Introduction
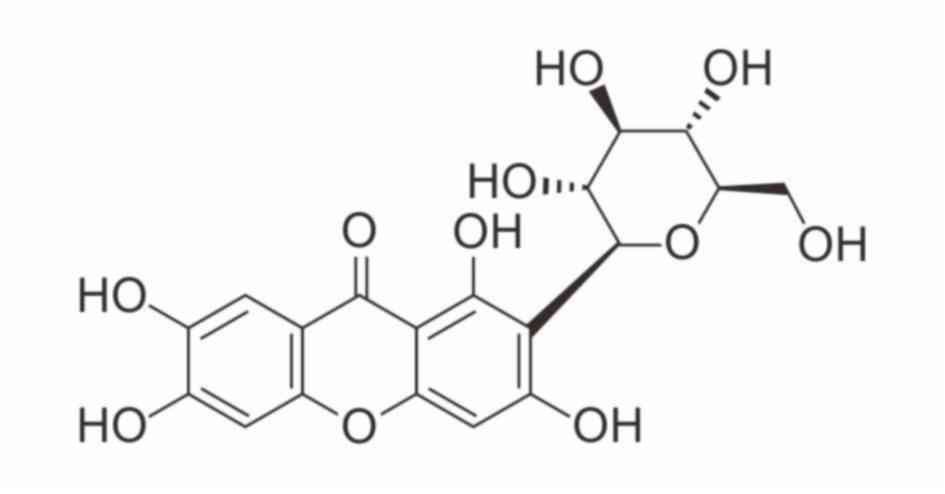
Mangiferin, a C-glucosyl xanthone with the formula
1,3,6,7-tetrahydroxyxanthone-C2-β-D-glucoside (Fig. 1), has been extensively studied both
in vivo and in vitro. It has been demonstrated to
possess numerous pharmacological activities, including
antioxidative, antiaging, antitumor, antibacterial, antiviral,
immunomodulatory, antidiabetic, hepatoprotective and analgesic
effects (1–3). Mangiferin has been detected in many
plant species and may be abundantly isolated from various parts of
Mangiferaindica (mango), including the leaves, stem bark,
fruit peels and root (4,5).
With advances in pharmacology and molecular biology,
research regarding mangiferin has increased and more
pharmacological mechanisms have been revealed, which provides
further information for the design and development of mangiferin as
a clinical therapeutic. However, Wang et al (6) reported that the solubility of
mangiferin was only 0.111 mg/ml. In addition, Han et al
(7) demonstrated that the oral
bioavailability of mangiferin was only 1.2%. This may be due to its
low lipophilicity properties, poor intestinal membrane permeability
and low oral absorption (8). These
experimental data collectively indicate that despite a wide range
of pharmacological activities, mangiferin has low solubility,
transmembrane permeability and bioavailability, which restricts the
clinical development and application of mangiferin. In order to
identify more effective therapeutic compounds, mangiferin
derivatives with improved solubility, bioavailability and potency
were obtained by chemical or biotransformation methods (9). Therefore, with the progress of
biotechnology and research practice, mangiferin may be identified
to represent a more promising, novel therapeutic drug in
clinic.
Based on previous studies concerning the structural
modification, pharmacological activities and therapeutic molecular
mechanisms of mangiferin in recent decades, the pharmacological
effects of mangiferin were reviewed herein, in order to provide
references for further drug research and development.
Potential application of mangiferin in
antidiabetic therapy
Prevalence and main features of
diabetes mellitus
Diabetes mellitus is a common endocrine and
metabolic disease that affects public health and quality of life
(10,11). Statistics from the International
Diabetes Federation Diabetes Atlas (http://www.diabetesatlas.org/resources/2017-atlas.html)
revealed that there were an estimated 425 million adult patients
with type 2 diabetes (T2D) in 2017, of whom 212 million were not
diagnosed (12). Notably, China
has the largest number of adult diabetic patients. The prevalence
of diabetes and prediabetes in China is 11.6 and 50.1%,
respectively, accounting for 113.9 million patients with diabetes
and 493.4 million individuals with prediabetes, according to
China's non-communicable disease surveillance group in 2013
(13). These data indicate that
diabetes has become a major public health problem worldwide.
The main feature of diabetes is hyperglycemia, which
is caused by disrupted glucose homeostasis (14,15).
β-cell deficiency in the pancreas results in insufficient insulin
secretion and type 1 diabetes, whereas insulin resistance may lead
to decreased insulin sensitivity in T2D. Glucose homeostasis
requires coordinated regulation of the pancreas and insulin target
organs, such as adipose tissue, muscle, the liver and the brain
(15). In the pancreas, α cells
secrete glucagon and β cells secrete insulin, which respond to
blood glucose alterations. High glucose stimulates insulin
secretion, which increases glucose uptake, use and storage in
adipose and muscle tissue. Low glucose stimulates glucagon release,
which promotes hepatic glucose production in the liver to increase
the blood glucose levels (16).
Fat cells release adipokines, including leptin, adiponectin and
cytokines, as well as free fatty acids (FFA) to regulate food
intake, insulin secretion and insulin sensitivity (17). The interaction between various
organs or tissues associated with blood glucose regulation is
essential. Disordered regulation of this system may lead to the
occurrence of diabetes (18).
Patients with type 1 and T2D may have progressive complications,
including heart disease, stroke, blindness, diabetic nephropathy
and kidney failure (19–22), if the disease is not effectively
controlled. Therefore, it is necessary prevent and treat diabetes
in the early stage.
Effect of mangiferin on diabetes and
diabetic complications
Although drugs with multiple pharmacological
mechanisms are the most effective regimens to alleviate the effects
of type 2 diabetes, they cannot completely prevent disease
progression (23,24). Therefore, novel and effective drugs
are urgently needed. It was recently reported that mangiferin
treatment was beneficial in streptozotocin (STZ)-induced diabetic
mice. Biochemical, toxicological and hematological parameters were
evaluated following oral treatment with 40 mg/kg mangiferin for 30
days. Compared with diabetic control mice, the levels of blood
glucose, glycosylated hemoglobin, aspartate aminotransferase (AST),
alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were
significantly reduced in mangiferin-treated diabetic mice.
Furthermore, the levels of red and white blood cells were notably
improved (25,26). Therefore, mangiferin was
demonstrated to possess anti-diabetic efficacy without toxicity in
chemically induced diabetic mice (Fig.
2A). Additionally, in a high-fat high fructose diet and
STZ-induced diabetic insulin-resistant rat model, mangiferin was
demonstrated to concurrently alleviate insulin resistance, improve
β-cell function, reduce serum levels of triglyceride (TG), total
cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C),
as well decrease the atherogenic index, liver TG and liver TC
content. Liver glycogen content was also enhanced (27). These results validated that
mangiferin may effectively improve insulin sensitivity in the
treatment of type 2 diabetes accompanied with metabolic disorders
(Fig. 2B). Similarly, Muruganandan
et al (28), Miura et
al (29,30) and Suman et al (31) verified that mangiferin exerted
significant antidiabetic, antihyperlipidemic and antiatherogenic
effects in diabetic rat models. As for the promotion of islet
function induced by mangiferin, our previous study performed 70%
partial pancreatectomy (PPx) in mice to elucidate the antidiabetic
mechanisms of mangiferin. The results indicated that mangiferin
facilitates β-cell proliferation and islet regeneration through the
regulation of crucial genes in cell cycle and islet regeneration
(Fig. 2C) (32). Furthermore, our upcoming report
successfully confirmed that mangiferin increases islet regeneration
in young and old diabetic mice (unpublished data). Taken together,
these data provide evidence that mangiferin has promising
application prospects in the treatment of diabetes.
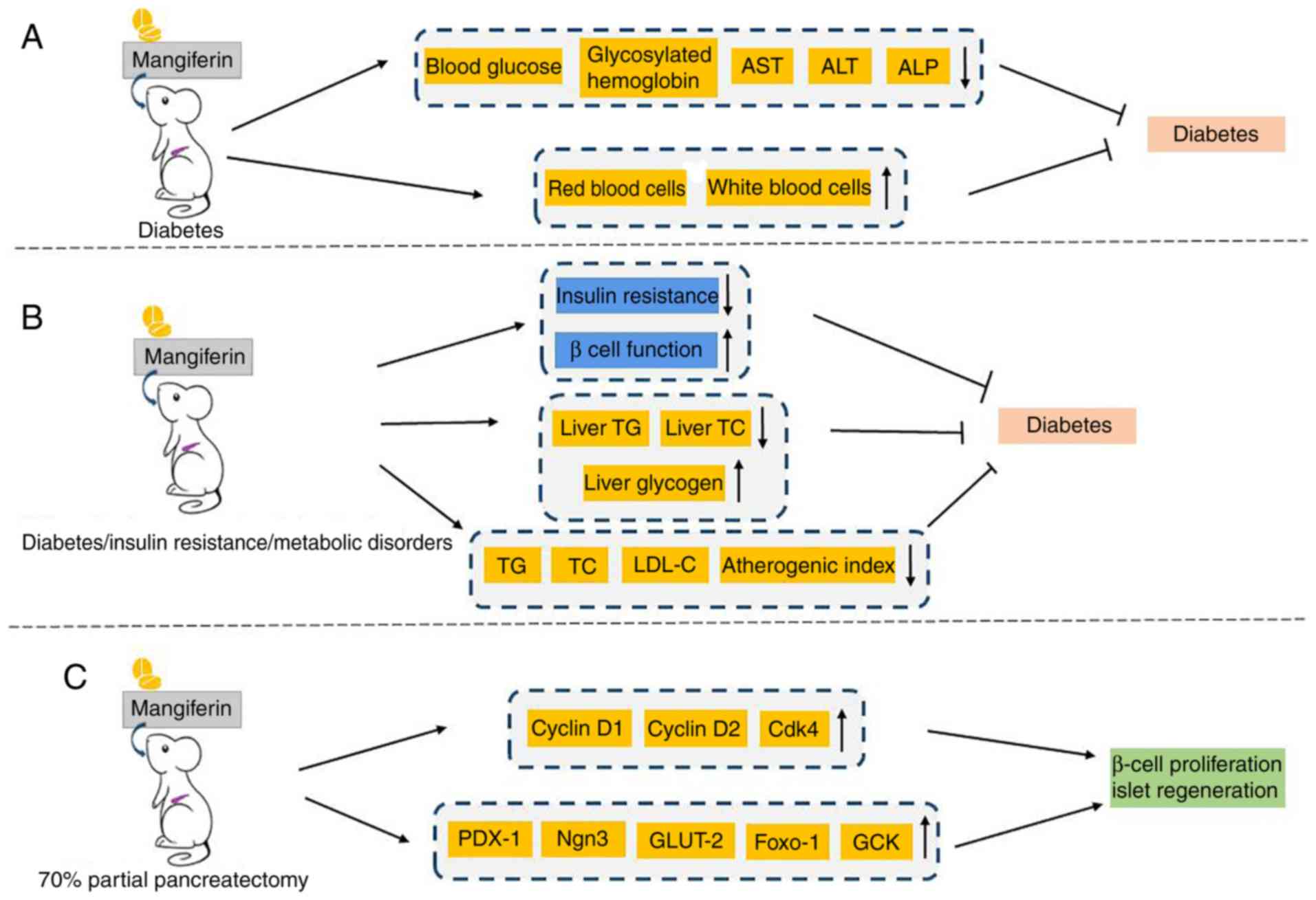 | Figure 2.Overview of the functions of
mangiferin in diabetes mellitus. (A) A significant reduction of
biochemical and toxicological parameters, as well as a marked
improvement in hematological parameters were simultaneously
observed in diabetic rats. (B) Mangiferin notably increased insulin
sensitivity and β cell function, and improved parameters associated
with metabolic disorders. (C) Mangiferin regulated the expression
of genes involved in cell cycle regulation and islet regeneration
to improve islet function. AST, aminotransferase; ALT, alanine
aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; TC,
total cholesterol; LDL-C, low density lipoprotein cholesterol;
Cdk4, cyclin-dependent kinase 4; PDX-1, pancreatic and duodenal
homeobox 1; Ngn3, neurogenin 3; GLUT-2, glucose transporter 2;
Foxo-1, forkhead box protein O1; GCK, glucokinase. |
As one of the most common and serious diabetic
complications, diabetic nephropathy (DN) is a renal impairment
caused by diabetes-induced microangiopathy and glomerulosclerosis.
Hypertension, proteinuria and hydropsy are the clinical
manifestations of DN (33).
Surprisingly, Pal et al (34) demonstrated that mangiferin could
protect kidneys from DN. They investigated the specific mechanisms
underlying the protective effects of mangiferin in a STZ-induced
diabetic nephropathy rat model and observed that mangiferin
markedly decreased plasma glucose, kidney to body weight ratio,
blood urea nitrogen (BUN), plasma creatinine, uric acid and urinary
albumin. Additionally, glomerular hypertrophy and hydropsy was
attenuated following mangiferin treatment at an oral dose of 40
mg/kg body weight/day in water for 30 days (34). Similarly, in animal experiments
performed by Liu et al (35) in an STZ-induced DN rat model,
evidential decreases in albuminuria, BUN, kidney weight index,
periodic acid-Schiff stain positive mesangial matrix area,
glomerular extracellular matrix expansion and accumulation and
glomerular basement membrane thickness were observed following oral
mangiferin treatment (15, 30 and 60 mg/kg) for 9 weeks, via
targeting of glyoxalase 1. These results validate the beneficial
actions of mangiferin in DN, although body weight and blood glucose
levels were not improved (35).
Furthermore, mangiferin (15, 30 and 60 mg/kg/day for 9 weeks)
effectively attenuated renal fibrosis through the inhibition of
osteopontin overproduction and inflammation in a STZ-induced rat
diabetic model (36).
Cardiovascular events may also occur in patients
with T2DM, including stroke, myocardial infarction and endothelial
dysfunction, which are major physical and economic burdens
(37–39). Hyperglycemia-induced oxidative
stress and inflammation are thought to be involved in the
development of cardiovascular disease. The activation of advanced
glycation end products-receptor for advanced glycation end products
(AGE-RAGE) results in increased oxidative stress, inflammation and
apoptosis in ischemia-reperfusion (IR)-induced myocardial injury in
diabetic rats (40). Notably,
mangiferin inhibits activation of the AGE-RAGE/mitogen-activated
protein kinase (MAPK), c-Jun N-terminal kinase (JNK) and p38
pathways (41). The expression of
extracellular regulated kinase 1/2 (ERK1/2) in the myocardium is
also increased (41). In addition,
chronic treatment with mangiferin (20 mg/kg for 16 weeks) decreased
the level of myocardial enzymes creatine kinase-MB and lactate
dehydrogenase (LDH), as well as the inflammatory mediators tumor
necrosis factor (TNF)-α and interleukin (IL)-1β in the serum and
left ventricular myocardium (41).
In diabetic cardiomyopathy (DCM) rats, mangiferin reduces AGE
production, and decreases the mRNA and protein expression of RAGE
(42), suggesting that mangiferin
treatment may be beneficial in DCM. A previous study determined the
effect of mangiferin on myocardial IR injury in diabetic rats and
further investigated the underlying mechanisms involved in its
cardioprotective effects. In a diabetic rat model induced by STZ,
mangiferin (60 mg/kg; oral gavage for 12 weeks) reduces blood
glucose and cardiomyocyte apoptosis, downregulates
inositol-requiring enzyme 1, apoptotic signal-regulating kinase and
JNK (43). This suggests that
mangiferin inhibits the apoptosis of myocardial cells in diabetic
rats via mechanisms associated with blood glucose regulation and
endoplasmic reticulum (ER) stress prevention. Endothelial
dysfunction, under conditions of ER stress and increased reactive
oxygen species (ROS) production, is tightly associated with
cardiovascular complications in diabetes (44). The expression of
thioredoxin-interacting protein (TXNIP), NLR family pyrin domain
containing 3 (NLRP3), IL-1β and IL-6, as well as the
phosphorylation of AMP-activated protein kinase (AMPK), was
attenuated in endothelial cells cultured with mangiferin at
concentrations of 0.1, 1 and 10 µM, demonstrating its inhibitory
effects on TXNIP/NLRP3 inflammasome activation and it ameliorative
effects on endothelial dysfunction (45).
Anti-tumor effects of mangiferin
The research and development of antitumor agents
with fewer adverse effects has become a popular topic of research.
As a monomer of traditional Chinese medicine, mangiferin has been
demonstrated to have direct and auxiliary roles in oncotherapy.
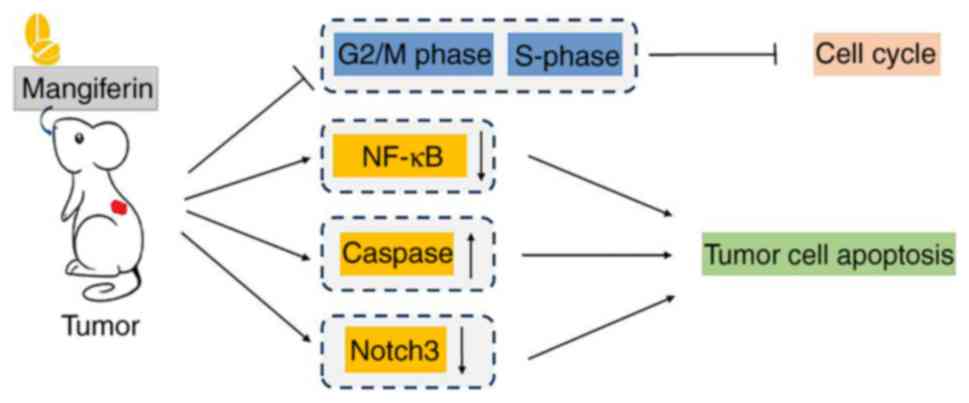
Mangiferin efficaciously inhibits tumor cell cycle progression. It
has been widely accepted that cell growth prior to cell division is
strictly controlled by the cyclin-dependent kinase 1 (CDK1)/cyclin
B1 complex (46–48). Notably, mangiferin has been
validated to trigger G2/M phase cell cycle arrest via regulation of
the CDK1-cyclin B1 signaling pathway (49–51).
DNA synthesis is accomplished during the S phase of the cell cycle,
and treatment with mangiferin causes S phase delay in colorectal
cancer HT29 cells and cervical cancer HeLa cells (52). In addition to its inhibitory
actions on cell cycle, mangiferin induces apoptosis in tumor cells.
Nuclear factor-κB (NF-κB) is a transcription factor that induces
the proliferation of cancer cells (53). RelA and RelB, important members of
NF-κB family, are transformed into activated NF-κB following
dimerization. It was demonstrated that mangiferin inhibits the
expression of RelA and RelB, activates inhibitor of NF-κB (IκB) and
suppresses the phosphorylation of NF-κB kinase, thereby inhibiting
the transcriptional activity of NF-κB and inducing apoptosis tumor
cells (54–56). IκB kinase α and IκB kinase
β-dependent IκB degradation and subsequent NF-κB activation have
been widely acknowledged as the canonical NF-κB activation pathway
(57). However, a collective
patent review on mangiferin unveiled that multiple signaling
pathways, including nuclear NF-κB signaling and cyclooxygenase-2
(COX-2) protein expression are involved in the antitumor effects of
mangiferin (56). This patent
review concluded that mangiferin is a candidate molecule in the
development of novel antitumor drugs. Furthermore, caspase
activation may participate in the apoptosis-inducing effects of
mangiferin in oncotherapy. A disturbance in the balance between
cell proliferation and apoptosis is known to initiate tumorigenesis
(58). Caspase activation serves a
critical role in apoptosis, particularly via the
mitochondria-initiated pathway (59). Pan et al (60) demonstrated that through regulation
of Bcl-2, apoptosis regulator (Bcl-2) and Bcl-2 associated X,
apoptosis regulator (Bax) expression, mangiferin potently inhibits
tumor cell proliferation and induces apoptosis in nasopharyngeal
carcinoma cells. Additionally, a study of the antitumor efficacy
and underlying mechanisms of mangiferin in human cervical carcinoma
HeLa cells demonstrated that the protein expression of BH3
interacting domain death agonist, Bcl-2 and pro-caspase-3 and −8 is
downregulated in response to mangiferin treatment, which results in
the activation of caspase-3, −7, −8 and −9, ultimately leading to
apoptosis (61).
Our previous studies contributed to the
understanding of the underling molecular mechanism of mangiferin in
oncotherapy (55,62,63).
In human breast carcinoma MCF-7 cells, mangiferin down regulates
the cyclin-dependent kinase 1-cyclin Bl signaling pathway and
induces G2/M phase cell-cycle arrest to inhibit tumor cell growth
(62). In addition, it was
demonstrated to inhibit the protein kinase C (PKC)-NF-κB pathway to
induce apoptotic cell death (62).
Furthermore, in vivo experiments performed in a MCF-7
×enograft rat model confirmed the in vitro results (62). Similarly, in human lung carcinoma
A549 cells, mangiferin exhibits antineoplastic properties, by
inducing G2/M phase cell cycle arrest via downregulation of the
cyclin-dependent kinase 1-cyclin B1 signaling pathway and inducing
apoptotic cell death by inhibiting the PKC-NF-κB pathway (55). In addition, in-depth research into
the underlying mechanisms of mangiferin successfully elucidated
that caspase-dependent apoptosis and obviously downregulated Notch3
expression occurs in mangiferin-treated human ovarian
adenocarcinoma OVCAR3 cells (63).
Based on these previous studies, it may be concluded that
mangiferin exhibits prominent anti-tumor action in certain
malignant neoplasms through multifactorial molecular mechanisms
(Fig. 3).
The neuroprotective properties of
mangiferin
Abundant research on the effects of mangiferin has
revealed the antioxidant and anti-inflammatory properties, due to
its C-glycosylxanthone structure (64). The C-glucosy l linkage and
polyhydroxy component in mangiferin contributes to its free
radical-scavenging activity (65).
Free radicals are highly reactive molecules that are implicated in
the pathology of traumatic brain injury and cerebral ischemia
through oxidative stress and inflammation (66–68).
In recent decades, the role of free radicals in the pathology of
traumatic brain injury and cerebral ischemia has been elucidated by
investigating oxidative stress and inflammation with other
pathogenic processes, such as excitotoxicity, calcium overload,
mitochondrial cytochrome c release, caspase activation and
apoptosis in trauma and ischemia of central nervous system (CNS)
(69). Furthermore, mangiferin is
capable of modulating the expression of proinflammatory signaling
molecules, including the expression of TNF-α and COX-2, as well as
regulating various transcription factors, such as NF-κB, and
NF-E2-related factor 2 (Nrf-2) (64). Research regarding the protective
effects of mangiferin in CNS injury, proinflammatory cytokine
expression, oxidative stress and neurobehavioral abnormalities
induced by lipopolysaccharide (LPS) in cerebrum are detailed
herein.
Accumulating evidence has revealed that mangiferin
is able to attenuate LPS-induced cognitive deficits caused by
neuroinflammation (70). Oral
mangiferin pre-treatment (50 mg/kg) and treatment (50 mg/kg)
following LPS injection in mice demonstrated that mangiferin
ameliorates cognitive deficits by decreasing LPS-induced IL-6
expression and increasing heme oxygenase-1 (HO-1) expression in
mice hippocampi. Similarly, in behavioral experiments, mice were
treated with LPS (0.83 mg/kg) intraperitoneal injection following
oral pretreatment with mangiferin (20 and 40 mg/kg, 14 days), which
subsequently attenuated depressive and anxiety-like behaviors
(71). Further research has
ascertained that mangiferin increases glutathione concentration,
superoxide dismutase (SOD) and catalase activity in mice; as well
as decreasing lipid peroxidation and nitrite level in the
hippocampus and prefrontal cortex (71). Furthermore, mangiferin reduces
LPS-induced IL-1β expression and oxidative stress when used in
depressive and anxiety disorders (71). Therefore, these studies indicated
that mangiferin greatly contributes to cerebral protection. By
decreasing COX-2 transcript stability, mangiferin potently reduces
LPS-induced prostaglandin E2 (PGE-2) synthesis and
8-iso-prostaglandin F2α (8-iso-PGF2α) production (72). Taken together, these data
demonstrated mangiferin exhibits potent antioxidant and
anti-inflammatory properties, by decreasing PGE-2 production, free
radical formation and COX-2 synthesis.
The underlying mechanisms of neuroinflammation and
oxidative damage in the brain are complicated. Young adult male
Wistar rats were exposed to a high-stress environment and
neurological and neuropsychiatric diseases associated with cell
damage and apoptosis were observed in their cerebrum, such as
neurodegenerative diseases, depression, and schizophrenia (73). It was demonstrated that mangiferin
administration (15, 30 and 60 mg/kg; oral gavage) prevents
hypothalamic/pituitary/adrenal (HPA) stress axis dysregulation,
neuroinflammation and oxidative damage. Pretreatment with
mangiferin inhibits an increase in the levels of glucocorticoids
(GCs), IL-1β, TNF-α, TNF receptor 1, NF-κB, inducible nitric oxide
synthase (iNOS) and COX-2 (73).
Furthermore, mangiferin effectively protects against early brain
injury (EBI) that is involved in the process of cerebral tissue
damage induced by subarachnoid hemorrhage (SAH) (74). It was demonstrated that mangiferin
decreases the mortality of animals with SAH, ameliorates
neurological deficits and brain edema, attenuates SAH-induced
oxidative stress and decreases cortical cell apoptosis in a
dose-dependent manner. Mechanism analysis of mangiferin
demonstrated that it exerts its neuroprotective effects against EBI
by promoting the nuclear factor erythroid 2-related factor 2
(Nrf2)/HO-1 cascade in the mitochondrial apoptosis pathway, as well
as through NLRP3 inflammasome activation and NF-κB inhibition
(74). In addition, in Wistar male
rats with cerebral IR injury, the activation of Nrf2/HO-1 cascade
is promoted in a dose-dependent manner by mangiferin (75). Furthermore, mangiferin ameliorates
the activities of SOD, glutathione and IL-10, which has a
protective role in oxidative stress induced by cerebral IR injury
(Fig. 4).
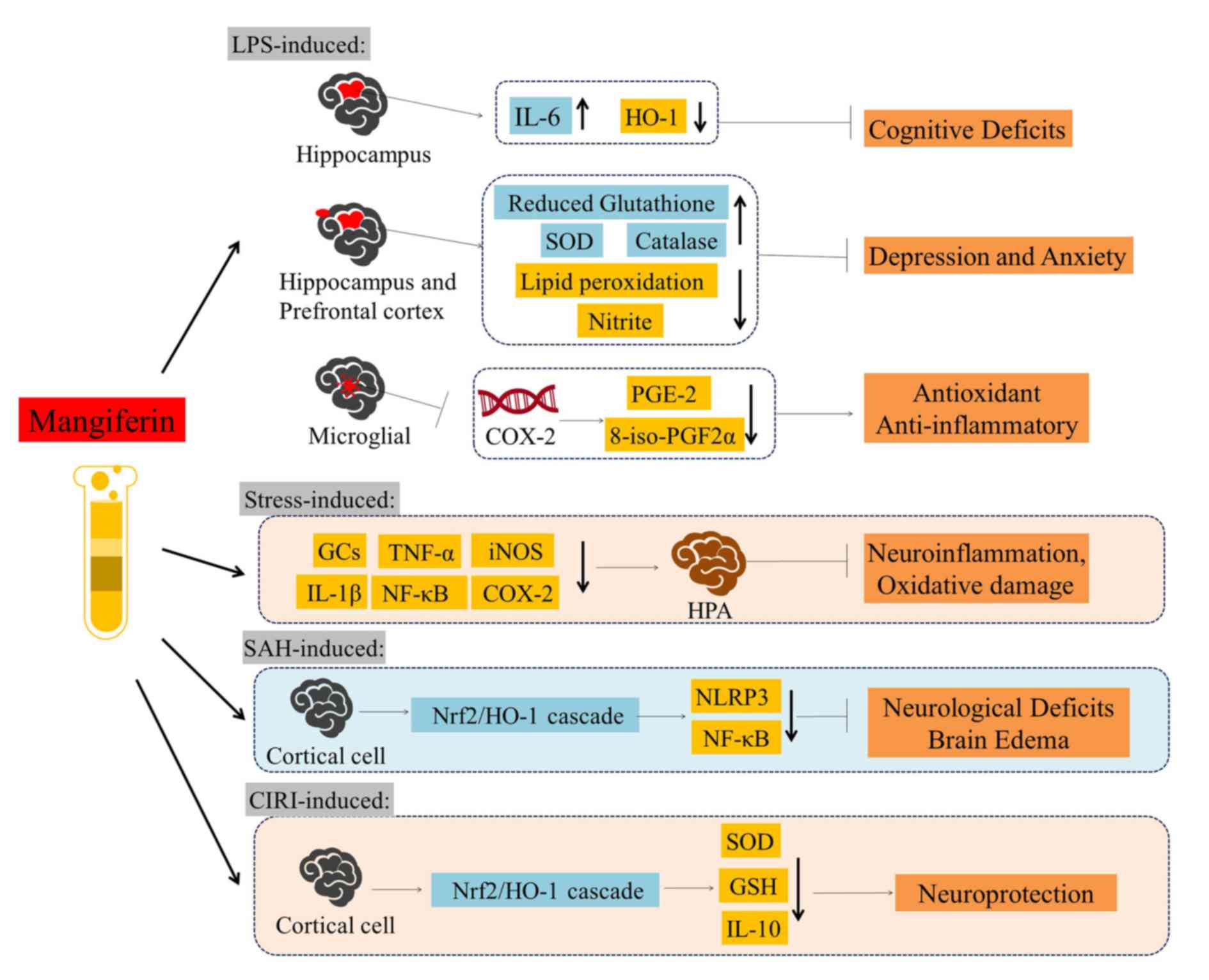 | Figure 4.Overview of the neuroprotective
activities of mangiferin against LPS-, stress-, SAH- and
CIRI-induced models. Mangiferin has been demonstrated to exert
protective effects against cognitive deficits, depression, anxiety,
neuroinflammation, oxidative damage, neurological deficits and
brain edema. LPS, lipopolysaccharide; SAH, subarachnoid hemorrhage;
CIRI, cerebral ischemia reperfusion injury; IL, interleukin; HO-1,
heme oxygenase-1; SOD, superoxide dismutase; PGE-2, prostaglandin
E2; 8-iso-PGF2α, 8-iso-prostaglandin F2α; GCs, glucocorticoids;
TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide
synthase; NF-κB, nuclear factor-κB; COX-2, cyclooxygenase-2; HPA,
hypothalamic-pituitary-adrenal axis; Nrf2, nuclear factor erythroid
2-related factor 2; NLRP3, NLR family pyrin domain containing 3;
GSH, glutathione. |
Cardiovascular effects of mangiferin
Cardioprotective effects of mangiferin. Increasing
amounts of evidence regarding the cardioprotective effects of
mangiferin in isoproterenol (ISPH)-induced myocardial infarction
(MI) rats has emerged. Prabhu et al (76) demonstrated that pretreatment with
mangiferin (100 mg/kg) for 28 days regulates the tissues defense
system against cardiac damage. Specifically, the activities of
heart tissue enzymic antioxidants, including superoxide dismutase,
catalase, glutathione peroxidase, glutathione transferase and
glutathione reductase, as well as non-enzymic antioxidants such as
cerruloplasmin, Vitamin C, Vitamin E and glutathione, were all
notably upregulated (76). It was
concluded that mangiferin exerts its beneficial effects due to its
antioxidant potential, which reduces myocardial oxygen consumption
and relieves angina pectoris (76). In addition, mangiferin protects the
myocardium against ISPH-induced MI by reducing lipid peroxide
formation and retaining the activities of myocardial marker
enzymes, including LDH, creatine phosphokinase (CPK), AST and ALT
(77). Those results suggested
that mangiferin effectively alleviates free radical release in the
ischemic myocardium and delays membrane lipid oxidation. In
addition, studies in myocardial cells under the condition of
ISPH-induced MI demonstrated mangiferin protects the structural
integrity of by reducing the effects of oxidative damage and
increasing mitochondrial energy metabolism (78). Mangiferin markedly increases the
activities of the tricarboxylic acid cycle and antioxidant defense
enzymes in MI (78). In addition,
mangiferin has been reported to prevent free radical-mediated lipid
peroxidation and increase lysosomal instability, thus alleviating
MI injury (79).
It has also been demonstrated that mangiferin
improved heart blood flow parameters and fiber disturbance in a
dose-dependent manner, following a single intravenous injection of
mangiferin (5, 10 or 20 mg/kg) in a MI experimental model (80). Mangiferin administration (20 mg/kg
for 4 weeks) restores the function of cardiac ejection, reduces the
accumulation of TNF-α and cleaved caspase-3, and upregulates Bcl-2
(80). In addition, mangiferin has
a therapeutic effect on post-MI left ventricular remodeling and
improves cardiac function. Mangiferin administration (50 or 100
mg/kg for 5 weeks) also protects against doxorubicin-induced
mortality and electrocardiogram abnormality, decreases the
expression of biochemical cardiac toxicity markers, such as
dehydrogenase and creatine phosphokinase isoenzyme (81). Histopathologically, mangiferin
treatment results in obvious reductions in inflammatory cell
number, fibrotic area and necrotic foci (81), which indicates that mangiferin may
have a preventive effect against ventricular hypertrophy and
fibrosis induced by MI. Therefore, mangiferin may be used in
combination with clinical treatments in the future, including with
surgery or other therapeutics (Fig.
5A).
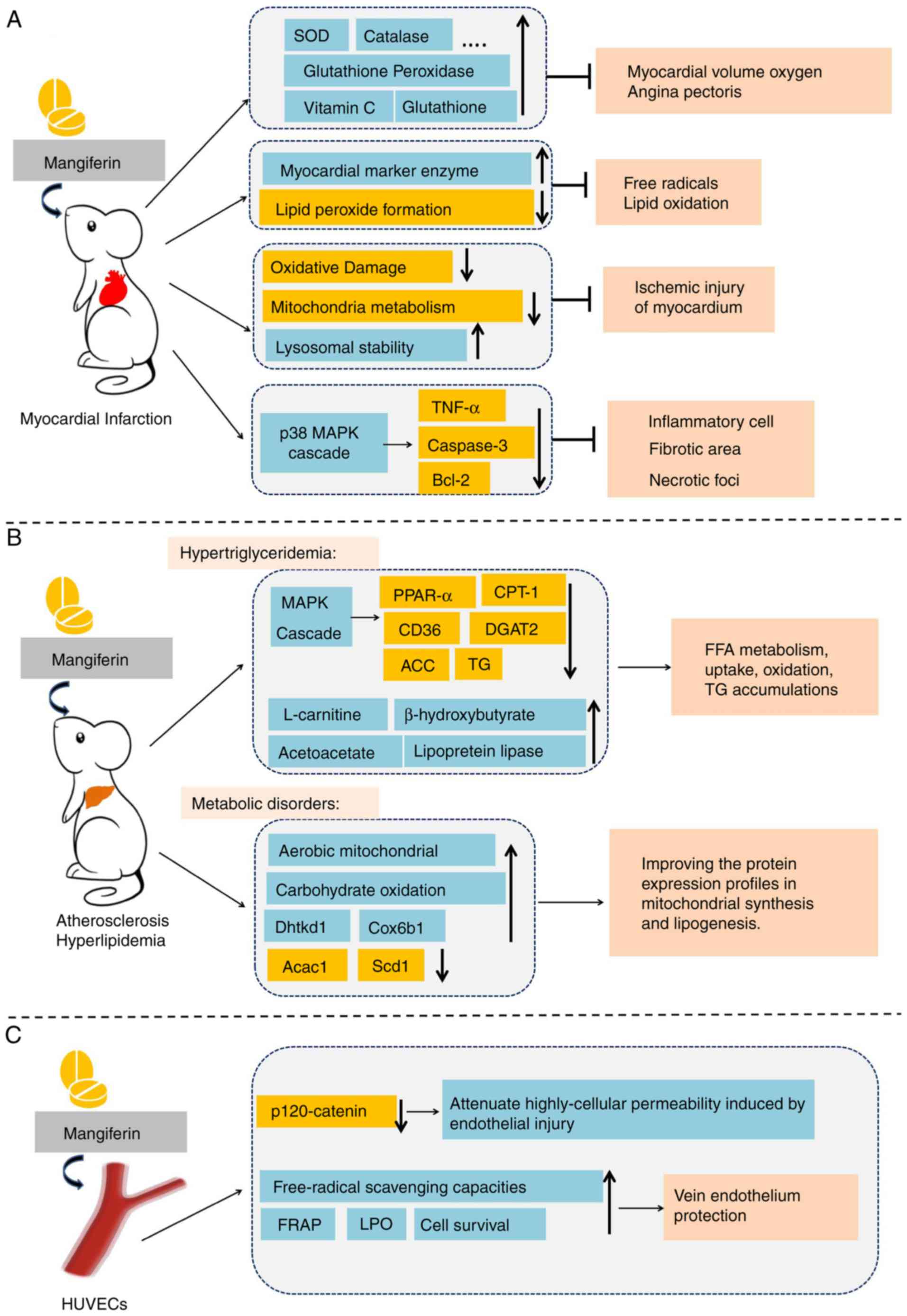 | Figure 5.Potential effects of mangiferin in
cardiovascular disease. (A) Mangiferin exhibited marked
cardioprotective effects in ISPH-induced myocardial infarction rat
models. (B) Mangiferin alleviated lipometabolic abnormalities in
the cardiovascular system. (C) Mangiferin protected vascular
endothelium function. SOD, TNF-α, Bcl-2, PPAR-α, peroxisome
proliferator-activated receptor-α; CPT-1, carnitine
palmitoyltransferase 1; DGAT2, diacylglycerol O-acyltransferase 2;
ACC, acetyl-CoA carboxylase; TG, triglyceride; FFA, free fatty
acid; Dhtkd1, dehydrogenase E1 and transketolase domain containing
1; Cox6b1, cytochrome C oxidase subunit 6B1; Acac1, acetyl-CoA
carboxylase 1; Scd1, stearoyl-CoA desaturase 1; FRAP, ferric
reducing ability of plasma; LPO, lipid peroxidation. |
Mangiferin in atherosclerosis and hyperlipemia.
Hyperlipidemia and elevated FFAs are risk factors for
atherosclerosis, hyperlipemia and cardiovascular disease (82). Therefore, research into the effects
of mangiferin on abnormal lipid metabolism in the cardiovascular
system is increasing (Fig. 5B).
Atherosclerosis is closely associated with abundant oxidative
events, including low-density lipoprotein (LDL) oxidation and
increased intracellular reactive ROS production. Mangiferin is a
polyphenol compound extracted from mango leaves and is the main
active ingredient of food supplement of the flood supplement
VIMANG, which is widely popular in Cuba (83). VIMANG is extracted from the bark of
Mangiferaindica and is typically administered as a tablet,
containing 300 mg of mango bark extract (83). Oral supplementation with VIMANG, or
its main polyphenol mangiferin, markedly reduces ROS generation in
isolated LDL receptor (−/−) liver mitochondria and spleen
lymphocytes (84). In addition,
treatment prevents mitochondrial nicotinamide-adenine dinucleotide
phosphate hydrate (NADPH)-linked substrate depletion and NADPH
spontaneous oxidation, making them suitable antioxidants with
potential use in atherosclerosis susceptible conditions (84).
Guo et al (85) discovered that mangiferin (50 and
150 mg/kg) ameliorates hypertriglyceridemia by modulating the
expression of genes involved in lipid oxidation and lipogenesis.
Body weight, liver weight, visceral fat-pad weight, serum TG, FFA
concentration, hepatic TG levels, hepatic FFA and muscle FFA
contents are immensely decreased following mangiferin treatment
(85). It was observed that
mangiferin upregulates the mRNA levels of peroxisome
proliferator-activated receptor-α, fatty acid translocase (CD36)
and carnitine palmitoyl transferase 1 (CPT-1) (86). Wistar rats were fed a high-fat diet
and administered mangiferin (50, 100, 150 mg/kg) simultaneously for
6 weeks, which confirmed that mangiferin improves FFA metabolism in
a dose-dependent manner through promoting FFA uptake and oxidation
(86). Similarly, it was also
observed in Wistar rats and HepG2 cells that levels of
intracellular FFA and TG accumulation was decreased in HepG2 cells.
Furthermore, the AMPK pathway is associated with this therapeutic
effect of mangiferin (86).
Mangiferin increases the AMP/ATP ratio upstream of AMPK, decreases
acyl-CoA:diacyl gycerol acyltransferase 2 (DGAT2) expression,
decreases acetyl-CoA carboxylase (ACC) activity. Furthermore,
mangiferin promotes AMPK phosphorylation, and upregulates the
expression of CD36 and CPT-1 (86). Based on the preliminary results of
cell and animal experiments, Na et al (87) conducted a 12-week double-blind
randomized clinical trial to evaluate the effects of mangiferin
(150 mg/day) on blood lipid profiles in overweight patients with
hyperlipidemia. Mangiferin supplementation substantially increases
the serum levels of mangiferin, high-density lipoprotein
cholesterol, L-carnitine, β-hydroxybutyrate and acetoacetate, and
increases lipoprotein lipase activity. However, mangiferin did not
effectively decrease serum levels of total cholesterol, low-density
lipoprotein cholesterol, serum glucose or insulin (87).
Apontes et al (88) administered mangiferin (400 mg/kg)
and a high fat diet (HFD) to C57BL6/J mice for 16 weeks,
demonstrating that mangiferin protects against HFD-induced weight
gain, promotes aerobic mitochondrial capacity and increases
thermogenesis. In addition, treatment with mangiferin in overweight
patients with hyperlipidemia stimulated carbohydrate oxidation and
improved glucose and insulin profiles (88). The activity of mangiferin on
lipogenesis regulation was further studied by proteomic analysis,
in which C57BL6/J mice were fed mixed granules containing
mangiferin and HFD for 18 weeks. It was demonstrated that out of
865 quantified proteins, 87 of them are differentially regulated by
mangiferin. Of these proteins, ~50% are involved in energy
metabolism and metabolite biosynthesis. Further classification
indicated that mangiferin increases the expression of proteins that
are crucial for mitochondrial biogenesis and oxidative activity,
including oxoglutarate dehydrogenase E1 and cytochrome c oxidase
subunit 6B1. In addition, mangiferin decreases the expression of
proteins that are critical for lipogenesis, including fatty acid
stearoyl-CoA desaturase 1 and acetyl-CoA carboxylase 1 (89). Taken together, these data suggest
that mangiferin may be used in the treatment of metabolic
disorders, by improving the protein expression profiles in
mitochondrial synthesis and lipogenesis.
Mangiferin in vein endothelium
The abnormal structure and function of vascular
endothelial cells are the pathological basis of many cardiovascular
diseases, and vascular endothelial cells are vulnerable to a series
of harmful factors (90). Under
the stimulation of high blood pressure, oxidative stress and high
levels of blood glucose/lipids, vascular endothelial cells
synthesize and release vasodilator factors and vasoconstrictor
factors, which have important roles in vascular homeostasis,
thrombosis and inflammation (91).
In recent decades, the protective effects of mangiferin on human
umbilical vein endothelial cells (HUVECs) have been extensively
demonstrated (Fig. 5C). It has
been demonstrated that mangiferin (20 µΜ) decreases high cellular
permeability and endothelial injury induced by paraquat
intoxication in HUVECs, through modulation of p120-catenin protein
(92). In addition, cell survival
increases in H2O2-treated HUVECs when
mangiferin (10 and 20 µΜ) is administered, through its free-radical
scavenging ability. A ferric reducing ability of plasma assay also
demonstrated it antioxidant capacity (93). Similarly, incubation of HUVECs with
glycated protein alone, or in combination with iron chelate,
resulted in increased lipid peroxidation (LPO), accompanied by
depletion of SOD, catalase, glutathione peroxidase and glutathione
reductase levels. These data suggest that mangiferin (5 and 10 µM)
has a protective effect against glycated protein-iron
chelate-induced toxicity (94),
which provides a promising perspective for the prevention of
oxidative stress and toxicant-associated disease.
Other properties of mangiferin
Mangiferin is used to prepare medicinal and food
supplements as a bioactive compound, where it may exert protective
effects against neurodegenerative disease, cancer, obesity,
cardiovascular disease and diabetes. However, mangiferin is also
associated with other miscellaneous properties.
Mangiferin in respiratory system
Mangiferin exerts protective effects against
bronchial asthma and other allergic diseases. Research has
demonstrated that mangiferin inhibits tracheal contractions induced
by distinct stimuli, including allergens, histamine,
5-hydroxytryptamine and carbachol, in a dose-dependent manner
(95). The antispasmodic effect of
mangiferin on allergic and non-allergic tracheal contraction of
guinea pig tracheal rings are a result of increased intracellular
levels of nitric oxide synthase 3 and cyclic GMP (95). In addition, in an allergic murine
experimental model, mice were orally treated with M. indica
extract (50, 100 or 250 mg/kg) or mangiferin (50 mg/kg) from day 0
to 24. The results revealed that mangiferin produces a remarkable
reduction in airway inflammation around vessels and bronchi,
decreases immunoglobulin (Ig)E levels and lymphocyte proliferation.
In addition, it was demonstrated that mangiferin inhibits IL-4 and
IL-5 cytokine production in bronchoalveolar lavage fluid and
lymphocyte culture supernatant (96). These experiments may be an
important part of pre-clinical research that is necessary for the
application of mangiferin in the treatment of respiratory diseases
(Fig. 6A).
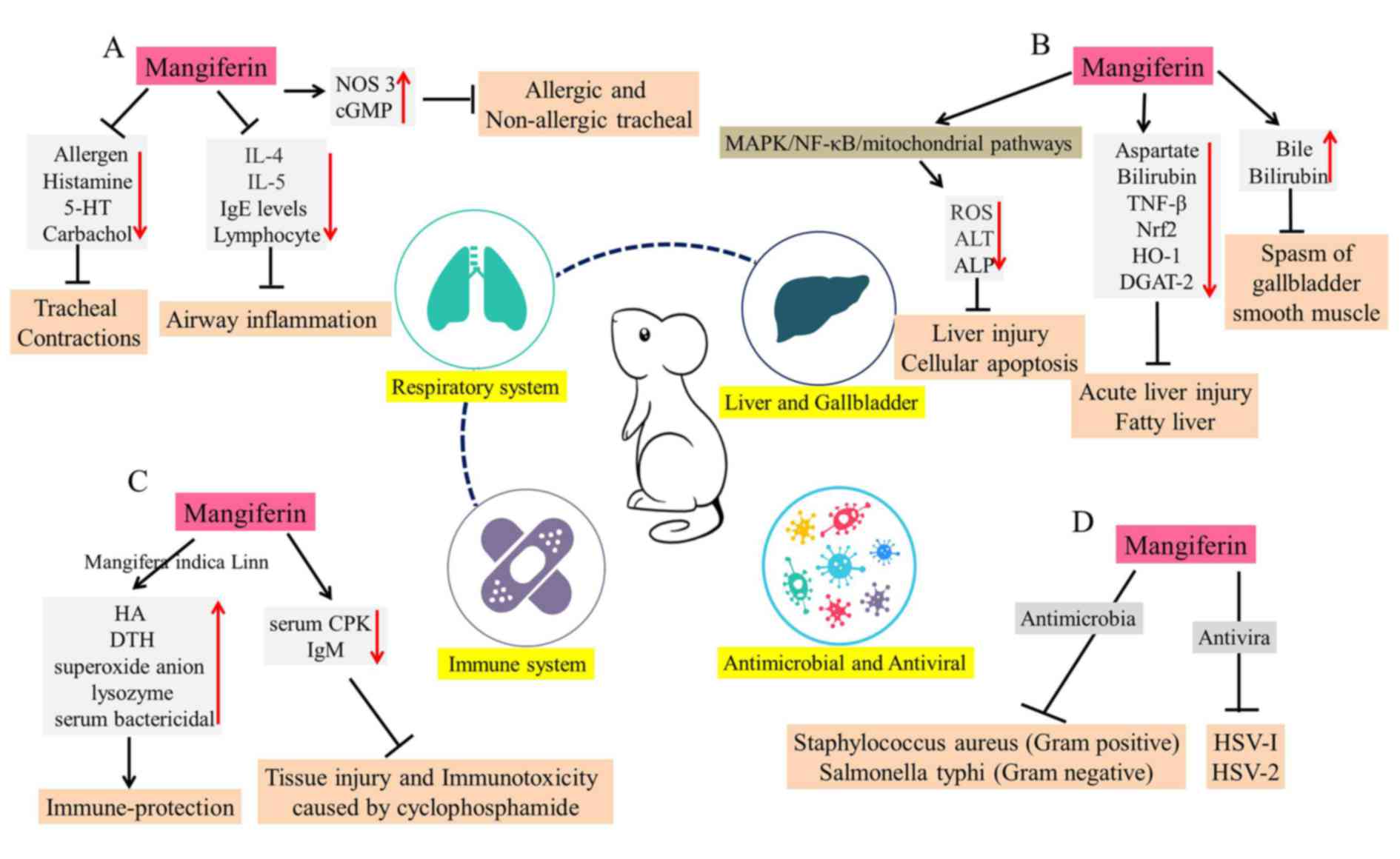 | Figure 6.Overview of the various actions of
mangiferin on (A) respiratory diseases, (B) liver and gallbladder
disorders, (C) immunological abnormalities and (D) pathogenic
microorganisms. 5-HT, 5-hydroxytryptamine; IL, interleukin; NOS 3,
nitric oxide synthase 3; cGMP, cyclic guanosine monophosphate;
MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB;
ROS, reactive oxygen species; ALT, alanine aminotransferase; ALP,
alkaline phosphatase; TNF-β, tumor necrosis factor-β; Nrf2, nuclear
factor, erythroid like 2; HO-1, heme oxygenase-1; DGAT-2,
diacylglycerol O-acyltransferase 2; HSV, herpes simplex virus; CPK,
creatine phosphokinase; HA, humoral antibody; DTH, delayed-type
hypersensitivity; Ig, immunoglobulin. |
Mangiferin in liver and gallbladder
disorders
Liver disease has become a major burden of human
health (97). Gentiopicroside and
mangiferin are regarded as two important medicinal monomers of
Swertia mussotii, a herb used in Tibetan folk medicine.
These may exert hepatoprotective and choleretic effects (Fig. 6B) (98,99).
Pal et al (100) investigated the molecular
mechanisms underlying the protective action of mangiferin against
lead-induced liver injury and cellular apoptosis (100). It was revealed that mangiferin
(100 mg/kg, orally for 6 days) inhibits ROS production and reduces
the levels of serum marker enzymes, such as ALT and ALP. Overall,
it was demonstrated that mangiferin exhibits both antioxidative and
antiapoptotic properties via MAPK/NF-κB/mitochondria-dependent
pathways (100). Compared with
silymarin, a standard hepatoprotective drug, the intraperitoneal
pretreatment of mangiferin (30 mg/kg) possesses an extensive
protective effect via reduction of serum aspartate and alanine
aminotransferases, alkaline phosphatase, bilirubin and inflammatory
mediator TNF-β (101). These
results suggest that mangiferin exhibits potent hepatoprotective
effects on CCl-4-induced liver damages in mice (101). In addition, in LPS and
D-galactosamine (D-GalN)-induced acute liver injury, mangiferin
upregulates the expression of Nrf2 and HO-1 in a dose-dependent
manner. Furthermore, mangiferin markedly inhibits
LPS/D-GalN-induced inflammatory factors, including IL-1β, TNF-α,
NLRP3, apoptosis-associated speck-like protein containing a CARD
and caspase-1 (102). Mangiferin
treatment ameliorates fatty liver in fructose-fed spontaneously
hypertensive rats (SHR) by inhibiting hepatic DGAT2, which
catalyzes the final step of triglyceride biosynthesis (103). Mangiferin may enhance de
novo fatty acid synthesis and oxidation in the treatment of
fatty liver. Furthermore, the experiment of bile duct drainage in
rats also demonstrated that mangiferin (20 mg/kg) markedly
increases bile secretion and bilirubin content. In addition,
gallbladder smooth muscle spasms are inhibited at a dose of 10
mg/kg (104).
Mangiferin and the immune system
The role of mangiferin in the immune system has
gained increasing attention (Fig.
6C). Mangiferaindica (extract containing 2.6%
mangiferin) has been investigated for its immunoprotective effects,
via increasing the frequency of delayed type hypersensitivity (DTH)
reactions and the humoral antibody (HA) titer in mice (105). Similarly, mangiferin stimulates
the immune systems and increases the resistance of Labeo
rohita to Aeromonas hydrophila infection, by promoting
superoxide anion, serum protein obtained from Labeo rohita
and albumin production, as well as lysozyme and serum bactericidal
activity (106). In order to
compare the immunoprotective effects of mangiferin (the major
polyphenol of Vimang) and VIMANG (an aqueous extract of Mangifera
indica) on mouse antibody responses induced by inoculation with
spores of microsporidian parasites, it was suggested that the
components of Mangifera indica extracts may be of potential
value for modulating the humoral response in different
immunopathological disorders (107). However, VIMANG contains other
extracts in addition to mangiferin polyphenol; therefore, the
researchers may have neglected to take into account the inhibitory
effects of other compounds isolated from VIMANG on the humoral
response (107). In addition,
mangiferin markedly suppresses tissue injury and immunotoxicity
caused by cyclophosphamide treatment, through decreasing serum CPK
activity and antigen-specific IgM levels (108). Thus, it was evident that
mangiferin exerts an immunoprotective role via inhibition of
reactive intermediate-induced oxidative stress in lymphocytes,
neutrophils and macrophages (108). These data suggest that mangiferin
has the potential to reduce the immunotoxicity of cyclophosphamide
in oncotherapy.
The antimicrobial and antiviral
effects of mangiferin
Anemarrhena asphodeloides, a plant widely
used in traditional Chinese medicine, has been reported to possess
antiviral and antibacterial activities (109). Mangiferin is the main effective
component of Anemarrhena asphodeloides (109). In addition to its antioxidative,
antidiabetic and antitumor properties, the antibacterial and
antiviral effects of mangiferin are also prominent (110). Mangiferin has been demonstrated
to exert antibacterial activity against two bacterial species:
Staphylococcus aureus (Gram positive) and Salmonella
typhi (Gram negative) (111).
Through tissue culture techniques, the antiviral effects of
mangiferin and isomangiferin on herpes simplex virus-1 (HSV-1) were
demonstrated, with average plaque reduction rates of 56.8 and
69.5%, respectively (112). The
EC50 of mangiferin against herpes simplex virus-2 (HSV-2) plaque
formation in HeLa cells was 111.7 mg, and the therapeutic index
(IC50/EC50) was 8.1 (113). Oral
mangiferin (50 mg/kg) suppresses the growth of nematode
Trichinella spiralis throughout the parasitic life cycle, by
inhibiting mast cell degranulation, decreasing the serum levels of
specific anti-Trichinella IgE and reducing the number of
parasitic larvae (114). These
studies confirm the antibacterial effects of isolated mangiferin,
which may further processed as an antibacterial agent (Fig. 6D).
The main barrier for xenobiotic absorption through
the skin is the stratum corneum. Research has revealed the ability
of mangiferin to reversibly inhibit elastase and collagenase
activity, as well as to permeate the stratum corneum and pass into
the epidermis and dermis (115).
As the fat and water distribution coefficient of mangiferin is
relatively high, oral absorption is low (9), which suggests that mangiferin may be
better absorbed through the skin.
Conclusions
Mangiferin has been demonstrated to possess several
beneficial properties, including antioxidant, antimicrobial,
antidiabetic, antiallergic, neuroprotective, cardiovascular
protective, anticancer, hypocholesterolemic and immunomodulatory
effects. Although it has been regarded as a compound with extensive
pharmacological activity, the pharmacodynamics of mangiferin remain
unclear. Mangiferin appears to have diverse pharmacological
effects. However, further clinical research into the pharmacology
and pharmacokinetics of mangiferin is required, as most of these
effects have only been demonstrated in in vivo and in
vitro experiments. The administration of mangiferin to animals
or humans will inevitably result in alterations in the body, at the
molecular, cellular, tissue and/or organs level, along with its
curative effects. Therefore, genomic, proteomic and metabolomic
analysis should be the main strategies utilized in order to
determine the pharmacodynamics of mangiferin.
The research and development of mangiferin is
expected to provide novel drugs for disease treatment. However,
among the numerous studies on the pharmacology of mangiferin,
researchers did not use a standardized therapeutic approach.
Specifically, the pharmacodynamic indexes of mangiferin in existing
researches, including dose, concentration and IC50, were not
compared and analyzed at the same base line; therefore, the
experimental results are not universal. Thus, the unified and
standardized evaluation of the efficacy of mangiferin is a central
component in the further exploration of pharmacodynamics and
quality assurance. Furthermore, in the clinical research of
mangiferin, researchers should focus on pharmacodynamic parameters
caused by its physicochemical properties, including
bioavailability, half-life, adverse reactions and toxicity. In
addition, further clinical trials should be implemented to allow
the broad application of this effective bioactive compound.
Acknowledgements
Not applicable.
Funding
This review was supported by the National Science
Foundation of China (grant no. 81802504), the Sichuan National
Science Research Funding (grant no. 2018JY0645) and Sichuan Health
and Family Planning Commission Funding (grant no. 16ZD0253), and
Dr. Yi Wang received funding from the Sichuan Provincial People's
Hospital and a Sichuan Scientific Research Grant for Returned
Overseas Chinese Scholars (grant no. 30305031014). The study was
also supported by the National Key Specialty Construction Project
of Clinical Pharmacy (grant no. 30305030698).
Availability of data and materials
Not applicable.
Authors' contributions
SD and HL wrote the manuscript. TL prepared the
figures. XX, HW and XH searched for the relevant literature. RT and
YW reviewed and edited the manuscript. All authors have read and
approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dar A, Faizi S, Naqvi S, Roome T,
Zikr-ur-Rehman S, Ali M, Firdous S and Moin ST: Analgesic and
antioxidant activity of mangiferin and its derivatives: The
structure activity relationship. Biol Pharm Bull. 28:596–600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duang XY, Wang Q, Zhou XD and Huang DM:
Mangiferin: A possible strategy for periodontal disease to therapy.
Med Hypotheses. 76:486–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guha S, Ghosal S and Chattopadhyay U:
Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a
naturally occurring glucosylxanthone. Chemotherapy. 42:443–451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ajila CM, Rao LJ and Rao UJ:
Characterization of bioactive compounds from raw and ripe Mangifera
indica L. peel extracts. Food Chem Toxicol. 48:3406–3411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iseda S: On mangiferin, the coloring
matter of mango (Mangifera indica Linn). V. Identification of sugar
component and the structure of mangiferin. Bull Chem Soc Jpn.
30:629–633. 1957. View Article : Google Scholar
|
|
6
|
Wang Z, Deng J, Wang Q, Li X and Wei H:
Improvement in the solubility of mangiferin by HP-β-CD inclusion.
Chin Tradit Patent Med. 2008.
|
|
7
|
Han D, Chen C, Cong Z, Yu Z and Xing T:
Determination of mangiferin in rat plasma by liquid-liquid
extraction with UPLC-MS/MS. J Pharm Biomed Anal. 51:260–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu R, Liu Z, Zhang C and Zhang B:
Gelucire44/14 as a novel absorption enhancer for drugs with
different hydrophilicities: In vitro and in vivo improvement on
transcorneal permeation. J Pharm Sci. 100:3186–3196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma H, Chen H, Sun L, Tong L and Zhang T:
Improving permeability and oral absorption of mangiferin by
phospholipid complexation. Fitoterapia. 93:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saleh F, Mumu SJ, Ara F, Hafez MA and Ali
L: Non-adherence to self-care practices & medication and health
related quality of life among patients with type 2 diabetes: A
cross-sectional study. BMC Public Health. 14:4312014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kueh YC, Morris T and Ismail AA: The
effect of diabetes knowledge and attitudes on self-management and
quality of life among people with type 2 diabetes. Psychol Health
Med. 22:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogurtsova K, da Rocha Fernandes JD, Huang
Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE and
Makaroff LE: IDF diabetes atlas: Global estimates for the
prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract.
128:40–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barker A, Langenberg C and Wareham NJ:
Genetic determinants of glucose homeostasis. Best Pract Res Clin
Endocrinol Metab. 26:159–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Göke B: Islet cell function: Alpha and
beta cells-partners towards normoglycaemia. Int J Clin Pract Suppl.
62:2–7. 2008. View Article : Google Scholar
|
|
17
|
Cusi K: The role of adipose tissue and
lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep.
10:306–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scheen AJ: Pathophysiology of type 2
diabetes. Acta Clin Belg. 58:335–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Avogaro A, Giorda C, Maggini M, Mannucci
E, Raschetti R, Lombardo F, Spila-Alegiani S, Turco S, Velussi M
and Ferrannini E: Diabetes and Informatics Study Group, Association
of Clinical Diabetologists, Istituto Superiore di Sanità: Incidence
of coronary heart disease in type 2 diabetic men and women: Impact
of microvascular complications, treatment, and geographic location.
Diabetes Care. 30:1241–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connelly K, Kelly D and Gilbert R:
Clinically relevant models of diabetic cardiac complications. Circ
Res. 101:e782007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Mattia G, Bravi MC, Laurenti O, Moretti
A, Cipriani R, Gatti A, Mandosi E and Morano S: Endothelial
dysfunction and oxidative stress in type 1 and type 2 diabetic
patients without clinical macrovascular complications. Diabetes Res
Clin Pract. 79:337–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Happich M, Breitscheidel L, Meisinger C,
Ulbig M, Falkenstein P, Benter U and Watkins J: Cross-sectional
analysis of adult diabetes type 1 and type 2 patients with diabetic
microvascular complications from a German retrospective
observational study. Curr Med Res Opin. 23:1367–1374. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen A and Horton ES: Progress in the
treatment of type 2 diabetes: New pharmacologic approaches to
improve glycemic control. Curr Med Res Opin. 23:905–917. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carpino PA and Goodwin B: Diabetes area
participation analysis: A review of companies and targets described
in the 2008–2008 patent literature. Expert Opin Ther Pat.
20:1627–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sellamuthu PS, Arulselvan P, Fakurazi S
and Kandasamy M: Beneficial effects of mangiferin isolated from
Salacia chinensis on biochemical and hematological parameters in
rats with streptozotocin-induced diabetes. Pak J Pharm Sci.
27:161–167. 2014.PubMed/NCBI
|
|
26
|
Sellamuthu PS, Muniappan BP, Perumal SM
and Kandasamy M: Antihyperglycemic effect of mangiferin in
streptozotocin induced diabetic rats. J Health Sci. 55:206–214.
2009. View Article : Google Scholar
|
|
27
|
Saleh S, El-Maraghy N, Reda E and Barakat
W: Modulation of diabetes and dyslipidemia in diabetic
insulin-resistant rats by mangiferin: Role of adiponectin and
TNF-α. An Acad Bras Cienc. 86:1935–1948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muruganandan S, Srinivasan K, Gupta S,
Gupta PK and Lal J: Effect of mangiferin on hyperglycemia and
atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol.
97:497–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miura T, Iwamoto N, Kato M, Ichiki H, Kubo
M, Komatsu Y, Ishida T, Okada M and Tanigawa K: The suppressive
effect of mangiferin with exercise on blood lipids in type 2
diabetes. Biol Pharm Bull. 24:1091–1092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miura T, Ichiki H, Hashimoto I, Iwamoto N,
Kato M, Kubo M, Ishihara E, Komatsu Y, Okada M, Ishida T and
Tanigawa K: Antidiabetic activity of a xanthone compound,
mangiferin. Phytomedicine. 8:85–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar SR, Ray MI, Ujwala M, Borde MK and
Deshmukh YA: Natural dipeptidyl peptidase-IV inhibitor mangiferin
mitigates diabetes- and metabolic syndrome-induced changes in
experimental rats. Diabetes Metab Syndr Obes. 9:261–272. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang HL, Li CY, Zhang B, Liu YD, Lu BM,
Shi Z, An N, Zhao LK, Zhang JJ, Bao JK and Wang Y: Mangiferin
facilitates islet regeneration and β-cell proliferation through
upregulation of cell cycle and β-cell regeneration regulators. Int
J Mol Sci. 15:9016–9035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pal PB, Sinha K and Sil PC: Mangiferin
attenuates diabetic nephropathy by inhibiting oxidative stress
mediated signaling cascade, TNFα related and mitochondrial
dependent apoptotic pathways in streptozotocin-induced diabetic
rats. PLoS One. 9:e1072202014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu YW, Zhu X, Zhang L, Lu Q, Wang JY,
Zhang F, Guo H, Yin JL and Yin XX: Up-regulation of glyoxalase 1 by
mangiferin prevents diabetic nephropathy progression in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 721:355–364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu X, Cheng YQ, Du L, Li Y, Zhang F, Guo
H, Liu YW and Yin XX: Mangiferin attenuates renal fibrosis through
down-regulation of osteopontin in diabetic rats. Phytother Res.
29:295–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gaede P, Vedel P, Larsen N, Jensen GV,
Parving HH and Pedersen O: Multifactorial intervention and
cardiovascular disease in patients with type 2 diabetes. N Engl J
Med. 348:383–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kannel WB and McGee DL: Diabetes and
cardiovascular disease: The Framingham Study. JAMA. 241:2035–2038.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manson JE, Colditz GA, Stampfer MJ,
Willett WC, Krolewski AS, Rosner B, Arky RA, Speizer FE and
Hennekens CH: A prospective study of maturity-onset diabetes
mellitus and risk of coronary heart disease and stroke in women.
Arch Intern Med. 151:1141–1147. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramasamy R, Yan SF and Schmidt AM:
Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis
of diabetes and its complications. Ann N Y Acad Sci. 1243:88–102.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suchal K, Malik S, Khan SI, Malhotra RK,
Goyal SN, Bhatia J, Kumari S, Ojha S and Arya DS: Protective effect
of mangiferin on myocardial ischemia-reperfusion injury in
streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK
pathways. Sci Rep. 7:420272017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hou J, Zheng D, Fung G, Deng H, Chen L,
Liang J, Jiang Y and Hu Y: Mangiferin suppressed advanced glycation
end products (AGEs) through NF-κB deactivation and displayed
anti-inflammatory effects in streptozotocin and high fat
diet-diabetic cardiomyopathy rats. Can J Physiol Pharmacol.
94:332–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hou J, Zheng D, Zhong G and Hu Y:
Mangiferin mitigates diabetic cardiomyopathy in
streptozotocin-diabetic rats. Can J Physiol Pharmacol. 91:759–763.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Basha B, Samuel SM, Triggle CR and Ding H:
Endothelial dysfunction in diabetes mellitus: possible involvement
of endoplasmic reticulum stress? Exp Diabetes Res. 2012:4818402012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Song J, Li J, Hou F, Wang X and Liu B:
Mangiferin inhibits endoplasmic reticulum stress-associated
thioredoxin-interacting protein/NLRP3 inflammasome activation with
regulation of AMPK in endothelial cells. Metabolism. 64:428–437.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barascu A, Besson P, Le Floch O, Bougnoux
P and Jourdan ML: CDK1-cyclin B1 mediates the inhibition of
proliferation induced by omega-3 fatty acids in MDA-MB-231 breast
cancer cells. Int J Biochem Cell Biol. 38:196–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyazaki T and Arai S: Two distinct
controls of mitotic cdk1/cyclin B1 activity requisite for cell
growth prior to cell division. Cell Cycle. 6:1419–1425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Porter LA and Donoghue DJ: Cyclin B1 and
CDK1: Nuclear localization and upstream regulators. Prog Cell Cycle
Res. 5:335–347. 2003.PubMed/NCBI
|
|
49
|
Hu X and Moscinski LC: Cdc2: A monopotent
or pluripotent CDK? Cell Prolif. 44:205–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng ZG, Yao YB, Yang J, Tang YL and Huang
X: Mangiferin induces cell cycle arrest at G2/M phase through
ATR-Chk1 pathway in HL-60 leukemia cells. Genet Mol Res.
14:4989–5002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yao YB, Peng ZG, Liu ZF, Yang J and Luo J:
Effects of mangiferin on cell cycle status and CDC2/Cyclin B1
expression of HL-60 cells. Zhong Yao Cai. 33:81–85. 2010.(In
Chinese). PubMed/NCBI
|
|
52
|
du Plessis-Stoman D, du Preez J and van de
Venter M: Combination treatment with oxaliplatin and mangiferin
causes increased apoptosis and downregulation of NFκB in cancer
cell lines. Afr J Tradit Complement Altern Med. 8:177–184.
2011.PubMed/NCBI
|
|
53
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Archiv. 446:475–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of Mangifera indica L. bark in MDA-MB231 breast cancer
cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Telang M, Dhulap S, Mandhare A and Hirwani
R: Therapeutic and cosmetic applications of mangiferin: A patent
review. Expert Opin Ther Pat. 23:1561–1580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pomerantz JL and Baltimore D: Two pathways
to NF-kappaB. Mol Cell. 10:693–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kawasaki H, Toyoda M, Shinohara H, Okuda
J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T and Tanigawa N:
Expression of survivin correlates with apoptosis, proliferation,
and angiogenesis during human colorectal tumorigenesis. Cancer.
91:2026–2032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim H, Kim H, Mosaddik A, Gyawali R, Ahn
KS and Cho SK: Induction of apoptosis by ethanolic extract of mango
peel and comparative analysis of the chemical constitutes of mango
peel and flesh. Food Chem. 133:416–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lv J, Wang Z, Zhang L, Wang HL, Liu Y, Li
C, Deng J, Wang Y and Bao JK: Mangiferin induces apoptosis and cell
cycle arrest in MCF-7 cells both in vitro and in vivo. J Anim Vet
Adv. 12:352–359. 2013.
|
|
63
|
Zou B, Wang H, Liu Y, Qi P, Lei T, Sun M
and Wang Y: Mangiferin induces apoptosis in human ovarian
adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol
Rep. 38:1431–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Saha S, Sadhukhan P and Sil PC:
Mangiferin: A xanthonoid with multipotent anti-inflammatory
potential. Biofactors. 42:459–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bera S, Chaudhuri S and Dutta D:
Assessment of free-radical scavenging activities of mangiferin from
Curcuma amada obtained by non-conventional extraction methods: A
comparative study. Indian J Biotechnol. 14:179–185. 2015.
|
|
66
|
Abdul-Muneer PM, Chandra N and Haorah J:
Interactions of oxidative stress and neurovascular inflammation in
the pathogenesis of traumatic brain injury. Mol Neurobiol.
51:966–979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fujiwara N, Som AT, Pham LD, Lee BJ,
Mandeville ET, Lo EH and Arai K: A free radical scavenger edaravone
suppresses systemic inflammatory responses in a rat transient focal
ischemia model. Neurosci Lett. 633:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ren Y, Wei B, Song X, An N, Zhou Y, Jin X
and Zhang Y: Edaravone's free radical scavenging mechanisms of
neuroprotection against cerebral ischemia: Review of the
literature. Int J Neurosci. 125:555–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lewén A, Matz P and Chan PH: Free radical
pathways in CNS injury. J Neurotrauma. 17:871–890. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fu Y, Liu H, Song C, Zhang F, Liu Y, Wu J,
Wen X, Liang C, Ma K, Li L, et al: Mangiferin regulates cognitive
deficits and heme oxygenase-1 induced by lipopolysaccharide in
mice. Int Immunopharmacol. 29:950–956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jangra A, Lukhi MM, Sulakhiya K, Baruah CC
and Lahkar M: Protective effect of mangiferin against
lipopolysaccharide-induced depressive and anxiety-like behaviour in
mice. Eur J Pharmacol. 740:337–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bhatia HS, Candelario-Jalil E, de Oliveira
AC, Olajide OA, Martínez-Sánchez G and Fiebich BL: Mangiferin
inhibits cyclooxygenase-2 expression and prostaglandin E 2
production in activated rat microglial cells. Arch Biochem Biophys.
477:253–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Márquez L, García-Bueno B, Madrigal JL and
Leza JC: Mangiferin decreases inflammation and oxidative damage in
rat brain after stress. Eur J Nutr. 51:729–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang Z, Guo S, Wang J, Shen Y, Zhang J and
Wu Q: Nrf2/HO-1 mediates the neuroprotective effect of mangiferin
on early brain injury after subarachnoid hemorrhage by attenuating
mitochondria-related apoptosis and neuroinflammation. Sci Rep.
7:118832017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang Z, Weian C, Susu H and Hanmin W:
Protective effects of mangiferin on cerebral ischemia-reperfusion
injury and its mechanisms. Eur J Pharmacol. 771:145–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Prabhu S, Jainu M, Sabitha KE and Devi CS:
Role of mangiferin on biochemical alterations and antioxidant
status in isoproterenol-induced myocardial infarction in rats. J
Ethnopharmacol. 107:126–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Prabhu S, Jainu M, Sabitha KE and Devi CS:
Cardioprotective effect of mangiferin on isoproterenol induced
myocardial infarction in rats. Indian J Exp Biol. 44:209–215.
2006.PubMed/NCBI
|
|
78
|
Prabhu S, Jainu M, Sabitha KE and Devi
Shyamala CS: Effect of mangiferin on mitochondrial energy
production in experimentally induced myocardial infarcted rats.
Vascul Pharmacol. 44:519–525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Prabhu S, Narayan S and Devi C: Mechanism
of protective action of mangiferin on suppression of inflammatory
response and lysosomal instability in rat model of myocardial
infarction. Phytother Res. 23:756–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zheng D, Hou J, Xiao Y, Zhao Z and Chen L:
Cardioprotective effect of mangiferin on left ventricular
remodeling in rats. Pharmacology. 90:78–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Arozal W, Suyatna FD, Juniantito V,
Rosdiana DS, Amurugam S, Aulia R, Monayo ER and Siswandi R: The
effects of mangiferin (Mangifera indica L) in doxorubicin-induced
cardiotoxicity in rats. Drug Res (Stuttg). 65:574–580. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nordestgaard BG and Varbo A: Triglycerides
and cardiovascular disease. Lancet. 384:626–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Alberto Nú?ez-Sellés J: Antioxidant
therapy: myth or reality? J Braz Chem Soc. 16:699–710. 2005.
View Article : Google Scholar
|
|
84
|
Pardo-Andreu GL, Paim BA, Castilho RF,
Velho JA, Delgado R, Vercesi AE and Oliveira HC: Mangifera indica
L: extract (Vimang) and its main polyphenol mangiferin prevent
mitochondrial oxidative stress in atherosclerosis-prone
hypercholesterolemic mouse. Pharmacol Res. 57:332–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Guo F, Huang C, Liao X, Wang Y, He Y, Feng
R, Li Y and Sun C: Beneficial effects of mangiferin on
hyperlipidemia in high-fat-fed hamsters. Mol Nutr Food Res.
55:1809–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Niu Y, Li S, Na L, Feng R, Liu L, Ying L
and Sun C: Mangiferin decreases plasma free fatty acids through
promoting its catabolism in liver by activation of AMPK. PLoS One.
7:e307822012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Na L, Zhang Q, Jiang S, Du S, Zhang W, Li
Y, Sun C and Niu Y: Mangiferin supplementation improves serum lipid
profiles in overweight patients with hyperlipidemia: A double-blind
randomized controlled trial. Sci Rep. 5:103442015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Apontes P, Liu Z, Su K, Benard O, Youn DY,
Li X, Li W, Mirza RH, Bastie CC, Jelicks LA, et al: Mangiferin
stimulates carbohydrate oxidation and protects against metabolic
disorders induced by high-fat diets. Diabetes. 63:3626–3636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lim J, Liu Z, Apontes P, Feng D, Pessin
JE, Sauve AA, Angeletti RH and Chi Y: Dual mode action of
mangiferin in mouse liver under high fat diet. PLoS One.
9:e901372014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Takagi A and Tada Y: Medical applicability
of cultured vascular endothelial cells in cardiovascular surgery.
Jpn J Cardiovasc Surg. 19:45–52. 1989. View Article : Google Scholar
|
|
91
|
Lahera V, Navarro-Cid J, Maeso R and
Cachofeiro V: Participation of endothelium-derived vasoconstrictor
factors in arterial hypertension. Rev Esp Cardiol. 52 Suppl
3:S4–S11. 1999.(In Spanish).
|
|
92
|
He Q, Ai J and Huang Y: Relationship
between endothelial damage and p120-catenin in paraquat
intoxication and the protective effect of mangiferin. Zhonghua Wei
Zhong Bing Ji Jiu Yi Xue. 26:369–373. 2014.(In Chinese). PubMed/NCBI
|
|
93
|
Luo F, Lv Q, Zhao Y, Hu G, Huang G, Zhang
J, Sun C, Li X and Chen K: Quantification and purification of
mangiferin from Chinese Mango (Mangifera indica L.) cultivars and
its protective effect on human umbilical vein endothelial cells
under H(2)O(2)-induced stress. Int J Mol Sci. 13:11260–11274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Venugopal R, Sakthisekaran D, Rajkapoor B
and Nishigaki I: In vitro protective effect of mangiferin against
glycated protein-iron chelate induced toxicity in human umbilical
vein endothelial cells. J Biol Sci. 7:1227–1232. 2007. View Article : Google Scholar
|
|
95
|
Vieira AB, Coelho LP, Insuela DB, Carvalho
VF, dos Santos MH, Silva PM and Martins MA: Mangiferin prevents
guinea pig tracheal contraction via activation of the nitric
oxide-cyclic GMP pathway. Plos One. 8:e717592013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Rivera DG, Hernández I, Merino N, Luque Y,
Álvarez A, Martín Y, Amador A, Nuevas L and Delgado R: Mangifera
indica L. extract (Vimang) and mangiferin reduce the airway
inflammation and Th2 cytokines in murine model of allergic asthma.
J Pharm Pharmacol. 63:1336–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cohen JC, Horton JD and Hobbs HH: Human
fatty liver disease. Old questions and new insights. Science.
332:1519–1523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Guo Y and Xia C: Research progress of
pharmacological action and clinical application in tibetan medicine
‘Zangyinchen’. Asia Pac Tradit Med. 2016.
|
|
99
|
Tan L, Feng-Zu HU and Dong Q: Simultaneous
determination of three iridoid glycosides and three flavonoids in
Swertia mussotii Franch. from Qinghai by UPLC. Chin J Pharm Anal.
2017.
|
|
100
|
Pal PB, Sinha K and Sil PC: Mangiferin, a
natural xanthone, protects murine liver in Pb(II) induced hepatic
damage and cell death via MAP kinase, NF-κB and mitochondria
dependent pathways. PLoS One. 8:e568942013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rasool M, Sabina EP, Mahinda PS and
Gnanaselvi BC: Mangiferin, a natural polyphenol protects the
hepatic damage in mice caused by CCl 4 intoxication. Comp Clin
Pathol. 21:865–872. 2012. View Article : Google Scholar
|
|
102
|
Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY,
Lin W, Jin LX and Xu CL: Mangiferin alleviates lipopolysaccharide
and D-galactosamine-induced acute liver injury by activating the
Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J
Pharmacol. 770:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xing X, Li D, Chen D, Zhou L, Chonan R,
Yamahara J, Wang J and Li Y: Mangiferin treatment inhibits hepatic
expression of acyl-coenzyme A: Diacylglycerol acyltransferase-2 in
fructose-fed spontaneously hypertensive rats: A link to
amelioration of fatty liver. Toxicol Appl Pharmacol. 280:207–215.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
He F, Zeng X, Wei G, Wen Y, Su H, Wei B,
et al: Choleretic action and spasmolysis of gallbladder smooth
muscle of mangiferin. China Pharmacist. 2014.
|
|
105
|
Makare N, Bodhankar S and Rangari V:
Immunomodulatory activity of alcoholic extract of Mangifera indica
L. in mice. J Ethnopharmacol. 78:133–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sahu S, Das BK, Pradhan J, Mohapatra BC,
Mishra BK and Sarangi N: Effect of Magnifera indica kernel as a
feed additive on immunity and resistance to Aeromonas hydrophila in
Labeo rohita fingerlings. Fish Shellfish Immunol. 23:109–118. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
García D, Leiro J, Delgado R, Sanmartín ML
and Ubeira FM: Mangifera indica L. extract (Vimang) and mangiferin
modulate mouse humoral immune responses. Phytother Res.
17:1182–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Muruganandan S, Lal J and Gupta PK:
Immunotherapeutic effects of mangiferin mediated by the inhibition
of oxidative stress to activated lymphocytes, neutrophils and
macrophages. Toxicology. 215:57–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Miura T, Ichiki H, Iwamoto N, Kato M, Kubo
M, Sasaki H, Okada M, Ishida T, Seino Y and Tanigawa K:
Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides
and active components, mangiferin and its glucoside. Biol Pharm
Bull. 24:1009–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim CY, Ahn MJ and Kim J: Preparative
isolation of mangiferin from anemarrhena asphodeloides rhizomes by
centrifugal partition chromatography. J Liq Chromatogr Relat
Technol. 29:869–875. 2006. View Article : Google Scholar
|
|
111
|
Maji HS, Maji S, Roy R, Sen A and Biswas
T: Isolation of mangiferin from flowering buds of mangifera indica
l and its evaluation of in vitro antibacterial activity. JPA.
4:49–56. 2015.
|
|
112
|
Zheng MS and Lu ZY: Antiviral effect of
mangiferin and isomangiferin on herpes simplex virus. Chin Med J
(Engl). 103:160–165. 1990.PubMed/NCBI
|
|
113
|
Zhu XM, Song JX, Huang ZZ, Wu YM and Yu
MJ: Antiviral activity of mangiferin against herpes simplex virus
type 2 in vitro. Zhongguo Yao Li Xue Bao. 14:452–454. 1993.(In
Chinese). PubMed/NCBI
|
|
114
|
García D, Escalante M, Delgado R, Ubeira
FM and Leiro J: Anthelminthic and antiallergic activities of
Mangifera indica L. stem bark components Vimang and mangiferin.
Phytother Res. 17:1203–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ochocka R, Hering A, Stefanowicz-Hajduk J,
Cal K and Barańska H: The effect of mangiferin on skin:
Penetration, permeation and inhibition of ECM enzymes. PLoS One.
12:e01815422017. View Article : Google Scholar : PubMed/NCBI
|