Introduction
A lot of studies have been performed to understand
the aging process and identify causal factors of aging. However,
the exact mechanism of aging has not been fully elucidated yet.
Other major research areas in aging are focused on finding ways to
slow down the aging process and retard age-related
pathophysiological changes. Among many genetic and environmental
interventions obtained from model organisms so far, the only
intervention that shows consistent lifespan extensions with
positive effects on health span in all experimental organisms
tested is dietary restriction (DR). The first report about the
effect of DR effect on aging was done with rats in 1917 (1). Dietary-restricted rats showed
increased lifespan and extended reproductive period compared to
not-restricted rats. Since then, the effect of DR has been
replicated in many organisms ranging from yeast to monkeys. In
Drosophila melanogaster, DR using dilution of yeast or sugar
has increased lifespan, imposing a reduced reproduction as a
trade-off (2). Mice fed with 40%
reduced calories have shown lifespan extension with reduced
incidence and/or delayed onset of age-related pathologies (3). A recent study with rhesus monkeys has
revealed that DR could significantly extend lifespan and delay the
onset of many age-related pathophysiological changes, including
incidence of cancer, diabetes mellitus, and brain atrophy (4). There are many different methods of DR
in C. elegans. Dilution of bacterial culture, a common food
source for C. elegance growth, can lead to lifespan
extension (5). Mutations in
eat-2 gene can lead to reduced pharyngeal food pumping rate
and confer a longevity phenotype (6). Worms grown in liquid synthetic medium
containing no bacteria also have an increased lifespan (7).
Despite conserved effect of DR on various organisms,
it is impracticable for humans because it requires long-term
restraint. The anti-aging effect of DR completely disappears within
a few days when dietary-restricted Drosophila melanogaster
are fed ad libitum (AL) (8). Therefore, people have attempted to
discover DR mimetics that can lead to DR response without food
restriction. Resveratrol, a polyphenol compound found in red wine,
has been shown to induce lifespan extension via activation of
sirtuin which is required for DR response (9). Genomic transcriptional profiling has
revealed that dietary supplementation with resveratrol causes
similar transcriptional changes observed with long-term DR
(10). Resveratrol also has
preventive effect against age-related neurodegenerative diseases,
including Alzheimer's disease (AD) and Parkinson's disease
(11). Metformin, a drug
prescribed for treatment of type 2 diabetes, can extend lifespan
and reduce the incidence of age-related diseases such as cancer,
chronic kidney disease, and cardiovascular diseases in mice
(12–14). The lifespan extension effect
conferred by metformin is dependent on the activation of
AMP-activated protein kinase (AMPK) known to modulates DR response
in C. elegans (15,16). In addition, the effect of metformin
on transcriptional profile overlaps with the effect of DR in mice
(17). A recent study has reported
that D-allulose, an isomer of D-fructose, can increase lifespan and
mimic DR response in C. elegans (18). The lifespan-extending effect of
D-allulose does not accompany a reduced food intake or require AMPK
(18). Further studies focusing on
underlying mechanisms of DR mimetics and the effect of DR mimetics
on age-related pathophysiological changes are necessary to identify
relevant DR mimetics.
Selenocysteine is a cysteine derivative in which
selenium substitutes sulfur in the side chain of cysteine.
Selenocysteine is incorporated into selenoproteins known to
modulate anti-oxidant defense and cellular redox regulation
(19). Lack of selenocysteine is
associated with a number of age-related diseases, including cancer,
cardiovascular diseases, and neurodegenerative diseases (20–22).
Selenocysteine-containing peptides show protective effects against
hepatic ischemia-reperfusion injury by decreasing free radicals and
apoptosis in rats (23).
Intraperitoneal injection of selenium can reduce oxidative toxicity
induced by scopolamine and confer neuroprotection in hippocampus of
rats (24). Mutations in
selenocysteine synthase gene encoding an enzyme required for
selenocysteine biosynthesis can lead to congenital cerebellar
atrophy in humans (25). In C.
elegans, organochalcogen containing selenium can inhibit
Mn-induced toxicity and induce increased expression of superoxide
dismutase-3 and nuclear localization of DAF-16 (26). Selenoprotein can also reverse
age-related decline of molting in C. elegans (27). Our previous study has shown that
dietary supplementation with selenocysteine can increase resistance
to environmental stressors such as oxidative stress and ultraviolet
irradiation in C. elegans (28). Selenocysteine can also extend
lifespan of C. elegans without reducing its fertility. It
can also delay age-related decline of its motility (28).
In the present study, we investigated the underlying
mechanism involved in the lifespan-extending effect of
selenocysteine using known long-lived genetic mutants of C.
elegans. The effect of selenocysteine on age-related
pathophysiological changes was then elucidated employing genetic or
nutritional interventions. Results of this study will broaden our
understanding of biological activities of selenocysteine and its
possible application to human health.
Materials and methods
Worm strains and maintenance
Wild-type N2 strain and all mutant strains were
purchased from C. elegans Genetics Center (CGC,
Minneapolis/St. Paul, MN, USA). Long-lived mutants age-1
(hx546), clk-1 (e2519), and eat-2
(ad465) were used to identify underlying mechanism for the
lifespan-extending effect of selenocysteine. CL4176 strain, the
genetic disease model of AD, contains human amyloid-β
(Aβ)1–42 [dvls27
(myo−3/Aβ1–42/let UTR, rol-6)]
transgene inducible in muscle tissues. TJ356 strain carries a
transgene daf-16 fused to GFP [zls356 IV
(daf-16p::daf-16a/b::GFP, rol-6)]. Worms were
maintained on Nematode Growth Media (NGM) plates (1.7% agar, 2.5
mg/ml peptone, 25 mM NaCl, 50 mM KH2PO4 pH
6.0, 5 µg/ml cholesterol, 1 mM CaCl2, and 1 mM
MgSO4) spotted with Escherichia coli OP50 as food
source.
Lifespan assay
Age-synchronization was accomplished by letting five
young adult worms lay eggs on a fresh NGM plate for 4 h at 20°C.
These eggs were then incubated at 20°C for 3 days. Among
age-synchronized young adult worms, 60 worms were randomly selected
for each group and transferred to fresh NGM plates. To inhibit
internal hatching, 5-fluoro-2′-deoxyruridine (12.5 mg/l) was added
to NGM plates. The number of worms alive, dead, or censored was
counted daily. Censored worms included killed, lost, or bagged
worms during assays. They were excluded from the statistical
analysis.
DR on solid NGM plates
DR was performed using bacterial dilution method.
After growing E. coli OP50 at 37°C, bacteria were prepared
at density of 5×109 bacteria/ml for AL group and
5×108 bacteria/ml for DR group using serial dilution.
Then 200 µl of each bacteria culture was spotted onto solid NGM
plates containing 5-fluoro-2′-deoxyuridine and ampicillin. The
survival of age-synchronized worms was monitored daily until all
worms were dead following the lifespan assay described
previously.
RNA interference (RNAi)
E. coli clones harboring each gene for RNAi
were obtained from Ahringer RNAi library (29). Their sequences were verified. The
expression of double-stranded RNA was induced by 0.4 mM
isopropyl-β-D-thio-galactoside (IPTG; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 4 h after OD600 reached 0.4.
Cultured medium was spotted onto NGM plates containing 100 µg/ml
ampicillin, 12.5 µg/ml tetracycline, 0.4 mM IPTG, and 0.5 mg/ml
5-fluoro-2′-deoxyuridine. The bacterial clone containing empty
vector (EV) was used as a negative control.
Aβ-induced toxicity assay
Five age-synchronized young adult CL4176 worms were
left to lay eggs for 5 h at 20°C. After removing five adult worms
from the plate, eggs were incubated for 5 d at 15°C. Thirty young
adult worms were then randomly selected and allowed to lay eggs for
2 h at 15°C. Their progeny were grown at 25°C for 24 h. Percentage
of paralyzed worms by the induction of Aβ in muscle was then
recorded (n=60).
Subcellular distribution of
DAF-16
Age-synchronized TJ356 worms were supplemented with
5 mM selenocysteine for 9 days at 20°C. Each worm (n=60) was
anaesthetized with 1 M sodium azide on a slide glass coated with 2%
agarose. Subcellular localization of DAF-16::GFP was then monitored
with a confocal microscope (Olympus FV10i; Olympus Corporation,
Tokyo, Japan). Each worm was distributed into three classes
according to subcellular localization of DAF-16::GFP: Cytosolic,
nucleus, or intermediate (both cytosolic and nuclear).
High-glucose-induced toxicity
assay
Randomly selected 60 age-synchronized worms were
transferred to a fresh NGM plate containing glucose (40 mM). The
survival of worms in untreated control group was compared to that
of glucose-treated group. The effect of 5 mM selenocysteine on
high-glucose-induced toxicity was measured at 20°C.
Determination of cellular reactive
oxygen species (ROS)
Three-day-old age-synchronized worms were treated
with or without 5 mM selenocysteine for 7 days at 20°C. Individual
worm was then transferred to a 96-well black plate containing 190
µl of PBST per well (n=20). After adding 10 µl of
H2DCF-DA (Sigma-Aldrich), fluorescence intensity of each
animal was determined using a fluorescence multi-reader (Infinite
F200; Tecan, Grodig, Austria).
Statistical analysis
For the lifespan assay and toxicity assay, we used
the log-rank test (30). The
log-rank test is a non-parametric Mantel-Cox test used for
statistical comparison between two survival curves. For subcellular
distribution of DAF-16 and cellular ROS levels, we employed the
standard two-tailed Student's t-test. Statistical analyses were
performed using Microsoft Excel 2016 (Microsoft Corporation,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Overlapping effect of selenocysteine
and eat-2 mutation on lifespan
After observing lifespan-extending effect of
selenocysteine in our previous study, we investigated the
underlying mechanisms involved in the longevity phenotype conferred
by selenocysteine in the present study (28). Three well-known long-lived mutants
representing different lifespan-extending pathways were employed.
Mutant age-1 has an increased lifespan due to reduced
insulin/IGF-1-like signaling (31). Mutation in clk-1 produces
less ROS due to reduced mitochondrial electron transport chain
reaction that can lead to lifespan extension (32). Mutant eat-2 is a genetic
model of DR. It intakes less food due to reduced pharyngeal pumping
rate (6). As previously reported,
dietary supplementation with selenocysteine increased the lifespan
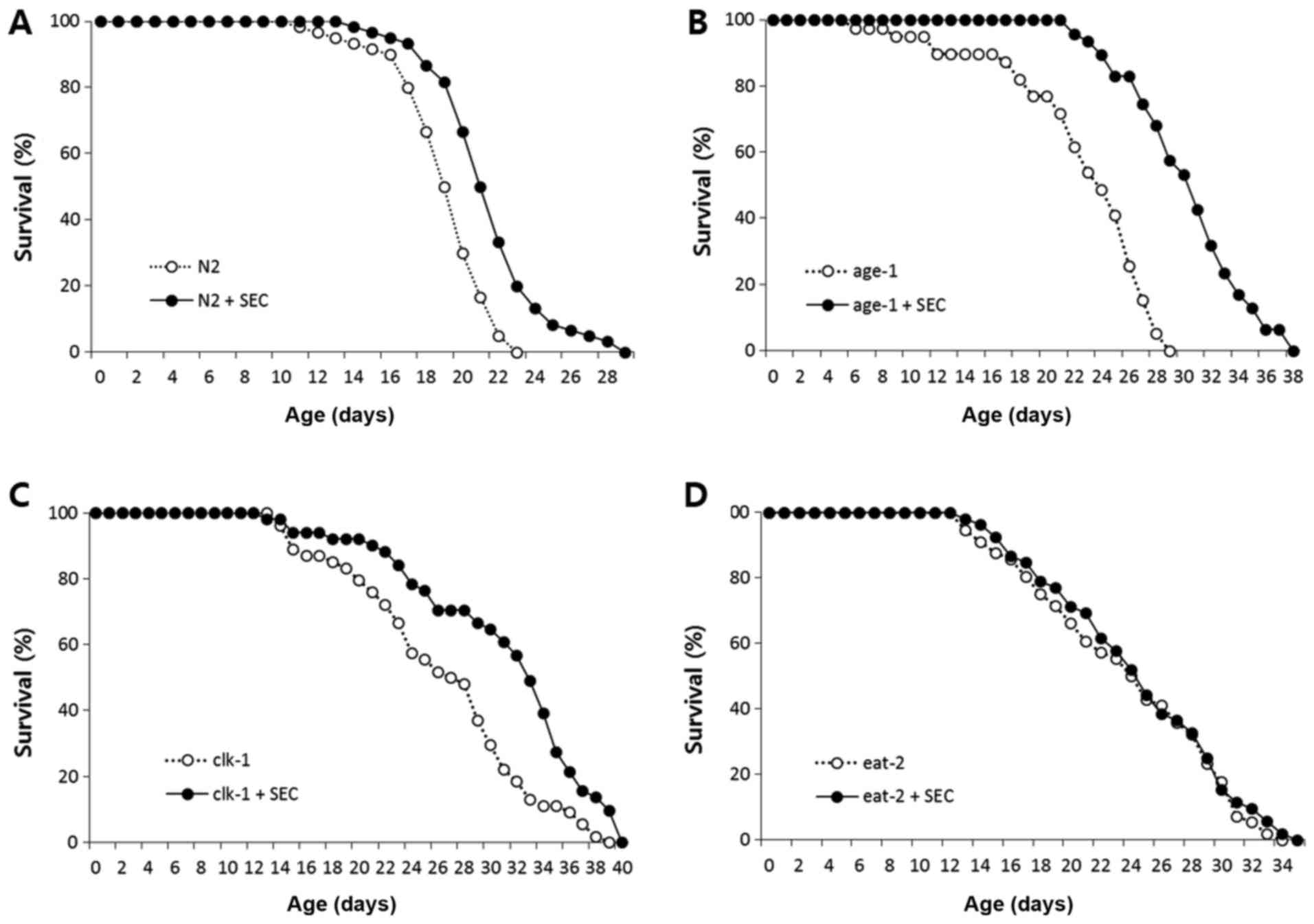
of wild-type control N2 (Fig. 1A).
Selenocysteine also significantly extended lifespans of long-lived
age-1 and clk-1 (Fig. 1B
and C). The mean lifespan of age-1 was 22.7 d and that
of age-1 treated with 5 mM selenocysteine was 30.4 d
(P<0.001). The mean lifespan of clk-1 was increased from
26.4 d in the untreated control to 31.1 d in the
selenocysteine-treated group (P<0.001). However, there was no
significant difference in the lifespan between untreated control
and selenocysteine-treated eat-2 mutants (Fig. 1D). Independent replicative
experiment also showed similar results (Table I). Results of these experiments
indicate that the effect of selenocystein on lifespan specifically
overlaps with that of eat-2 mutation, a genetic model of
DR.
 | Table I.Effect of selenocysteine on the
lifespan of the wild-type N2 strain and long-lived mutants. |
Table I.
Effect of selenocysteine on the
lifespan of the wild-type N2 strain and long-lived mutants.
|
|
| Mean lifespan
(day) |
|
|---|
|
|
|
|
|
|---|
| Group | Experiment no. | Control | Selenocysteine (5
mM) | P-value |
|---|
| N2 | 1st experiment | 21.0 | 29.8 | 0.004 |
|
| 2nd experiment | 20.2 | 21.6 | 0.027 |
| age-1 | 1st experiment | 22.7 | 30.4 | <0.001 |
|
| 2nd experiment | 24.2 | 28.9 | 0.016 |
| clk-1 | 1st experiment | 26.4 | 31.1 | 0.001 |
|
| 2nd experiment | 16.6 | 19.8 | 0.009 |
| eat-2 | 1st experiment | 23.8 | 24.5 | 0.557 |
|
| 2nd experiment | 18.6 | 19.1 | 0.728 |
Selenocysteine mimics DR and requires
SKN-1 for lifespan extension
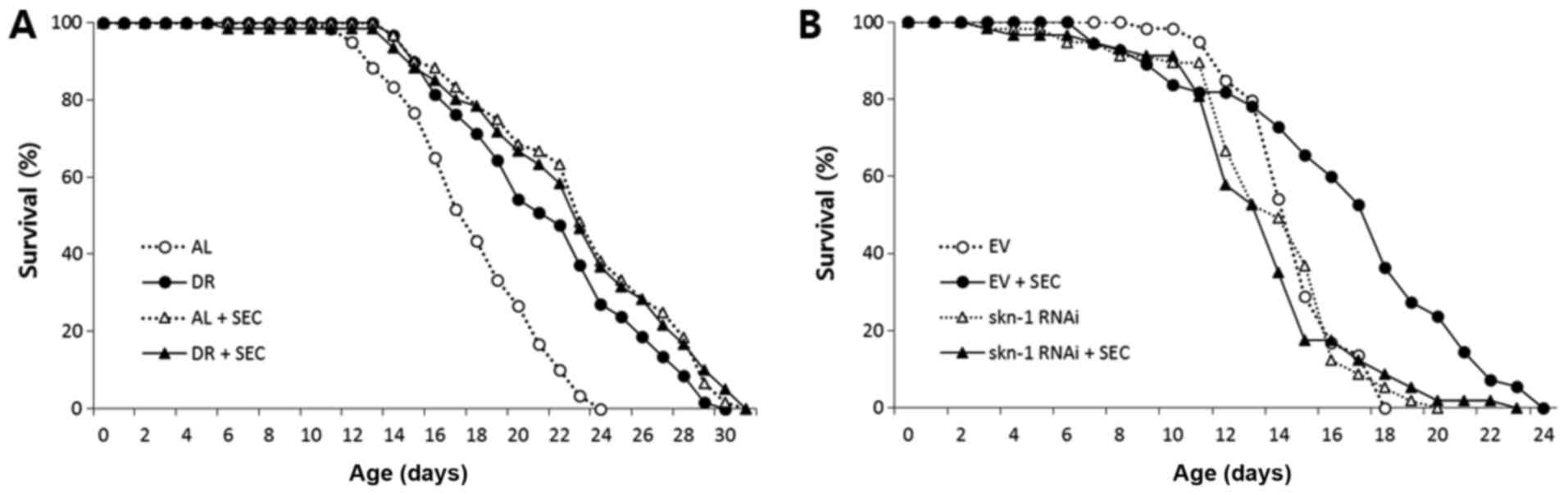
To confirm the overlapping effect of selenocysteine
and DR on lifespan, we examined the effect of selenocysteine on
lifespan of dietary-restricted worms. Compared to AL worms whose
mean lifespan was 17.9 days, the mean lifespan of
dietary-restricted worms was increased to 21.5 days (P<0.001;
Fig. 2A). Supplementation with
selenocysteine also significantly extended its lifespan (mean
lifespan: 22.6 days). However, simultaneous intervention with DR
and selenocysteine failed to further increase lifespan. The mean
lifespan of worms treated with both DR and selenocysteine was 20.7
days, which was not significantly different from the mean lifespan
of worms treated with either DR or selenocysteine (Fig. 2A and Table II). Next, we determined whether
lifespan extended by selenocysteine was mediated by the same factor
that modulated DR response. SKN-1 is a transcription factor known
to regulate responses to oxidative stress and DR-induced lifespan
extension in C. elegans (33). RNAi of skn-1 induces cell
fate transformation during development and modulates oxidative
stress response in adult worms (34,35).
Interestingly, the extended lifespan conferred by selenocysteine
was completely blocked by gene knockdown of skn-1 using RNAi
(Fig. 2B). Mean lifespans of worms
treated with and without selenocysteine were 16.7 and 14.7 days,
respectively (P<0.001). The mean lifespan of worms treated with
both skn-1 RNAi and selenocysteine (13.5 days) was not
significantly different from that of worms treated with
skn-1 RNAi alone (13.8 days) (Table III). Another well-known
DR-mediating factor is DAF-16, a FOXO transcription factor that can
modulate stress response. Gene knockdown of daf-16 by RNAi
reduces resistance to oxidative stress and decreases lifespan in
C. elegans (36,37) However, knockdown of daf-16
did not significantly affect the lifespan-extending effect of
selenocysteine (Table III).
These findings validate the effect of selenocysteine as a DR
mimetic, suggesting that lifespan extension by selenocysteine
requires SKN-1. However, it is not dependent on DAF-16.
 | Table II.Effect of selenocysteine and dietary
restrictions on the lifespan of the wild-type N2 strain. |
Table II.
Effect of selenocysteine and dietary
restrictions on the lifespan of the wild-type N2 strain.
| A, First
experiment |
|---|
|
|---|
| Group | Mean lifespan
(day) | P-value |
|---|
| AL | 17.9 |
|
| DR | 21.5 |
<0.001a |
| AL+SEC | 22.6 |
<0.001a |
| DR+SEC | 20.7 | 0.514b |
|
| B, Second
experiment |
|
| Group | Mean lifespan
(day) | P-value |
|
| AL | 14.6 |
|
| DR | 17.1 |
<0.001a |
| AL+SEC | 16.7 |
<0.001a |
| DR+SEC | 18.1 | 0.171b |
 | Table III.Effect of skn-1 or
daf-16 RNAi on the lifespan extension induced by
selenocysteine. |
Table III.
Effect of skn-1 or
daf-16 RNAi on the lifespan extension induced by
selenocysteine.
|
|
| Mean lifespan
(days) |
|
|---|
|
|
|
|
|
|---|
| Group | Experiment no. | Control | Selenocysteine (5
mM) | P-value |
|---|
| EV | 1st experiment | 14.7 | 16.7 | <0.001 |
|
| 2nd experiment | 13.7 | 16.7 | <0.001 |
| skn-1
RNAi | 1st experiment | 13.8 | 13.5 | 0.633 |
|
| 2nd experiment | 21.4 | 18.9 | 0.254 |
| daf-16
RNAi | 1st experiment | 12.5 | 15.6 | <0.001 |
|
| 2nd experiment | 12.8 | 14.8 | <0.001 |
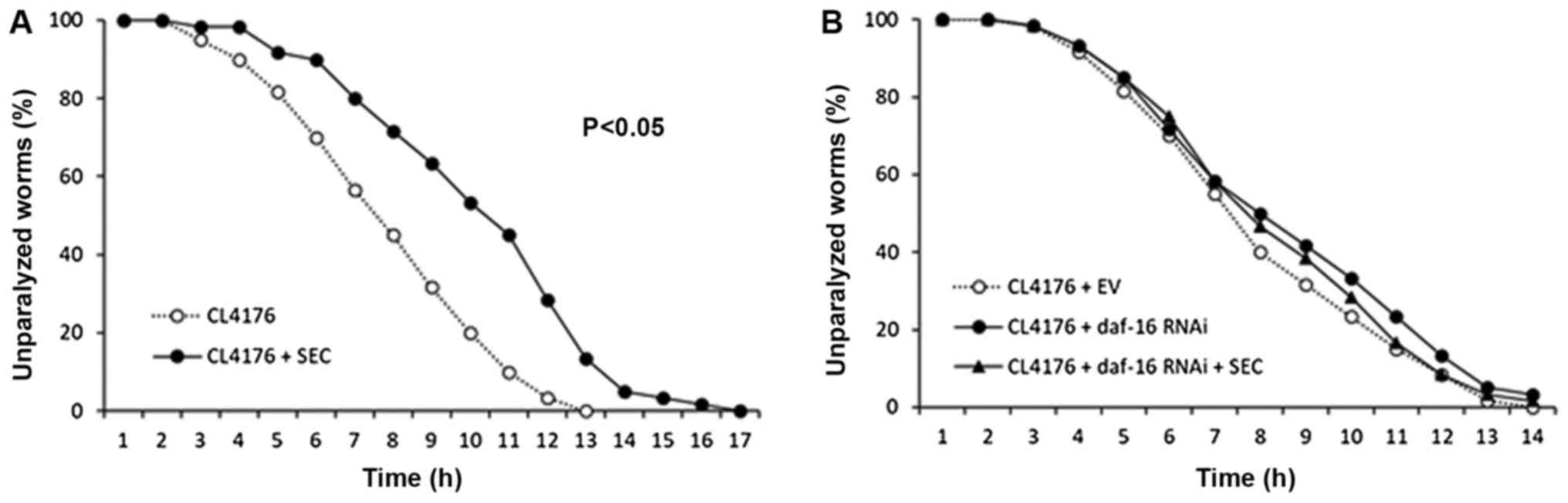
Selenocysteine decreases Aβ-induced
toxicity through DAF-16
Previous studies have shown that DR can retard many
age-related pathophysiological changes (38). We tested the effect of
selenocysteine on Aβ-induced toxicity using genetic AD animal
model. Induction of transgenic human Aβ gene in muscle tissues
caused paralysis in C. elegans (mean survival time 7.0 h).
However, dietary supplementation with selenocysteine markedly
suppressed the rate of paralysis (Fig.
3A). Mean survival time was increased to 9.4 h, which was
increased by 34% when compared to that of untreated control
(P<0.001; Table II). Next, we
examined the effect of daf-16 knockdown on Aβ-induced
paralysis. DAF-16 is known to be required for protection against Aβ
toxicity (39). Surprisingly, the
inhibitory effect of selenocysteine on Aβ-induced paralysis
disappeared when the expression of daf-16 was specifically
knocked down by RNAi (Fig. 2B).
Mean survival time was not significantly different between EV
control group (7.2 h) and daf-16 RNAi group (7.9 h).
Supplementation with selenocysteine failed to increase mean
survival time without DAF-16 (7.8 h) (Table IV). Based on previous finding that
the lifespan-extending effect of selenocystein required SKN-1, we
also examined the effect of skn-1 knockdown on Aβ-induced
paralysis. Unlike results obtained with daf-16 RNAi,
selenocysteine significantly increased survival time after Aβ
induction with skn-1 RNAi (Table IV). Our data showed that the
lifespan-extending effect of selenocysteine could retard
pathophysiological changes in AD animal model. In addition, DAF-16,
but not SKN-1, was necessary for the preventive effect of
selenocysteine against Aβ-induced toxicity in C.
elegans.
 | Table IV.Effect of daf-16 or
skn-1 RNAi on reduced amyloid-β toxicity by
selenocysteine. |
Table IV.
Effect of daf-16 or
skn-1 RNAi on reduced amyloid-β toxicity by
selenocysteine.
|
|
| Mean survival time
(h) |
|
|---|
|
|
|
|
|
|---|
| Group | Experiment no. | Control | Selenocysteine (5
mM) | P-value |
|---|
| EV | 1st experiment | 7.0 | 9.4 | <0.001 |
|
| 2nd experiment | 7.4 | 10.5 | <0.001 |
| daf-16
RNAi | 1st experiment | 7.9 | 7.8 | 0.836 |
|
| 2nd experiment | 7.6 | 7.9 | 0.461 |
| skn-1
RNAi | 1st experiment | 7.0 | 9.2 | 0.017 |
|
| 2nd experiment | 8.6 | 10.6 | 0.015 |
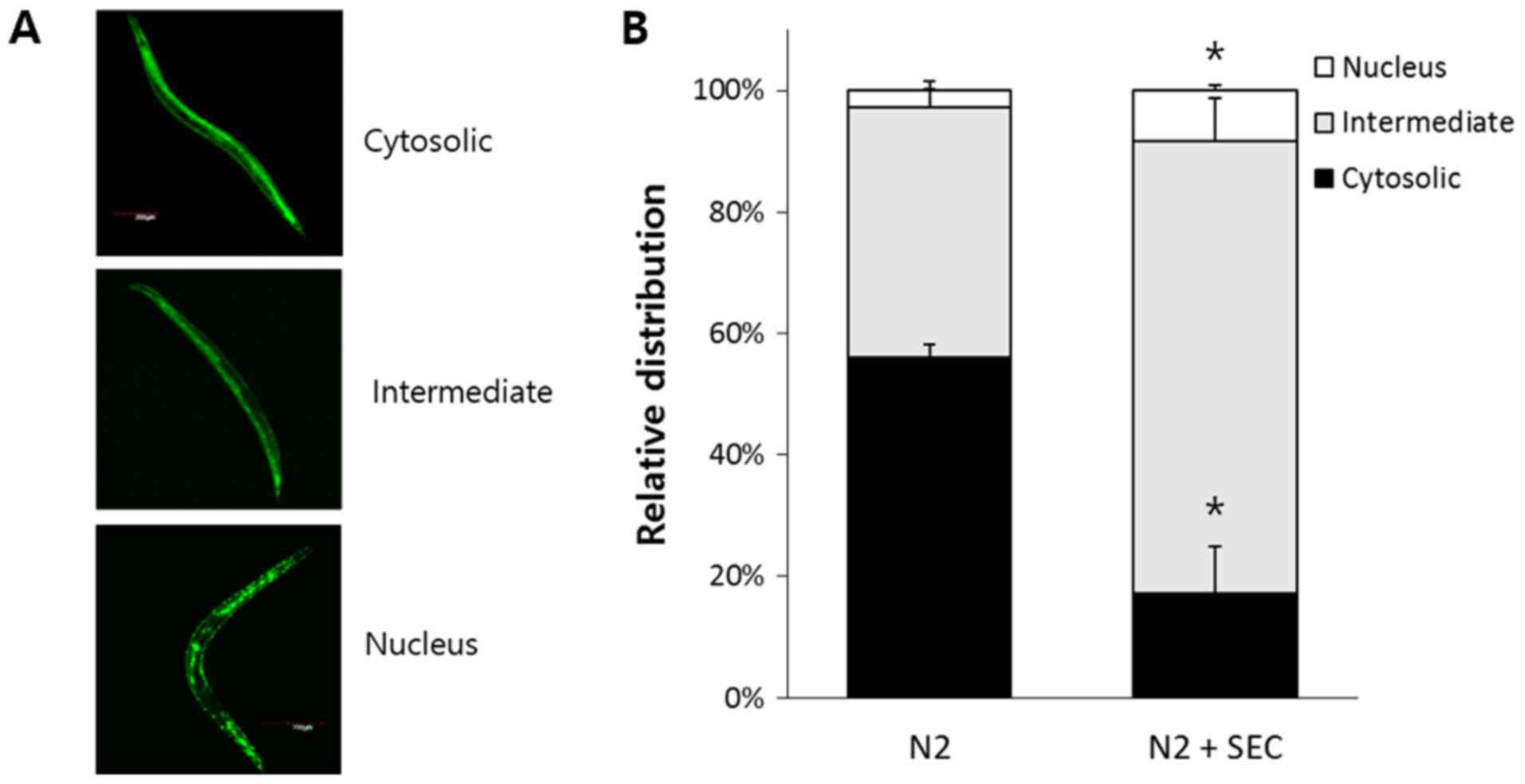
Selenocysteine induces nuclear
localization of DAF-16
DAF-16 is transferred to nucleus in response to
environmental stressors such as oxidative stress, heat shock, and
UV irradiation (40). DR also
induces nuclear localization of DAF-16 (40,41).
Based on the result that DAF-16 was required for increased
resistance to Aβ-induced toxicity by selenocysteine, we
hypothesized that selenocysteine might induce nuclear localization
of DAF-16. Using DAF-16::GFP transgene, we classified subcellular
distribution of DAF-16 into three categories: Cytosolic,
intermediate, and nucleus (Fig.
4A). Dietary supplementation with selenocysteine induced
nuclear localization of DAF-16 without any environmental stimuli.
In the untreated control group, 56.1±2.00% (mean ± SEM) of worms
showed cytosolic distribution of DAF-16 while only 17.2±7.78% of
worms showed cytosolic distribution of DAF-16 in
selenocysteine-treated worms (P=0.008). The percent of intermediate
distribution which showed both cytosolic and nucleus localization
of DAF-16 was increased from 41.1±0.012% in the untreated control
group to 74.5±6.96% in the group with supplementation of
selenocysteine (P=0.012). In addition, higher number of animals
showed nuclear localization of DAF-16 in the selenocysteine-treated
group compared to that in the untreated control group. Percent of
nuclear distribution was 2.8±1.47% in the untreated control group
and 8.3±0.96% in selenocysteine-treated group (P=0.034; Fig. 4B). Therefore, selenocysteine could
confer resistance to Aβ-induced toxicity through induction of
nuclear localization of DAF-16 in C. elegans.
Selenocysteine reduces
high-glucose-induced toxicity and cellular ROS
DR can improve glucose homeostasis and ameliorates
diabetes mellitus (42).
Metformin, a widely-used medicine for type 2 diabetes, can mimic DR
responses in C. elegans (16). We thus determined whether
DR-mimicking selenocysteine could reduce high-glucose-induced
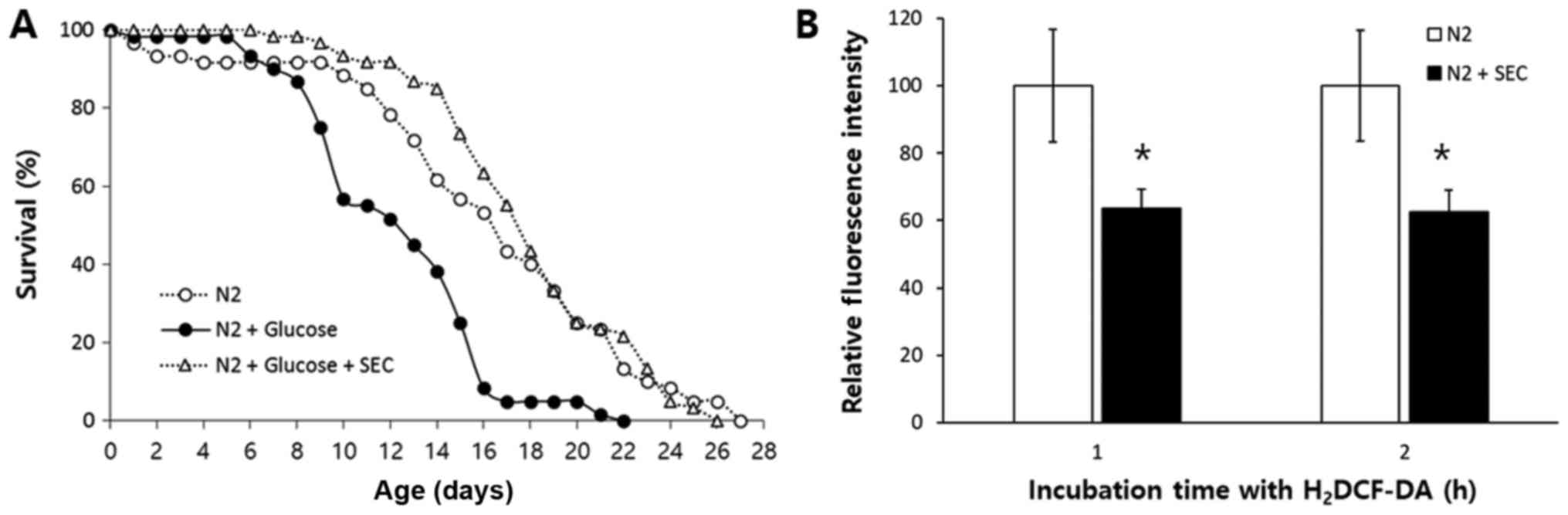
toxicity. As shown in Fig. 5A,
high glucose increased mortality of C. elegans. Mean
lifespan was decreased from 16.4 days in the untreated control
group to 12.4 days in the high-glucose-treated group, which was a
decrease of 24% (P<0.001). However, the reduced lifespan due to
high-glucose-toxicity was completely recovered when selenocysteine
was simultaneously treated with high glucose. Triple replicative
experiments also showed similar results (Table V). A previous study has shown that
high-glucose-diet can induce an increase in ROS level while
chicoric acid, an anti-diabetic molecule, can increase lifespan and
reduce cellular ROS level (43).
We then measured cellular ROS levels in the untreated control group
and selenocysteine-treated group. Fluorescence intensity
representing cellular ROS level was significantly decreased after
supplementation with selenocysteine (Fig. 5B). After 1 h of incubation with
H2DCF-DA, relative fluorescence intensity was decreased
to 64±5.7% (mean ± SEM) in the group with supplementation of
selenocysteinein compared to that of the untreated control group
(100±16.8%, P=0.047). There was also a significant decrease in
relative fluorescence intensity by supplementation with
selenocysteine after 2 h of incubation with H2DCF-DA
[100±16.5 and 63±6.4% in the untreated control and
selenocysteine-treated group, respectively (P=0.042)].
Independently repeated experiment also showed similar results.
Relative fluorescence intensity was decreased to 67±6.8% (P=0.026)
after 1 h of incubation with H2DCF-DA and 66±6.6%
(P=0.050) after 2 h of incubation with H2DCF-DA. Taken
together, these results suggest that selenocysteine can suppress
high-glucose-induced toxicity by lowering cellular ROS levels.
 | Table V.The effect of a high-gluocse diet and
selenocysteine on the lifespan of the wild-type N2 strain. |
Table V.
The effect of a high-gluocse diet and
selenocysteine on the lifespan of the wild-type N2 strain.
| Experiment | Mean lifespan
(days) | P-value |
|---|
| 1st experiment |
|
|
| N2 | 16.4 |
|
|
N2+Glucose | 12.4 |
<0.001a |
|
N2+Glucose+SEC | 18.0 |
<0.001b |
| 2nd experiment |
|
|
| N2 | 17.0 |
|
|
N2+Glucose | 12.0 |
<0.001a |
|
N2+Glucose+SEC | 17.7 |
<0.001b |
| 3rd experiment |
|
|
| N2 | 15.0 |
|
|
N2+Glucose | 13.0 | 0.001a |
|
N2+Glucose+SEC | 16.0 |
<0.001b |
Discussion
Positive impact of DR on lifespan and age-related
diseases has been reported in many organisms. In human,
centenarians live in Okinawa have less intake of calories compared
to people live in other areas of Japan. They show lower incidence
of cancer, Parkinson's disease, and AD (44). In the present study, the
lifespan-extending effect of selenocysteine was not observed in
eat-2 mutant. However, lifespans of age-1 mutant and
clk-1 mutant were significantly increased by selenocysteine.
These findings suggest that the effect of selecocysteine on
lifespan overlaps with that of DR. Previously, novel DR-specific
genes have been identified using lifespan assay with the same three
long-lived mutants. Genetic knockout of nlp-7 or
cup-4 specifically abolished the longevity phenotype
conferred by eat-2 mutation, but not by age-1 or
clk-1 mutation (45). In
C. elegans, there are several methods of DR. Each DR method
seems to involve independent and overlapping cellular signaling
pathways. For example, eat-2 mutation, a genetic model of
DR, requires transcription factors SKN-1 and PHA-4. However, it is
not dependent on DAF-16 for lifespan extension (46). The longevity phenotype caused by
dilution of bacteria or peptone involves AMPK pathway and requires
DAF-16 (15). Food deprivation
extends lifespan via HSF-1 while lifespan extension by intermittent
fasting is mediated by RHEB-1 in C. elegans (47,48).
Results of the present study showed that selenocysteine mimicked
the effect of DR through SKN-1 without requiring DAF-16. Additional
studies are needed to determine the effect of selenocysteine on
other methods of DR and identify downstream targets of
selenocyteine for DR response. Such studies can broaden our
understanding of cellular mechanisms involved in the effect of
selenocysteine on lifespan.
Due to a difficulty of life-long restraint on food
intake, people have searched DR mimetics that can induce DR
response without restricting diet. Leading compounds that can mimic
DR are sirtuin activators. Sirtuin is a NAD-dependent histone
deacetylase. Activation of SIRT1, a mammalian sirtuin, is known to
be necessary for DR-induced long lifespan (48). Dietary supplementation with
resveratrol increases both mean and maximum lifespans in yeast,
nematode, and fruit fly. Such effect of resveratrol is dependent on
sirtuin activation (9,49). Nicotinamide adenine dinucleotide
(NAD+, a cofactor of sirtuin) and oxaloacetic acid that
can increase NAD+ level are also strong candidates of DR
mimetics that can modulate sirtuin activity (50). Mammalian target of rapamycin (mTOR)
signaling that senses cellular nutrient status is known to be
reduced by DR (51). Reduced mTOR
signaling using genetic interventions or diet of rapamycin can
increase lifespan in animal models (51). Rapamycin also has positive effects
on age-related decline in cognitive function and cardiac function
(52,53). Insulin/IGF-1-like signaling is one
of most studied lifespan-modulating pathways conserved from
invertebrates to vertebrates (54). Reduced plasma insulin observed in
dietary-restricted animals is regarded as a biomarker of longevity
(42). Metformin, a drug that can
reduce plasma insulin level, can extend lifespan of C.
elegans (16). Incidence of
age-related diseases including cancer and cardiovascular disease
can be markedly reduced by metformin (12,13).
In mice, dietary supplementation with metformin can lead to genomic
transcriptional profile resembling that of DR (17). A recent study has reported that
dietary supplementation with N-acetyl-L-cysteine, a cysteine
derivative with anti-oxidant activity, can mimic the effect of DR
on lifespan and reduce Aβ-induced toxicity in C. elegans
(55). Here, we demonstrated that
the lifespan-extending effect of selenocysteine was achieved by
mimicking DR response. S-allycysteine, another cysteine derivative,
has also shown health span-promoting and lifespan-extending
activities in vivo (56).
Taken together, these results suggest that cysteine derivatives are
a novel category of putative DR mimetics. The effect of cysteine
derivatives including selenocysteine on aging process and onset of
age-related diseases in higher organisms should be followed to
evaluate cysteine derivatives as strong DR mimetics.
In addition to lifespan extension, DR can reduce
incidence of many age-related diseases and retard various
pathophysiological changes observed in aging mammals. DR has
protective effects against AD, amyotropic lateral sclerosis, and
various cancers (57,58). The progression of tumors can be
delayed by DR in rodents while glucose intolerance and the severity
of diabetes in mice can be improved by DR (34,42).
A variety of age-related pathologies including cataracts, cognitive
impairments, and muscle dysfunction can be attenuated by DR
(38,59,60).
A recent study of DR in rhesus monkeys has revealed that the
incidence of cancer, type 2 diabetes, and cardiovascular disease is
lower in DR animals compared to that in their counterparts fed with
AL (4). The present study also
showed that dietary supplementation of selenocysteine resulted in
decreased susceptibility to Aβ-induced toxicity in AD genetic
model. Such effect required DAF-6, but not SKN-1. Taken together,
these results suggest that the increased lifespan and extended
survival under Aβ-induced toxicity by supplementation with
selenocysteine might be mediated by independent cellular pathways.
Reduced survival due to high-glucose-diet was also completely
recovered by supplementation with selenocysteine. These findings
suggest that selenocysteine can ameliorate age-related
pathophysiological changes and increase lifespan, achieving the
same effect of DR. Our results provide strong evidence for the
development of DR mimetics using selenocysteine. Further studies
focusing on the effect of selenocysteine on age-related diseases
such as AD, Parkinson's disease, and diabetes mellitus in mammalian
disease models and the molecular basis of the effect of
selenocysteine are necessary for the understanding of in
vivo activity of selenocysteine.
The significance of this study is the identification
of a novel DR mimetics. Further studies focusing on the cellular
pathways involved and the longevity phenotype in other model
organisms should be followed in a near future to compare the effect
of selenocysteine with other previously reported DR mimetics, such
as metformin and resveratrol. So far, many DR mimetics has been
reported in many model organisms. Each DR mimetic has both positive
and not significant effect on lifespan and healthspan. For example,
the effect of metformin on lifespan is not universal (no effect on
lifespan of rats) and sex-dependent in fruit fly (61). A recent meta-analysis studies
reports no effect of metformin on over-all mortality (62). The effect of resveratrol on
lifespan extension was not observed in invertebrate models
(63). Therefore, it is important
to understand the mechanisms involved with each DR mimetics and to
propose a possible combination of DR mimetics that are effective on
multiple species. It is unlikely to find perfect and safe DR
mimetics in the near future since the exact mechanism of DR itself
is still illusive. However, due to universality of DR effect and
difficulty in implementing DR, researches focusing on the
identification of novel DR mimetics and elucidation of underlying
mechanisms of DR mimetics are invaluable to human health. They are
drawing increasing attention in the field of aging.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Soonchunhyang
University Research Fund and the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. 2015R1D1A1A01057435).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHK performed all of the experiments in the present
study and wrote the first manuscript. BKK analyzed the effect of
selenocysteine on mutants and the DR effect. SKP designed the
experiments, and reviewed and edited the final version of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Osborne TB, Mendel LB and Ferry EL: The
effect of retardation of growth upon the breeding period and
duration of life of rats. Science. 45:294–295. 1917. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chippindale AK, Leroi AM, Kim SB and Rose
MR: Phenotypic plasticity and selection in Drosophila life
history evolution. I. Nutrition and the cost of reproduction. J
Evol Biol. 6:171–193. 1993. View Article : Google Scholar
|
|
3
|
Sohal RS, Ku HH, Agarwal S, Forster MJ and
Lal H: Oxidative damage, mitochondrial oxidant generation and
antioxidant defenses during aging and in response to food
restriction in the mouse. Mech Ageing Dev. 74:121–133. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colman RJ, Anderson RM, Johnson SC,
Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons
HA, Kemnitz JW and Weindruch R: Caloric restriction delays disease
onset and mortality in rhesus monkeys. Science. 325:201–204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klass MR: Aging in the nematode
Caenorhabditis elegans: Major biological and environmental
factors influencing life span. Mech Ageing Dev. 6:413–429. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lakowski B and Hekimi S: The genetics of
caloric restriction in Caenorhabditis elegans. Proc Natl
Acad Sci USA. 95:13091–13096. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szewczyk NJ, Udranszky IA, Kozak E, Sunga
J, Kim SK, Jacobson LA and Conley CA: Delayed development and
lifespan extension as features of metabolic lifestyle alteration in
C. elegans under dietary restriction. J Exp Biol.
209:4129–4139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mair W, Goymer P, Pletcher SD and
Partridge L: Demography of dietary restriction and death in
Drosophila. Science. 301:1731–1733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barger JL, Kayo T, Vann JM, Arias EB, Wang
J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, et
al: A low dose of dietary resveratrol partially mimics caloric
restriction and retards aging parameters in mice. PLoS One.
3:e22642008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caruana M, Cauchi R and Vassallo N:
Putative role of red wine polyphenols against brain pathology in
Alzheimer's and Parkinson's disease. Front Nutr. 3:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: A new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papanas N and Maltezos E: Oral
antidiabetic agents: Anti-atherosclerotic properties beyond glucose
lowering? Curr Pharm Des. 15:3179–3192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pilmore HL: Review: Metformin: Potential
benefits and use in chronic kidney disease. Nephrology (Carlton).
15:412–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greer EL, Dowlatshahi D, Banko MR, Villen
J, Hoang K, Blanchard D, Gygi SP and Brunet A: An AMPK-FOXO pathway
mediates longevity induced by a novel method of dietary restriction
in C. elegans. Curr Biol. 17:1646–1656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Onken B and Driscoll M: Metformin induces
a dietary restriction-like state and the oxidative stress response
to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1.
PLoS One. 5:e87582010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhahbi JM, Mote PL, Fahy GM and Spindler
SR: Identification of potential caloric restriction mimetics by
microarray profiling. Physiol Genomics. 23:343–350. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shintani T, Sakoguchi H, Yoshihara A,
Izumori K and Sato M: d-Allulose, a stereoisomer of d-fructose,
extends Caenorhabditis elegans lifespan through a dietary
restriction mechanism: A new candidate dietary restriction mimetic.
Biochem Biophys Res Commun. 493:1528–1533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Lutz PB, Pepelyayeva Y, Arnér ES,
Bayse CA and Rozovsky S: Redox active motifs in selenoproteins.
Proc Natl Acad Sci USA. 111:6976–6981. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brenneisen P, Steinbrenner H and Sies H:
Selenium, oxidative stress, and health aspects. Mol Aspects Med.
26:256–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kleinman WA and Richie JP Jr: Status of
glutathione and other thiols and disulfides in human plasma.
Biochem Pharmacol. 60:19–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tapiero H, Townsend DM and Tew KD: The
antioxidant role of selenium and seleno-compounds. Biomed
Pharmacother. 57:134–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Q, Pan Y, Cheng Y and Li H and Li H:
Protection of rat liver against hepatic ischemia-reperfusion injury
by a novel selenocysteine-containing 7-mer peptide. Mol Med Rep.
14:2007–2015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balaban H, Nazıroğlu M, Demirci K and Övey
İS: The protective role of selenium on scopolamine-induced memory
impairment, oxidative stress, and apoptosis in aged rats: The
involvement of TRPM2 and TRPV1 channels. Mol Neurobiol.
54:2852–2868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Puppala AK, French RL, Matthies D, Baxa U,
Subramaniam S and Simonović M: Structural basis for early-onset
neurological disorders caused by mutations in human selenocysteine
synthase. Sci Rep. 6:325632016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wollenhaupt SG, Soares AT, Salgueiro WG,
Noremberg S, Reis G, Viana C, Gubert P, Soares FA, Affeldt RF,
Lüdtke DS, et al: Seleno- and telluro-xylofuranosides attenuate
Mn-induced toxicity in C. elegans via the DAF-16/FOXO
pathway. Food Chem Toxicol. 64:192–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stenvall J, Fierro-González JC, Swoboda P,
Saamarthy K, Cheng Q, Cacho-Valadez B, Arnér ES, Persson OP,
Miranda-Vizuete A and Tuck S: Selenoprotein TRXR-1 and GSR-1 are
essential for removal of old cuticle during molting in
Caenorhabditis elegans. Proc Natl Acad Sci USA.
108:1064–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JS, Kim SH and Park SK: Selenocysteine
modulates resistance to environmental stress and confers anti-aging
effects in C. elegans. Clinics (Sao Paulo). 72:491–498.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kamath RS, Fraser AG, Dong Y, Poulin G,
Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et
al: Systematic functional analysis of the Caenorhabditis
elegans genome using RNAi. Nature. 421:231–237. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peto R and Peto J: Asymptotically
efficient rank invariant test procedures. JSTOR. 135:pp185–207.
1972.
|
|
31
|
Johnson TE: Increased life-span of age-1
mutants in Caenorhabditis elegans and lower Gompertz rate of
aging. Science. 249:908–912. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong A, Boutis P and Hekimi S: Mutations
in the clk-1 gene of Caenorhabditis elegans affect
developmental and behavioral timing. Genetics. 139:1247–1259.
1995.PubMed/NCBI
|
|
33
|
Bishop NA and Guarente L: Two neurons
mediate diet-restriction-induced longevity in C. elegans.
Nature. 447:545–549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du Z, Santella A, He F, Tiongson M and Bao
Z: De novo inference of systems-level mechanistic models of
development from live-imaging-based phenotype analysis. Cell.
156:359–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SK, Tedesco PM and Johnson TE:
Oxidative stress and longevity in Caenorhabditis elegans as
mediated by SKN-1. Aging Cell. 8:258–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang B, Ren C, Li Y, Lu Y, Li W, Wu Y,
Gao Y, Ratcliffe PJ, Liu H and Zhang C: Sodium sulfite is a
potential hypoxia inducer that mimics hypoxic stress in
Caenorhabditis elegans. J Biol Inorg Chem. 16:267–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masse I, Molin L, Billaud M and Solari F:
Lifespan and dauer regulation by tissue-specific activities of
Caenorhabditis elegans DAF-18. Dev Biol. 286:91–101. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barger JL, Walford RL and Weindruch R: The
retardation of aging by caloric restriction: Its significance in
the transgenic era. Exp Gerontol. 38:1343–1351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cohen E and Dillin A: The insulin paradox:
Aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci.
9:759–767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henderson ST and Johnson TE: daf-16
integrates developmental and environmental inputs to mediate aging
in the nematode Caenorhabditis elegans. Curr Biol.
11:1975–1980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ihara A, Uno M, Miyatake K, Honjoh S and
Nishida E: Cholesterol regulates DAF-16 nuclear localization and
fasting-induced longevity in C. elegans. Exp Gerontol.
87:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mayurasakorn K, Hasanah N, Homma T, Homma
M, Rangel IK, Garza AE, Romero JR, Adler GK, Williams GH and Pojoga
LH: Caloric restriction improves glucose homeostasis, yet increases
cardiometabolic risk in caveolin-1-deficient mice. Metabolism.
83:92–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schlernitzauer A, Oiry C, Hamad R, Galas
S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G,
Feillet-Coudray C, et al: Chicoric acid is an antioxidant molecule
that stimulates AMP kinase pathway in L6 myotubes and extends
lifespan in Caenorhabditis elegans. PLoS One. 8:e787882013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Willcox BJ, Willcox DC and Suzuki M:
Demographic, phenotypic, and genetic characteristics of
centenarians in Okinawa and Japan: Part 1-centenarians in Okinawa.
Mech Ageing Dev. 165:75–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SK, Link CD and Johnson TE: Life-span
extension by dietary restriction is mediated by NLP-7 signaling and
coelomocyte endocytosis in C. elegans. FASEB J. 24:383–392.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greer EL and Brunet A: Different dietary
restriction regimens extend lifespan by both independent and
overlapping genetic pathways in C. elegans. Aging Cell.
8:113–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Honjoh S, Yamamoto T, Uno M and Nishida E:
Signalling through RHEB-1 mediates intermittent fasting-induced
longevity in C. elegans. Nature. 457:726–730. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baur JA: Resveratrol, sirtuins, and the
promise of a DR mimetic. Mech Ageing Dev. 131:261–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mouchiroud L, Houtkooper RH, Moullan N,
Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M,
Schoonjans K, et al: The NAD(+)/Sirtuin pathway modulates longevity
through activation of mitochondrial UPR and FOXO signaling. Cell.
154:430–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blagosklonny MV: Calorie restriction:
Decelerating mTOR-driven aging from cells to organisms (including
humans). Cell Cycle. 9:683–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Flynn JM, O'Leary MN, Zambataro CA,
Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong
R, Rosen CJ, et al: Late-life rapamycin treatment reverses
age-related heart dysfunction. Aging Cell. 12:851–862. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Halloran J, Hussong SA, Burbank R,
Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R,
Richardson A, Hart MJ and Galvan V: Chronic inhibition of mammalian
target of rapamycin by rapamycin modulates cognitive and
non-cognitive components of behavior throughout lifespan in mice.
Neuroscience. 223:102–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tatar M, Bartke A and Antebi A: The
endocrine regulation of aging by insulin-like signals. Science.
299:1346–1351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oh SI and Park SK: N-acetyl-l-cysteine
mimics the effect of dietary restriction on lifespan and reduces
amyloid beta-induced toxicity in Caenorhabditis elegans.
Food Sci Biotechnol. 26:783–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim JS and Park SK: Supplementation of
S-ALLYL cysteine improves health span in Caenorhabditis
elegans. Biosci J. 33:411–421. 2017. View Article : Google Scholar
|
|
57
|
Halagappa VK, Guo Z, Pearson M, Matsuoka
Y, Cutler RG, Laferla FM and Mattson MP: Intermittent fasting and
caloric restriction ameliorate age-related behavioral deficits in
the triple-transgenic mouse model of Alzheimer's disease. Neurobiol
Dis. 26:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miller BF, Robinson MM, Reuland DJ, Drake
JC, Peelor FF III, Bruss MD, Hellerstein MK and Hamilton KL:
Calorie restriction does not increase short-term or long-term
protein synthesis. J Gerontol A Biol Sci Med Sci. 68:530–538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Masoro EJ, Yu BP and Bertrand HA: Action
of food restriction in delaying the aging process. Proc Natl Acad
Sci USA. 79:4239–4241. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
McKiernan SH, Bua E, McGorray J and Aiken
J: Early-onset calorie restriction conserves fiber number in aging
rat skeletal muscle. FASEB J. 18:580–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Selman C: Dietary restriction and the
pursuit of effective mimetics. Proc Nutr Soc. 73:260–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Boussageon R, Supper I, Bejan-Angoulvant
T, Kellou N, Cucherat M, Boissel JP, Kassai B, Moreau A, Gueyffier
F and Cornu C: Reappraisal of metformin efficacy in the treatment
of type 2 diabetes: A meta-analysis of randomised controlled
trials. PLoS Med. 9:e10012042012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bass TM, Weinkove D, Houthoofd K, Gems D
and Partridge L: Effects of resveratrol on lifespan in
Drosophila melanogaster and Caenorhabditis elegans.
Mech Ageing Dev. 128:546–552. 2007. View Article : Google Scholar : PubMed/NCBI
|