Introduction
General anesthesia has a great impact on the
development of infants and young children (1), and may contribute to the development
of learning disabilities (2,3). At
the early stage of development, anesthesia and surgery may produce
significant cell apoptosis, which reduces hippocampal long-term
potentiation (4,5), an important cellular mode of learning
and memory (6). A number of
anesthetics have been used for the general anesthesia of children.
Isoflurane has neuroprotective properties, rapidly induces
anesthesia and is metabolized (7,8).
Therefore, isoflurane is widely accepted for use in children.
However, neurotoxic effects of isoflurane have also been reported.
Spatial memory in young and adult animals was impaired following
isoflurane anesthesia (9,10). In an animal study, isoflurane
administration resulted in the appearance of a large number of
apoptotic cells and subsequent cognitive impairment (11). Therefore, it is of clinical
significance to identify compounds with the potential to inhibit
isoflurane toxicity.
Angiotensin II receptor type 2 (AT2R) is widely
expressed in the fetus and in newborns (12). In experimental autoimmune
encephalomyelitis models, AT2R is downregulated and an AT2R agonist
ameliorates the function of AT2R, which suggests that AT2R is
involved in the pathological processes of autoimmune
encephalomyelitis (13). Compound
(C) 21 is a non-peptide agonist of AT2R, which functions in blood
vessel endothelium dilation, inflammatory reaction inhibition and
the promotion neuronal repair and regeneration (14–16).
However, whether C21 inhibits cell apoptosis caused by isoflurane
in neonatal rats has not yet been confirmed.
The BCL2, apoptosis regulator (Bcl-2) is an oncogene
which is involved in mitochondria-dependent apoptosis regulation.
The expression of Bcl-2 inhibits apoptosis elicited by multiple
cytotoxic factors (17,18). In the current study, the protective
effects of C21 on isoflurane-induced apoptosis in neonatal rats
were investigated, and the underlying mechanisms were assessed. The
data revealed that C21 inhibited general anesthesia-induced
neuronal apoptosis, likely through promoting Bcl-2 expression.
Materials and methods
Animals and groups
Specific pathogen-free Sprague Dawley rats (n=8; 1:1
male:female; weight, ~252 g) were purchased from Shanghai Super
B&K Laboratory Animal Co., Ltd. (license number 2013-0016;
Shanghai, China) and maintained in a temperature-controlled
environment (25°C) and a humidity of 40–60% with a standard 12 h
light/dark cycle and ad libitum access to food and water.
All experimental procedures were approved by the ethics committee
of Guizhou Medical University (Guiyang, China). A total of 18 pups
from four litters were randomly divided into three groups (n=6 in
each group; 1:1 male:female): i) Control group, ii) isoflurane
group and iii) a C21 treatment group. Post-natal day 7 rats inhaled
1.3% isoflurane (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
3 h each day for a consecutive 3 days. Following the administration
of isoflurane, rats in the C21 treatment group received 0.1 ml C21
(1 µg/kg; cat. no. C160; Sigma-Aldrich; Merck KGaA)
intraperitoneally each day for a consecutive 4 days, while the rats
in the isoflurane group received a similar volume of saline.
Following treatment, the rats were anesthetized with 1% sodium
pentobarbital (45 mg/kg; intraperitoneal injection) and
decapitated. Rats weighed 10–15 g at the time of sacrifice. Fresh
brain tissues were collected for flow cytometry, enzyme-linked
immunosorbent assay (ELISA), reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis,
while the brain tissues were fixed for transmission electron
microscopy (TEM) and the terminal
deoxynucleotidyl-transferase-mediated dUTP nick end-labelling
(TUNEL) assay.
Flow cytometry
The cortex, hippocampus, amygdala and hypothalamus
were isolated and ground. A single cell suspension was prepared
following trypsinization. A metal mesh was applied to isolate the
single cells from the homogenates. Cells were centrifuged at 375 ×
g for 2 min at 4°C, and 5×105 cells were collected for
flow cytometry. Apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
kit [cat. no. AP101-100-kit; Hanzhou Multi Sciences (Lianke)
Biotech Co., Ltd., Hangzhou, China] according to the manufacturer's
protocol. Following staining with Annexin V-FITC (5 µl) and PI (10
µl) together for 5 min at room temperature, cells were detected
using a flow cytometer (NovoCyte 2060R, ACEA Biosciences, Inc., San
Diego, CA, USA) with excitation at 488 nm and emission at 530 nm.
Data were analyzed using FlowJo 10 (FlowJo LLC, Ashland, OR,
USA).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end-labelling
(TUNEL) staining
Collected brain tissues were fixed in 10% formalin
at 4°C for 24 h for the TUNEL assay. Cortex, hippocampus, amygdala
and hypothalamus were subsequently separated and cryoprotected in
30% sucrose for 1 h at 4°C, prior to slicing into 20 µm sections
with a freezing microtome. TUNEL staining (2 µl) was performed
using the ApopTag In Situ Apoptosis Detection kit (cat. no.
C1089; Beyotime Institute of Biotechnology, Shanghai, China) at
45°C for 2 h, following the manufacturer's protocol. Following
this, 3 drops of mounting medium containing
4′,6-diamidino-2-phenylindole (cat. no. ab104139; Abcam, Cambridge,
UK) was added and the slides were then covered. After staining, the
sections were imaged using fluorescence microscopy (Olympus
Corporation, Tokyo, Japan). Four fields in each slice were analyzed
and apoptotic cells were counted.
TEM
The bilateral hippocampus was fixed in 2.5%
glutaraldehyde at 4°C for 2 h, dehydrated in 70, 75, 80, 85 and 95%
ethanol, embedded in paraffin, solidified, and sectioned into 70 nm
slices. Following this, sections were stained with 3% uranyl
acetate and 3% lead citrate for 7 min at room temperature and
imaged using transmission electron microscopy (80 kV; JEM-1230;
JEOL, Ltd., Tokyo, Japan).
ELISA
Peroxisome proliferator-activated receptor (PPAR)-α
levels were detected with a PPAR-α ELISA kit (cat. no. ml003331;
MLbio, Shanghai, China) according to the manufacturer's protocol.
The reagents in the kit were kept at room temperature for 30 min. A
standard curve was established. Cortex, hippocampus, hypothalamus
and amygdala tissues were homogenized and centrifuged at 11,000 × g
for 10 min at 4°C and the supernatants were used in the ELISA. All
standard samples and test samples required three duplicates. A
blank control, without sample or enzyme reagent, was used. The
absorbance was detected at 450 nm using a microplate reader
(RT-6100; Rayto Life and Analytical Sciences Co., Ltd., Shenzen,
China).
RT-qPCR
mRNA was extracted from rat cortex, hippocampus,
hypothalamus and amygdala using a TRIzol® assay kit
(Thermo Fisher Scientific, Inc.). The mRNA was transcribed into
cDNA using a PrimeScript™ RT-PCR Kit (cat. no. DRR014A; Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol (25°C for 9 min, 37°C for 121 min and 85°C
for 5 min). SYBR Green (cat. no. HY-K0501; MedChemExpress, Monmouth
Junction, NJ, USA) was used to detect the expression level of Bcl-2
using cDNA as a template. qPCR was performed using the following
thermocycling conditions: Initial denaturation at 95°C for 10 min;
followed by 36 cycles at 95°C for 14 sec and 58°C for 1 min. The
2−ΔΔCq method was used to quantify the results as
previously described (19), and
relative expression was normalized to GAPDH. The primer sequences
are listed in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence
(5′-3′) | Primer length
(bp) | Product length
(bp) | Annealing temperature
(°C) |
|---|
| Bcl-2 | Forward |
GGCATCTTCTCCTTCCAGC | 19 | 265 | 58 |
|
| Reverse |
AGAGTTCCTCCACCACCGT | 19 |
|
|
| GAPDH | Forward |
GCAAGTTCAACGGCACAG | 18 | 141 | 58 |
|
| Reverse |
CGCCAGTAGACTCCACGAC | 19 |
|
|
Western blot analysis
Homogenates from the cortex, hippocampus, amygdala
and hypothalamus were obtained from each group and lysed using an
illustra triplePrep Kit (cat. no. 28-9425-44; GE Healthcare Life
Sciences). The protein levels were detected using a bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology).
Protein samples were separated by 12% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with
Tris-buffered saline containing 0.1% Tween 20 and 5% fat-free milk
for 2 h at room temperature, and were subsequently incubated
overnight at 4°C with rabbit antibodies against Bcl-2 (1:1,500;
cat. no. bs-0032R; BIOSS, Beijing, China) and GAPDH (cat. no. A007;
1:1,000; ABclonal Biotech Co., Ltd., Woburn, MA, USA). Following
this, membranes were incubated with peroxidase-conjugated goat
anti-rabbit immunoglobulin G (1:5,000; cat. no. TA140003; OriGene
Technologies, Inc., Beijing, China) for 2 h at room temperature. A
chemiluminescent substrate detection reagent (cat. no. RPN2133; GE
Healthcare Life Sciences) was applied to assist with staining. The
target band was analyzed using Quantity One version 1.4.6 (Bio-Rad
Laboratories, Inc.) for densitometric analysis.
Data analysis
The data are expressed as the mean ± standard
deviation and were statistically analyzed using SPSS 19 (IBM Corp.,
Armonk, NY, USA). One-way analysis of variance followed by
Newman-Keuls post-hoc test was applied to determine statistical
significance. All experiments were repeated six times. P<0.05
was considered to indicate a statistically significant
difference.
Results
C21 prevents isoflurane-induced
neuronal apoptosis
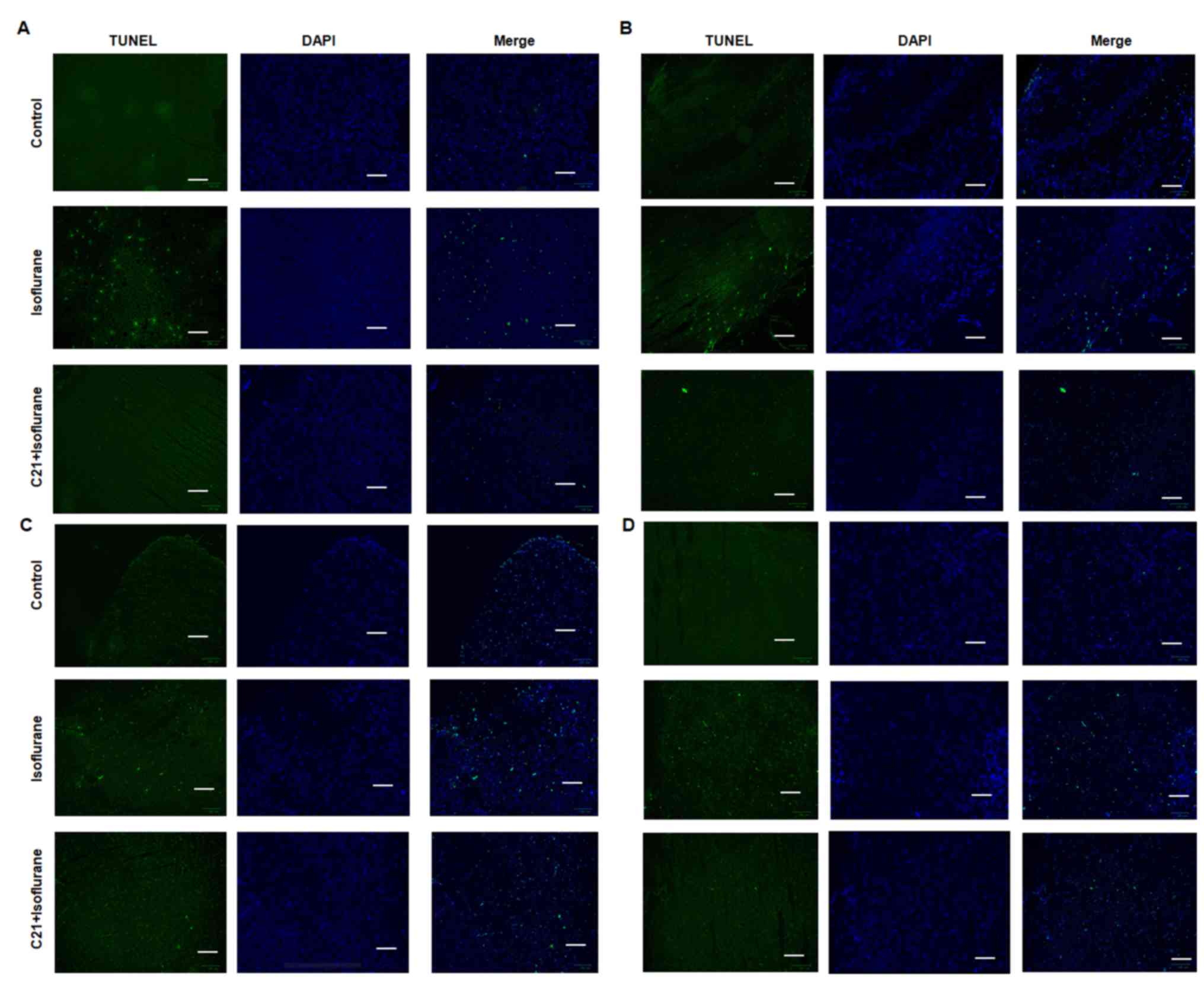
The TUNEL assay demonstrated that postnatal
administration of isoflurane elicited marked apoptosis in the
cortex, hippocampus, amygdala and hypothalamus. By contrast, C21
treatment appeared to reduce apoptosis in these four regions
(Fig. 1). The apoptotic rates in
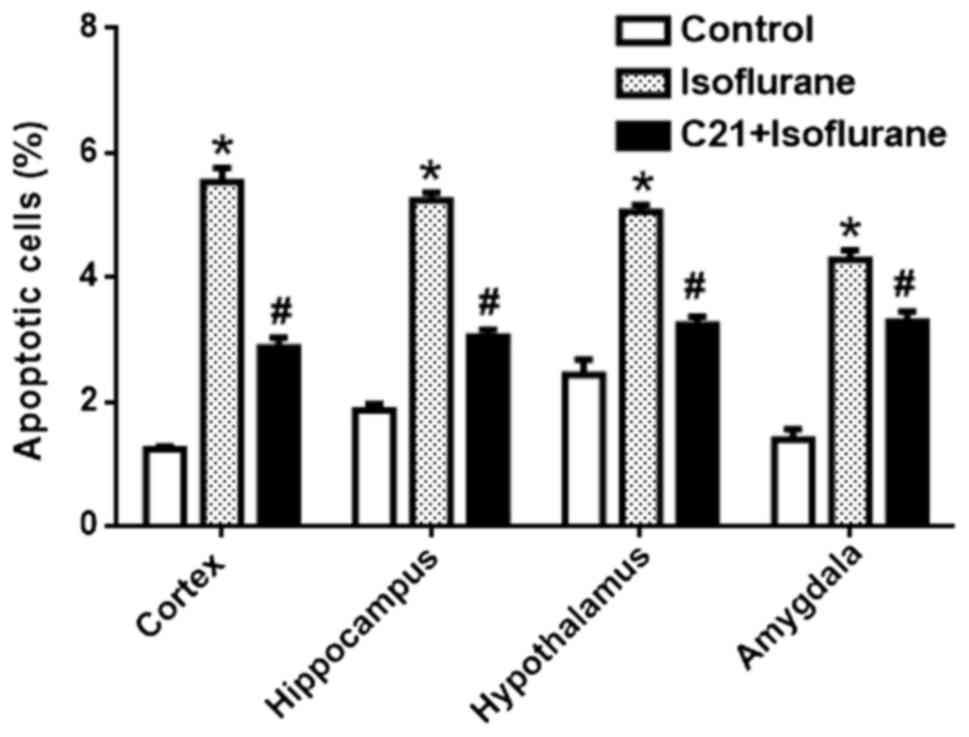
each group were calculated (Fig.
2). In the control groups, the apoptotic rates were 1.23%
(cortex), 1.86% (hippocampus), 2.43% (hypothalamus) and 1.40%
(amygdala). The apoptotic rates in the isoflurane group were 5.53%
(cortex), 5.24% (hippocampus), 5.05% (hypothalamus) and 4.28%
(amygdala). The apoptotic rates in the C21+Isoflurane group were
2.87% (cortex), 3.05% (hippocampus), 3.15% (hypothalamus) and 3.29%
(amygdala).
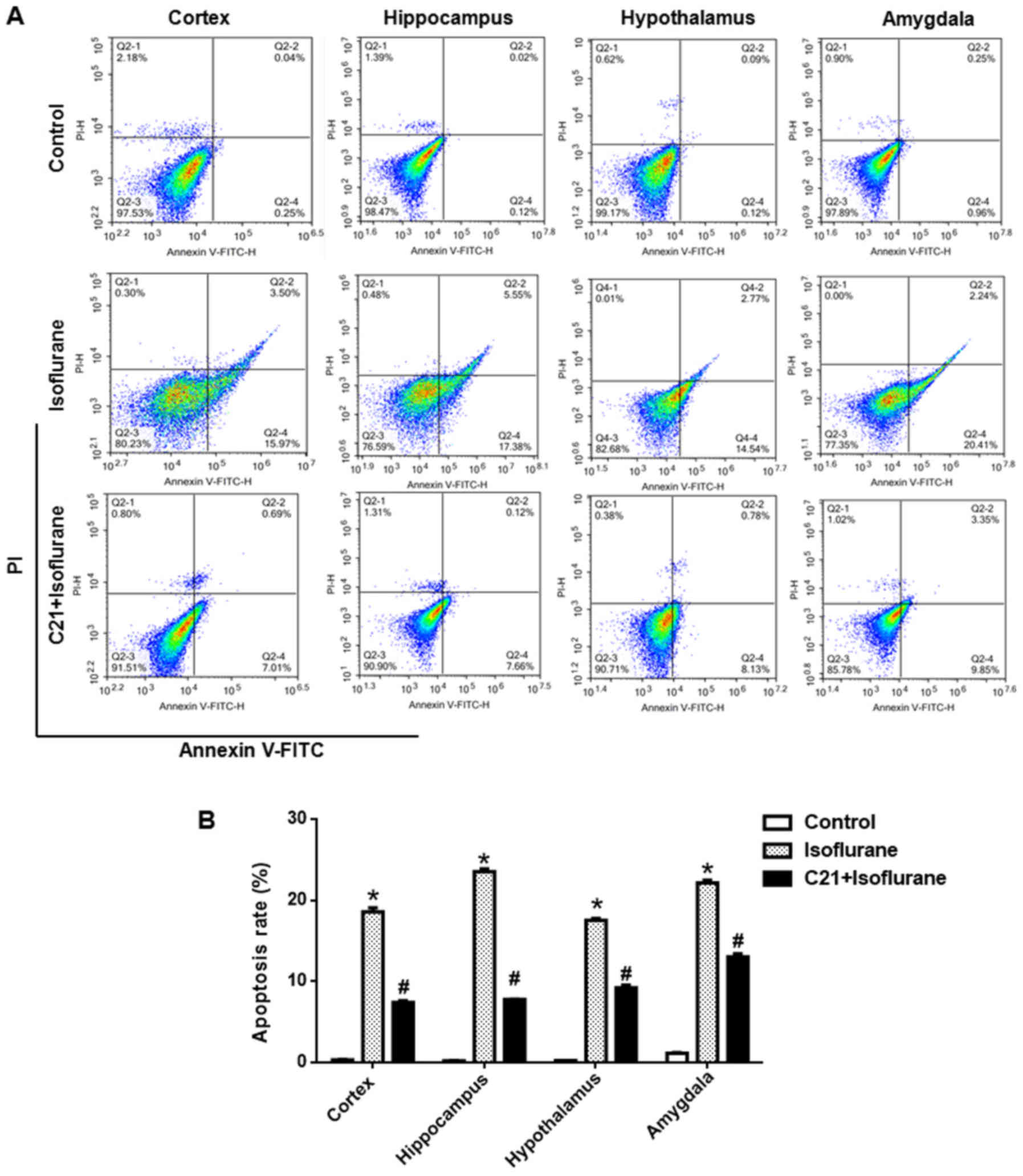
Apoptosis was further confirmed by flow cytometry.
As shown in Fig. 3, approximately
20% of cells were apoptotic in the isoflurane group, which was
significantly reduced by C21 treatment (~8%).
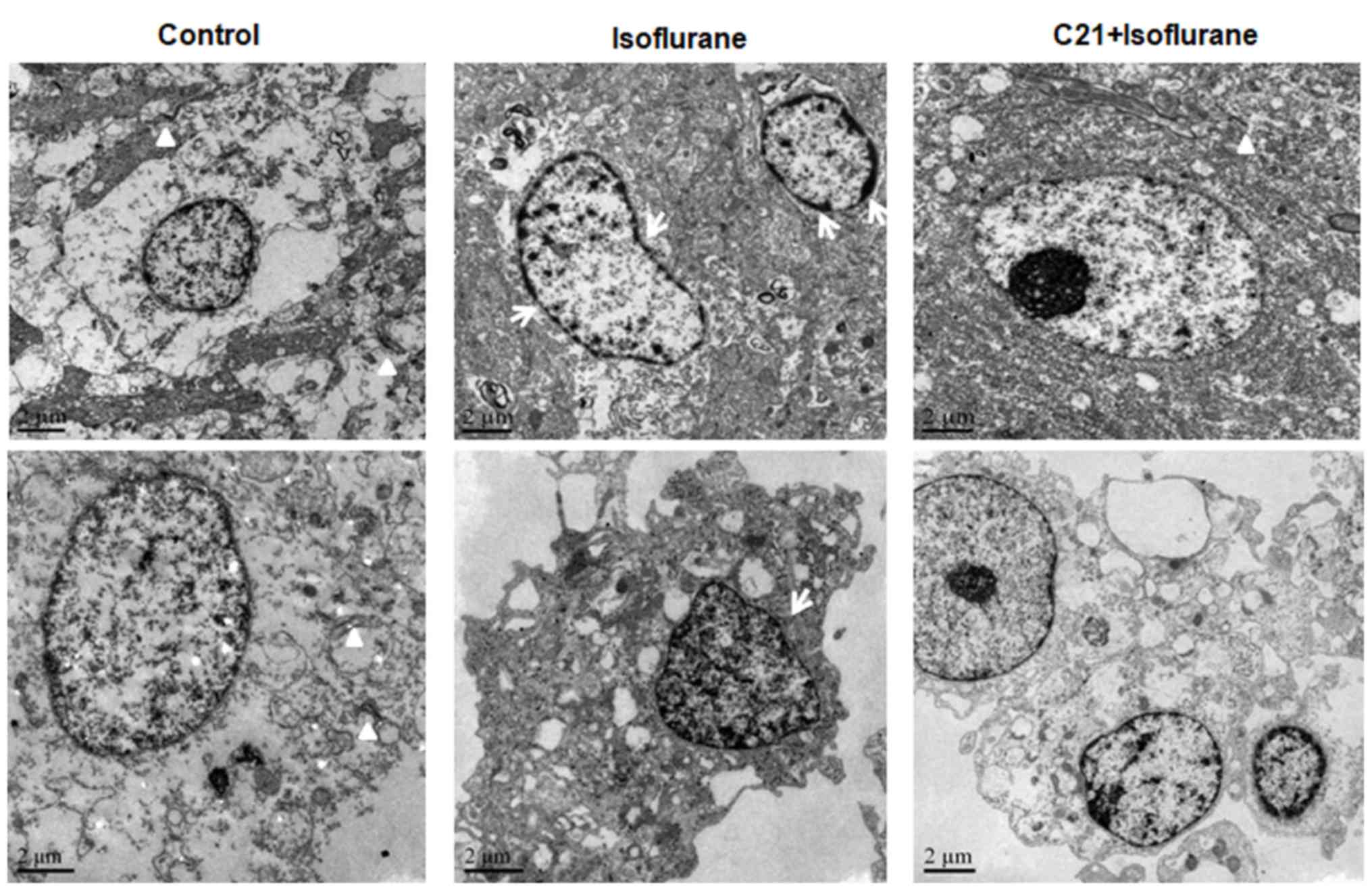
TEM further confirmed that isoflurane induced
apoptosis in the hippocampus, which was ameliorated by C21
treatment (Fig. 4). The nuclei of
the cells in the control group were round and transparent. The
nuclei of the cells in the isoflurane group were shrunken, and
condensed chromatin was evident. A marked reduction in synapse
numbers was observed in the isoflurane group, but not in the
control group. By contrast, the nuclear shrinkage and decrease in
synapse number was less prominent in the C21 group.
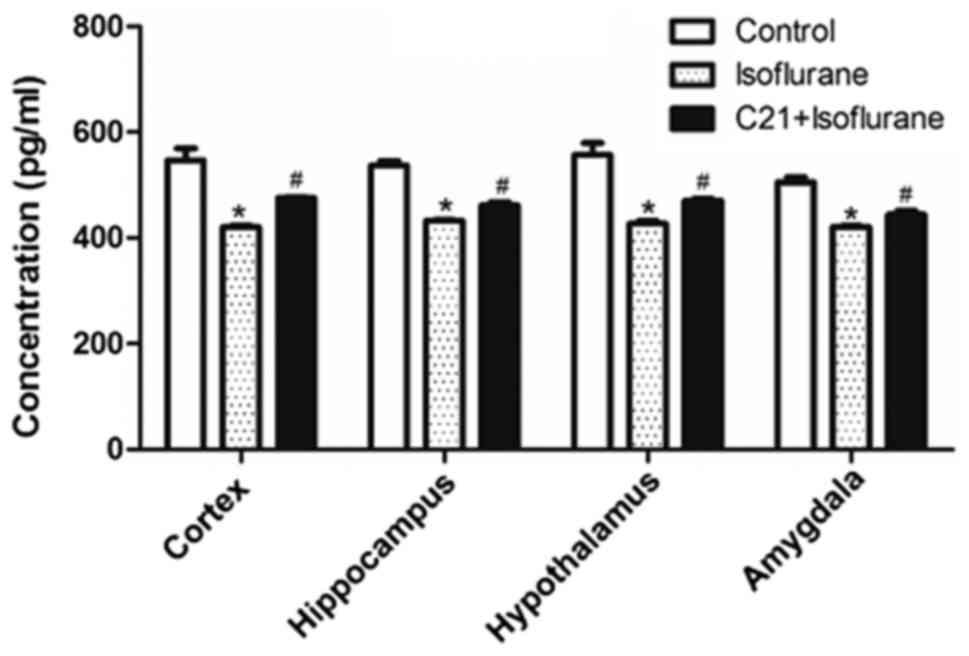
C21 increases PPAR-α expression
PPAR-α levels in several brain regions were measured
using ELISA. As shown in Fig. 5,
isoflurane exposure significantly reduced PPAR-α levels in the four
selected regions, compared with the control. By contrast, C21
treatment significantly elevated PPAR-α levels compared with the
isoflurane group.
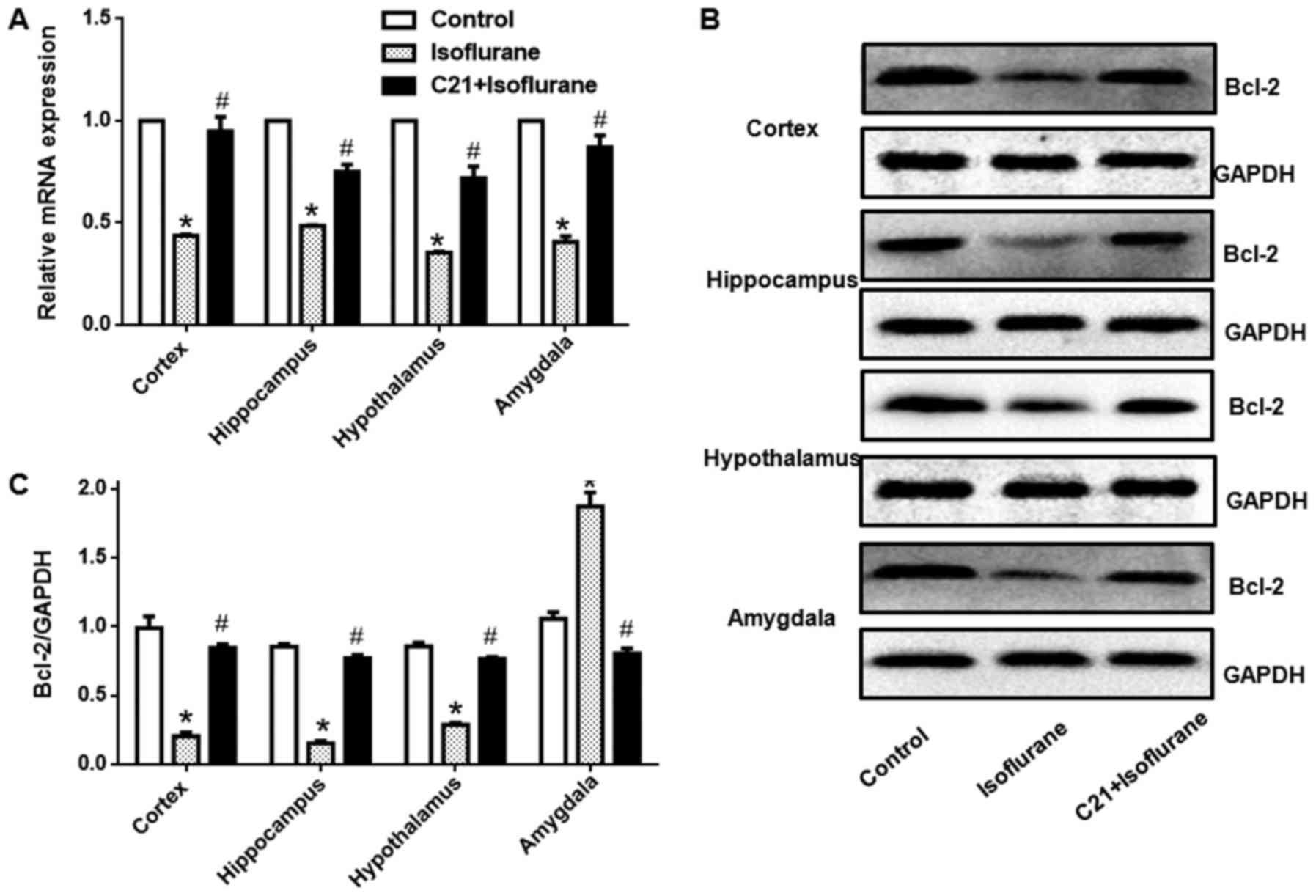
C21 increases Bcl-2 expression
Bcl-2 expression in different regions was detected
at both the mRNA and protein level. Fig. 6 shows that, isoflurane exposure
significantly reduced Bcl-2 mRNA levels in the four selected
regions. Additionally, C21 treatment significantly elevated Bcl-2
mRNA expression. Western blot analysis also demonstrated that
isoflurane exposure significantly reduced Bcl-2 protein expression
in the four selected regions. Furthermore, C21 treatment
significantly increased Bcl-2 protein expression.
Discussion
General anesthetics, not only affect the development
of neurons, but also damage them (4,5).
Previous findings have demonstrated that general anesthetics cause
extensive cell death in the brain, reduce the number of brain cells
and decrease synaptic function, resulting in cognitive impairment,
and this damage extends to adulthood (20,21).
Neonatal rats are the most commonly used model to investigate
neurodevelopment (22). The
present study investigated the therapeutic effect of C21 on
isoflurane-induced apoptosis in neonatal rats. The results revealed
that isoflurane exposure caused marked apoptosis in the cortex,
hippocampus, amygdala and hypothalamus.
C21 is an AT2R agonist, which has vasodilatory
activity, and may protect neuronal function (23). C21-mediated neuroprotection has
been widely reported in ischemic injury, vascular dementia and
diabetes (24–26). In addition, this neuroprotective
effect is likely mediated through an AT2R-dependent pathway or an
AT2R-independent pathway (15). In
the current study, AT2R expression was not directly measured, in
part due to the limitations of available techniques to localize
this receptor. However, the neuroprotective activity of C21 in
general anesthesia-induced cerebral injury was observed. Synapse
number is closely associated with synaptic function, as well as
learning and memory (27). TEM
analysis revealed that the number of synapses was reduced by
isoflurane treatment. C21 treatment increased the number of
synapses, which suggested that C21 improved impaired axon and
synapse development, which was induced by isoflurane exposure.
Neuronal apoptosis is an important component of neurotoxicity
(28,29). In immature animal neurons, general
anesthesia produces severe cytotoxicity, particularly in synaptic
growth (20,21). In the present study, two different
methods were utilized to detect apoptosis. A similar trend in each
group was observed in the two methods, with C21 treatment
attenuating the apoptosis induced by isoflurane. However, the
apoptotic rates detected via the two methods were inconsistent,
which may have been caused by the different analysis methods
utilized in the present study. Flow cytometry may be more effective
in determining total apoptosis.
Mitochondrial structure and Bcl-2 family protein
expression at the outer membrane destroys mitochondrial
permeability transition and mitochondrial pore function. Isoflurane
activates the mitochondria-dependent apoptosis pathway and
downregulates anti-apoptotic protein B cell lymphoma-extra large
expression in the immature brain (30,31).
In the current study, TUNEL and flow cytometry were used to detect
apoptosis in the cerebral cortex, hippocampus, amygdala and
hypothalamus, and apoptosis in the isoflurane group was
significantly increased. These results suggested that apoptosis may
have been a major cause of the impairment of neurodevelopment
following isoflurane exposure. Notably, apoptosis was ameliorated
by C21 treatment. These results suggested that C21 had a protective
effect against neuronal apoptosis induced by isoflurane. Previous
studies have also demonstrated that isoflurane exposure reduces
Bcl-2 expression and elicits apoptosis in cerebral tissue (32). Consistent with these findings, the
results of the current study also confirmed that the expression of
Bcl-2 in the isoflurane groups was significantly decreased. In
addition, the present results suggested that C21 protected neonatal
rat brain tissue by increasing the expression of Bcl-2.
PPAR-α is a recently discovered target for vascular
pathological alterations (33). It
is a ligand-activated transcription factor that belongs to the
nuclear hormone receptor superfamily. When PPAR binds with its
ligand, it promotes the gene transcription of target cells, and
regulates cell cycle, differentiation and apoptosis in various cell
types, thereby regulating blood glucose, lipid metabolism and cell
differentiation (34). Certain
studies have demonstrated that PPAR-α has anti-inflammatory and
anti-apoptotic effects (35,36).
The results of the current study demonstrated that isoflurane
decreased PPAR-α levels in the cerebral cortex, hippocampus,
amygdala and hypothalamus. This revealed that isoflurane may have
impaired neurodevelopment through PPAR-α expression regulation.
Notably, C21 treatment elevated PPAR-α levels in the cerebral
cortex, hippocampus, amygdala and hypothalamus. However, the
mechanism through which C21 activated PPAR-α still requires further
clarification. Based on these data, C21 may be effective in
clinically preventing the adverse effects induced by isoflurane
anesthesia. However, further studies using animal models as well as
clinical studies are required prior to the administration of C21 to
children.
In summary, isoflurane damaged the neonatal rat
brain by altering the expression of Bcl-2 in the brain, and this
was ameliorated by C21 administration. The present study provided a
theoretical basis for the safer clinical use of anesthetics.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology fund of Guizhou Province (grant no. LH-2015-7394).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JY, LY, JW and HX performed the experiments and
analyzed the data. QZ designed the study and wrote the
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
ethics committee of Guizhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng M, Hofacer RD, Jiang C, Joseph B,
Hughes EA, Jia B, Danzer SC and Loepke AW: Brain regional
vulnerability to anaesthesia-induced neuroapoptosis shifts with age
at exposure and extends into adulthood for some regions. Br J
Anaesth. 113:443–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Dong Y, Zhou C, Zhang Y and Xie
Z: Anesthetic sevoflurane reduces levels of hippocalcin and
postsynaptic density protein 95. Mol Neurobiol. 51:853–863. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao T, Li Y, Wei W, Savage S, Zhou L and
Ma D: Ketamine administered to pregnant rats in the second
trimester causes long-lasting behavioral disorders in offspring.
Neurobiol Dis. 68:145–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng Y, He L, Prasad V, Wang S and Levy
RJ: Anesthesia-induced neuronal apoptosis in the developing retina:
A window of opportunity. Anesth Analg. 121:1325–1335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noguchi KK, Johnson SA, Dissen GA, Martin
LD, Manzella FM, Schenning KJ, Olney JW and Brambrink AM:
Isoflurane exposure for three hour triggers apoptotic cell death in
neonatal macaque brain. Br J Anaesth. 119:524–531. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu G, Liu Y, Wang Y, Bi X and Baudry M:
Different patterns of electrical activity lead to long-term
potentiation by activating different intracellular pathways. J
Neurosci. 35:621–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao DA, Bi LY, Huang Q, Zhang FM and Han
ZM: Isoflurane provides neuroprotection in neonatal hypoxic
ischemic brain injury by suppressing apoptosis. Braz J Anesthesiol.
66:613–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sosunov SA, Ameer X, Niatsetskaya ZV,
Utkina-Sosunova I, Ratner VI and Ten VS: Isoflurane anesthesia
initiated at the onset of reperfusion attenuates oxidative and
hypoxic-ischemic brain injury. PLoS One. 10:e01204562015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalkman CJ, Peelen L, Moons KG, Veenhuizen
M, Bruens M, Sinnema G and de Jong TP: Behavior and development in
children and age at the time of first anesthetic exposure.
Anesthesiology. 110:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flick RP, Wilder RT, Sprung J, Katusic SK,
Voigt R, Colligan R, Schroeder DR, Weaver AL and Warner DO:
Anesthesia and cognitive performance in children: No evidence for a
causal relationship. Are the conclusions justified by the data?
Response to Bartels et al, 2009. Twin Res Hum Genet.
12:611–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Wei K, Hu R, Zhang B, Li L, Wan L,
Zhang C and Yao W: Upregulation of Cdh1 attenuates
isoflurane-induced neuronal apoptosis and long-term cognitive
impairments in developing rats. Front Cell Neurosci. 11:3682017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Villela D, Leonhardt J, Patel N, Joseph J,
Kirsch S, Hallberg A, Unger T, Bader M, Santos RA, Sumners C and
Steckelings UM: Angiotensin type 2 receptor (AT2R) and receptor
Mas: A complex liaison. Clin Sci. 128:227–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valero-Esquitino V, Lucht K, Namsolleck P,
Monnet-Tschudi F, Stubbe T, Lucht F, Liu M, Ebner F, Brandt C,
Danyel LA, et al: Direct angiotensin type 2 receptor (AT2R)
stimulation attenuates T-cell and microglia activation and prevents
demyelination in experimental autoimmune encephalomyelitis in mice.
Clin Sci (Lond). 128:95–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Del Borgo M, Lee HW, Baraldi D,
Hirmiz B, Gaspari TA, Denton KM, Aguilar MI, Samuel CS and Widdop
RE: Anti-fibrotic potential of AT2 receptor agonists.
Front Pharmacol. 8:5642017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennion DM, Isenberg JD, Harmel AT, DeMars
K, Dang AN, Jones CH, Pignataro ME, Graham JT, Steckelings UM,
Alexander JC, et al: Post-stroke angiotensin II type 2 receptor
activation provides long-term neuroprotection in aged rats. PLoS
One. 12:e01807382017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandey A and Gaikwad AB: Compound 21 and
Telmisartan combination mitigates type 2 diabetic nephropathy
through amelioration of caspase mediated apoptosis. Biochem Biophys
Res Commun. 487:827–833. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Um HD: Bcl-2 family proteins as regulators
of cancer cell invasion and metastasis: A review focusing on
mitochondrial respiration and reactive oxygen species. Oncotarget.
7:5193–5203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng JH, Follis Viacava A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YL, Chen X and Wang ZP: Detrimental
effects of postnatal exposure to propofol on memory and hippocampal
LTP in mice. Brain Res. 1622:321–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YL, Li F and Chen X: Pten
inhibitor-bpV ameliorates early postnatal propofol exposure-induced
memory deficit and impairment of hippocampal LTP. Neurochem Res.
40:1593–1599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Yang S and Zhu G: Postnatal calpain
inhibition elicits cerebellar cell death and motor dysfunction.
Oncotarget. 8:87997–88007. 2017.PubMed/NCBI
|
|
23
|
Koulis C, Chow BS, McKelvey M, Steckelings
UM, Unger T, Thallas-Bonke V, Thomas MC, Cooper ME, Jandeleit-Dahm
KA and Allen TJ: AT2R agonist, compound 21, is reno-protective
against type 1 diabetic nephropathy. Hypertension. 65:1073–1081.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwanami J, Mogi M, Tsukuda K, Steckelings
UM, Unger T, Thallas-Bonke V, Thomas MC, Cooper ME, Jandeleit-Dahm
KA, Allen TJ, et al: Possible synergistic effect of direct
angiotensin II type 2 receptor stimulation by compound 21 with
memantine on prevention of cognitive decline in type 2 diabetic
mice. Eur J Pharmacol. 724:9–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima
K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, et al: Direct
stimulation of angiotensin II type 2 receptor initiated after
stroke ameliorates ischemic brain damage. Am J Hypertens.
27:1036–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwanami J, Mogi M, Tsukuda K, Wang XL,
Nakaoka H, Kan-no H, Chisaka T, Bai HY, Shan BS, Kukida M and
Horiuchi M: Direct angiotensin II type 2 receptor stimulation by
compound 21 prevents vascular dementia. J Am Soc Hypertens.
9:250–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu G, Wang Y, Li J and Wang J: Chronic
treatment with ginsenoside Rg1 promotes memory and hippocampal
long-term potentiation in middle-aged mice. Neuroscience.
292:81–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozaki M, Deshpande SS, Angkeow P, Bellan
J, Lowenstein CJ, Dinauer MC, Goldschmidt-Clermont PJ and Irani K:
Inhibition of the Rac1 GTPase protects against nonlethal
ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB
J. 14:418–429. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lennon SV, Martin SJ and Cotter TG:
Dose-dependent induction of apoptosis in human tumour cell lines by
widely diverging stimuli. Cell Prolif. 24:203–214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Li DY, Zhao SM, Zheng ZJ, Hu J, Li
ZZ and Xiong SB: Rutin attenuates isoflurane-induced neuroapoptosis
via modulating JNK and p38 MAPK pathways in the hippocampi of
neonatal rats. Exp Ther Med. 13:2056–2064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monsalve FA, Pyarasani RD, Delgado-Lopez F
and Moore-Carrasco R: Peroxisome proliferator-activated receptor
targets for the treatment of metabolic diseases. Mediators Inflamm.
2013:5496272013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian G, Fan W, Ahlemeyer B, Karnati S and
Baumgart-Vogt E: Peroxisomes in different skeletal cell types
during intramembranous and endochondral ossification and their
regulation during osteoblast differentiation by distinct peroxisome
proliferator-activated receptors. PLoS One. 10:e01434392015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fong WH, Tsai HD, Chen YC, Wu JS and Lin
TN: Anti-apoptotic actions of PPAR-gamma against ischemic stroke.
Mol Neurobiol. 41:180–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crisafulli C, Bruscoli S, Esposito E,
Mazzon E, Di Paola R, Genovese T, Bramanti P, Migliorati G and
Cuzzocrea S: PPAR-α contributes to the anti-inflammatory activity
of 17β-estradiol. J Pharmacol Exp Ther. 331:796–807. 2009.
View Article : Google Scholar : PubMed/NCBI
|