Introduction
Due to its easy access and sufficient length, the
great saphenous vein remains widely used in coronary artery bypass
surgery (1,2). However, the long-term effect of the
vein graft is restricted by its decreased patency rate. The present
study focused on the effect and molecular mechanism of medical
cyanoacrylate glue on the long-term patency of rabbit vein
grafts.
When vein grafts are transplanted into the arterial
system, dilatation occurs under arterial pressure, which causes the
vascular wall to stretch, triggering remodeling of the vascular
wall. Once the vein bridge in the blood vessel dilates, the
diameter of the artery and vein blood vessel is increased, which
causes a further mismatch in the arteriovenous anastomosis. In
addition, blood flow is disordered in the anastomosis and the
venous vascular segment. When laminar blood flows through the blood
vessels, the flow has a protective effect on the inner wall of the
blood vessels. In disease, the abnormal and disordered blood flow
leads to vascular endothelial cell damage and dysfunction (3–5).
Mechanotransduction is involved in the cytokine release of
sensitive cells and signaling activation by mechanical stress
(6,7). The activated signaling pathway is
transformed into a cellular chemical signaling, and eventually
regulates the cellular functioning of the effector cells by
altering gene and protein expression levels (8–10).
Platelet endothelial cell adhesion molecule 1
(PECAM-1) is a type of mechanosensor, which serves important roles
in promoting the release and delivery of biological signals via the
signaling pathways with which it interacts (11). Proliferating cell nuclear antigen
(PCNA) is a marker for the assessment of cell proliferation
(12,13). It was demonstrated that vascular
cell adhesion protein 1 (VCAM-1), ERK1/2 and endothelial nitric
oxide synthase (eNOS) expression has important roles in injured
arteries (14–16). In the present study, 36 New Zealand
white rabbits were divided into three groups at random, with 12 in
each group: The first group received no surgery (normal group); one
group was subjected to transplantation of the left external jugular
vein to the ipsilateral common carotid artery (surgery group); the
other was subjected to transplantation of left external jugular
vein to the common carotid artery on the labial side followed by
the application of medical adhesive spray (medical adhesive spray
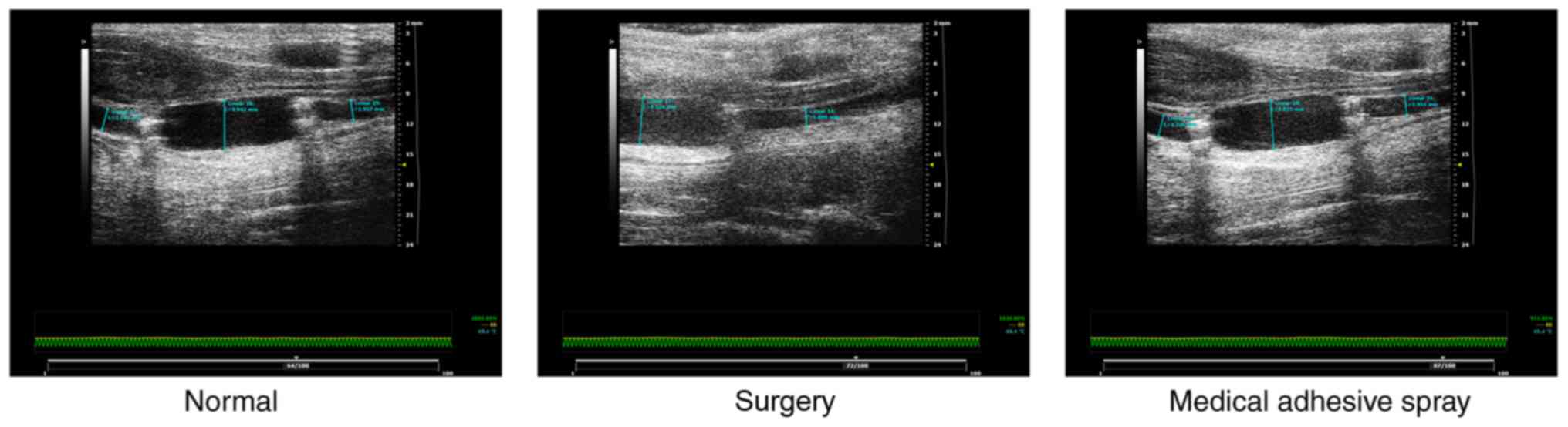
group). A total of 4 weeks post-surgery, vascular ultrasound was
performed to measure blood vessel diameter and vessel blood flow
velocity. For pathological examination, the vein graft was removed,
the thickness and area of the intima and media of the vessel were
measured based via images, and alterations in the expression of
PCNA, PECAM-1, VCAM-1, extracellular signal-regulated kinase
(ERK)1/2 and eNOS were detected by immunohistochemical staining,
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis and western blotting.
Materials and methods
Animals
A total of 36 New Zealand white rabbits were
randomly selected and divided into three groups, 12 rabbits/group.
Rabbits were provided by Capital Medical University (Beijing,
China). Of the three groups, one received no surgery (normal
group), one received transplantation of the left external jugular
vein to the ipsilateral common carotid artery (surgery group), and
the other received the transplantation of the left external jugular
vein to the common carotid artery on the labial side followed by
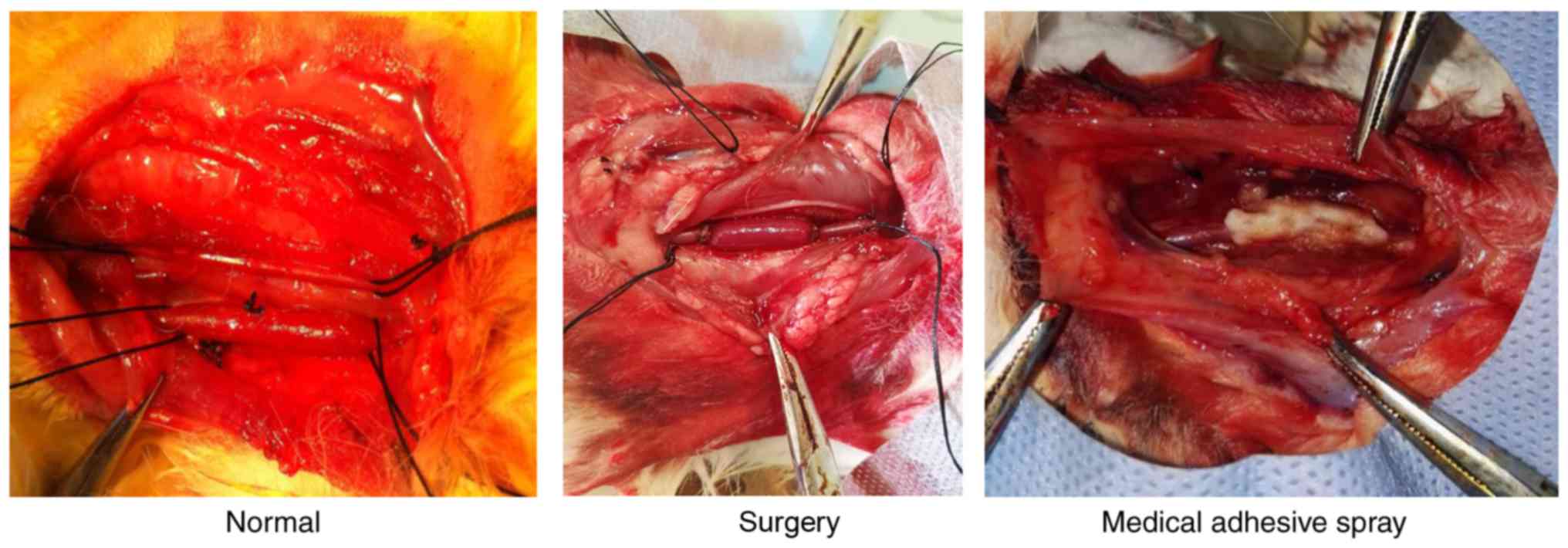
medical adhesive spray (medical adhesive spray group) (Fig. 1). Animals were maintained at 22°C
with 55% humidity on a standard 12 h light/dark cycle with food and
water available ad libitum. Animal experiments were in
accordance with the ethics standards of animal research. The
present study was approved by the Animal Experiments and
Experimental Animal Welfare Committee of Capital Medical University
(approval no. AEEI-2015-144).
Establishment of the model of rabbit
external jugular vein graft in the common carotid artery
The autologous vein graft model was established. The
rabbits were treated with fasting and water for 4 h prior to the
surgery, the ear edge vein channel was established, and 30 g/l
amobarbital anesthesia was administered intravenously with 30 mg/kg
concentration. Subsequently, rabbits were fixed in a supine
position. Longitudinal incisions were made from the anterior median
of the neck skin and 30–50 mm of the jugular vein was cut, washed
and soaked in heparin saline. The right common carotid artery was
dissociated, and an ear vein injection of 10 g/l heparin (1 ml/kg)
as given. Carotid artery catheterization was performed and a
Philips V24E monitor (Philips Medical Systems, Inc., Bothell, WA,
USA) was used to monitor the mean arterial blood pressure.
Following occlusion of the proximal and distal resection of the
carotid artery, the appropriate vein length was cut and inverted.
An 8-0 nylon monofilament line was used for end-to-end anastomosis
with an 8–10 needle, followed by opening the ends of the artery
clamps to exhaust. Once the patency of the vein bridge and a lack
of bleeding was ensured, the suture incision was performed layer by
layer. An ear margin vein injection of 10 g/l heparin (1 ml/kg) was
subsequently administered to prevent thrombosis, and an injection
of penicillin (800,000 units) was administered to prevent
infection. On the same day as the surgery, or the following day,
blood vessel specimens were examined using the Philips IU22 color
Doppler ultrasonic detector (Philips Medical Systems, Inc.) to
ensure the patency of the vein bridge. Images of blood flow were
acquired via postoperative ultrasonic measurement. The rabbits were
constantly fed for 4 weeks. Daily observations of the animals were
made and the data recorded.
Specimen collection and
immunohistochemical detection
Immunohistochemical examination, RT-qPCR analysis
and western blotting were used to detect the expression of PCNA,
PECAM-1, VCAM-1, ERK1/2 and eNOS in the vein bridge. A total of 4
weeks subsequent to surgery, 30 g/l amobarbital anesthesia was used
to treat the animals. Following anesthesia, the average arterial
pressure was detected via left carotid artery catheterization. The
right vein graft was separated and the required graft tissue was
rapidly cut. The external jugular vein graft bridges of the three
groups of rabbits were fixed in 4% polyformaldehyde at 4°C for 1 h.
Following dehydration and permeabilization with 0.01% Triton X-100,
the sections were embedded in paraffin. The thickness was 4 µm.
Following embedding in paraffin, sections were blocked with 1%
bovine serum albumin (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 20°C for 1 h and stained with
hematoxylin-eosin at room temperature for 4 h. Sections were
subsequently incubated with primary antibodies against PCNA (1:100;
cat. no. ab29; Abcam, Cambridge, MA, USA), PECAM-1 (1:100; cat. no.
ab28364; Abcam), VCAM-1 (1:100; cat. no. ab106777; Abcam), ERK1/2
(1:100; cat. no. ab196883; Abcam) at 20°C for 2 h, followed by
incubation with horseradish peroxidase-conjugated goat anti-rabbit
and anti-mouse secondary antibodies (1:500; cat. nos. S004 and
S001, respectively; Beijing TDY Biotech Co., Ltd., Beijing, China)
at room temperature for 1 h. Sections were sealed and observed by
light microscopy at a magnification of ×100. Image analysis was
performed using an Olympus BX50 microscope (Olympus Corporation,
Tokyo, Japan). The microscope was used to observe the tissue
sections, and Image Pro Plus software 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) was used to perform image acquisition and image
analysis. The alterations in average arterial pressure prior to and
following surgery, and the thickness and area of the graft vein
intima and media were detected.
The RT-qPCR analysis and western blotting were
performed by previously described methods (9,17).
The specific primer sequences for each gene, including the
reference gene were (5′-3′): PCNA forward, GGACTTAGATGTTGAACAGCTTGG
and reverse, TTCTCCACTGGCGGAAAACTT; PECAM-1 forward,
AACCATGCCATGAAGCCAGTA and reverse, ACCGAGGACACTTCCACTTCTG; ERK1/2
forward, ATAAAAAACCCCGCCGAGA and reverse, CAGCCAACCAGCAAAATCCA;
eNOS forward, CACCCAGCATCTACCCAGGA and reverse, AGGGCACGGGGTCTCCA;
and β-actin forward, AAGTGCGACGTGGACATCCG and reverse,
GGGCGGTGATCTCCTTCTGC. For western blot analysis, primary antibodies
against PCNA (1:500; cat. no. ab29; Abcam), PECAM-1 (1:500; cat.
no. ab28364; Abcam), VCAM-1 (1:500; cat. no. ab106777; Abcam),
ERK1/2 (1:500; cat. no. ab196883; Abcam), eNOS (1:500; cat. no.
YM3164; ImmunoWay Biotechnology Company, Plano, TX, USA) and
β-actin (1:1,000; cat. no. ab8227; Abcam) were incubated with the
blots overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated goat anti-rabbit and anti-mouse secondary
antibodies (1:1,000; cat. nos. S004 and S001, respectively; Beijing
TDY Biotech Co., Ltd.) at room temperature for 1 h.
Statistical analysis
SPSS 21.0 statistical analysis software (IBM Corp.,
Armonk, NY, USA) was applied, using the Student's t-test for two
independent groups or one-way analysis of variance followed by
Tukey's post-hoc test. All data are expressed as mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pathological examination of vein
grafts
The results of the present study demonstrated that
the graft vascular wall was thickened in the surgery group,
although this was not apparent in the normal and medical adhesive
spray groups, illustrating that medical adhesive spray led to a
positive outcome in the patency of the rabbit vein graft (Fig. 2).
A total of 4 weeks post-surgery, the thickness and
area of the intima and media of vessel were measured on
formalin-fixed, paraffin wax-embedded pathological sections
(Table I). Based on
hematoxylin-eosin staining, it was observed that the graft intima
was markedly thickened in the normal group (Table I). The lumen was narrowed, with the
appearance of a large number of proliferated smooth muscle cells
under the intima. The smooth muscle cells were in a disordered
arrangement, presenting as the typical phenotype of secretory
cells. Collagen fibers around the cell membrane were slightly
thickened.
 | Table I.Alterations in thickness and area of
the intima and media in the normal control, surgery and medical
adhesive spray groups, as measured via hematoxylin-eosin staining
images. |
Table I.
Alterations in thickness and area of
the intima and media in the normal control, surgery and medical
adhesive spray groups, as measured via hematoxylin-eosin staining
images.
|
| Intima | Media |
|---|
|
|
|
|
|---|
| Group | Thickness, µm | Area,
µm2 | Thickness, mm | Area,
mm2 |
|---|
| Normal control | 30.17±1.78 | 0.41±0.04 | 40.37±2.36 | 0.51±0.05 |
| Surgery | 107.50±12.73 | 1.03±0.04 | 115.04±15.42 | 1.09±0.05 |
| Medical adhesive
spray |
41.34±14.53a |
0.53±0.02a | 103.38±11.13 | 1.05±0.03 |
In the medical adhesive spray group, the intimal
thickening was weakened compared with that in the normal group. The
intima was notably small compared with the normal group. There was
no obvious thickening of the media (Table I).
The intimal thicknesses of the normal group and the
medical adhesive spray group were 107.50±12.73 and 41.34±14.53 µm
(P<0.01), respectively. Intimal areas were 1.031±0.043 and
0.533±0.022 µm2 (P<0.01), respectively. The
thicknesses of the media of the vein graft in the normal group and
the medical adhesive spray group were 115.04±15.42 and 103.38±11.13
mm (P>0.05), respectively. The areas of the media were
1.092±0.051 and 1.054±0.030 mm2 (P>0.05),
respectively.
Medical adhesive spray inhibits the
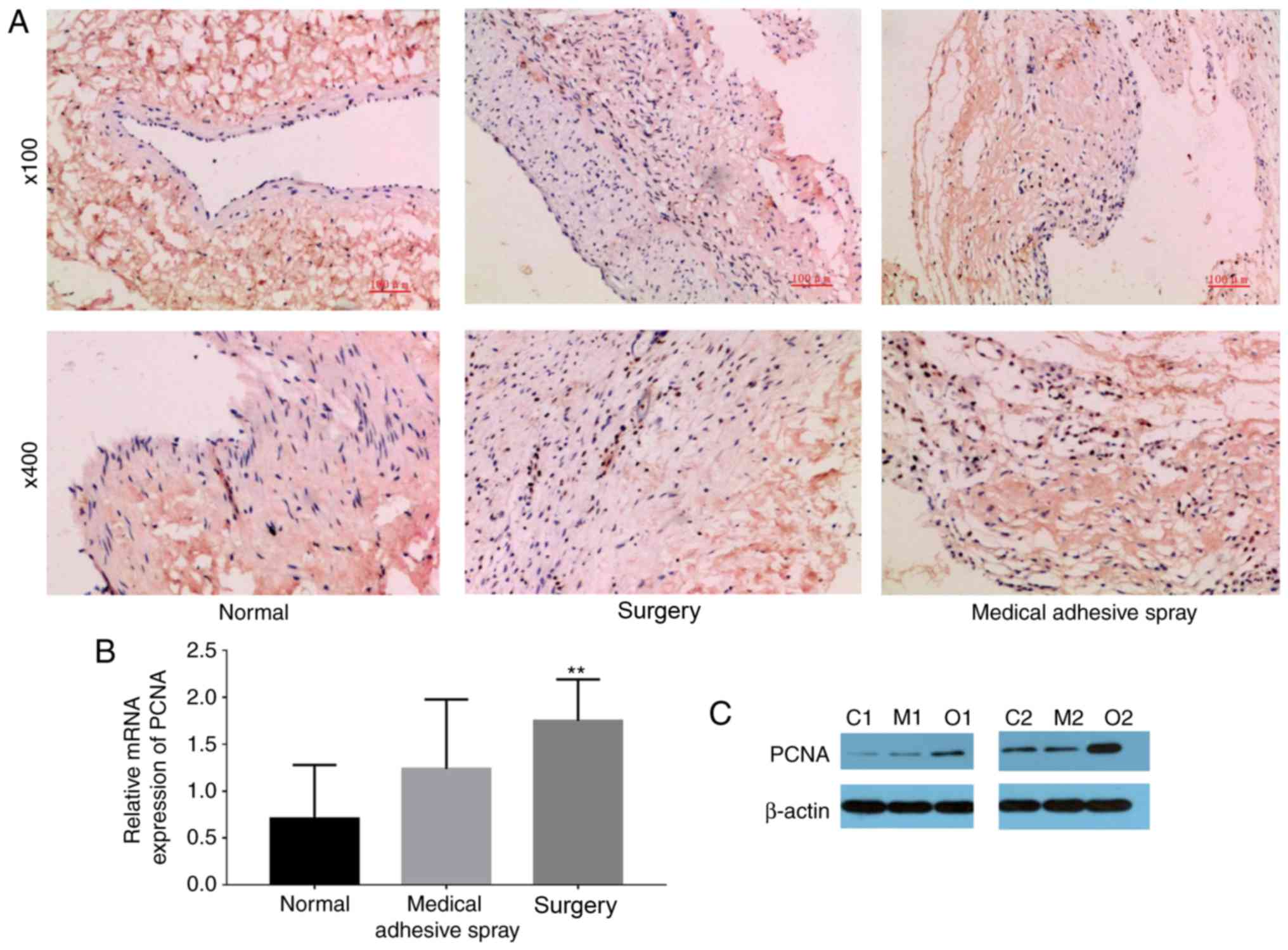
injury-induced expression of PCNA
In order to examine the proliferation
characteristics of vascular intimal tissues, immunohistochemical
staining was performed using the monoclonal antibody of PCNA to
test the level of PCNA in vascular wall tissues.
It was observed that yellow-brown particles in the
vascular endothelial cell nuclei were positive for PCNA staining
(Fig. 3). The expression of PCNA
was significantly increased in the surgery group compared with the
normal and medical adhesive spray groups (P<0.01).
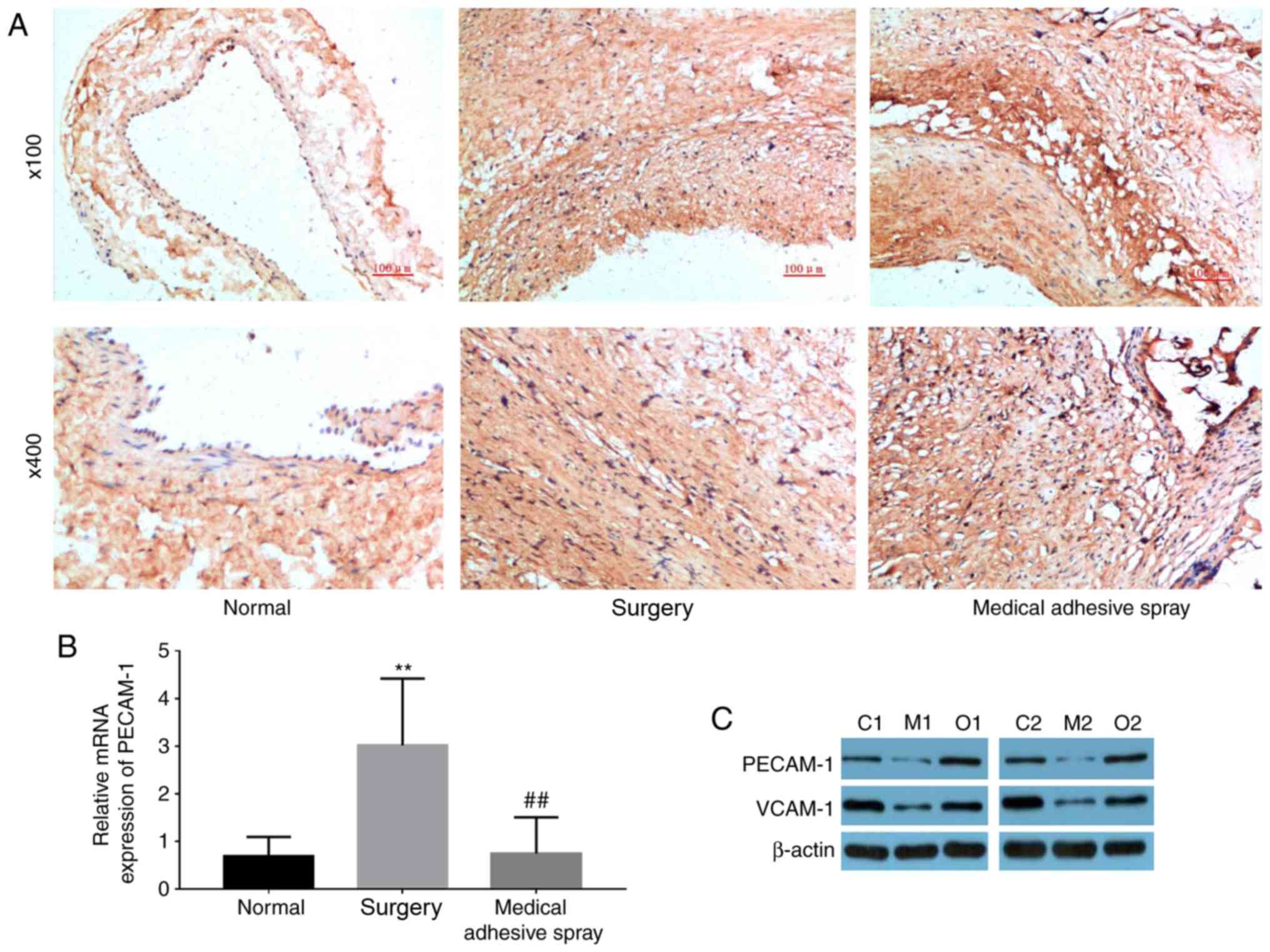
Medical adhesive spray inhibits the
injury-induced expression of PECAM-1 and VCAM-1
PECAM-1 was primarily located at the connection
between endothelial cells, and was stained dark brown (Fig. 4A). PECAM-1 and VCAM-1 were more
highly expressed in the surgery groups compared with the normal
group (P<0.01). However, the use of medical adhesive inhibited
the increase in PECAM-1 and VCAM-1.
Medical adhesive spray inhibits the
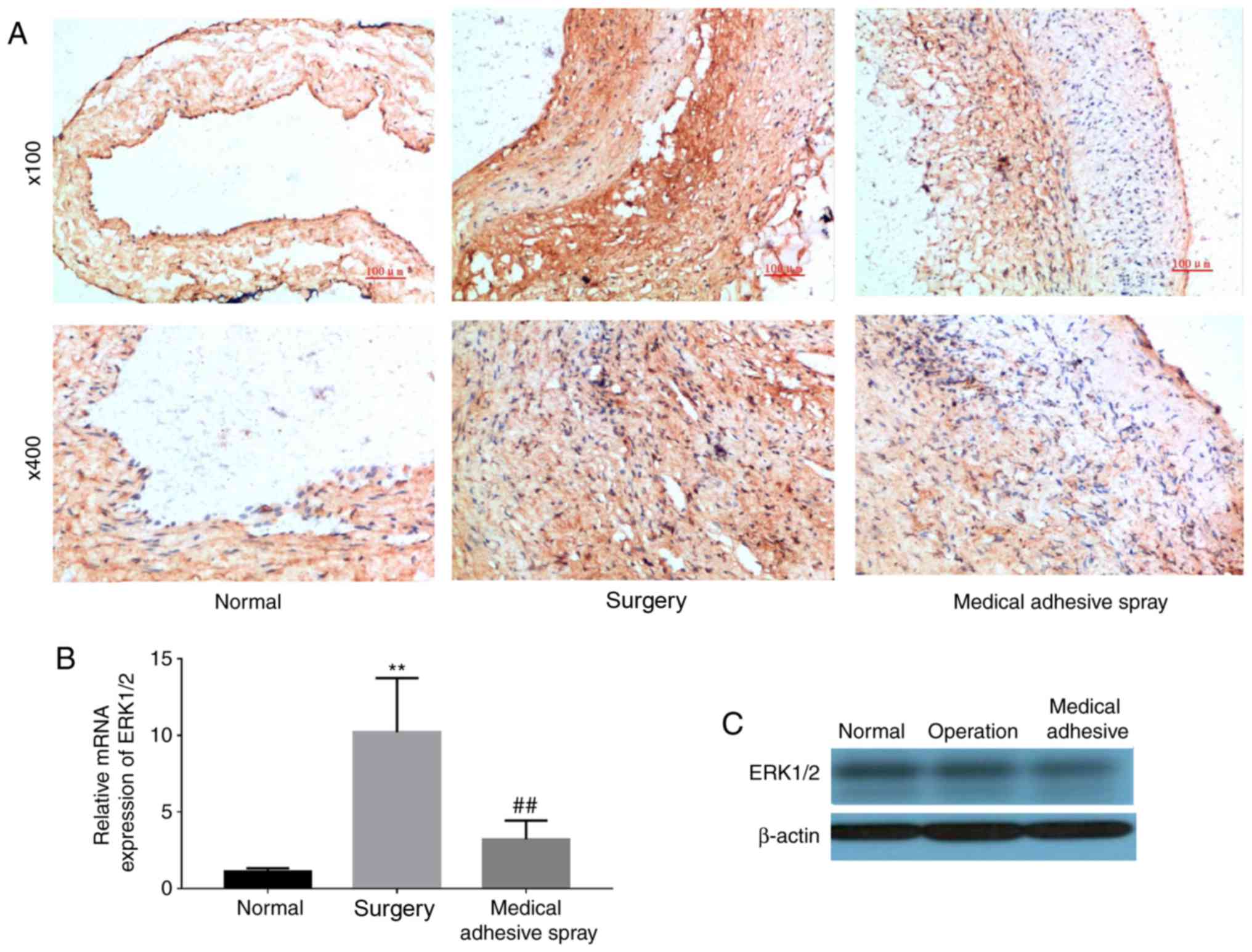
injury-induced expression of ERK1/2
The expression of ERK1/2 was additionally detected
(Fig. 5). Similar to the
expression of PECAM-1 and VCAM-1, ERK1/2 was more highly expressed
in the surgery groups compared with the normal group (P<0.01).
However, the use of medical adhesive inhibited the increase in
ERK1/2.
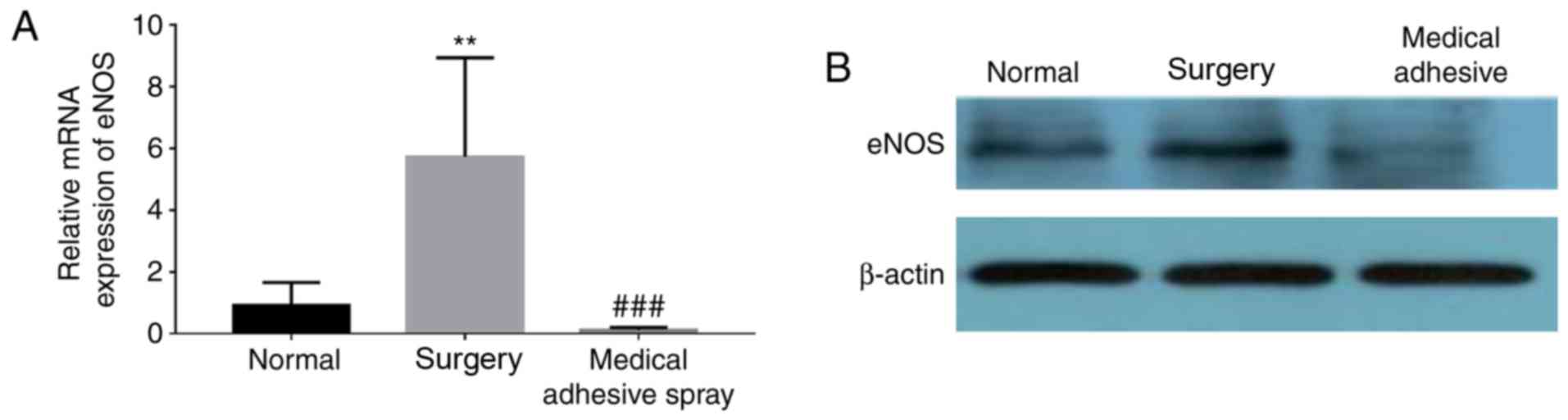
Medical adhesive spray inhibits the
injury-induced expression of eNOS
The expression of eNOS was additionally detected
(Fig. 6). Similar to the
expression of ERK1/2, eNOS was more highly expressed in the surgery
groups compared with the normal group (P<0.01). However, the use
of medical adhesive inhibited the increase in eNOS.
Discussion
The excessive proliferation of smooth muscle cells
and migration toward the intima, in addition to the abnormal
deposition of vascular wall interstitial cells, results in vascular
remodeling, vascular wall thickening, atherosclerosis and stenosis
of the lumen (6,7). This process has been defined as vein
arterialization (3–5). The present study demonstrated that
the thickening of the venous wall was primarily caused by the
proliferation of smooth muscle, which occurred over 4 weeks.
Following transplantation of the vascular graft into the arterial
environment, the vascular expansion resulted in an increased
mechanical force (mechanical expansion and traction) with increased
arterial pressure, causing damage to endothelial cells and smooth
muscle cells, and triggering the release of various growth
factors.
PCNA is an acidic polypeptide only synthesized and
expressed in proliferating cells, and is additionally termed
cyclin. As the auxiliary protein of DNA polymerase δ, PCNA is an
important factor during cell synthesis (18–20).
The expression of PCNA has been demonstrated to be associated with
cell proliferation, and it acts as an indicator of cell
proliferation (19). The most
important function of PCNA is that it acts as an auxiliary protein
of DNA polymerase 6 during nucleic acid metabolism (21). PCNA is, therefore, essential for
DNA synthesis. DNA synthesis is the key event of the cell
proliferation cycle. PCNA is a cell proliferation marker antigen
(22). In the cell cycle, the
synthesis of PCNA is important for the entry of cells into the S
phase from the G0 and G1 phases. PCNA expression has been
demonstrated to be increased in the late stages of cell entry into
the G1 phase, reaches its peak in the S phase, and decreases in the
G2 and M phases (23). The results
of the present study demonstrated that expression of PCNA was
significantly increased in the surgery group compared with the
normal and the medical adhesive spray groups, indicating that the
medical adhesive inhibited cell proliferation following
surgery.
The present study demonstrated that a stent may
reduce tension injury to the vein graft wall in the arterial
environment, prevent the continuous expansion of the vein graft at
arterial pressure, and reduce mechanical damage to the vascular
endothelial cells and smooth muscle cells. Medical adhesive has
been further demonstrated to prevent the excessive expansion of
vein grafts, accelerate the blood flow rate, and decrease leukocyte
adhesion, fibrin deposition, and thrombus formation and platelet
aggregation (3–5). It has been demonstrated that laminar
shear stress inhibits the proliferation of endothelial cells
through the induction of cell cycle dependent kinase inhibitors,
although disordered fluid shear stress may remove this inhibition
and consequently promote cell proliferation (24). ERK1/2 and eNOS are involved in the
expansion of vascular wall (6,25).
Medical adhesive spray may inhibit the expansion of the vascular
wall via inhibition of the ERK1/2 and eNOS signaling pathways.
PECAM-1 and VCAM-1 are primarily distributed in
blood cells and vascular endothelial cells. PECAM-1 and VCAM-1 are
involved in the signal transduction of adhesion molecules, and may
additionally regulate the function of endothelial cells (26,27).
The cell-cell interactions between endothelial cells are the
structural foundation for the maintenance of vascular integrity and
the regulation of leukocyte infiltration, in addition to vascular
permeability (28,29).
PECAM-1 belongs to a superfamily of immunoglobulin
adhesion molecules. PECAM-1 is expressed in activated platelet
membrane and vascular endothelial cells, and may induce the initial
stages of inflammation and stimulate the migration of leukocytes by
participating in the transition of extracellular signals to the
intracellular environment (30).
PECAM-1 is able to control the activity of platelets, regulate the
migration of leukocytes to the vascular intima and maintain the
integrity of the vessel wall. If endothelial cells are injured,
their function is decreased, leading to adverse consequences
(31,32). The results of the present study
suggested that PECAM-1 and VCAM-1 may serve important roles in the
regulation of endometrial hyperplasia by medical adhesive.
In conclusion, the application of medical adhesive
significantly inhibited intimal hyperplasia, which may be
associated with restriction of the over-distension of the vein
graft by downregulating the ERK1/2 and eNOS levels, reducing the
injury to the vascular intima and inhibiting the signaling pathways
involved in intimal hyperplasia. However, the exact mechanism
underlying the role of medical adhesive in the inhibition of
intimal hyperplasia requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
SC and CG conceived and designed the experiments.
SC, YY, HL, MG and LB performed the experiments. SC produced the
manuscript. SC and YY conducted data analysis.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experiments and Experimental Animal Welfare Committee of Capital
Medical University (approval no. AEEI-2015-144).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shoab SS, Lowry D and Tiwari A: Effect of
treated length in endovenous laser ablation of great saphenous vein
on early outcomes. J Vasc Surg Venous Lymphat Disord. 4:416–421.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fattoum M, Kennel S, Knez P, Schmitz-Rixen
T, Khout H and Tenholt MH: Lower extremity arterial
revascularization using conditioned small-diameter great saphenous
vein. J Vasc Surg. 64:819–823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehoux S and Jones EA: Shear stress,
arterial identity and atherosclerosis. Thromb Haemost. 115:467–473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heo KS, Fujiwara K and Abe J: Shear stress
and atherosclerosis. Mol Cells. 37:435–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham KS and Gotlieb AI: The role of
shear stress in the pathogenesis of atherosclerosis. Lab Invest.
85:9–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng Y and Liu J: Role of glypican-1 in
endothelial NOS activation under various steady shear stress
magnitudes. Exp Cell Res. 348:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Y, Waters M, Andrews A, Honarmandi P,
Ebong EE, Rizzo V and Tarbell JM: Fluid shear stress induces the
clustering of heparan sulfate via mobility of glypican-1 in lipid
rafts. Am J Physiol Heart Circ Physiol. 305:H811–H820. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dunn J, Simmons R, Thabet S and Jo H: The
role of epigenetics in the endothelial cell shear stress response
and atherosclerosis. Int J Biochem Cell Biol. 67:167–176. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JX, Yan ZP, Zhang YY, Wu J, Liu XH and
Zeng Y: Hemodynamic shear stress regulates the transcriptional
expression of heparan sulfate proteoglycans in human umbilical vein
endothelial cell. Cell Mol Biol (Noisy-le-grand). 62:1–34.
2016.
|
|
10
|
Nigro P, Abe J and Berk BC: Flow shear
stress and atherosclerosis: A matter of site specificity. Antioxid
Redox Signal. 15:1405–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vogel V: Mechanotransduction involving
multimodular proteins: Converting force into biochemical signals.
Annu Rev Biophys Biomol Struct. 35:459–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boehm EM, Powers KT, Kondratick CM, Spies
M, Houtman JC and Washington MT: The proliferating cell nuclear
antigen (PCNA)-interacting protein (PIP) motif of DNA polymerase
eta mediates its interaction with the C-terminal domain of Rev1. J
Biol Chem. 291:8735–8744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MacNeill SA: PCNA-binding proteins in the
archaea: Novel functionality beyond the conserved core. Curr Genet.
62:527–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krejcy K, Schwarzacher S, Ferber W, Plesch
C, Cybulsky MI and Weidinger FF: Expression of VCAM-1 in rabbit
iliac arteries is associated with vasodilator dysfunction of
regenerated endothelium following balloon injury. Atherosclerosis.
122:59–67. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Ding Z, Wu M, Xu W, Qian M, Du Q,
Zhang L, Cui Y, Zheng J, Chang H, et al: The apolipoprotein A-I
mimetic peptide, D-4F, alleviates ox-LDL-induced oxidative stress
and promotes endothelial repair through the eNOS/HO-1 pathway. J
Mol Cell Cardiol. 105:77–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Chen L, Fan Y, Jiang J and Wan J:
Atorvastatin increases endothelial progenitor cells in
balloon-injured mouse carotid artery. Can J Physiol Pharmacol.
92:369–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawasoe Y, Tsurimoto T, Nakagawa T,
Masukata H and Takahashi TS: MutSalpha maintains the mismatch
repair capability by inhibiting PCNA unloading. Elife.
5:e151552016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juríková M, Danihel L', Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang F, Abmayr SM and Workman JL:
Limiting PCNA-unloading at the G1/S transition. Cell Cycle.
153001–3002. 2016.
|
|
21
|
Boehm EM, Spies M and Washington MT: PCNA
tool belts and polymerase bridges form during translesion
synthesis. Nucleic Acids Res. 44:8250–8260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Álvarez V, Viñas L, Gallego-Sánchez A,
Andrés S, Sacristán MP and Bueno A: Orderly progression through
S-phase requires dynamic ubiquitylation and deubiquitylation of
PCNA. Sci Rep. 6:255132016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokoyama R, Hirakawa T, Hayashi S,
Sakamoto T and Matsunaga S: Dynamics of plant DNA replication based
on PCNA visualization. Sci Rep. 6:296572016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akimoto S, Mitsumata M, Sasaguri T and
Yoshida Y: Laminar shear stress inhibits vascular endothelial cell
proliferation by inducing cyclin-dependent kinase inhibitor
p21(Sdi1/Cip1/Waf1). Circ Res. 86:185–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Liao B, Li M, Cheng M, Fu Y, Liu
Q, Chen Q, Liu H, Fang Y, Zhang G and Yu F: Shear stress regulates
endothelial cell function through SRB1-eNOS signaling pathway.
Cardiovasc Ther. 34:308–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng YM, Chen XH and Zhang X: Roles of
PECAM-1 in cell function and disease progression. Eur Rev Med
Pharmacol Sci. 20:4082–4088. 2016.PubMed/NCBI
|
|
27
|
Paddock C, Zhou D, Lertkiatmongkol P,
Newman PJ and Zhu J: Structural basis for PECAM-1 homophilic
binding. Blood. 127:1052–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu P, Theiss A, Han J and Feagins LA:
Increased cell adhesion molecules, PECAM-1, ICAM-3, or VCAM-1,
predict increased risk for flare in patients with quiescent
inflammatory bowel disease. J Clin Gastroenterol. 51:522–527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Lin L, Li R, Yuan C, Xu M, Huang
JH and Huang M: Dimer conformation of soluble PECAM-1, an
endothelial marker. Int J Biochem Cell Biol. 77:102–108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial PECAM-1 and its function in vascular physiology and
atherogenic pathology. Exp Mol Pathol. 100:409–415. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Lei L, Cai XJ, Chen LY, Yuan M,
Yang G, Huang P and Wang X: A preliminary study of pamidronic acid
downregulation of angiogenic factors IGF-1/PECAM-1 expression in
circulating level in bone metastatic breast cancer patients. Onco
Targets Ther. 9:3147–3152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lertkiatmongkol P, Paddock C, Newman DK,
Zhu J, Thomas MJ and Newman PJ: The role of sialylated glycans in
human PECAM-1-mediated trans-homophilic interactions and
endothelial cell barrier function. J Biol Chem. 291:26216–26225.
2016. View Article : Google Scholar : PubMed/NCBI
|