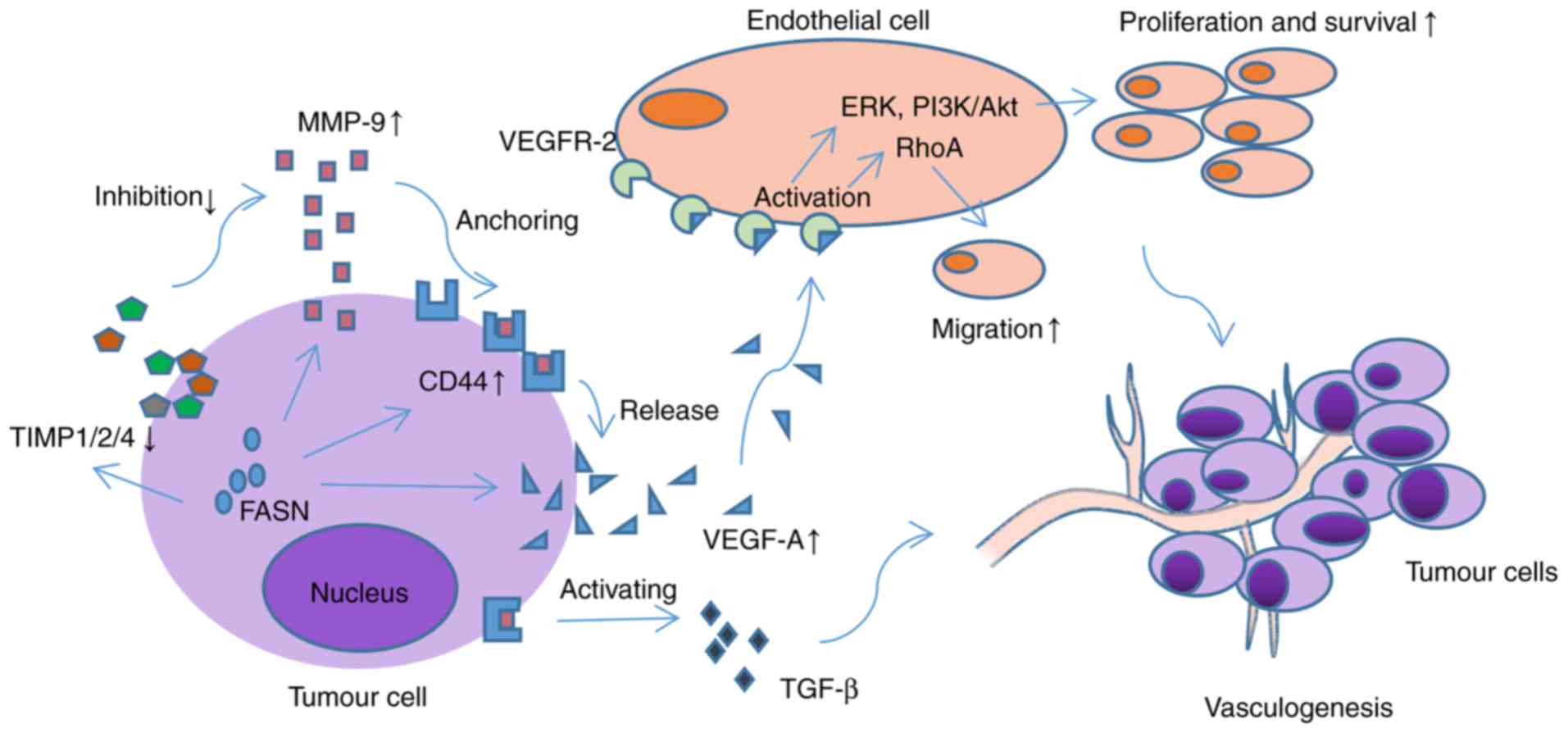
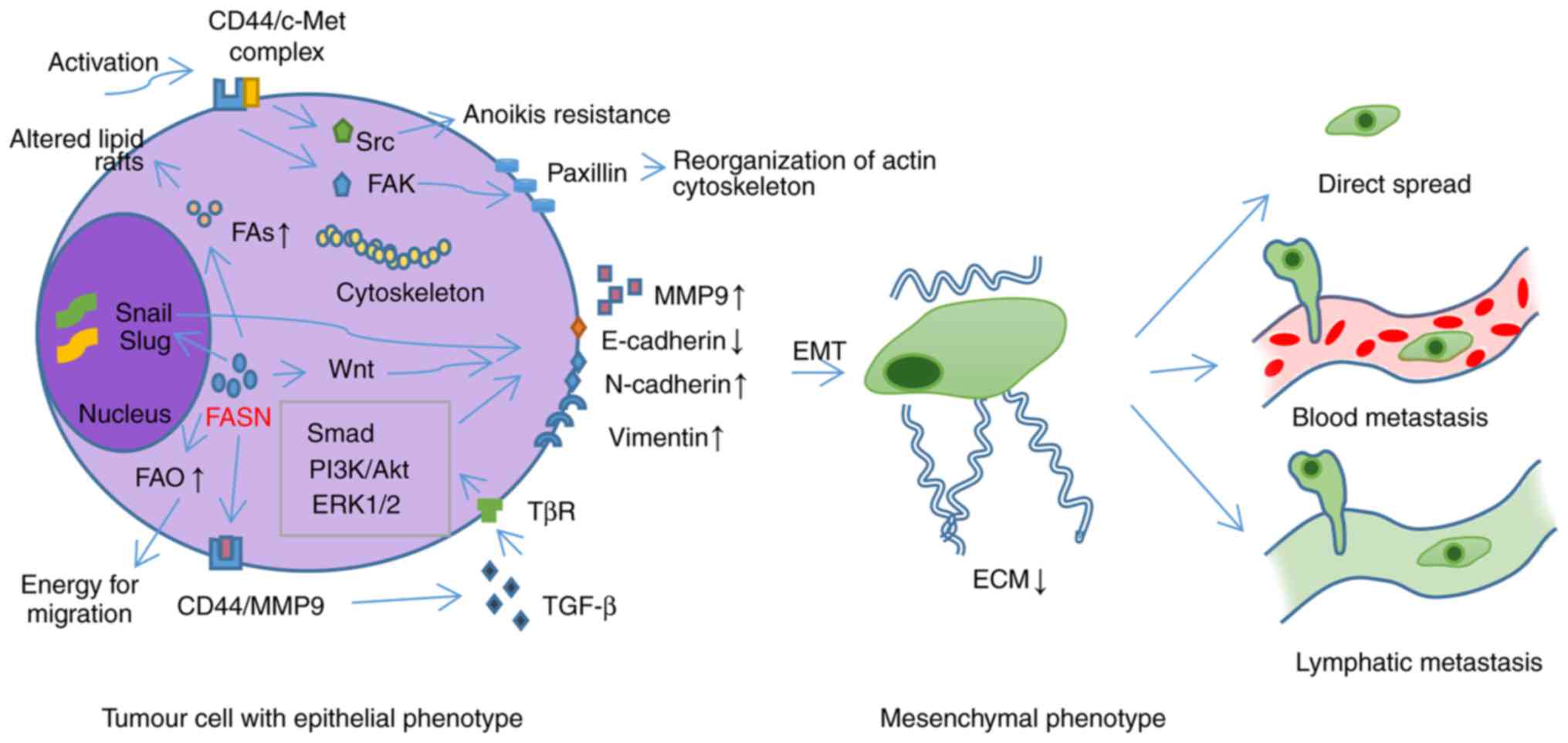
|
1
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konerding MA, Fait E and Gaumann A: 3D
microvascular architecture of pre-cancerous lesions and invasive
carcinomas of the colon. Br J Cancer. 84:1354–1362. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mcintyre A and Harris AL: Metabolic and
hypoxic adaptation to anti-angiogenic therapy: A target for induced
essentiality. EMBO Mol Med. 7:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaytseva YY, Elliott VA, Rychahou P,
Mustain WC, Kim JT, Valentino J, Gao T, O'Connor KL, Neltner JM,
Lee EY, et al: Cancer cell-associated fatty acid synthase activates
endothelial cells and promotes angiogenesis in colorectal cancer.
Carcinogenesis. 35:1341–1351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Hua L: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Browne CD, Hindmarsh EJ and Smith JW:
Inhibition of endothelial cell proliferation and angiogenesis by
orlistat, a fatty acid synthase inhibitor. FASEB J. 20:2027–2035.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Jin G, Mi R, Zhang J, Zhang J, Xu
H, Cheng S, Zhang Y, Song W and Liu F: Inhibition of fatty acid
synthase suppresses neovascularization via regulating the
expression of VEGF-A in glioma. J Cancer Res Clin Oncol.
142:2447–2459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seguin F, Carvalho MA, Bastos DC, Agostini
M, Zecchin KG, Alvarez-Flores MP, Chudzinski-Tavassi AM, Coletta RD
and Graner E: The fatty acid synthase inhibitor orlistat reduces
experimental metastases and angiogenesis in B16-F10 melanomas. Br J
Cancer. 107:977–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 6 Suppl 16:S15–S18. 2002.
View Article : Google Scholar
|
|
11
|
Cantelmo AR, Brajic A and Carmeliet P:
Endothelial metabolism driving angiogenesis: Emerging concepts and
principles. Cancer J. 21:244–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schoors S, Bruning U, Missiaen R, Queiroz
KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia
J, et al: Fatty acid carbon is essential for dNTP synthesis in
endothelial cells. Nature. 520:192–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elmasri H, Ghelfi E, Yu C, Traphagen S,
Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G and
Cataltepe S: Endothelial cell-fatty acid binding protein 4 promotes
angiogenesis: Role of stem cell factor/c-kit pathway. Angiogenesis.
15:457–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ku CY, Liu YH, Lin HY, Lu SC and Lin JY:
Liver fatty acid-binding protein (L-FABP) promotes cellular
angiogenesis and migration in hepatocellular carcinoma. Oncotarget.
7:18229–18246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kazlauskas A: Lysophosphatidic acid
contributes to angiogenic homeostasis. Exp Cell Res. 333:166–170.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:412017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nath A, Li I, Roberts LR and Chan C:
Elevated free fatty acid uptake via CD36 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma. Sci
Rep. 5:147522015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo
Y, Li L, Tan Z, Chen X, Mani SA and Chang JT: Candidate
anti-metastasis drugs suppress the metastatic capacity of breast
cancer cells by reducing membrane fluidity. Cancer Res.
76:2037–2049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang L, Wang H, Li J, Fang X, Pan H, Yuan
X and Zhang P: Up-regulated FASN expression promotes transcoelomic
metastasis of ovarian cancer cell through epithelial-mesenchymal
transition. Int J Mol Sci. 15:11539–11554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang L, Xiao L, Sugiura H, Huang X, Ali
A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY and Evers BM: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalezguerrico AM, Espinoza I, Schroeder
B, Park CH, Kvp CM, Khurana A, Corominas-Faja B, Cuyàs E, Alarcón
T, Kleer C, et al: Suppression of endogenous lipogenesis induces
reversion of the malignant phenotype and normalized differentiation
in breast cancer. Oncotarget. 7:71151–71168. 2016.PubMed/NCBI
|
|
29
|
Ruan HY, Yang C, Tao XM, He J, Wang T,
Wang H, Wang C, Jin GZ, Jin HJ and Qin WX: Downregulation of ACSM3
promotes metastasis and predicts poor prognosis in hepatocellular
carcinoma. Am J Cancer Res. 7:543–553. 2017.PubMed/NCBI
|
|
30
|
Sun L, Kong Y, Cao M, Zhou H, Li H, Cui Y,
Fang F, Zhang W, Li J, Zhu X, et al: Decreased expression of
acetyl-CoA synthase 2 promotes metastasis and predicts poor
prognosis in hepatocellular carcinoma. Cancer Sci. 108:1338–1346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanai JI, Doro N, Seth P and Sukhatme VP:
ATP citrate lyase knockdown impacts cancer stem cells in vitro.
Cell Death Dis. 4:e6962013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanai J, Doro N, Sasaki AT, Kobayashi S,
Cantley LC, Seth P and Sukhatme VP: Inhibition of lung cancer
growth: ATP citrate lyase knockdown and statin treatment leads to
dual blockade of mitogen-activated protein kinase (MAPK) and
phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol.
227:1709–1720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Huang J, Xin W, Chen L, Zhao X, Lv
Z, Liu Y and Wan Q: Lipid accumulation is ahead of
epithelial-to-mesenchymal transition and therapeutic intervention
by acetyl-CoA carboxylase 2 silence in diabetic nephropathy.
Metabolism. 63:716–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SW, Chou CT, Chang CC, Li YJ, Chen
ST, Lin IC, Kok SH, Cheng SJ, Lee JJ, Wu TS, et al: HMGCS2 enhances
invasion and metastasis via direct interaction with PPARα to
activate Src signaling in colorectal cancer and oral cancer.
Oncotarget. 8:22460–22476. 2017.PubMed/NCBI
|
|
35
|
Koichiro K, Shogo S, Chiaki K, Yuki K, Ke
Y and Hiroshi F: High expression of fatty acid-binding protein 5
promotes cell growth and metastatic potential of colorectal cancer
cells. FEBS Open Bio. 6:190–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muehlberg FL, Song YH, Krohn A, Pinilla
SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM,
Devarajan E, et al: Tissue-resident stem cells promote breast
cancer growth and metastasis. Carcinogenesis. 30:589–597. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishi K, Suzuki K, Sawamoto J, Tokizawa Y,
Iwase Y, Yumita N and Ikeda T: Inhibition of fatty acid synthesis
induces apoptosis of human pancreatic cancer cells. Anticancer Res.
36:4655–4660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ventura R, Mordec K, Waszczuk J, Wang Z,
Lai J, Fridlib M, Buckley D, Kemble G and Heuer TS: Inhibition of
de novo palmitate synthesis by fatty acid synthase induces
apoptosis in tumor cells by remodeling cell membranes, inhibiting
signaling pathways, and reprogramming gene expression.
EBioMedicine. 2:806–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bandyopadhyay S, Zhan R, Wang Y, Pai SK,
Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, Iiizumi M, et al:
Mechanism of apoptosis induced by the inhibition of fatty acid
synthase in breast cancer cells. Cancer Res. 66:5934–5940. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui Y, Xing P, Wang Y, Liu M, Qiu L, Ying
G and Li B: NADPH accumulation is responsible for apoptosis in
breast cancer cells induced by fatty acid synthase inhibition.
Oncotarget. 8:32576–32585. 2017.PubMed/NCBI
|
|
47
|
Samudio I, Harmancey R, Fiegl M,
Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W,
Duvvuri S, Taegtmeyer H and Andreeff M: Pharmacologic inhibition of
fatty acid oxidation sensitizes human leukemia cells to apoptosis
induction. J Clin Invest. 120:142–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boren J and Brindle KM: Apoptosis-induced
mitochondrial dysfunction causes cytoplasmic lipid droplet
formation. Cell Death Differ. 19:1561–1570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia SN, Lin C, Chen DF, Li AQ, Dai L,
Zhang L, Zhao LL, Yang JS, Yang F and Yang WJ: The transcription
factor p8 regulates autophagy in response to palmitic acid stress
via a mammalian target of rapamycin (mTOR)-independent signaling
pathway. J Biol Chem. 291:4462–4472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wen YA, Xing X, Harris JW, Zaytseva YY,
Mitov MI, Napier DL, Weiss HL, Mark Evers B and Gao T: Adipocytes
activate mitochondrial fatty acid oxidation and autophagy to
promote tumor growth in colon cancer. Cell Death Dis. 8:e25932017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Niso-Santano M, Malik SA, Pietrocola F,
Pedro Bravo-San JM, Mariño G, Cianfanelli V, Ben-Younès A, Troncoso
R, Markaki M, Sica V, et al: Unsaturated fatty acids induce
non-canonical autophagy. EMBO J. 34:1025–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lochner M, Berod L and Sparwasser T: Fatty
acid metabolism in the regulation of T cell function. Trends
Immunol. 36:81–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kleinfeld AM and Okada C: Free fatty acid
release from human breast cancer tissue inhibits cytotoxic
T-lymphocyte-mediated killing. J Lipid Res. 46:1983–1990. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hossain F, Al-Khami AA, Wyczechowska D,
Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T,
Zou W, et al: Inhibition of fatty acid oxidation modulates
immunosuppressive functions of myeloid-derived suppressor cells and
enhances cancer therapies. Cancer Immunol Res. 3:1236–1247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao W and Gabrilovich D: Abstract 3649:
Contribution of fatty acid accumulation to myeloid-derived
suppressor cell function in cancer. Cancer Res. 71:36492011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harris DT: Changes in plasma membrane
phospholipids inhibit antibody-mediated lysis. Biochem Biophys Res
Commun. 417:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shaikh SR and Edidin M: Immunosuppressive
effects of polyunsaturated fatty acids on antigen presentation by
human leukocyte antigen class I molecules. J Lipid Res. 48:127–138.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harris DT: Alterations in target cell
membrane phospholipids alter T cell but not NK cell killing.
Immunobiology. 218:21–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yoo TJ, Kuo CY, Spector AA, Denning GM,
Floyd R, Whiteaker S, Kim H, Kim J, Abbas M and Budd TW: Effect of
fatty acid modification of cultured hepatoma cells on
susceptibility to natural killer cells. Cancer Res. 42:3596–3600.
1982.PubMed/NCBI
|
|
63
|
Nomura M, Liu J, Rovira II,
Gonzalez-Hurtado E, Lee J, Wolfgang MJ and Finkel T: Fatty acid
oxidation in macrophage polarization. Nat Immunol. 17:216–217.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Luan B, Yoon YS, Le LJ, Kaestner KH,
Hedrick S and Montminy M: CREB pathway links PGE2 signaling with
macrophage polarization. Proc Natl Acad Sci USA. 112:15642–15647.
2015.PubMed/NCBI
|
|
65
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ford JH: Saturated fatty acid metabolism
is key link between cell division, cancer, and senescence in
cellular and whole organism aging. Age (Dordr). 32:231–237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maeda M, Scaglia N and Igal RA: Regulation
of fatty acid synthesis and Delta9-desaturation in senescence of
human fibroblasts. Life Sci. 84:119–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen Y, Wang Y, Huang Y, Zeng H, Hu B,
Guan L, Zhang H, Yu AM, Johnson CH, Gonzalez FJ, et al: PPARα
regulates tumor cell proliferation and senescence via a novel
target gene carnitine palmitoyltransferase 1C. Carcinogenesis.
38:474–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ponnusamy S, Alderson NL, Hama H,
Bielawski J, Jiang JC, Bhandari R, Snyder SH, Jazwinski SM and
Ogretmen B: Regulation of telomere length by fatty acid elongase 3
in yeast. Involvement of inositol phosphate metabolism and Ku70/80
function. J Biol Chem. 283:27514–27524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eitsuka T, Nakagawa K, Suzuki T and
Miyazawa T: Polyunsaturated fatty acids inhibit telomerase activity
in DLD-1 human colorectal adenocarcinoma cells: A dual mechanism
approach. Biochim Biophys Acta. 1737:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eitsuka T, Nakagawa K and Miyazawa T: Dual
mechanisms for telomerase inhibition in DLD-1 human colorectal
adenocarcinoma cells by polyunsaturated fatty acids. Biofactors.
21:19–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mizushina Y, Takeuchi T, Sugawara F and
Yoshida H: Anti-cancer targeting telomerase inhibitors:
β-rubromycin and oleic acid. Mini Rev Med Chem. 12:1135–1143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Scaglia N, Tyekucheva S, Zadra G,
Photopoulos C and Loda M: De novo fatty acid synthesis at the
mitotic exit is required to complete cellular division. Cell Cycle.
13:859–868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cai L and Tu BP: Acetyl-CoA drives the
transcriptional growth program in yeast. Cell Cycle. 10:3045–3046.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mauvoisin D, Charfi C, Lounis AM, Rassart
E and Mounier C: Decreasing stearoyl-CoA desaturase-1 expression
inhibits β-catenin signaling in breast cancer cells. Cancer Sci.
104:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee D, Wada K, Taniguchi Y, Al-Shareef H,
Masuda T, Usami Y, Aikawa T, Okura M, Kamisaki Y and Kogo M:
Expression of fatty acid binding protein 4 is involved in the cell
growth of oral squamous cell carcinoma. Oncol Rep. 31:1116–1120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT and
Ding J: Growth arrest induced by C75, A fatty acid synthase
inhibitor, was partially modulated by p38 MAPK but not by p53 in
human hepatocellular carcinoma. Cancer Biol Ther. 5:978–985. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pan J, Zhou S, Xiang R, Zhao Z, Liu S,
Ding N, Gong S, Lin Y, Li X, Bai X, et al: An Ω-3 fatty acid
desaturase-expressing gene attenuates prostate cancer proliferation
by cell cycle regulation. Oncol Lett. 13:3717–3721. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Saadi H, Seillier M and Carrier A: The
stress protein TP53INP1 plays a tumor suppressive role by
regulating metabolic homeostasis. Biochimie. 118:44–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Parrales A and Iwakuma T: p53 as a
regulator of lipid metabolism in cancer. Int J Mol Sci.
17:E20742016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Furuta E, Pai SK, Zhan R, Bandyopadhyay S,
Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, et al:
Fatty acid synthase gene is up-regulated by hypoxia via activation
of Akt and sterol regulatory element binding protein-1. Cancer Res.
68:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Huang D, Li T, Li X, Zhang L, Sun L, He X,
Zhong X, Jia D, Song L, Semenza GL, et al: HIF-1-mediated
suppression of acyl-CoA dehydrogenases and fatty acid oxidation is
critical for cancer progression. Cell Rep. 8:1930–1942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang Y, Wang H, Zhang J, Lv J and Huang
Y: Positive feedback loop and synergistic effects between
hypoxia-inducible factor-2α and stearoyl-CoA desaturase-1 promote
tumorigenesis in clear cell renal cell carcinoma. Cancer Sci.
104:416–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Patterson WL III and Georgel PT: Breaking
the cycle: The role of omega-3 polyunsaturated fatty acids in
inflammation-driven cancers. Biochem Cell Biol. 92:321–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Calder PC: n-3 Polyunsaturated fatty
acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83
Suppl 6:S1505–S1519. 2006. View Article : Google Scholar
|
|
88
|
Fazio C, Piazzi G, Vitaglione P, Fogliano
V, Munarini A, Prossomariti A, Milazzo M, D'Angelo L, Napolitano M,
Chieco P, et al: Inflammation increases NOTCH1 activity via MMP9
and is counteracted by Eicosapentaenoic acid-free fatty acid in
colon cancer cells. Sci Rep. 6:206702016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Williams-Bey Y, Boularan C, Vural A, Huang
NN, Hwang IY, Shan-Shi C and Kehrl JH: Omega-3 free fatty acids
suppress macrophage inflammasome activation by inhibiting NF-κB
activation and enhancing autophagy. PLoS One. 9:e979572014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hansen KJ and Houten BV: Investigating the
metabolic relationship between ovarian cancer cells and adipocytes:
The role of fatty acid beta-oxidation. Gynecol Oncol. 137 Suppl
1:S1102015. View Article : Google Scholar
|
|
91
|
Lazar I, Clement E, Dauvillier S, Milhas
D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S,
et al: Adipocyte exosomes promote melanoma aggressiveness through
fatty acid oxidation: A novel mechanism linking obesity and cancer.
Cancer Res. 76:4051–4057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Loftus TM, Jaworsky DE, Frehywot GL,
Townsend CA, Ronnett GV, Lane MD and Kuhajda FP: Reduced food
intake and body weight in mice treated with fatty acid synthase
inhibitors. Science. 288:2379–2381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kridel SJ, Axelrod F, Rozenkrantz N and
Smith JW: Orlistat is a novel inhibitor of fatty acid synthase with
antitumor activity. Cancer Res. 64:2070–2075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hoover HS, Blankman JL, Niessen S and
Cravatt BF: Selectivity of inhibitors of endocannabinoid
biosynthesis evaluated by activity-based protein profiling. Bioorg
Med Chem Lett. 18:5838–5841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Puig T, Benhamu B, Turrado C, Relat J,
Ortega-Gutierrez S, Casals G, Marrero PF, Haro D, Brunet J,
Lopez-Rodriguez ML and Colomer R: Novel poliphenolic inhibitors of
fatty acid synthase (FASN) have potential as anticancer agents.
Cancer Res. 68:2008.
|
|
97
|
Infante J, Patel M, Hoff DV, Brenner A,
Rubino C, McCulloch W, Zhukova-Harrill V and Parsey M: 3LBA Initial
report of a first-in-human study of the first-in-class fatty acid
synthase (FASN) inhibitor, TVB-2640. Eur J Cancer. 50 Suppl
6:S195–S196. 2014. View Article : Google Scholar
|
|
98
|
Vázquez MJ, Leavens W, Liu R, Rodríguez B,
Read M, Richards S, Winegar D and Domínguez JM: Discovery of
GSK837149A, an inhibitor of human fatty acid synthase targeting the
beta-ketoacyl reductase reaction. FEBS J. 275:1556–1567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Linehan WM, Srinivasan R and Schmidt LS:
The genetic basis of kidney cancer: A metabolic disease. Nat Rev
Urol. 7:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wishart DS: Is cancer a genetic disease or
a metabolic disease? EBioMedicine. 2:478–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen T and Li H: Fatty acid metabolism and
prospects for targeted therapy of cancer. Eur J Lipid Sci Tec.
119:2017.
|
|
103
|
Mariette G, Anne T, Pierre A,
Clavel-Chapelon F and Nicole C: Dietary fat, fatty acid composition
and risk of cancer. Eur J Lipid Sci Tec. 107:540–559. 2010.
|
|
104
|
Balaban S, Lee LS, Schreuder M and Hoy AJ:
Obesity and cancer progression: Is there a role of fatty acid
metabolism? Biomed Res Int. 2015:2745852015. View Article : Google Scholar : PubMed/NCBI
|