Introduction
In the course of human tumour pathogenesis, normal
cells progressively acquire a sequence of biological abilities,
known as the hallmarks of cancer, which comprise the six
established hallmarks of sustaining proliferative signalling,
evading growth suppressors, resisting cell death, enabling
replicative immortality, inducing angiogenesis, and activating
invasion and metastasis. There are also two emerging hallmarks,
namely the programming of energy metabolism and evading immune
destruction (1,2).
In order to meet the needs of biosynthesis during
high levels of proliferation, the reprogramming of fatty acid (FA)
metabolism becomes essential in cancer cells. At present, a number
of previous studies have investigated the effects of cancer on FA
metabolism during progression, demonstrating the increased de
novo biogenesis and β-oxidation of FAs in these transformed
cells. However, whether this influence is unilateral remains to be
fully elucidated.
In the present review, evidence regarding the
contribution of the change in FAs in cells during the acquisition
and development of the hallmarks of cancer is discussed. In
addition, the review provides a comprehensive insight into the
underlying roles of FAs in tumourigenesis that go beyond their
function in membrane phospholipid synthesis, signal transduction
and energy production. Due to the diversity of cancer phenotypes
and limitations of existing evidence, results on the associations
may not be universal. However, the aim of the present review was to
provide a diverse perspective in order to obtain a better
understanding of the pathogenesis of cancer and to highlight
potential novel treatment targets and therapies.
FA metabolism and angiogenesis
The induction of angiogenesis, including the
sprouting of existing blood vessels and tubular arrangement of
endothelial cells (ECs), is crucial for metastatic spread and for
primary and metastatic tumour growth. The overstimulation of
angiogenic growth by induced signals in tumour progression results
in various tumour vasculature abnormalities (3), which in turn induces hypoxia,
nutrient deficiency, reduced drug delivery, and more aggressive
tumour growth (4); this forms a
vicious circle of events. Tumour angiogenesis has long been
recognised as an important target for anticancer therapy, and
evaluation from the perspective of FA metabolism is an interesting
area to evaluate first.
FA synthase (FASN), an enzyme associated with the
endogenous synthesis of palmitic acid, is overexpressed in various
types of human cancer, and its expression level is associated with
prognosis and invasion depth. Increased knowledge of its connection
with angiogenesis is gradually being obtained. FASN can affect the
expression of a series of vasculogenesis-related factors, including
promotive growth-regulated protein family members, angiogenin and
interleukin (IL)-6, and suppressants tissue inhibitor of
metalloproteinase-1 (TIMP-1) and tissue inhibitor of TIMP-2
(5). It has been reported that
FASN promotes angiogenesis in colorectal cancer by stimulating the
secretion of angiogenic factors and the proliferation of ECs
(5); significantly increased
expression of FASN has also been positively correlated with
elevated vascular endothelial growth factor (VEGF) levels (Fig. 1) (6). The significant impact of FASN
inhibitors on angiogenesis has also been demonstrated in previous
studies; by inhibiting the proliferation of ECs, they induce the
inhibitory effect of angiogenesis (7). The same effect has also been observed
in melanoma and glioma, supporting the key role of FASN in the
neovascularization of tumours (8,9).
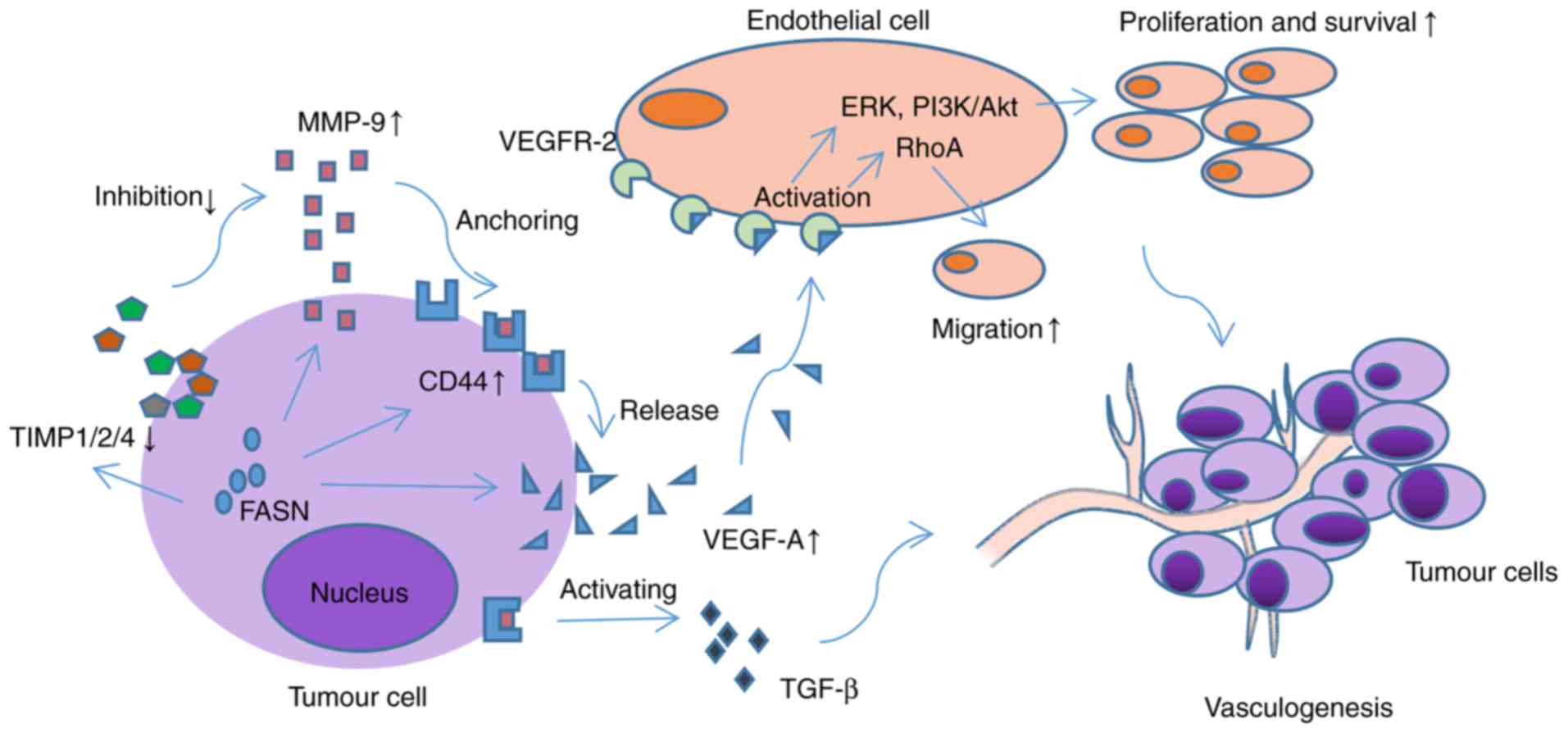 | Figure 1.Mechanism by which FASN promotes
angiogenesis through the regulation of VEGFA. By regulating the
expression of TIMP1/2/4, MMP-9 and CD-44, FASN affects the
synthesis and release of VEGFA, and the activation of VEGFR2. By
activating the downstream ERK, PI3K/Akt and RhoA signalling
pathways, the proliferation and migration of vascular endothelial
cells and angiogenesis of the tumour are enhanced. In addition, the
CD44/MMP9 complex can activate proangiogenic TGF-β precursors and
promote tumour vasculogenesis. FASN, fatty acid synthase; VEGFR,
vascular endothelial growth factor receptor; TIMP, tissue inhibitor
of metalloproteinase; MMP, matrix metalloproteinase; CD, cluster of
differentiation; ERK, extracellular signal-regulated kinase; PI3K,
phosphoinositide 3 kinase; Akt, protein kinase B; TGF-β,
transforming growth factor-β. |
The role of another factor closely associated with
angiogenesis, FA oxidation (FAO), is gradually being elucidated.
During vessel sprouting, FAO is involved in the growth and
differentiation of vascular ECs (10). Although not imperative for the
production of energy in ECs, FAO supplies carbon for glutamic acid
to facilitate the synthesis of deoxyribonucleotides (11,12);
this is significant as the carbon source required for this
synthesis is predominantly from glucose and glutamine (13). In addition, as the rate limiting
enzyme of FAO, the pharmacological inhibition of carnitine
palmitoyl transferase 1 (CPT1) or genetic loss of CPT1 in ECs can
markedly reduce pathologic angiogenesis (11,12).
FA-binding protein (FABP), an intracellular FA
carrier protein, has also been observed to correlate with this
hallmark of cancer. Elmasri et al (14) identified that FABP4 was closely
associated with the proliferation and migration of ECs, in addition
to vessel sprouting. Mechanically, a previous study suggested that
FABP may be associated with increased VEGFA and neovascularization
by binding with VEGF receptor 2 on the cell membrane (15). Kazlauskas (16) hypothesised that the expression of
VEGF may be enhanced through mechanisms associated with FABP5 and
peroxisome proliferator activated receptor-γ in prostate cancer
cells following the uptake of exogenous FAs, indicating that there
may be a link between the expression of VEGF and FABP. In addition,
signal transduction lipids, including phenyl glycidyl ether 2
(PGE2) and lysophosphatidic acid, are important for the vascular
growth and mobilisation of immunocytes, particularly macrophages
that accelerate angiogenesis in tumours.
These results are indicative of an important
association between FA metabolism and angiogenesis; they provide a
basis for novel potential therapeutic strategies for targeting
angiogenesis in cancer at different stages.
FA metabolism and the activation of invasion
and metastasis
Several types of cancer gradually obtain the
characteristics of invasion and metastasis with deterioration, an
ability that is associated with the development and poor prognosis
of cancer. Through an invasion-metastasis cascade, cancer cells
infiltrate nearby blood vessels and lymphatic ducts, or are
transferred to distant tissues (17). During this progression, an
important mode is epithelial-mesenchymal transition (EMT). Through
this process, cancer cells reverse to an undifferentiated state and
possess the abilities of invasion, transmission and resistance to
apoptosis. In this reversible process, the majority of enzymes
associated with these epigenetic modifications require the
involvement of cofactors including acetyl coenzyme A (acetyl-CoA),
the intermediate products in FA metabolism, which causes EMT to
become susceptible to the changing intracellular metabolite levels
(18).
FA transporter [FAT; also known as cluster of
differentiation (CD)-36], a membrane glycoprotein involved in
transporting FAs, provides certain groups of cancer cells with
unique metastasis-initiating potential and, particularly in human
squamous cell carcinoma, CD36 has been identified as the inducer of
distant metastasis (19,20). CD36 is also involved with poor,
long-term outcomes in several types of cancer, including melanoma
and breast carcinoma, due to its prometastatic characteristics
(19). The expression of FAT is
linked with that of Wnt and transforming growth factor (TGF)-β
pathway-related genes, which are two potential activators of EMT
(21). Nath et al (20) also demonstrated that the uptake of
free FA (FFA), elevated by CD36, activated TGF-β signalling
pathways, which in turn activated EMT. Correspondingly, due to the
correlation between CD36 and metastasis, the suppression of CD36
also compromises the development of metastasis. A previous study
suggested that inhibiting CD36 using neutralising antibodies
resulted in a decrease in metastasis in mice with deficient immune
systems (19). Furthermore, the
induction of EMT requires complex remodelling of cellular lipid
composition in order to alter the membrane fluidity required for
cell migration (22), thereby
highlighting the possibility of targeting membrane fluidity for the
suppression of metastasis.
Considering the potential dependence of cells with a
high energy demand following EMT on FA metabolism, several concerns
have been raised regarding the correlation between FASN and EMT.
Polyak and Weinberg (23) revealed
that FASN was upregulated in EMT cells and, following silencing the
expression of FASN using short hairpin RNAs, EMT reversal was
induced. This upregulation has also been demonstrated in peritoneal
metastasis of oophoroma; FASN was observed to promote EMT via the
transcriptional regulation of different cadherins (24). Other studies have reported that
FASN may exert its influence on invasion and metastasis by
regulating the Wnt or TGF-β signalling pathways (25). By promoting the synthesis of FAs,
FASN alters the composition of lipid rafts and activates the
CD44/c-Met complex, inducing the activation of Src, focal adhesion
kinase and paxillin, in addition to reorganisation of the actin
cytoskeleton, resulting in changes in cell morphology and increased
mobility (Fig. 2) (26). In addition, the suppression of FASN
was reported to be connected with the abrogation of EMT induced by
hepatocyte growth factors, and reverse the poor differentiation and
malignant characteristics in transformed cells (27,28).
Taken together, these results suggest that there may be a positive
correlation between FASN and EMT.
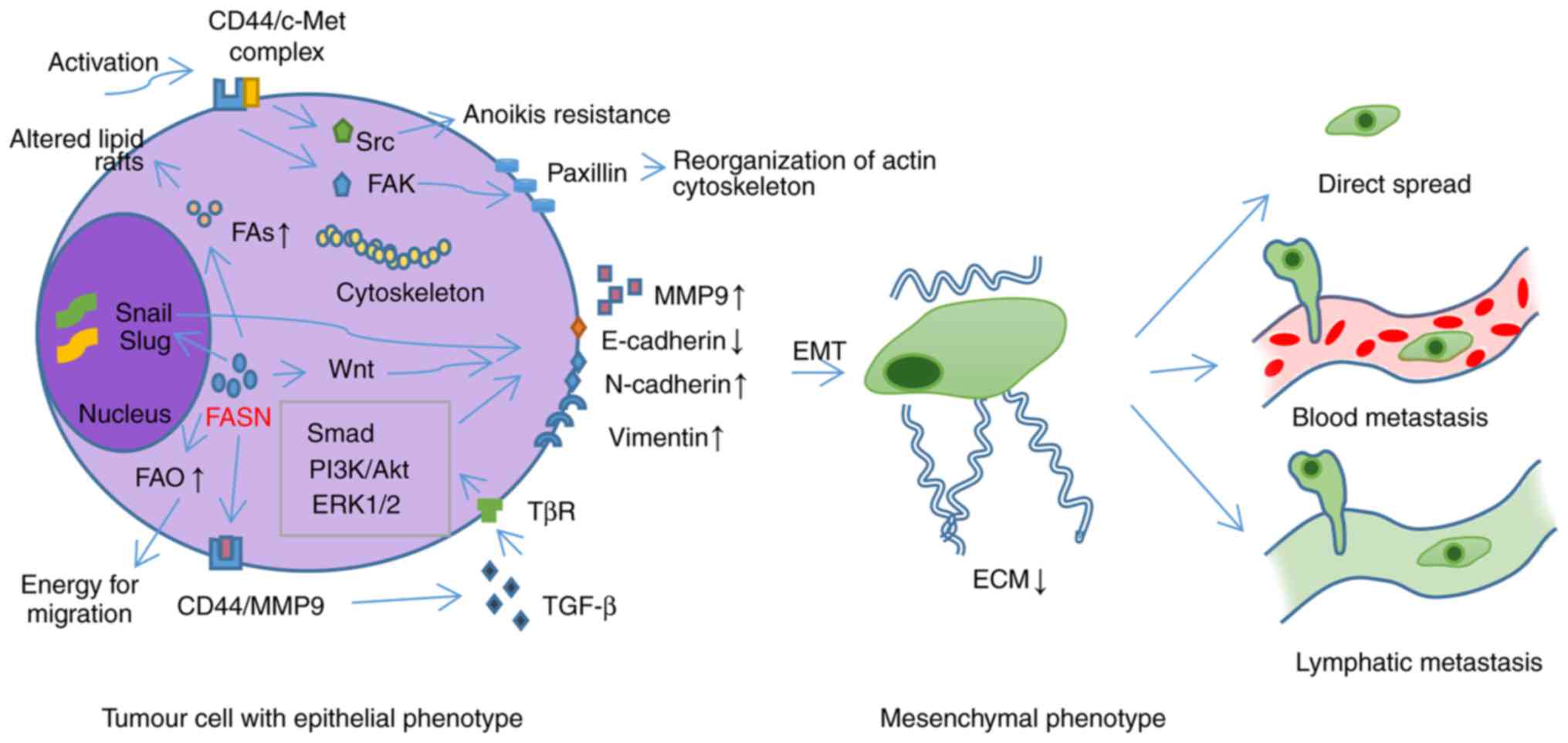 | Figure 2.FASN promotes EMT conversion and the
metastasis of tumour cells through various mechanisms. By promoting
the synthesis of FAs, FASN alters the composition of lipid rafts
and activates the CD44/c-Met complex, inducing the activation of
Src, FAK and paxillin, and reorganisation of the actin
cytoskeleton, resulting in changes in cell morphology and increased
mobility. FASN can also regulate the expression of MMP9,
E-cadherin, N-cadherin and Vimentin by activating the Wnt and TGF-β
signalling pathways, and promoting the expression of Snail and Slug
transcription factors; this triggers the EMT of tumour cells. FAO
can also be promoted to provide sufficient energy for tumour
migration. FAs, fatty acids; FASN, fatty acid synthase; EMT,
epithelial-mesenchymal transition; FAK, focal adhesion kinase; FAO,
fatty acid oxidation; CD, cluster of differentiation; PI3K,
phosphoinositide 3 kinase; Akt, protein kinase B; ERK,
extracellular signal-regulated kinase; TGF-β, transforming growth
factor-β. |
Other enzymes associated with FA metabolism also
have a number of associations with this translation. The
overexpression of acetyl-CoA synthase and stearoyl-CoA desaturase-1
(SCD1) can activate EMT in colorectal cancer; however, acetyl-CoA
synthase (ACS)2 acetylises hypoxia-inducible factor (HIF)-2α, one
of the key inducers of EMT, to induce a prohibitive effect, and the
overexpression of acyl-CoA medium-chain synthetase 3 also impairs
the migration and invasion of hepatocellular carcinoma (HCC) cells
(29,30). In addition, a high expression level
of adenosine triphosphate citrate lyase (ACL) is responsible for
lymphatic metastasis in gastric adenocarcinoma, and silencing ACL
reverses EMT in lung cancer accompanied by the downregulation of
Snail, which can promote EMT in cancer (31,32).
Furthermore, acetyl-CoA carboxylase 2 silencing has a protective
effect on the EMT transformation induced by glucose stress,
triglyceride deposition and the accumulation of malonyl-CoA in the
kidneys (33). Mitochondrial
3-hydroxy-3-methylglutaryl-CoA synthase, a key enzyme in lipid
synthesis, also increases the metastatic potential of colorectal
cancer cells by activating Src signalling (34).
In addition to these enzymes, FAs and the
corresponding receptors are involved in the regulation of EMT.
Elevated FFA levels can promote the metastatic progression of HCC
via the transcriptional activation of EMT (20). Furthermore, a high expression level
of FABP, which is responsible for the combination and transmission
of long chain FAs, has been also reported to enhance metastasis in
colorectal cancer (35).
The interactions in the tumour microenvironment are
also key in this transformation. 27-Hydroxycholesterol, produced by
tumour-associated macrophages or cholesterol metabolism, increases
lung metastasis by acting through liver X receptor secondary to
effects on EMT (36). Triglyceride
catabolism in adipocytes drives the homing, migration and invasion
of ovarian cancer cells by supplying FAs as energy substrates
(37). In addition, adipose
tissue-derived stem cells can be stimulated by breast cancer cells
to secrete stromal cell-derived factor-1, and in turn, these
paracrine factors enable cancer cells to migrate, invade and
metastasise (38). In such a
microenvironment, the actions of tumour cells are not isolated, but
are the result of the combined effects of intracellular and
extracellular resistance factors and attributing factors.
In addition, metabolism in cells with EMT is
reprogrammed to meet energy requirements. Lipid metabolism can be
regulated by EMT transcription factors, including the repressor of
E-cadherin, which also affects the signal cascade required for the
complete activation EMT (39,40).
Therefore, taken together, the aforementioned
evidence indicates that there are multiple associations between FA
metabolism and the metastasis of cancer, which are associated with
advanced stage, poor prognosis and malignant characteristics,
including drug resistance. Furthermore, due to the interactions
between the various factors that induce EMT and feedback
regulation, the complex signalling network for cell invasion and
metastasis presents difficulties in the understanding, prevention
and treatment of cancer. Further investigation into FA metastasis
may assist in this understanding.
FA metabolism and resisting cell death
Apoptosis
Apoptosis, a mechanism that maintains homeostasis
through programmed cell death controlled by genes, has long been
considered the biggest challenge in the onset of cancer since it
was first suggested by Kerr et al in 1972 (41). With regulation of different B-cell
lymphoma 2 (Bcl-2) members, cells can initiate this program in
response to DNA damage and insufficient growth factors (42). Correspondingly, cancer evades this
cell death mechanism via p53 deletion, thereby lowering the
expression of pro-apoptotic factors or increasing the expression of
anti-apoptotic factors and survival signals. FA metabolism is also
involved in this transformation.
ACL and FASN, important enzymes and the primary
obstacles in FA synthesis, indirectly affect the development and
apoptosis of cancer cells. Nishi et al (43) demonstrated that the use of
5-(tetradecyloxy)-2-furoic acid, an acetyl-CoA carboxylase
inhibitor, increased the number of apoptotic cells by increasing
the activity of caspase3, and the addition of palmitate attenuated
this, suggesting that there may be a link between FA and apoptosis.
The use of selective FASN inhibitors to promote apoptosis also
supports this (44). Mechanically,
Bandyopadhyay et al (45)
reported that the increase in malonyl-CoA and the induction of
pro-apoptotic genes tumour necrosis factor-related
apoptosis-inducing ligand, Bcl-2-interacting protein 3 and
death-associated protein kinase 2, induced by the inhibition of
FASN, may be the primary causes. It is also suggested that the
accumulation of NADPH in this process may have a strong correlation
with apoptosis (46). Therefore,
there is a clear association between FA synthesis and
apoptosis.
In addition, as expected, successful FAO is
associated with the anti-apoptotic ability of cancer. Samudio et
al (47) revealed that human
leukaemia cells with inhibited FAO were more susceptible to
apoptosis induced by ABT-737, an activator of Bcl-2, resulting from
the fact that FAO acts as a regulator of Bcl-2 antagonist/killer
1-dependent mitochondrial permeability. Furthermore, the main cause
of the rapid accumulation of lipid droplets, one of the
characteristics of apoptosis, is the suppression of FAO accompanied
by an enhancement in the activity of acyl-CoA synthase (48). The cooperation of FA metabolism is
an important strategy to consider in future investigations.
Autophagy
Autophagy, an important form of cell death, is a
self-digestive process in which intracellular organelles or
proteins are degraded in order to meet the needs of cell metabolism
or the reprogramming of certain organelles. However, the role of
autophagy in the development of tumours is complex and
multifaceted. At present, autophagy is considered to function as a
tumour suppressive process in the early stages of tumourigenesis by
eliminating cytotoxic substrates, however, it also contributes to
tumour cell resistance to apoptosis under energy stress conditions
in established tumour autophagy (49). The triggering mechanism of
autophagy in tumours is complicated, and FA metabolism is involved
in this process.
As important products in FA synthesis, the
accumulation of palmitic acids in cells can trigger autophagy
through lipotoxic effects via the mammalian target of rapamycin
(mTOR)-independent signalling pathway (50). In addition, FAs released by
adipocytes in the tumour microenvironment can also trigger
autophagy to promote cell survival in adverse environments via
absorption by tumour cells and the activation of adenosine
monophosphate-activated protein kinase (AMPK) signals (51). A novel hypothesis is that different
types of FAs stimulate autophagic responses through distinct
molecular mechanisms: Saturated FAs may trigger autophagy by
activating the AMPK, protein kinase R, c-Jun N-terminal kinase 1
and lipid kinase complex, whereas unsaturated FAs may induce this
process via an intact Golgi apparatus (52).
FA metabolism and avoiding immune
destruction
The immune system is considered to be the greatest
challenge for tumours to overcome in order to develop. The
well-established theory of immune surveillance states that, through
constant examination, the immune system is responsible for the
recognition and elimination of nascent transformed cells via
various means of monitoring, which is vital for prevention of the
emergence, development and metastasis of cancer. However, through
immunoediting processes, in which weak immunogenic tumour cells are
allowed to evade and undergo further expansion while the immune
system inhibits tumour growth (53), or the absence or inhibition of a
link in the immune system, this mechanism is avoided and solid
tumours form. In this process, FA metabolism leads to the promotion
of immune evasion through effects in different sites.
Previous studies have demonstrated that FA
metabolism is important in the development, differentiation,
function and distribution of different T cell subsets (54); supporting the hypothesis that FA
metabolism regulation has an effect on the normal function and
failure of the immune system. Kleinfeld and Okada (55) reported that the cytotoxicity of T
lymphocytes can be reduced by the FFA released by breast cancer
tissues to evade immune injury. In addition, the accumulation of
linoleic acid in non-alcoholic fatty liver disease is associated
with the loss of CD4+ T cells via the induction of
mitochondrial damage, leading to the occurrence of HCC (56). Myeloid-derived suppressor cells
(MDSCs), associated with immune deficiency in cancer through the
production of immunosuppressive cytokines and the inhibition of T
lymphocytes, can be arrested by the pharmacological inhibition of
FAO (57). A previous study also
revealed that MDSCs with FA accumulation induced by the
overexpression of fatty acid transport protein 4 have a higher
prohibitive effect on immune cells than normal MDSCs (58). All of the above evidence is
suggestive of a promotion of FA metabolism in the development of
immune system evasion.
Alterations in FAs are also closely associated with
the sensitivity of cancer cells to immune system-induced cell
death. In humoral immunity, regulation of the biosynthesis of cell
membrane FAs and lipids can affect the sensitivity of tumour cells
to antibody-mediated lysis (59).
In cellular immunity, Shaikh andEdidin (60) indicated that the sensitivity of
target cells to effector T cells can be markedly reduced by the
addition of polyunsaturated FAs; this effect is reported to arise
during the identification phase (61). In addition, immunity against the
cytotoxicity mediated by natural killer cells can be enhanced by an
increase in oleic acid and linoleic acid in the membrane of HCC
cells (62).
Macrophages, including tumour-prohibitive M1 type
and tumour-promoting M2 type macrophages, are involved in the
phagocytosis, antigen presentation and the secretion of cytokines
and are important parts of the immune system. The M2 polarization
of tumour-associated macrophages, an immunosuppressive phenotype,
is known to be associated with an increase in FAO (63). Furthermore, the PGE2 released by
cancer cells can transform tumour-associated macrophages from the
tumour-suppressing M1 phenotype to the tumour-promoting M2
phenotype, resulting in immune system evasion (64). PGE2 can also inhibit the immune
response in cancer by inducing the immunosuppressive cytokine IL-10
(65). As an important part of the
immune system, functional changes in macrophages induced by FA
metabolism are attributable to this evasion.
FA metabolism and enabling replicative
immortality
In the ongoing replication process, the telomere
length of normal cells gradually reduces and aging or cell death
can be induced to a certain extent. Therefore, for the majority of
cancer cells, this mechanism requires avoidance in order to permit
infinite reproduction. In the regulation of the appearance of the
senescence state, the formation and activity of telomerase is the
most promising method. During this progression, FAs are directly or
indirectly involved in the emergence of this important
hallmark.
Cellular aging, an irreversible state in which the
physiological function and proliferation and differentiation
abilities gradually decline, is an important obstacle that requires
avoidance by tumour cells in order to survive. Previous studies
have suggested that the appearance of senescence is closely
associated with the de novo synthesis and desaturation of
FAs. The inhibition of FASN and SCD1, induced by telomere
shortening and the subsequent activation of P53, results in
increased levels of palmitic acid, reduced levels of
monounsaturated FAs and phospholipids, and the induction of
senescence (66,67). Therefore, it is hypothesised that
the modification of FA metabolism is vital in the aging process. In
addition, carnitine palmitoyl transferase 1C, an enzyme associated
with the transportation and oxidation of FAs, is also associated
with proliferation and senescence (68); therefore, altering the metabolism
of FAs to delay or eliminate cell aging is beneficial for cancer
progression.
The gradual reduction of telomeres, which protect
chromosomal ends, is the greatest challenge for the immortal
replication of cancer cells. Ponnusamy et al (69) demonstrated that the expression of
FA elongase 3 is involved in determination of the length of
telomeres. As the pivotal enzyme in lengthening telomeres, the
activation of telomerase is also essential in the progression of
cancer. Previous studies have revealed that polyunsaturated FAs
(PUFAs), but not saturated FAs or trans-FAs, can significantly
inhibit telomerase activity, and the enhancement of this effect is
accompanied by an increase in the number of double bonds (70,71).
Furthermore, oleic acid, a monounsaturated FA, can competitively
inhibit telomerase activity through its specific structure and
molecular length (72).
The ability to duplicate without limit is essential
for tumour development. Therefore, regulating FA metabolism or
using unsaturated FAs to treat a wide variety of solid tumours and
malignancies may be an effective strategy.
FA metabolism and sustaining proliferative
signalling
The maintenance of proliferative signalling is a
prerequisite for the formation, development and deterioration of
tumours. Intracellular or extracellular ligands activate a sequence
of signalling pathways, including the mitogen-activated protein
kinase (MAPK) kinase/extracellular signal-regulated kinase (ERK) or
phosphoinositide 3 kinase (PI3K)/protein kinase B (Akt)/mTOR
signalling pathways, to have a regulatory function in the
proliferation of transformed cells, mainly via the regulation of
cell cycle. These mitogenic signals are controlled to produce,
release, activate and degrade specific factors, thereby maintaining
signalling balance and intracellular homeostasis. However, the
abnormal secretion of ligands or constitutive activation of a
signalling pathway, which FA metabolism is known to be involved in,
can also result in the continuous proliferation of cancer cells
(2).
Previous studies have indicated that the de
novo synthesis of FAs is critical for completion of the cell
cycle at the G2/M phase, further highlighting the potential of
targeting FA metabolism to eradicate excessive proliferation
(73). Of note, several phases of
abnormal FA anabolism are closely associated with this hallmark of
cancer. Cai and Tu (74)
demonstrated that the upregulation of acetyl-CoA, which is
important for the production of FAs, can facilitate cell entry into
the cell cycle by promoting the acetylation of growth-related
genomic proteins. SCD1, a key enzyme in catalysing saturated fatty
acyl-CoA into its monounsaturated state, is associated with the
activation of ERK1/2 and the expression of cell cycle-related genes
(75). FABP4 has also been shown
to be vital in the induction of Akt and MAPK signalling cascades in
several malignancies, including breast cancer and oral squamous
cell carcinoma (76). The
pharmacological inhibition of FASN has also been confirmed to be
closely associated with pro-proliferative pathways (77). In addition, most notably, the
antiproliferative effects of omega-3 FAs are also as a result of
the regulation of cell cycle (78).
FA metabolism and evading growth
suppressors
In the process of tumour progression, in order to
combat potent negative regulators, tumour cells must obtain the
ability to evade growth inhibitive factors, which are mainly
composed of proteins encoded by tumour suppressor genes, the
inhibition mechanism and TGF signalling pathways (2). Through the absence of associated gene
expression or the destruction of inhibitory programs, tumours are
able to evade the reactions that are responsible for the response
to internal and external signals and the subsequent regulation of
proliferation, senescence and apoptosis.
P53 protein, which is expressed by the p53 gene, is
an important established tumour suppressor and is an important
growth inhibitive factor as it functions as a regulator of
transcription and the cell cycle; the loss of expression or
mutations in P53 are known to contribute to the development of
several types of cancer (79). A
number of experiments have demonstrated that p53 is also closely
associated with the metabolism of FAs. Saadi et al (80) demonstrated that p53 is involved in
mitophagy and inhibits the production of reactive oxygen species
which favour lipid accumulation. In addition, wild-type p53 exerts
a negative effect on transformed cells, whereas mutant p53 may lead
to the development and deterioration of tumours. Through the
activation of sterol regulatory element binding proteins (SREBPs)
and the upregulation of cholesterol production, the growth of
breast cancer cells is accelerated (81). Therefore, p53 is able to regulate
lipid metabolism and respond to stressors.
FA metabolism and the tumour
microenvironment
The tumour microenvironment is important for
transformed cells. The effect of the microenvironment on tumour
metabolism is an important factor to consider. Hypoxia,
inflammation, and the metabolism of adjacent cells can affect the
occurrence and development of cancer. In this process, FA
metabolism is synergistically or negatively involved in the
interactions between tumours and the microenvironment.
Hypoxia
Due to the rapid growth of cancer cells and
uncontrolled angiogenesis, hypoxia represents a significant
environmental state. Furuta et al (82) demonstrated that hypoxia upregulates
the expression of SREBP-1, an important transcription factor in the
anabolism of FAs, which in turn promotes breast cancer progression.
The inhibition of hypoxia in FAO also facilitates the survival,
proliferation and metastasis of cancer cells (83). In addition, the synergistic effect
of hypoxia and FA metabolism has been verified. Zhang et al
(84) reported that the
PI3K/Akt-mediated crosstalk between SCD1 and HIF-2α contributes to
cancer progression. Therefore, the promotion of metabolic changes
in FAs under hypoxic conditions may be an area of interest for
future investigations into cancer development.
Inflammation
Cancer-associated inflammation, which is usually
caused by the necrosis of tumour cells or oncogenic changes and the
subsequent chronic stimulation induced by immune cells, is a
favourable environment for the growth or malignant transformation
of tumour cells (85); tumour
cells are also able to tolerate cell necrosis for the increase in
growth factors during the immune response. The production of
mediators that contribute to inflammation are also associated with
the metabolism of FAs.
It has been suggested that n-3 PUFAs can be
transformed into anti-inflammatory molecules by lipoxygenase,
whereasn-6 PUFAs are primarily converted into the pro-inflammatory
form by cyclooxygenase (86). In
addition to the inhibition of the arachidonic acid metabolism and
influencing the expression of inflammatory genes, n-3 PUFAs can
also produce mediators, known as resolvins, which possess
anti-inflammatory properties (87). Fazio et al (88) demonstrated that the EMT stimulated
by inflammation in colorectal cancer can be inhibited by
eicosapentaenoic acid, an n-3 PUFA. In addition, n-3 PUFAs can
reduce the activation of macrophage inflammasomes to have an
inhibitory effect (89). Further
investigations into the links between inflammation and metabolism
and regulating FA metabolism to alter the carcinogenic inflammatory
microenvironment are required to potentially develop novel cancer
therapies.
Stromal cells
Cancer progression is associated with interactions
between stromal and cancer cells. As an important part of the
tumour microenvironment, stromal cells promote the proliferation
and invasion of cancer cells by providing metabolic substrates or
signalling molecules. A previous study on colon cancer revealed
that transformed cells are able to absorb the FFAs released by
surrounding adipocytes to activate FAO and autophagy in order to
facilitate cancer development (51). Similar auxo-action has been
confirmed in ovarian cancer (90).
In addition, in terms of the changing modalities, exosomes derived
from adipocytes are considered to be associated with the invasion
of melanoma through FAO (91).
Potential drug targets in cancer
therapy
Targeting abnormal tumour metabolism is an
attractive potential avenue for future direct medicines. Due the
importance of FA metabolism in protein modification, the synthesis
of the cell membrane and the localization of oncogenic molecules,
pharmacological inhibitors that target the key enzymes in these
processes may be effective in future therapies.
The early generation of FASN inhibitors, including
cerulenin and C75, are limited in their application, despite their
confirmed induction of apoptosis in cancer cells, due to side
effects associated with weight and appetite (92,93).
As an anti-obesity drug, orlistat has also been shown to exert
inhibitory effects on lipid metabolism and a certain degree of
tumour suppression; however, its poor selectivity and membrane
permeability prevent it from being an ideal antineoplastic agent
clinically (94,95). In addition, several novel
generations of molecules targeting FASN, including GSK837149A,
TVB2640, and plant-derived polyphenols, are currently in
development; among these, TVB2640 has now moved into the human
trials phase (96–98). Therefore, the accurate and
efficient inhibition of tumour lipid metabolism offers promise as a
novel therapeutic strategy.
Concluding remarks
The previous few decades of experimental results
have indicated that cancer is similar to a metabolic disease that
involves disordered energy metabolism in tumour cells (99,100). The abnormal metabolism of FAs is
known to be crucial in cancer biology and pathology (101). In general, any limitations on the
essential capabilities of cancer cells can have an inhibitory
effect on tumourigenesis and tumour progression. However, due to
the diversity of cancer types and characteristics, the same
therapeutic method tends to have different effects between
different types of cancer. Further investigations are required on
the association between FA metabolism and the various hallmarks of
different types of cancer in order to determine the angiogenesis
tendency or how cancer invasion and metastasisis facilitated.
Targeting the corresponding intermediate products of a signalling
pathway may have a good response, which in turn may highlight novel
strategies and potential therapies (102). This targeted treatment is likely
to be more effective and have fewer toxic side effects towards
normal tissues, therefore, non-toxic metabolic therapy may become
the primary method for treating cancer in the future. Furthermore,
due to the correlation between FAs and cancer, other sources of
FAs, including dietary habits, obesity and hyperlipidaemia, also
require consideration in addition to the regulation of metabolic
processes (103,104). In future precision medicine, FA
metabolism offers significant potential. However, due to the
diversity of tumour types, the flexibility of tumour metabolism and
complex interactions in the tumour microenvironment, drugs or
therapies that may be effective and appropriate for clinical
treatments require further verification.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Program
on Key Research Project of China (grant no. 2016YFC0902700), the
National Natural Science Foundation of China (grant nos. 81672672,
81572650, 81772891, 81502357 and 81621062), the Natural Science
Foundation of Zhejiang Province (grant no. Q142114001) and the
State Key Laboratory of Oral Diseases Special Funded Projects.
Availability of data and materials
Not applicable.
Authors' contributions
X-HY and X-HR wrote the manuscript. Y-LT and X-HL
were involved in the conception of the aims of the review, provided
suggestions for the outline and structure of the manuscript, and
performed the final corrections. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FA
|
fatty acid
|
|
FASN
|
fatty acid synthase
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
FAO
|
fatty acid oxidation
|
|
ECs
|
endothelial cells
|
|
CPT1
|
carnitine palmitoyl transferase 1
|
|
PGE2
|
phenyl glycidyl ether 2
|
|
FABP
|
fatty acid-binding protein
|
|
PPARγ
|
peroxisome proliferator activated
receptor γ
|
|
EMT
|
epithelial-mesenchymal transition
|
|
acetyl-CoA
|
acetyl coenzyme A
|
|
FAT
|
fatty acid transporter
|
|
FFA
|
free fatty acid
|
|
SCD1
|
stearoyl-CoA desaturase-1
|
|
ACS
|
acetyl-CoA synthase
|
|
HIF
|
hypoxia-inducible factor
|
|
ACL
|
ATP citrate lyase
|
|
HCC
|
hepatocellular carcinoma
|
|
27HC
|
27-hydroxycholesterol
|
|
LXR
|
liver X receptor
|
|
NAFLD
|
nonalcoholic fatty liver disease
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
PUFAs
|
polyunsaturated fatty acids
|
|
ROS
|
reactive oxygen species
|
|
SREBPs
|
sterol regulatory element binding
proteins
|
|
TIMP1
|
tissue inhibitor of
metalloproteinase-1
|
|
MMP
|
matrix metalloproteinase
|
|
CD
|
cluster of differentiation
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
PI3K
|
phosphoinositide 3 kinase
|
|
Akt
|
protein kinase B
|
|
RhoA
|
Ras homolog family member A
|
|
TGF
|
transforming growth factor
|
|
FAK
|
focal adhesion kinase
|
References
|
1
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konerding MA, Fait E and Gaumann A: 3D
microvascular architecture of pre-cancerous lesions and invasive
carcinomas of the colon. Br J Cancer. 84:1354–1362. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mcintyre A and Harris AL: Metabolic and
hypoxic adaptation to anti-angiogenic therapy: A target for induced
essentiality. EMBO Mol Med. 7:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaytseva YY, Elliott VA, Rychahou P,
Mustain WC, Kim JT, Valentino J, Gao T, O'Connor KL, Neltner JM,
Lee EY, et al: Cancer cell-associated fatty acid synthase activates
endothelial cells and promotes angiogenesis in colorectal cancer.
Carcinogenesis. 35:1341–1351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Hua L: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Browne CD, Hindmarsh EJ and Smith JW:
Inhibition of endothelial cell proliferation and angiogenesis by
orlistat, a fatty acid synthase inhibitor. FASEB J. 20:2027–2035.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Jin G, Mi R, Zhang J, Zhang J, Xu
H, Cheng S, Zhang Y, Song W and Liu F: Inhibition of fatty acid
synthase suppresses neovascularization via regulating the
expression of VEGF-A in glioma. J Cancer Res Clin Oncol.
142:2447–2459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seguin F, Carvalho MA, Bastos DC, Agostini
M, Zecchin KG, Alvarez-Flores MP, Chudzinski-Tavassi AM, Coletta RD
and Graner E: The fatty acid synthase inhibitor orlistat reduces
experimental metastases and angiogenesis in B16-F10 melanomas. Br J
Cancer. 107:977–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 6 Suppl 16:S15–S18. 2002.
View Article : Google Scholar
|
|
11
|
Cantelmo AR, Brajic A and Carmeliet P:
Endothelial metabolism driving angiogenesis: Emerging concepts and
principles. Cancer J. 21:244–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schoors S, Bruning U, Missiaen R, Queiroz
KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia
J, et al: Fatty acid carbon is essential for dNTP synthesis in
endothelial cells. Nature. 520:192–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elmasri H, Ghelfi E, Yu C, Traphagen S,
Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G and
Cataltepe S: Endothelial cell-fatty acid binding protein 4 promotes
angiogenesis: Role of stem cell factor/c-kit pathway. Angiogenesis.
15:457–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ku CY, Liu YH, Lin HY, Lu SC and Lin JY:
Liver fatty acid-binding protein (L-FABP) promotes cellular
angiogenesis and migration in hepatocellular carcinoma. Oncotarget.
7:18229–18246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kazlauskas A: Lysophosphatidic acid
contributes to angiogenic homeostasis. Exp Cell Res. 333:166–170.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:412017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nath A, Li I, Roberts LR and Chan C:
Elevated free fatty acid uptake via CD36 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma. Sci
Rep. 5:147522015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo
Y, Li L, Tan Z, Chen X, Mani SA and Chang JT: Candidate
anti-metastasis drugs suppress the metastatic capacity of breast
cancer cells by reducing membrane fluidity. Cancer Res.
76:2037–2049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang L, Wang H, Li J, Fang X, Pan H, Yuan
X and Zhang P: Up-regulated FASN expression promotes transcoelomic
metastasis of ovarian cancer cell through epithelial-mesenchymal
transition. Int J Mol Sci. 15:11539–11554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang L, Xiao L, Sugiura H, Huang X, Ali
A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY and Evers BM: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalezguerrico AM, Espinoza I, Schroeder
B, Park CH, Kvp CM, Khurana A, Corominas-Faja B, Cuyàs E, Alarcón
T, Kleer C, et al: Suppression of endogenous lipogenesis induces
reversion of the malignant phenotype and normalized differentiation
in breast cancer. Oncotarget. 7:71151–71168. 2016.PubMed/NCBI
|
|
29
|
Ruan HY, Yang C, Tao XM, He J, Wang T,
Wang H, Wang C, Jin GZ, Jin HJ and Qin WX: Downregulation of ACSM3
promotes metastasis and predicts poor prognosis in hepatocellular
carcinoma. Am J Cancer Res. 7:543–553. 2017.PubMed/NCBI
|
|
30
|
Sun L, Kong Y, Cao M, Zhou H, Li H, Cui Y,
Fang F, Zhang W, Li J, Zhu X, et al: Decreased expression of
acetyl-CoA synthase 2 promotes metastasis and predicts poor
prognosis in hepatocellular carcinoma. Cancer Sci. 108:1338–1346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanai JI, Doro N, Seth P and Sukhatme VP:
ATP citrate lyase knockdown impacts cancer stem cells in vitro.
Cell Death Dis. 4:e6962013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanai J, Doro N, Sasaki AT, Kobayashi S,
Cantley LC, Seth P and Sukhatme VP: Inhibition of lung cancer
growth: ATP citrate lyase knockdown and statin treatment leads to
dual blockade of mitogen-activated protein kinase (MAPK) and
phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol.
227:1709–1720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Huang J, Xin W, Chen L, Zhao X, Lv
Z, Liu Y and Wan Q: Lipid accumulation is ahead of
epithelial-to-mesenchymal transition and therapeutic intervention
by acetyl-CoA carboxylase 2 silence in diabetic nephropathy.
Metabolism. 63:716–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SW, Chou CT, Chang CC, Li YJ, Chen
ST, Lin IC, Kok SH, Cheng SJ, Lee JJ, Wu TS, et al: HMGCS2 enhances
invasion and metastasis via direct interaction with PPARα to
activate Src signaling in colorectal cancer and oral cancer.
Oncotarget. 8:22460–22476. 2017.PubMed/NCBI
|
|
35
|
Koichiro K, Shogo S, Chiaki K, Yuki K, Ke
Y and Hiroshi F: High expression of fatty acid-binding protein 5
promotes cell growth and metastatic potential of colorectal cancer
cells. FEBS Open Bio. 6:190–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muehlberg FL, Song YH, Krohn A, Pinilla
SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM,
Devarajan E, et al: Tissue-resident stem cells promote breast
cancer growth and metastasis. Carcinogenesis. 30:589–597. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishi K, Suzuki K, Sawamoto J, Tokizawa Y,
Iwase Y, Yumita N and Ikeda T: Inhibition of fatty acid synthesis
induces apoptosis of human pancreatic cancer cells. Anticancer Res.
36:4655–4660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ventura R, Mordec K, Waszczuk J, Wang Z,
Lai J, Fridlib M, Buckley D, Kemble G and Heuer TS: Inhibition of
de novo palmitate synthesis by fatty acid synthase induces
apoptosis in tumor cells by remodeling cell membranes, inhibiting
signaling pathways, and reprogramming gene expression.
EBioMedicine. 2:806–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bandyopadhyay S, Zhan R, Wang Y, Pai SK,
Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, Iiizumi M, et al:
Mechanism of apoptosis induced by the inhibition of fatty acid
synthase in breast cancer cells. Cancer Res. 66:5934–5940. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui Y, Xing P, Wang Y, Liu M, Qiu L, Ying
G and Li B: NADPH accumulation is responsible for apoptosis in
breast cancer cells induced by fatty acid synthase inhibition.
Oncotarget. 8:32576–32585. 2017.PubMed/NCBI
|
|
47
|
Samudio I, Harmancey R, Fiegl M,
Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W,
Duvvuri S, Taegtmeyer H and Andreeff M: Pharmacologic inhibition of
fatty acid oxidation sensitizes human leukemia cells to apoptosis
induction. J Clin Invest. 120:142–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boren J and Brindle KM: Apoptosis-induced
mitochondrial dysfunction causes cytoplasmic lipid droplet
formation. Cell Death Differ. 19:1561–1570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia SN, Lin C, Chen DF, Li AQ, Dai L,
Zhang L, Zhao LL, Yang JS, Yang F and Yang WJ: The transcription
factor p8 regulates autophagy in response to palmitic acid stress
via a mammalian target of rapamycin (mTOR)-independent signaling
pathway. J Biol Chem. 291:4462–4472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wen YA, Xing X, Harris JW, Zaytseva YY,
Mitov MI, Napier DL, Weiss HL, Mark Evers B and Gao T: Adipocytes
activate mitochondrial fatty acid oxidation and autophagy to
promote tumor growth in colon cancer. Cell Death Dis. 8:e25932017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Niso-Santano M, Malik SA, Pietrocola F,
Pedro Bravo-San JM, Mariño G, Cianfanelli V, Ben-Younès A, Troncoso
R, Markaki M, Sica V, et al: Unsaturated fatty acids induce
non-canonical autophagy. EMBO J. 34:1025–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lochner M, Berod L and Sparwasser T: Fatty
acid metabolism in the regulation of T cell function. Trends
Immunol. 36:81–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kleinfeld AM and Okada C: Free fatty acid
release from human breast cancer tissue inhibits cytotoxic
T-lymphocyte-mediated killing. J Lipid Res. 46:1983–1990. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hossain F, Al-Khami AA, Wyczechowska D,
Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T,
Zou W, et al: Inhibition of fatty acid oxidation modulates
immunosuppressive functions of myeloid-derived suppressor cells and
enhances cancer therapies. Cancer Immunol Res. 3:1236–1247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao W and Gabrilovich D: Abstract 3649:
Contribution of fatty acid accumulation to myeloid-derived
suppressor cell function in cancer. Cancer Res. 71:36492011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harris DT: Changes in plasma membrane
phospholipids inhibit antibody-mediated lysis. Biochem Biophys Res
Commun. 417:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shaikh SR and Edidin M: Immunosuppressive
effects of polyunsaturated fatty acids on antigen presentation by
human leukocyte antigen class I molecules. J Lipid Res. 48:127–138.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harris DT: Alterations in target cell
membrane phospholipids alter T cell but not NK cell killing.
Immunobiology. 218:21–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yoo TJ, Kuo CY, Spector AA, Denning GM,
Floyd R, Whiteaker S, Kim H, Kim J, Abbas M and Budd TW: Effect of
fatty acid modification of cultured hepatoma cells on
susceptibility to natural killer cells. Cancer Res. 42:3596–3600.
1982.PubMed/NCBI
|
|
63
|
Nomura M, Liu J, Rovira II,
Gonzalez-Hurtado E, Lee J, Wolfgang MJ and Finkel T: Fatty acid
oxidation in macrophage polarization. Nat Immunol. 17:216–217.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Luan B, Yoon YS, Le LJ, Kaestner KH,
Hedrick S and Montminy M: CREB pathway links PGE2 signaling with
macrophage polarization. Proc Natl Acad Sci USA. 112:15642–15647.
2015.PubMed/NCBI
|
|
65
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ford JH: Saturated fatty acid metabolism
is key link between cell division, cancer, and senescence in
cellular and whole organism aging. Age (Dordr). 32:231–237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maeda M, Scaglia N and Igal RA: Regulation
of fatty acid synthesis and Delta9-desaturation in senescence of
human fibroblasts. Life Sci. 84:119–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen Y, Wang Y, Huang Y, Zeng H, Hu B,
Guan L, Zhang H, Yu AM, Johnson CH, Gonzalez FJ, et al: PPARα
regulates tumor cell proliferation and senescence via a novel
target gene carnitine palmitoyltransferase 1C. Carcinogenesis.
38:474–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ponnusamy S, Alderson NL, Hama H,
Bielawski J, Jiang JC, Bhandari R, Snyder SH, Jazwinski SM and
Ogretmen B: Regulation of telomere length by fatty acid elongase 3
in yeast. Involvement of inositol phosphate metabolism and Ku70/80
function. J Biol Chem. 283:27514–27524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eitsuka T, Nakagawa K, Suzuki T and
Miyazawa T: Polyunsaturated fatty acids inhibit telomerase activity
in DLD-1 human colorectal adenocarcinoma cells: A dual mechanism
approach. Biochim Biophys Acta. 1737:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eitsuka T, Nakagawa K and Miyazawa T: Dual
mechanisms for telomerase inhibition in DLD-1 human colorectal
adenocarcinoma cells by polyunsaturated fatty acids. Biofactors.
21:19–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mizushina Y, Takeuchi T, Sugawara F and
Yoshida H: Anti-cancer targeting telomerase inhibitors:
β-rubromycin and oleic acid. Mini Rev Med Chem. 12:1135–1143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Scaglia N, Tyekucheva S, Zadra G,
Photopoulos C and Loda M: De novo fatty acid synthesis at the
mitotic exit is required to complete cellular division. Cell Cycle.
13:859–868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cai L and Tu BP: Acetyl-CoA drives the
transcriptional growth program in yeast. Cell Cycle. 10:3045–3046.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mauvoisin D, Charfi C, Lounis AM, Rassart
E and Mounier C: Decreasing stearoyl-CoA desaturase-1 expression
inhibits β-catenin signaling in breast cancer cells. Cancer Sci.
104:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee D, Wada K, Taniguchi Y, Al-Shareef H,
Masuda T, Usami Y, Aikawa T, Okura M, Kamisaki Y and Kogo M:
Expression of fatty acid binding protein 4 is involved in the cell
growth of oral squamous cell carcinoma. Oncol Rep. 31:1116–1120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT and
Ding J: Growth arrest induced by C75, A fatty acid synthase
inhibitor, was partially modulated by p38 MAPK but not by p53 in
human hepatocellular carcinoma. Cancer Biol Ther. 5:978–985. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pan J, Zhou S, Xiang R, Zhao Z, Liu S,
Ding N, Gong S, Lin Y, Li X, Bai X, et al: An Ω-3 fatty acid
desaturase-expressing gene attenuates prostate cancer proliferation
by cell cycle regulation. Oncol Lett. 13:3717–3721. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Saadi H, Seillier M and Carrier A: The
stress protein TP53INP1 plays a tumor suppressive role by
regulating metabolic homeostasis. Biochimie. 118:44–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Parrales A and Iwakuma T: p53 as a
regulator of lipid metabolism in cancer. Int J Mol Sci.
17:E20742016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Furuta E, Pai SK, Zhan R, Bandyopadhyay S,
Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, et al:
Fatty acid synthase gene is up-regulated by hypoxia via activation
of Akt and sterol regulatory element binding protein-1. Cancer Res.
68:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Huang D, Li T, Li X, Zhang L, Sun L, He X,
Zhong X, Jia D, Song L, Semenza GL, et al: HIF-1-mediated
suppression of acyl-CoA dehydrogenases and fatty acid oxidation is
critical for cancer progression. Cell Rep. 8:1930–1942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang Y, Wang H, Zhang J, Lv J and Huang
Y: Positive feedback loop and synergistic effects between
hypoxia-inducible factor-2α and stearoyl-CoA desaturase-1 promote
tumorigenesis in clear cell renal cell carcinoma. Cancer Sci.
104:416–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Patterson WL III and Georgel PT: Breaking
the cycle: The role of omega-3 polyunsaturated fatty acids in
inflammation-driven cancers. Biochem Cell Biol. 92:321–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Calder PC: n-3 Polyunsaturated fatty
acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83
Suppl 6:S1505–S1519. 2006. View Article : Google Scholar
|
|
88
|
Fazio C, Piazzi G, Vitaglione P, Fogliano
V, Munarini A, Prossomariti A, Milazzo M, D'Angelo L, Napolitano M,
Chieco P, et al: Inflammation increases NOTCH1 activity via MMP9
and is counteracted by Eicosapentaenoic acid-free fatty acid in
colon cancer cells. Sci Rep. 6:206702016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Williams-Bey Y, Boularan C, Vural A, Huang
NN, Hwang IY, Shan-Shi C and Kehrl JH: Omega-3 free fatty acids
suppress macrophage inflammasome activation by inhibiting NF-κB
activation and enhancing autophagy. PLoS One. 9:e979572014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hansen KJ and Houten BV: Investigating the
metabolic relationship between ovarian cancer cells and adipocytes:
The role of fatty acid beta-oxidation. Gynecol Oncol. 137 Suppl
1:S1102015. View Article : Google Scholar
|
|
91
|
Lazar I, Clement E, Dauvillier S, Milhas
D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S,
et al: Adipocyte exosomes promote melanoma aggressiveness through
fatty acid oxidation: A novel mechanism linking obesity and cancer.
Cancer Res. 76:4051–4057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Loftus TM, Jaworsky DE, Frehywot GL,
Townsend CA, Ronnett GV, Lane MD and Kuhajda FP: Reduced food
intake and body weight in mice treated with fatty acid synthase
inhibitors. Science. 288:2379–2381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kridel SJ, Axelrod F, Rozenkrantz N and
Smith JW: Orlistat is a novel inhibitor of fatty acid synthase with
antitumor activity. Cancer Res. 64:2070–2075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hoover HS, Blankman JL, Niessen S and
Cravatt BF: Selectivity of inhibitors of endocannabinoid
biosynthesis evaluated by activity-based protein profiling. Bioorg
Med Chem Lett. 18:5838–5841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Puig T, Benhamu B, Turrado C, Relat J,
Ortega-Gutierrez S, Casals G, Marrero PF, Haro D, Brunet J,
Lopez-Rodriguez ML and Colomer R: Novel poliphenolic inhibitors of
fatty acid synthase (FASN) have potential as anticancer agents.
Cancer Res. 68:2008.
|
|
97
|
Infante J, Patel M, Hoff DV, Brenner A,
Rubino C, McCulloch W, Zhukova-Harrill V and Parsey M: 3LBA Initial
report of a first-in-human study of the first-in-class fatty acid
synthase (FASN) inhibitor, TVB-2640. Eur J Cancer. 50 Suppl
6:S195–S196. 2014. View Article : Google Scholar
|
|
98
|
Vázquez MJ, Leavens W, Liu R, Rodríguez B,
Read M, Richards S, Winegar D and Domínguez JM: Discovery of
GSK837149A, an inhibitor of human fatty acid synthase targeting the
beta-ketoacyl reductase reaction. FEBS J. 275:1556–1567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Linehan WM, Srinivasan R and Schmidt LS:
The genetic basis of kidney cancer: A metabolic disease. Nat Rev
Urol. 7:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wishart DS: Is cancer a genetic disease or
a metabolic disease? EBioMedicine. 2:478–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen T and Li H: Fatty acid metabolism and
prospects for targeted therapy of cancer. Eur J Lipid Sci Tec.
119:2017.
|
|
103
|
Mariette G, Anne T, Pierre A,
Clavel-Chapelon F and Nicole C: Dietary fat, fatty acid composition
and risk of cancer. Eur J Lipid Sci Tec. 107:540–559. 2010.
|
|
104
|
Balaban S, Lee LS, Schreuder M and Hoy AJ:
Obesity and cancer progression: Is there a role of fatty acid
metabolism? Biomed Res Int. 2015:2745852015. View Article : Google Scholar : PubMed/NCBI
|
















