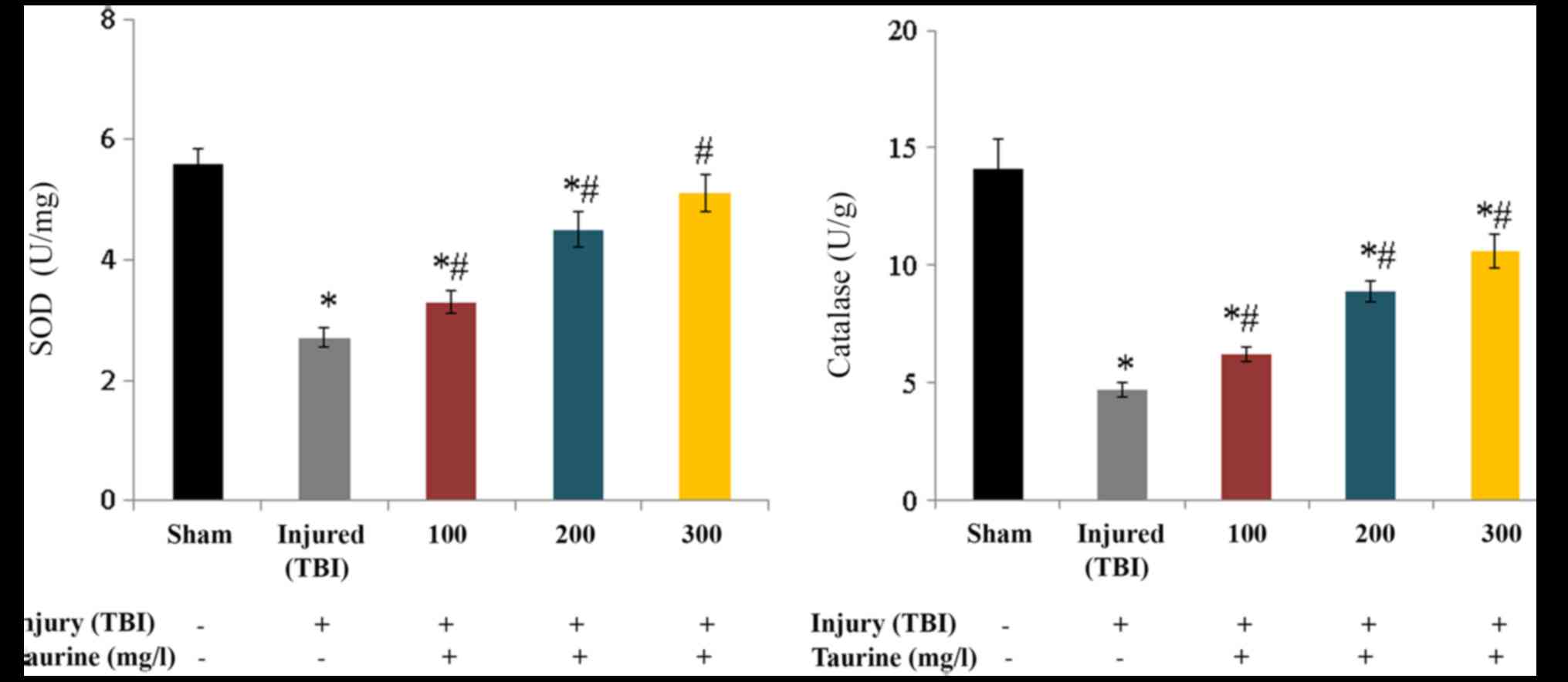
SOD activity was substantially reduced to 2.7 U/mg
in injured brain cells. Taurine supplementation significantly
increased SOD activity to 3.3 (100 mg/l), 4.5 (200 mg/l), and 5.1
U/mg (300 mg/l) in injured brain cells (P<0.05; Fig. 3). Catalase activity was
substantially reduced to 4.7 U/g in injured brain cells. Taurine
supplementation significantly increased catalase activity to 6.2
(100 mg/l), 8.9 (200 mg/l), and 10.6 U/g (300 mg/l) in injured
brain cells (P<0.05; Fig. 3).
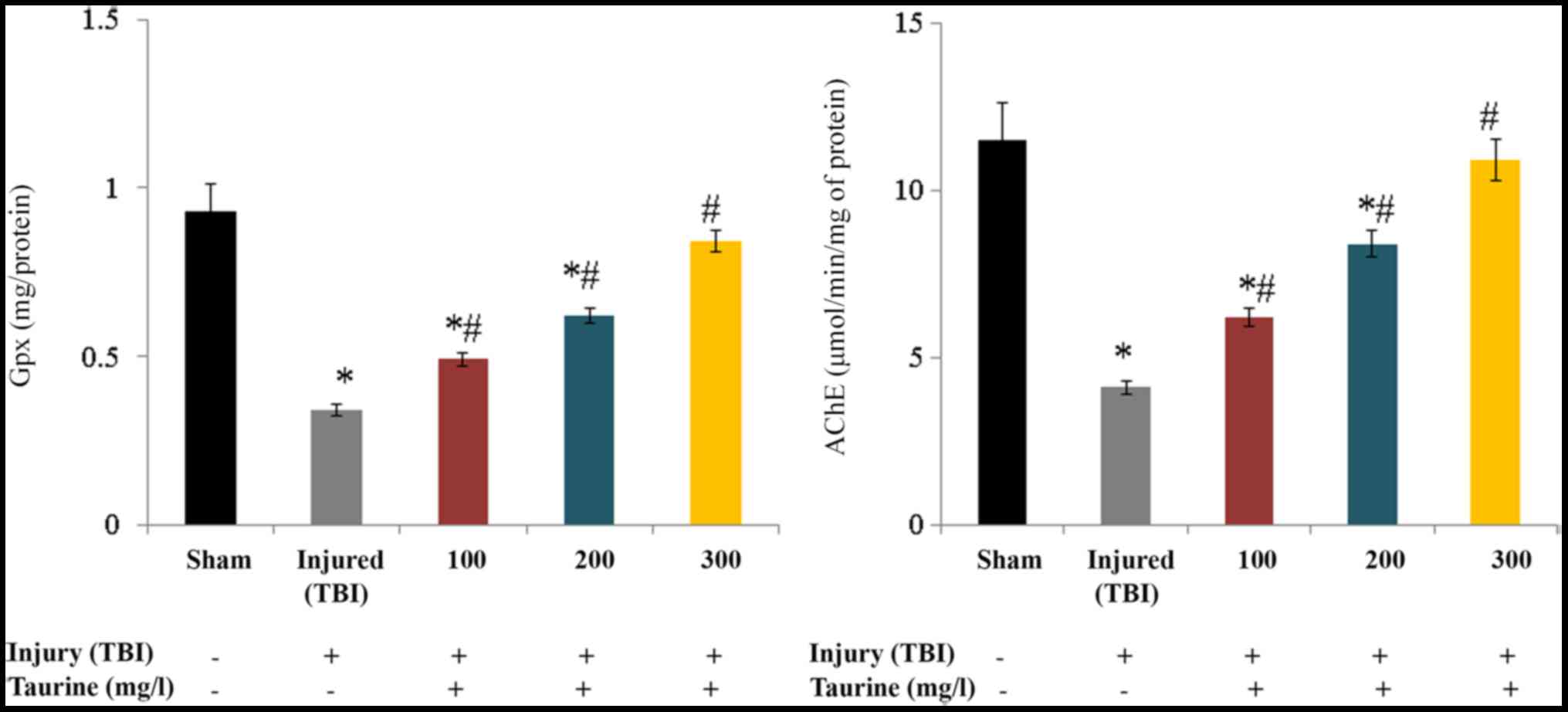
Gpx activity was substantially reduced to 0.34 mg/protein in
injured brain cells. Taurine supplementation significantly
increased Gpx activity to 0.49 (100 mg/l), 0.62 (200 mg/l), and
0.84 mg/protein (300 mg/l) in injured brain cells (P<0.05;
Fig. 4). AChE activity was
substantially reduced to 4.1 µmol/min/mg of protein in injured
brain cells. Taurine supplementation significantly increased AChE
activity to 6.2 (100 mg/l), 8.4 (200 mg/l), and 10.9 µmol/min/mg of
protein (300 mg/l) in injured brain cells (P<0.05; Fig. 4).
Effect of taurine on inflammatory
markers
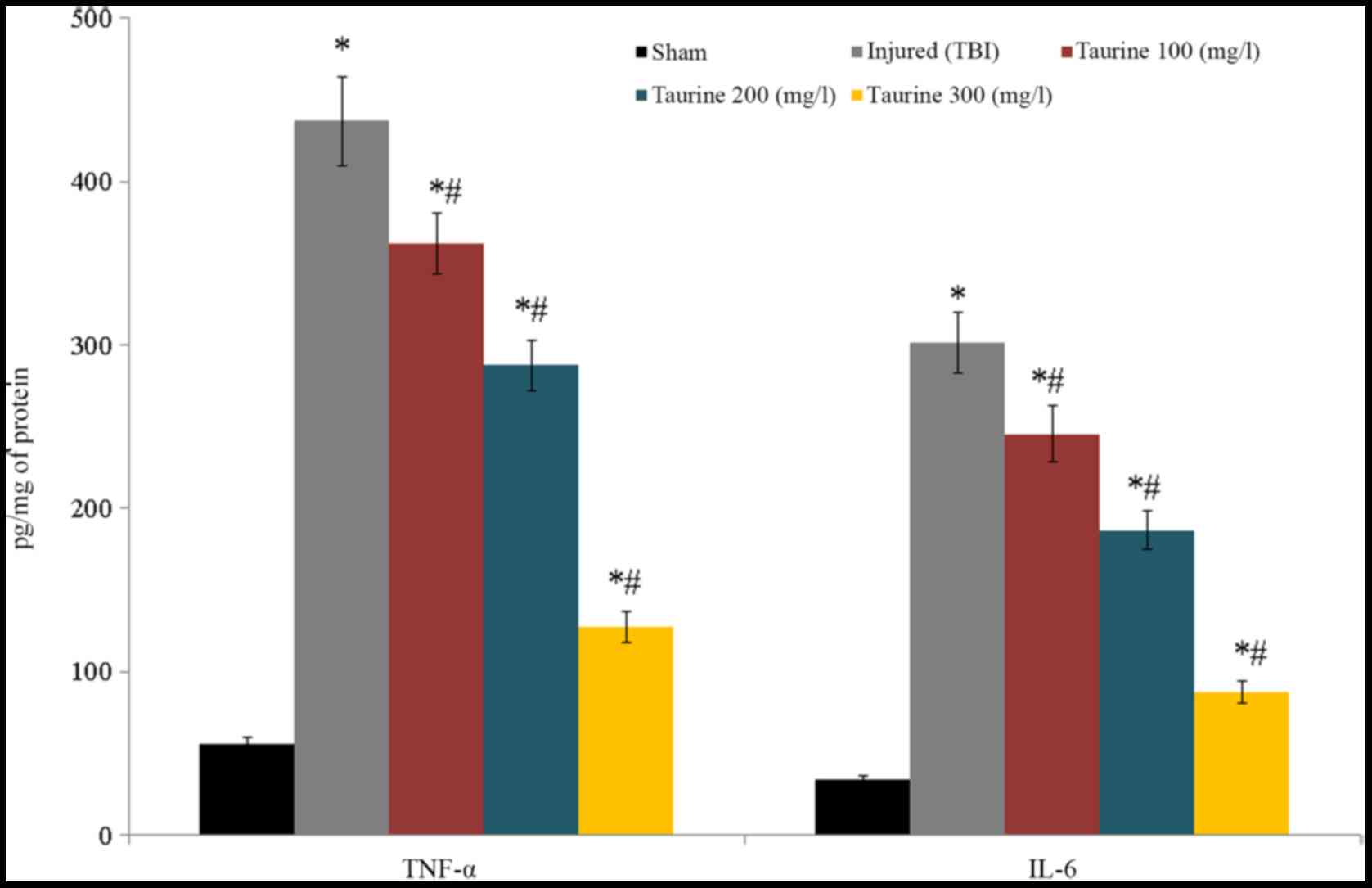
TNF-α and IL-6 levels were substantially reduced to
437.12 and 301.5 pg/mg of protein, respectively, in injured brain
cells. Following taurine treatment, TNF-α levels were decreased
[362.11 (100 mg/l), 287.45 (200 mg/l), and 127.25 pg/mg of protein
(300 mg/l)], while IL-6 levels were increased [245.6 (100 mg/l),
186.5 (200 mg/l), and 87.5 pg/mg of protein (300 mg/l)] in injured
brain cells (P<0.05; Fig.
5).
Effect of taurine on apoptosis
markers
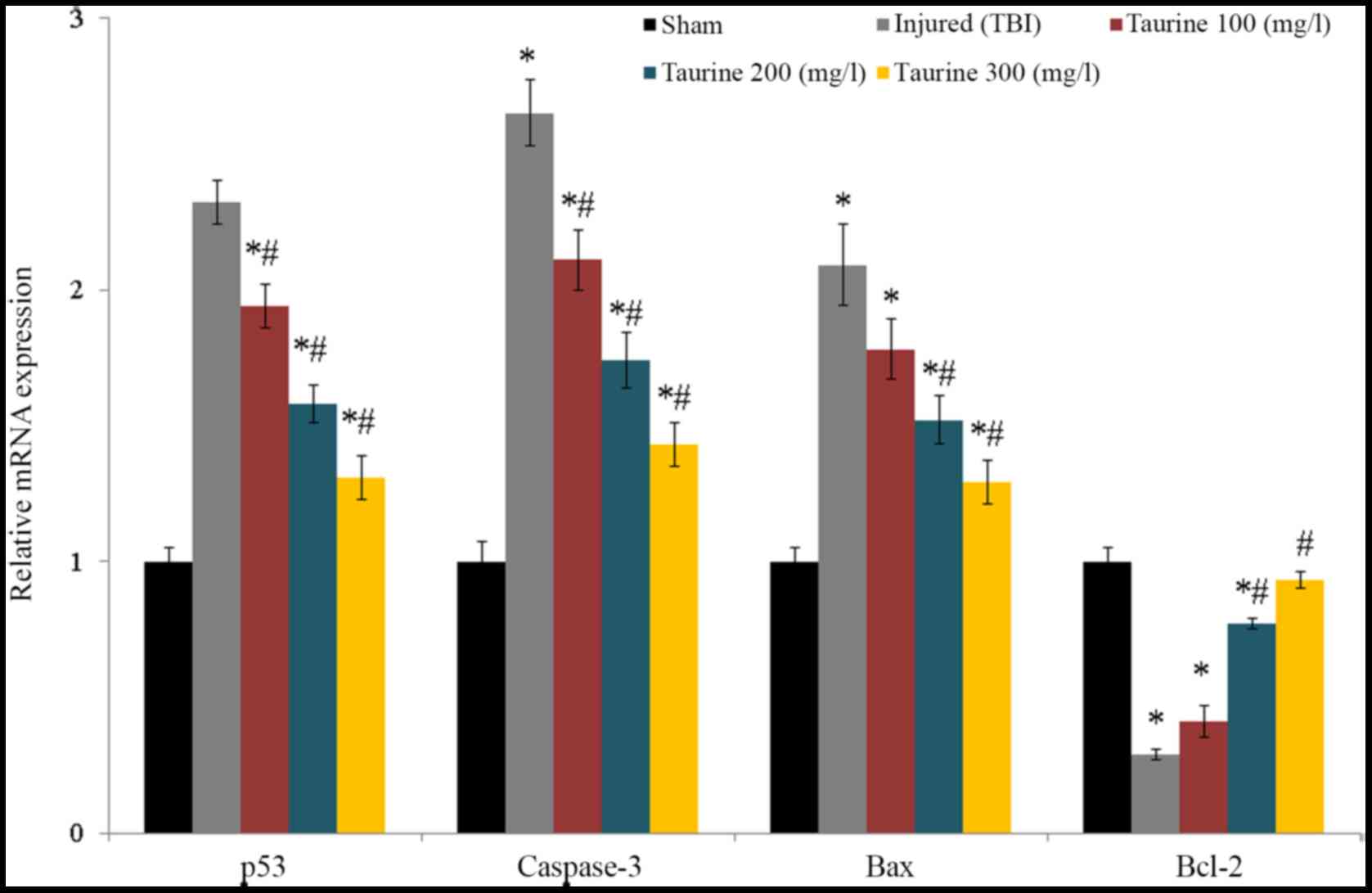
Taurine supplementation significantly reduced p53,
caspase-3, and bax mRNA expression and increased bcl-2 mRNA
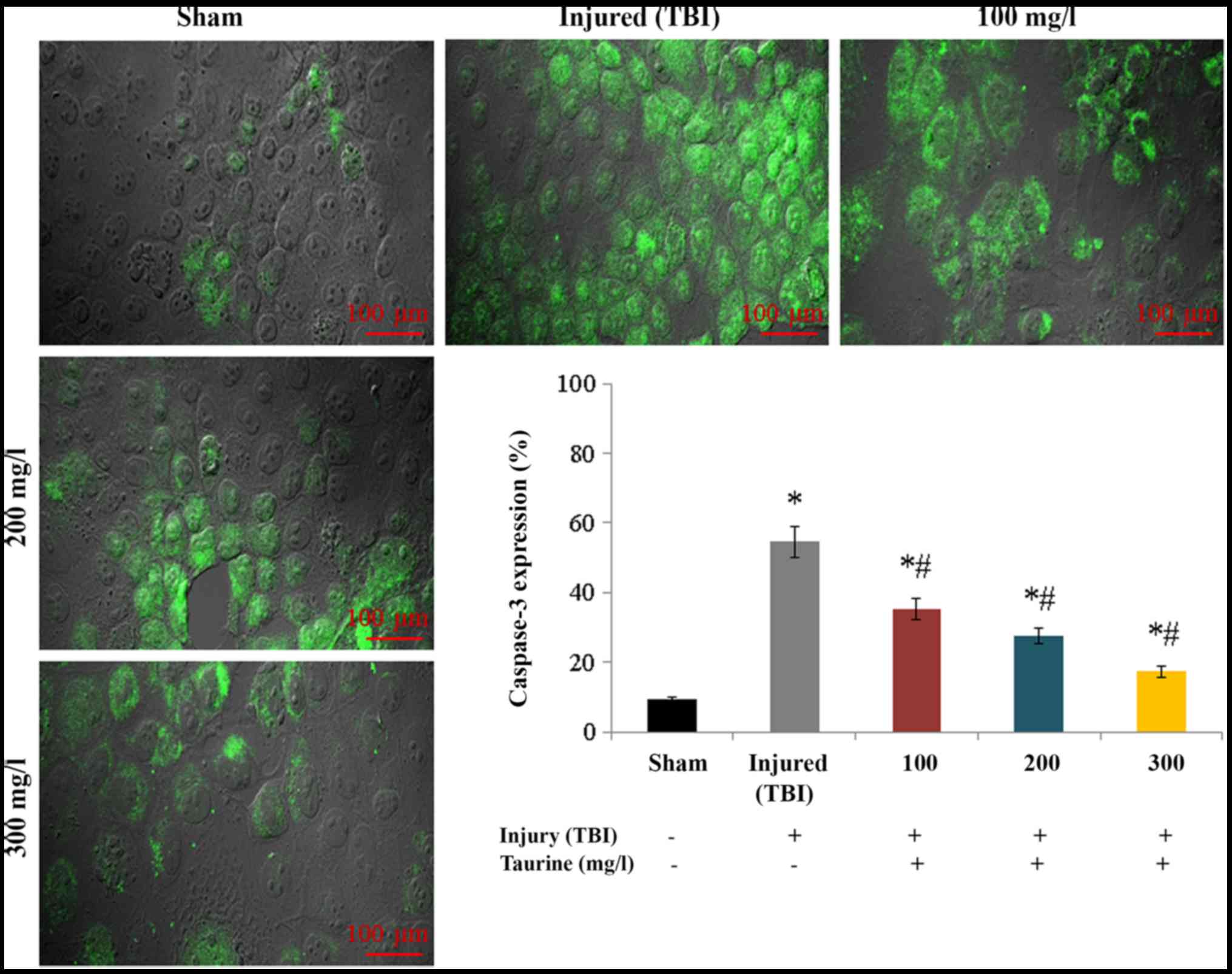
expression in injured brain cells (P<0.05; Fig. 6). Protein expression of caspase-3
increased to 54.51% in injured brain cells compared to normal brain
cells. Taurine supplementation substantially reduced caspase-3
protein expression to 35.31 (100 mg/l), 27.48 (200 mg/l), and 17.3%
(300 mg/l) (P<0.05; Fig.
7).
Discussion
This study investigated the protective effect of
taurine against inflammation, apoptosis, and oxidative stress in
TBI. Taurine supplementation has been shown to substantially reduce
infarct volume, brain swelling, cell death, and neurological
deficits in a stroke-induced rat model (32). Taurine also significantly reduced
apoptosis in cardiomyocytes of rats (33). Sun et al (34) also reported a protective effect of
taurine against head injury. Several researchers have associated
mitochondrial dysfunction with increased ROS and superoxide
production, glutathione oxidation, and reduced antioxidant enzymes
(2).
Taurine increases antioxidant activity by reducing
superoxide production, which leads to improved mitochondrial
function (35). Taurine also plays
a crucial role in protein synthesis in mitochondria, and increases
electron transport chain (ETC) activity (36). Our experimental results indicate
that taurine increases antioxidant levels through increased
mitochondrial ETC activity in TBI.
Mitochondrial dysfunction leads to increased
production of oxidants, which leads to neuronal apoptosis and
necrosis. Mitochondrial respiratory chains present on the inner
mitochondrial membrane contain four transmembrane protein
complexes. Chen and Chan (37)
observed energy metabolism dysfunction associated with pathological
changes in mitochondria following TBI. Several researchers have
found that respiratory enzyme levels were decreased following
traumatic and ischemic brain injury (38,39).
Zhu et al (40) have
illustrated the incidence of gastrointestinal dysfunction in TBI.
Mitochondrial dysfunction can increase the production of oxidants,
which play a crucial role in apoptosis and necrosis of neurons.
Proapoptotic markers, such as bcl-2, increase in response to
increased oxidants produced in brain injury. Apoptosis is induced
through increased oxidants and misfolded proteins (41). Increased production of reactive
oxygen species (ROS) and superoxide, glutathione oxidation, and
reduced antioxidant enzymes have been associated with mitochondrial
dysfunction (2,42–45).
Vlodavsky et al (46)
postulated that post-traumatic cytotoxic edema is associated with
mitochondrial function. Sun et al (47,48)
found that taurine increases respiratory chain complex activity and
mitochondrial-mediated apoptosis and necrosis, and reduces free
radical and oxidative stress.
The proapoptotic marker bcl-2 has been shown to
increase following brain injury in response to increased oxidants,
and apoptosis has been shown to be induced due to increased levels
of oxidants and misfolded proteins. In this study, we investigated
the expression of various anti-apoptotic markers including p53,
caspase-3, and bax. Taurine supplementation substantially reduced
expression of these markers in vitro Several studies have
reported that taurine is effective against calcium overload and
oxidative stress (41). Lotocki
et al (18) indicated that
inflammation is a well-known critical event in TBI, and
inflammation may be induced by the secretion of cytokines and
activation of glial cells. Taurine supplementation has been shown
to reduce inflammatory cytokines such as TNF-α, IL-6, IL-1α, and
IL-1β in spinal cord injury and TBI (48). In this study, taurine significantly
reduced TNF-α and IL-6 levels.
Taurine supplementation was found to be effective
against oxidative stress, apoptosis, and inflammation in injured
brain cells.
Acknowledgements
The present study was supported by the Key Research
and Development Project of Shaanxi Province (grant no.
2017SF-180).
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XN, SZ, HL and SL were involved in the experimental
design, data acquisition, data analysis and interpretation, and
manuscript preparation. XN performed the experiments and SZ
performed the review of the literature. HL conducted data analysis
and SL was a major contributor in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving rats were monitored and
approved by the Ethics Committee of The Second Affiliated Hospital
of Xi'an Jiaotong University (reference no. 2o14/2Tx1221).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schuller-Levis GB and Park E: Taurine: New
implications for an old amino acid. FEMS Microbiol Lett.
226:195–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Q, Fan W, Cai Y, Wu Q, Mo L, Huang Z
and Huang H: Protective effects of taurine in traumatic brain
injury via mitochondria and cerebral blood flow. Amino Acids.
48:2169–2177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rak K, Völker J, Jürgens L, Scherzad A,
Schendzielorz P, Radeloff A, Jablonka S, Mlynski R and Hagen R:
Neurotrophic effects of taurine on spiral ganglion neurons in
vitro. Neuroreport. 25:1250–1254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Izumi I, Kagamimori S, Sokejima
S, Yamagami T, Liu Z and Qi B: Role of taurine supplementation to
prevent exercise-induced oxidative stress in healthy young men.
Amino Acids. 26:203–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagita T, Han SY, Hu Y, Nagao K,
Kitajima H and Murakami S: Taurine reduces the secretion of
apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis.
7:382008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olive MF: Interactions between taurine and
ethanol in the central nervous system. Amino Acids. 23:345–357.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dominy J Jr, Thinschmidt JS, Peris J,
Dawson R Jr and Papke RL: Taurine-induced long-lasting potentiation
in the rat hippocampus shows a partial dissociation from total
hippocampal taurine content and independence from activation of
known taurine transporters. J Neurochem. 89:1195–1205. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuboyama-Kasaoka N, Shozawa C, Sano K,
Kamei Y, Kasaoka S, Hosokawa Y and Ezaki O: Taurine
(2-aminoethanesulfonic acid) deficiency creates a vicious circle
promoting obesity. Endocrinology. 147:3276–3284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Birdsall TC: Therapeutic applications of
taurine. Altern Med Rev. 3:128–136. 1998.PubMed/NCBI
|
|
11
|
Foos TM and Wu JY: The role of taurine in
the central nervous system and the modulation of intracellular
calcium homeostasis. Neurochem Res. 27:21–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leon R, Wu H, Jin Y, Wei J, Buddhala C,
Prentice H and Wu JY: Protective function of taurine in
glutamate-induced apoptosis in cultured neurons. J Neurosci Res.
87:1185–1194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El Idrissi A, Messing J, Scalia J and
Trenkner E: Prevention of epileptic seizures by taurine. Adv Exp
Med Biol. 526:515–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maas AI, Stocchetti N and Bullock R:
Moderate and severe traumatic brain injury in adults. Lancet
Neurol. 7:728–741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins C and Dean J: Acquired brain
injuryOccupational Therapy and Physical Dysfunction: Principles,
Skills and Practice. Turner A, Foster M and Johnson SE: Churchill
Livingstone; Edinburgh: pp. 395–396. 1996
|
|
16
|
McIntosh TK: Neurochemical sequelae of
traumatic brain injury: Therapeutic implications. Cerebrovasc Brain
Metab Rev. 6:109–162. 1994.PubMed/NCBI
|
|
17
|
Werner C and Engelhard K: Pathophysiology
of traumatic brain injury. Br J Anaesth. 99:4–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lotocki G, de Rivero Vaccari JP, Perez ER,
Sanchez-Molano J, Furones-Alonso O, Bramlett HM and Dietrich WD:
Alterations in blood-brain barrier permeability to large and small
molecules and leukocyte accumulation after traumatic brain injury:
Effects of post-traumatic hypothermia. J Neurotrauma. 26:1123–1134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakajima Y, Osuka K, Seki Y, Gupta RC,
Hara M, Takayasu M and Wakabayashi T: Taurine reduces inflammatory
responses after spinal cord injury. J Neurotrauma. 27:403–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heidari R, Jamshidzadeh A, Niknahad H,
Mardani E, Ommati MM, Azarpira N, Khodaei F, Zarei A, Ayarzadeh M,
Mousavi S, et al: Effect of taurine on chronic and acute liver
injury: Focus on blood and brain ammonia. Toxicol Rep. 3:870–879.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Jung J, Fini ME and Lo EH:
Mechanical injury in rat cortical cultures activates MAPK signaling
pathways and induces secretion of matrix metalloproteinase-2 and
−9. J Cereb Blood Flow Metab. 21:S2642001.
|
|
22
|
Katano H, Fulita K, Kato T, Asai K,
Kawamura Y, Masago A and Yamada K: Traumatic injury in vitro
induces IEG mRNA in cultured glial cells, suppressed by co-culture
with neurons. Neuroreport. 10:2439–2448. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lau LT and Yu AC: Astrocytes produce and
release interleukin-1, interleukin-6, tumor necrosis factor alpha
and interferon-gamma following traumatic and metabolic injury. J
Neurotrauma. 18:351–359. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaddour T, Omar K, Oussama AT, Nouria H,
Iméne B and Abdelkader A: Aluminium-induced acute neurotoxicity in
rats: Treatment with aqueous extract of Arthrophytum (Hammada
scoparia). J Acute Dis. 5:470–482. 2016. View Article : Google Scholar
|
|
25
|
Erden Inal M, Akgün A and Kahraman A: The
effects of exogenous glutathione on reduced glutathione level,
glutathione peroxidase and glutathione reductase activities of rats
with different ages and gender after whole-body Γ-irradiation. J Am
Aging Assoc. 26:55–58. 2003.PubMed/NCBI
|
|
26
|
Madakkannu B and Ravichandran R: In vivo
immunoprotective role of Indigofera tinctoria and Scoparia dulcis
aqueous extracts against chronic noise stress induced immune
abnormalities in Wistar albino rats. Toxicol Rep. 4:484–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Afshari JT, Ghomian N, Shameli A, Shakeri
MT, Fahmidehkar MA, Mahajer E, Khoshnavaz R and Emadzadeh M:
Determination of interleukin-6 and tumor necrosis factor-alpha
concentrations in Iranian-Khorasanian patients with preeclampsia.
BMC Pregnancy Childbirth. 5:142005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Medhat D, Hussein J, El-Naggar ME, Attia
MF, Anwar M, Latif YA, Booles HF, Morsy S, Farrag AR, Khalil WKB
and El-Khayat Z: Effect of Au-dextran NPs as anti-tumor agent
against EAC and solid tumor in mice by biochemical evaluations and
histopathological investigations. Biomed Pharmacother.
91:1006–1016. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaheen TI, El-Naggar MI, Hussein JS,
El-Bana M, Emara E, El-Khayat Z, Fouda MMG, Ebaid H and Hebeish A:
Antidiabetic assessment; in vivo study of gold and core-shell
silver-gold nanoparticles on streptozotocin-induced diabetic rats.
Biomed Pharmacother. 83:865–875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lobos E, Gebhardt C, Kluge A and
Spanel-Borowski K: Expression of nerve growth factor (NGF) isoforms
in the rat uterus during pregnancy: Accumulation of precursor
proNGF. Endocrinology. 146:1922–1929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun M, Zhao Y, Gu Y and Xu C:
Anti-inflammatory mechanism of taurine against ischemic stroke is
related to down-regulation of PARP and NF-κB. Amino Acids.
42:1735–1747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Zhang Y, Liu X, Zuo J, Wang K, Liu
W and Ge J: Exogenous Taurine attenuates mitochondrial oxidative
stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta
Biochim Biophys Sin (Shanghai). 45:359–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun M, Zhao Y, Gu Y and Zhang Y:
Protective effects of taurine against closed head injury in rats. J
Neurotrauma. 32:66–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schaffer SW, Jong CJ, Ito T and Azuma J:
Effect of taurine on ischemia-reperfusion injury. Amino Acids.
46:21–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimada K, Jong CJ, Takahashi K and
Schaffer SW: Role of ROS production and turnover in the antioxidant
activity of taurine. Adv Exp Med Biol. 803:581–596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen H and Chan DC: Mitochondrial
dynamics-fusion, fission, movement, and mitophagy-in
neurodegenerative diseases. Hum Mol Genet. 18:169–176. 2009.
View Article : Google Scholar
|
|
38
|
Xiong Y, Gu Q, Peterson PL, Muizelaar JP
and Lee CP: Mitochondrial dysfunction and calcium perturbation
induced by traumatic brain injury. J Neurotrauma. 14:23–34. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keelan J, Timothy EB and Clark BJ:
Heightened resistance of the neonatal brain to ischemia-reperfusion
involves a lack of mitochondrial damage in the nerve terminal.
Brain Res. 821:124–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu KJ, Huang H, Chu H, Yu H and Zhang SM:
Alterations in enterocyte mitochondrial respiratory function and
enzyme activities in gastrointestinal dysfunction following brain
injury. World J Gastroenterol. 20:9585–9591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prentice H, Modi JP and Wu JY: Mechanisms
of neuronal protection against excitotoxicity, endoplasmic
reticulum stress, and mitochondrial dysfunction in stroke and
neurodegenerative diseases. Oxid Med Cell Longev. 2015:9645182015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gong H, Li H, Li J and Wang C: Myricetin
inhibition on murine glioma GL261 cell line. Farmacia. 65:1–7.
2017.
|
|
43
|
Tătăranu LG, Georgescu AM, Buteică SA,
Siloși I, Mogoșanu GD, Purcaru SO, Alexandru O, Stovicek OP,
Brîndușa C, Doșa M, et al: Ligustrum vulgare hydroalcoholic extract
induces apoptotic cell death in human primary brain tumour cells.
Farmacia. 65:766–771. 2017.
|
|
44
|
Boda D, Negrei C, Nicolescu F and Bălălău
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
45
|
Negrei C, Arsene AL, Toderescu CD, Boda D
and Ilie M: Acitretin treatment in psoriazis may influence the cell
membrane fluidity. Farmacia. 60:767–772. 2012.
|
|
46
|
Vlodavsky E, Palzur E, Shehadeh M and
Soustiel JF: Posttraumatic cytotoxic edema is directly related to
mitochondrial function. J Cereb Blood Flow Metab. 37:166–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun M, Gu Y, Zhao Y and Xu C: Protective
functions of taurine against experimental stroke through depressing
mitochondria-mediated cell death in rats. Amino Acids.
40:1419–1429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Q, Hu H, Wang W, Jin H, Feng G and Jia
N: Taurine attenuates amyloid β1-42-induced mitochondrial
dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem
Biophys Res Commun. 447:485–489. 2014. View Article : Google Scholar : PubMed/NCBI
|