Introduction
Osteoclasts are multinucleated, exclusively
bone-absorbing cells, which share the same precursor cells with
macrophages and dendritic cells (1). Imbalance of osteoclastic bone
resorption and osteoblastic bone formation are associated with
multiple pathological disorders, including osteoporosis,
osteopenia, Crohn's disease, rheumatoid arthritis (RA) and
psoriatic arthritis (2–5). Immune cells profoundly influence the
pathogenesis of these diseases in multiple processes, as macrophage
precursors are able to differentiate into osteoclasts and multiple
inflammatory cells provide cytokines for the progression of
osteolysis (6,7). Since vital signaling factors
including interleukin (IL)-1β, tumor necrosis factor-α (TNF-α),
IL-6 and IL-17 have been revealed to give rise to osteoclast
differentiation and activation (8,9), the
strong association between inflammation and bone degradation at the
molecular signaling level has received extensive attention.
Bacterial lipopolysaccharide (LPS), one of the key
components of the outer membrane of Gram-negative bacteria, is able
to stimulate the innate immune system to initiate inflammatory
responses, exclusively through its pattern recognition receptor
Toll-like receptor 4 (TLR4) (10).
It is reported that LPS treatment substantially increases the mRNA
and protein expression levels of the osteoclast-associated genes
receptor activator of nuclear factor κ-B (RANK), TNF
receptor-associated factor 6 (TRAF6) and cyclooxygenase-2 in
Raw264.7 cells (11). Furthermore,
the activation of TLRs is able to suppress expression of RANK in
human primary cell cultures, therefore attenuating the activity of
RANK ligand (RANKL) and macrophage colony-stimulating factor
mediated pathways, consequently resulting in the inhibition of
osteoclastogenesis (12). The
intriguing results concerning the function of TLRs in addition to
other inflammatory factors in osteoclastogenesis indicates a
homeostasis of these molecules and their feedback regulation
(13).
MicroRNAs (miRNAs/miRs) are a group of highly
conserved, single-stranded, small noncoding RNA molecules that
regulate gene expression in a post-transcriptional manner.
Accumulating evidence has demonstrated that miRNAs serve a critical
role in regulating immune responses and inflammation (14,15).
Previously, a number of studies have revealed that miRNAs are
involved in the development and differentiation of bone cells. For
example, miR-223, which is almost exclusively expressed in mouse
bone marrow, is regulated by the enhancer modulators PU.1 and CAAT
(16). In addition, miR-125b,
miR-26a, miR-133 and miR-135 have all been implicated in the
differentiation of osteoblasts (17–19).
miR-146a is a typical multi-functional microRNA that
serves an important role in regulating multiple biological
processes in various types of cells (20,21).
The aberrant expression of miR-146a is frequently observed in
various diseases, for example rheumatoid arthritis (RA) (22). In the present study, the functions
of LPS and miR-146a in the development of osteoclast induced by
RANKL were investigated.
Materials and methods
Materials
LPS from Escherichia coli O55:B5 was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Recombinant murine
RANKL was purchased from PeproTech EC Ltd., (London, UK). A
tartrate-resistant acid phosphatase (TRAP) staining kit was
obtained from Kamiya Biomedical Company (Seattle, WA, USA).
Phosphorylated (Ser) p65, p65, phosphorylated (Ser) IKBα and TRAF6
antibodies were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA). IRAK1 and GAPDH antibodies were purchased from
Abcam (Cambridge, UK). Primers were synthesized by Sangon Biotech
Co., Ltd., (Shanghai, China) and the miR-146a mimic was purchased
from Shanghai GenePharma, Co., Ltd., (Shanghai, China). TaqMan
dye-labeled probes were purchased from Applied Biosystems (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture and transfection. The murine macrophage
cell line Raw264.7 was obtained from the American Type Culture
Collection (Manassas, VA, USA) and maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% heat
inactivated fetal calf serum (HIFCS) penicillin (100 U/ml) and
streptomycin (100 µg/ml; all from Gibco; Thermo Fisher scientific,
Inc.) at 37°C under 5% CO2. For osteoclast cultures,
cells were cultured in α-Minimum Essential Medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc.) with 10% HIFCS in the presence of
RANKL (30 ng/ml), LPS (100 ng/ml), or together.
For transfection, the synthetic miR-146a mimics,
miR-146a inhibitor, or negative control were transfected into cells
using Lipofectamine® RNAiMAX (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Sequences of the
miR-146a mimics were 5′-UGAGAACUGAAUUCCAUGGGUU-3′ and
5′-CCCAUGGAAUUCAGUUCUCAUU-3′ (antisense), negative control sequence
were 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGGAATT-3′
(antisense). miR-146a inhibitor was a 29-O methylated
single-stranded miR-146a antisense oligonucleotides, the sequence
was 5′-AACCCAUGGAAUUCAGUUCUCA-3′, and that of the negative control
for the inhibitor was 5′-CAGUACUUUUGUGUAGUACAA-3′. In brief, 5×103
cells were seeded into a 48-well plate 24 h prior to transfection.
The next day, 200 nM miRNA mimic, miRNA inhibitor, or negative
control were transfected into cells. Cells were then cultured for
an additional 3 days with RANKL (30 ng/ml) and LPS (100 ng/ml) to
collect RNA, protein or observe osteoclast differentiation.
TRAP staining assays
Using the K-ASSAY TRAP Staining kit, TRAP staining
was performed according to the manufacturer's protocol. In brief,
the medium was removed from the cells, which were then washed and
fixed for 5 min at room temperature. Then, the chromogenic
substrate was added to each well and cells were incubated at 37°C
for 60 min, washed by ddH2O. Cells were observed using a
Leica DM IRB inverted microscope. TRAP-positive multinuclear cells
containing >3 nuclei were classified as osteoclasts. Cell images
were obtained using LAS EZ software version 3.4.0.272 (Leica DMIL
LED Inverted microscope; Leica Microsystems GmbH, Wetzlar,
Germany). TRAP-positive cells were counted as the mean number of
positive cells of 6 fields of view under the microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
For RNA extraction, Raw264.7 cells were plated in a
6-well plate at a density of 5×104 cells/ml in α-MEM with 10%
HIFCS. Cells were treated with 30 ng/ml RANKL, 100 ng/ml LPS, a
combination or 200 nM miR-146a mimic. Total RNA was extracted using
the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For TNF-α, IL-6 and IL-1β
genes analysis, 300 ng total RNA was converted to cDNA using
PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. qPCR was performed
for different genes in separate wells (Singleplex assay) of a
384-well plate in a reaction volume of 5 µl using TB Green™ Premix
Ex Taq™ (Takara Biotechnology Co., Ltd., Dalian, China) and
reaction condition as following: 95°C for 15 sec, 1 cycle; 95°C for
5 sec, 60°C for 30 sec, 40 cycles. GAPDH served as the endogenous
control for all experiments. The primers used were as follows:
GAPDH forward, 5′-TGGATTTGGACGCATTGGTC-3′ and reverse,
5′-TTTGCACTGGTACGTGTTGAT-3′; 5′-ATCTTTTGGGGTCCGTCAACT-3′; TNF-α
forward, 5′-CCCTCACACTCAGATCATCTTCT-3′ and reverse,
5′-GCTACGACGTGGGCTACAG-3′; IL-6 forward,
5′-TAGTCCTTCCTACCCCAATTTCC-3′ and reverse,
5′-TTGGTCCTTAGCCACTCCTTC-3′; TRAF6 forward,
5′-AAAGCGAGAGATTCTTTCCCTG-3′ and reverse,
5′-ACTGGGGACAATTCACTAGAGC-3′; IRAK1 forward,
5′-AGCCGAGGTCTGCATTACATT-3′ and reverse,
5′-TGGCAGTCTGGATAACTGATGA-3′. For miRNA analysis, 50 ng total RNA
was converted to miRNA using the TaqMan™ MicroRNA Reverse
Transcription kit (Invitrogen; Thermo Fisher Scientific, USA)
according to the manufacturer's protocol. qPCR was performed for
different miRNA in separate wells (Singleplex assay) of a 384-well
plate in a reaction volume of 5 µl. Sno202 served as the endogenous
control for all experiments. TaqMan dye-labeled probes were
purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.).
The PCR reaction mixture was performed in an Applied Biosystems ABI
Prism 7300 sequence detection system instrument using reaction
condition as follows: 50°C for 2 min, 95°C for 10 min, 1 cycle;
95°C for 15 sec, 60°C for 1 min, 40 cycles. Analysis of relative
gene expression data was made using real-time quantitative PCR and
the 2−ΔΔCq method (23).
Western blotting
Cells were lysed using RIPA buffer (Thermo Fisher
Scientific, Inc.). Then, 20 µg lysate proteins were separated using
10% SDS-PAGE, transferred to polyvinylidene fluoride membranes,
blocked with 5% BSA in TBS solution at room temperature for 1 h and
probed with primary antibodies directed against phosphorylated
(Ser) p65 (cat. no. 3033; 1:1,000), p65 cat. no. 8242; 1:1,000),
phosphorylated (Ser) IKBα (cat. no. 2859; 1:1,000), TRAF6 (cat. no.
8028; 1:1,000; all Cell Signaling Technology, Inc.), IRAK1 (Abcam;
cat. no. ab238; 1:1,000) and GAPDH (cat. no. ab9485; 1:5,000;
Abcam) at 4°C overnight. The next day, the membranes were incubated
with anti-mouse IgG (cat. no. 7076; 1:5,000) or anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074; 1:5,000; both Cell Signaling
Technology, Inc.) at room temperature for 1 h and visualized with a
SuperSignal West Pico kit (Thermo Fisher Scientific, Inc.). All
results were normalized against GAPDH as loading controls.
Statistical analysis
GraphPad Prism version 5.0 (Graphpad Software, Inc.,
La Jolla, CA, USA) was used for data analysis. The reproducibility
of each experiment was confirmed by three independent experiments.
Bars represent the mean ± standard error of the mean. Differences
between groups were analyzed for statistical significance using an
unpaired Student's t-test or two-way analysis of variance followed
by Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
LPS exerts a synergic function in
promoting RANKL-induced osteoclast differentiation
RANKL is an essential regulator of osteoclast
differentiation and activation (24). LPS, the key component of the
Gram-negative bacterial cell wall, is a well-known stimulator of
inflammatory bone loss (25). To
investigate whether there is a synergic effect of LPS on
RANKL-induced osteoclast differentiation, murine macrophage
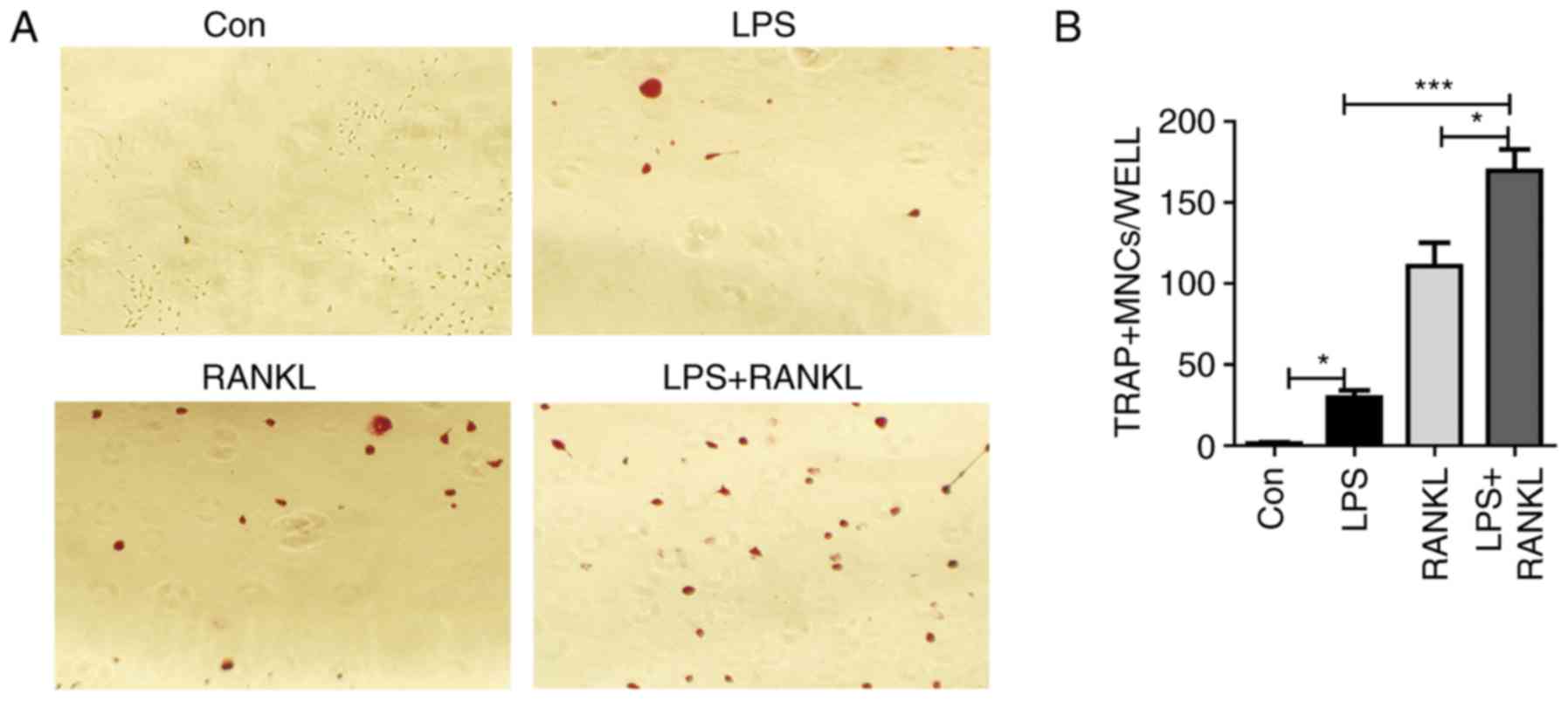
Raw264.7 cells were treated with LPS and/or RANKL. Consistent with
previous studies (11,26), Raw264.7 cells were able to form
multinucleated cells in response to either RANKL or LPS stimulation
(Fig. 1A). Results from
histological staining with TRAP confirmed the presence of
osteoclasts (Fig. 1A). In
addition, the number of osteoclasts generated from LPS and RANKL
combined treatment was significantly (LPS + RANKL vs. RANKL,
P<0.05; LPS + RANKL vs. LPS, P<0.001) increased compared with
stimulation with either treatment alone (Fig. 1B).
LPS stimulation triggers
pro-inflammatory cytokine production during osteoclastogenesis
One previous study has revealed that
pro-inflammatory cytokines serve a critical role in osteoclast
development and bone loss (27).
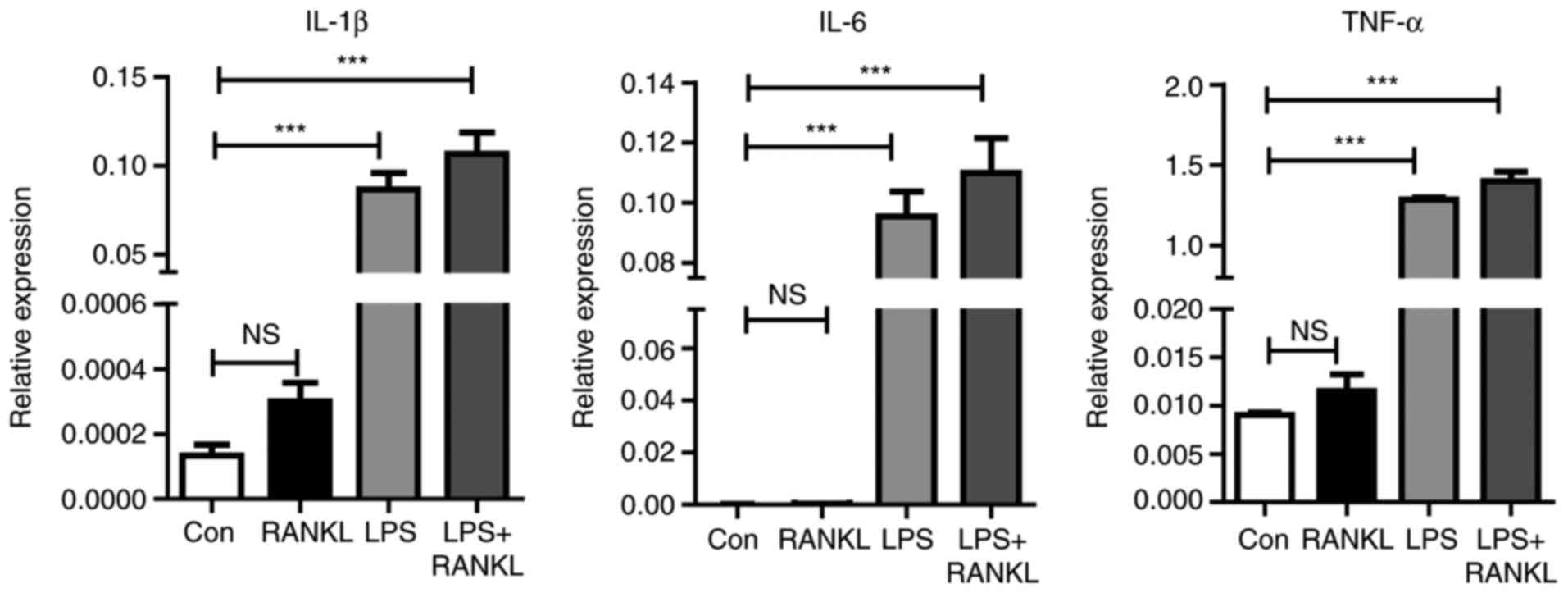
Therefore, to understand how LPS enhances osteoclast
differentiation induced by RANKL, cytokine expression during
osteoclastogenesis was assessed. As presented in Fig. 2, the expression levels of
inflammatory mediators, including IL-6, TNF-α and IL-1β were
significantly (P<0.001) increased following LPS stimulation
compared with the control.
miR-146a is highly induced during
osteoclastogenesis
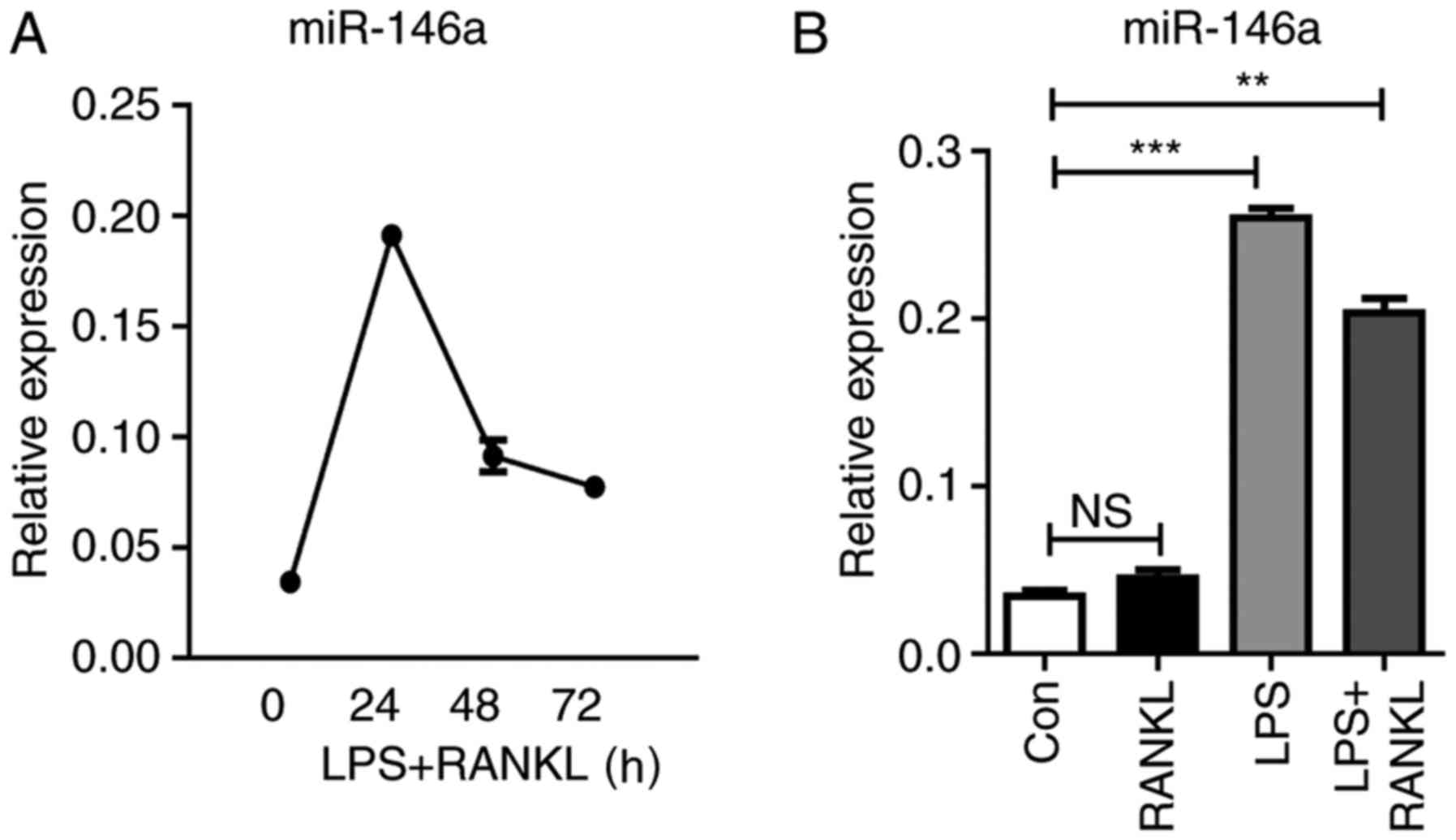
In response to LPS stimulation, Raw264.7 cells
express various types of miRNAs. Among them, miR-146a is highly
induced and serves a negative regulatory role in the TLR4 signaling
pathway (28). To investigate the
function of miR-146a in the development of osteoclasts, the present
study analyzed the kinetic change of miR-146a expression during
osteoclastogenesis. The expression levels of miR-146a were
continuously elevated within 24 h following LPS and RANKL
treatment, which then subsequently declined. In addition, it was
revealed that the combined treatment of LPS and RANKL reduced
miR-146a expression compared with LPS stimulation alone (Fig. 3).
miR-146a inhibits osteoclast
differentiation
To further elucidate the role of miR-146a in
osteoclast differentiation, the expression levels of miR-146a in
Raw264.7 cells were manipulated to demonstrate whether it could
affect the outcomes.
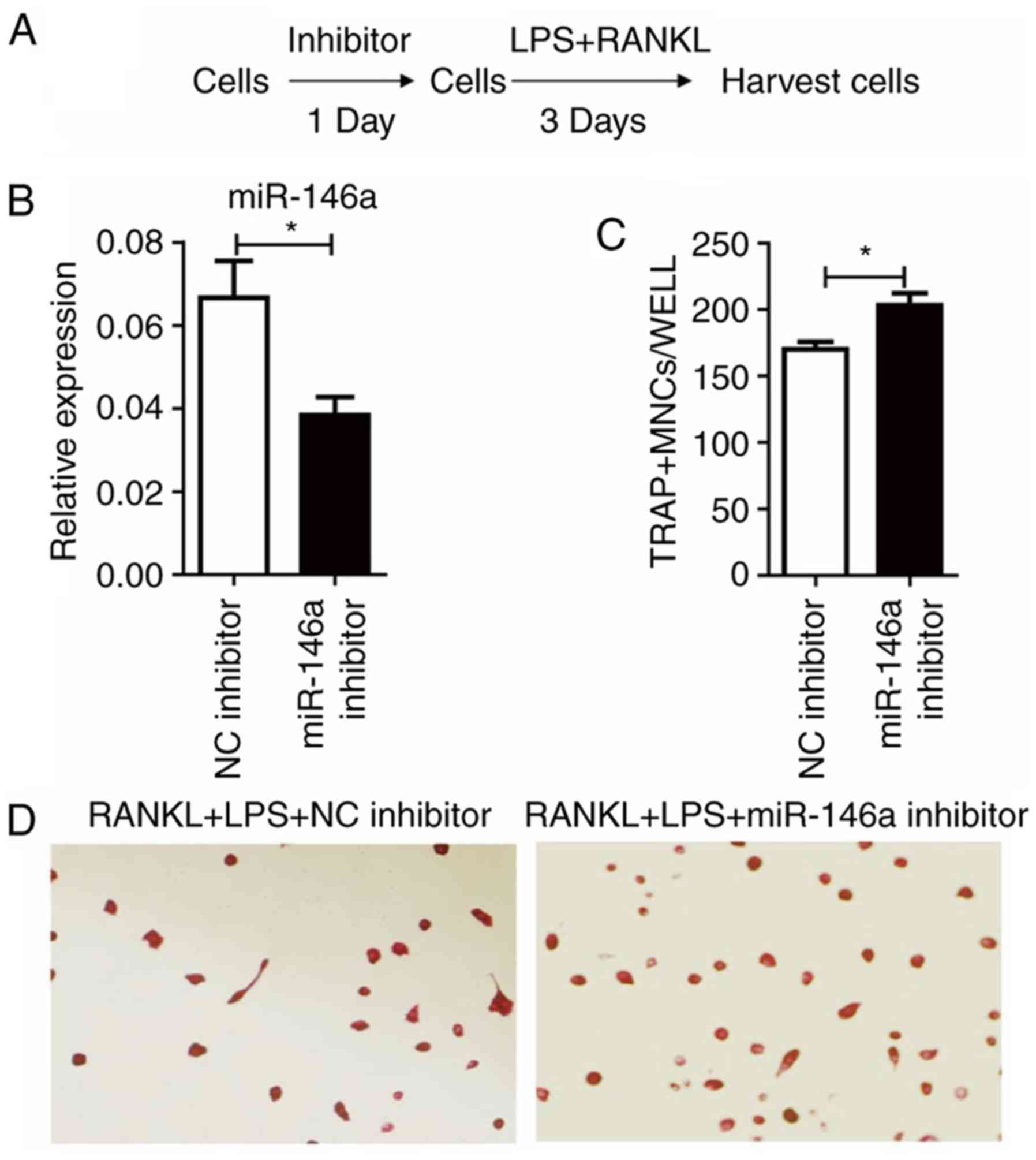
As miR-146a was upregulated following LPS and RANKL
treatment, the effect of miR-146a inhibitor was tested (Fig. 4A). Treatment of miR-146a inhibitor
24 h prior to LPS and RANKL administration significantly
(P<0.05) reduced the levels of miR-146a, compared with the NC
inhibitor group (Fig. 4B).
Notably, it was demonstrated that miR-146a inhibition significantly
increased the number of TRAP+ cells induced by LPS and RANKL
(P<0.05; Fig. 4C and D).
Next, if overexpression of miR-146a could prevent
osteoclast differentiation was tested. Two strategies were
designed, named ‘Prophylactic’ and ‘Therapeutic’ (Fig. 5A). In the ‘Prophylactic’ strategy,
Raw264.7 cells were transfected with miR-146a mimics one day prior
to LPS and RANKL treatment; in the ‘Therapeutic’ strategy, Raw264.7
cells received miR-146a mimic transfection one day following LPS
and RANKL treatment. Then, cells were harvested 3 days following
miR-146a mimic transfection, with LPS and RANKL stimulation. Data
from RT-qPCR results revealed that miR-146a expression was
significantly (P<0.001) elevated in both ‘Prophylactic’ and
‘Therapeutic’ groups, compared with the negative controls (Fig. 5B). Pre-treatment with the miR-146a
mimic revealed a potent inhibitory role in osteoclast
differentiation, with a 37% decrease compared with the control
group (Fig. 5C and D).
Furthermore, treatment with the miR-146a mimic during the process
of osteoclast development was able to prevent osteoclast formation
by 24% (Fig. 5C).
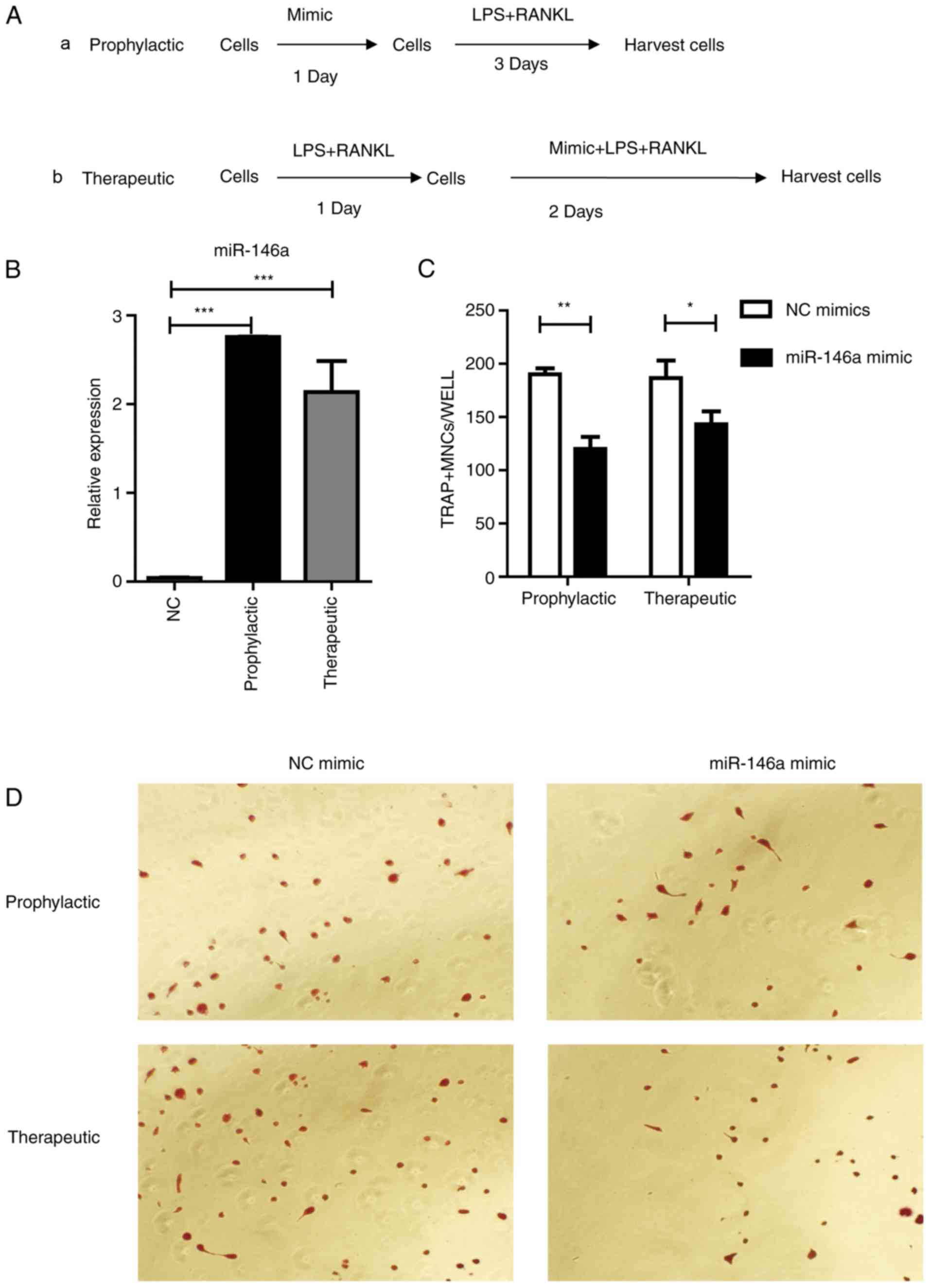 | Figure 5.miR-146a inhibits osteoclast
differentiation in Raw264.7 cells. (A) Study design of the
experiment. (a) Cells were treated with 200 nm mimic for 24 h, then
30 ng/ml RANKL and 100 ng/ml LPS were added and incubated for 3
days, (b) or cells were treated with 30 ng/ml RANKL and 100 ng/ml
LPS for 24 h followed by the addition of 200 nM mimic for 2 days.
(B) miR-146a expression subsequent to treatment with mimic as
aforementioned. (C) Numbers of osteoclasts were determined by
averaging the positive cell number of six fields of view under a
microscope. Bars represent the mean ± standard error of the mean.
(D) Subsequent to culturing, the cells were fixed and stained for
TRAP. TRAP+ cells with >3 nuclei were classified as osteoclasts.
Magnification, ×200. *P<0.05, **P<0.01 and ***P<0.001.
miR, microRNA; LPS, lipopolysaccharide; RANKL, receptor activator
of nuclear factor κβ ligand; TRAP, tartrate-resistant acid
phosphatase; NC, negative control. |
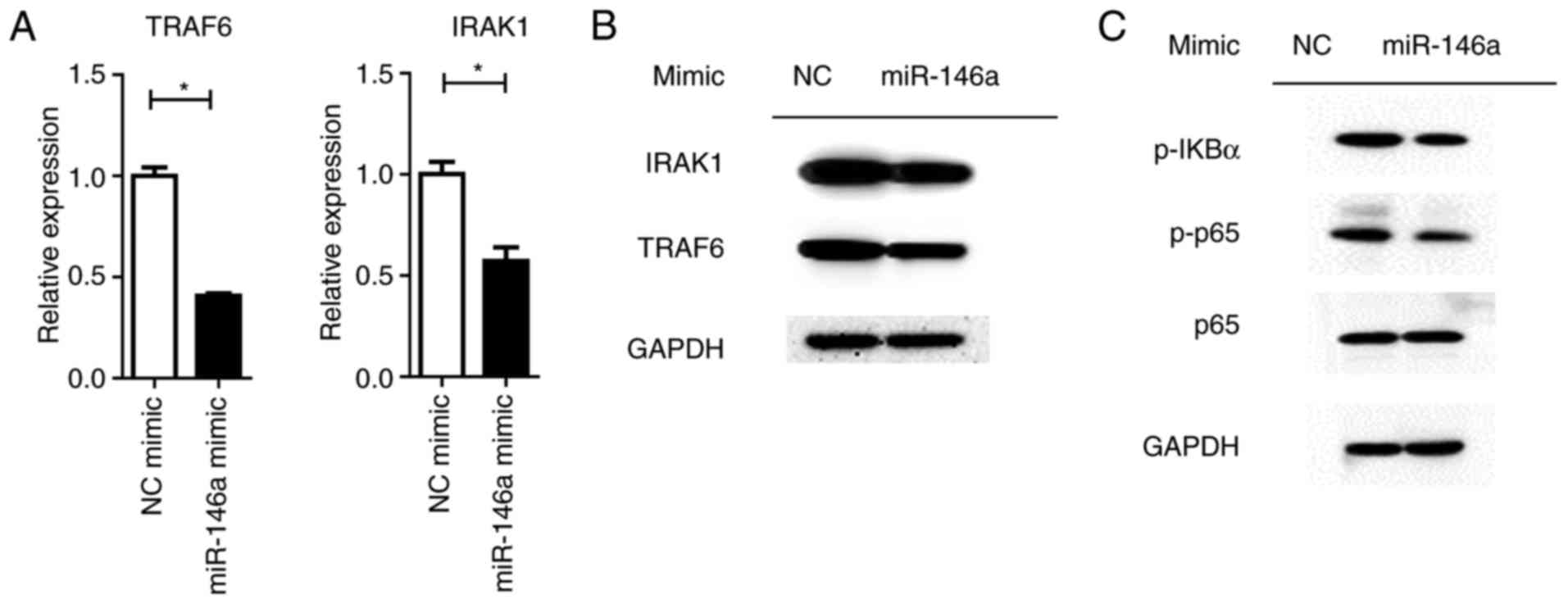
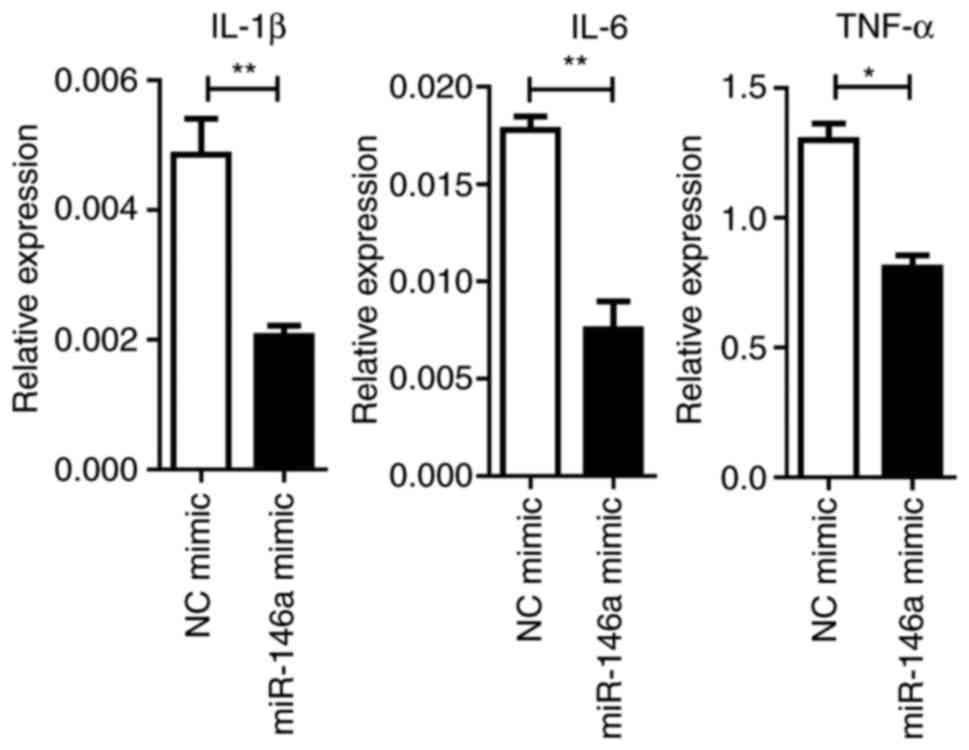
Overexpression of miR-146a reduces
NF-κβ activation and cytokine production
MiRNAs control a variety of biological processes by
negatively regulating the expression of multiple genes. TRAF6 and
IRAK1 are reported to be targets of miR-146a, which are the key
components upstream of NF-κβ signaling (28). It was revealed that the
overexpression of miR-146a significantly repressed TRAF6 and IRAK1
expression at the mRNA and protein levels (P<0.05; Fig. 6A). Downstream NF-κβ activation was
dampened by the miR-146a mimic, as assayed by the phosphorylation
levels of the p65 protein (Fig.
6B). Furthermore, the expression levels of pro-inflammatory
cytokines were significantly (IL1-β and IL-6, P<0.01; TNF-α,
P<0.05) reduced by miR-146a overexpression (Fig. 7).
Discussion
Inflammation is well known to be involved in
osteoclast-induced bone loss under pathological circumstances. For
instance, chronic infectious inflammation in the periodontium
enhances local osteoclast activation and destroys the
tooth-supporting alveolar bone, eventually resulting in periodontal
disease (29). Systemic bone
erosion occurs in RA as a consequence of distorted bone remodeling
resulting from overactive osteoclastogenesis in the context of
chronic inflammation (30). The
mechanisms underlying the tight association between bone resorption
and inflammation or infection have been studied for decades
(31), yet the intricate and
multi-factorial aspects involved in signal transduction have made
it difficult to develop a comprehensive understanding of the
different types of mediators, in addition to determining their
active location in source cells, their upstream and downstream
effectors, and the potential of how to control them. Further
research is required to develop novel therapeutic strategies,
therefore resulting in advancements in the treatment for
osteoclast-induced dysfunctions.
The ability of LPS to augment cytokine synthesis and
release by macrophages, fibroblasts and other cells has made LPS a
potent inducer of osteoclast activation (32–34).
Additionally, osteoclast differentiation, survival and fusion are
also orchestrated by LPS-dependent cytokines IL-1, IL-6 and TNF-α
in vivo and in vitro (35–38).
Takami et al (36) once
noted the mixed outcomes of LPS in osteoclastogenic assays in the
presence or absence of stromal cells and osteoblasts. The induction
of osteoclast differentiation by LPS requires the prior priming of
RANKL, otherwise the inclusion of LPS may block the
osteoclastogenic activity in primary bone marrow monocytes
(39). In the present study, it
was revealed that LPS exhibits a combined effect of enhancing
RANKL-induced osteoclast differentiation. Additionally, in
accordance with the previous reports (40–42),
inflammatory mediators IL-6, TNF-α and IL-1β were elevated
following LPS stimulation during the differentiation of
osteoclasts.
miR-146a, which is located in the LOC285628 gene on
human chromosome 5, was first discovered in the human acute
monocytic leukemia cell line THP-1 (28). The elevated expression of miR-146a
may be induced by LPS administration through a negative feedback
loop involving the TLR and NF-κβ pathways (43,44).
TRAF6 and IL-1 receptor-associated kinase 1 are directly
downregulated by miR-146a in this mediation initiated following LPS
treatment (17). Nakasa et
al (45) revealed the
suppressive role of miR-146a in the differentiation process of
peripheral blood mononuclear cells into osteoclasts in a
dose-dependent manner. In the present study, the high expression of
miR-146a in response to LPS and RANKL stimulation in Raw264.7 cells
was observed. miR-146a not only prevents osteoclast formation
induced by either LPS or RANKL (data not shown), but also
demonstrates a strong inhibition on LPS and RANKL co-stimulation.
The inhibitory effect of miR-146a on osteoclastogenesis is further
verified in the mouse primary bone marrow derived macrophages (data
not shown). Therefore, these results suggest a protective role of
miR-146a in inflammation-associated bone loss disorders. However,
another study revealed that miR-146a facilitates osteoarthritis by
regulating cartilage homeostasis (46). Further in vivo studies,
particularly investigating cell type specific miR-146a knockout,
should be conducted to elucidate this point.
The negative feedback loop of miR-146a and LPS
stimulation through the TLR and NF-κβ pathway serves as an
important regulatory mechanism in LPS-induced immune responses
(47,48). The regulatory axis of
miR-146a-NF-κβ has been observed in various kinds of diseases,
including cancer (49,50). Considering that NF-κβ signaling is
also highly responsive to osteoclastogenesis and osteoclast
precursor activation, TRAF6 and IRAK1, two crucial upstream
inducers of the NF-κβ pathway, were analyzed following the forced
expression of miR-146a. It was revealed that the expression of
TRAF6 and IRAK1 in response to the overexpression of miR-146a was
decreased at the mRNA and protein levels. Furthermore, the
activation of NF-κβ signaling was also inhibited by the miR-146a
mimic. The aforementioned results demonstrated that miR-146a serves
as a negative modulator in the process of osteoclast
differentiation and therefore limits osteoclast number in a proper
range and subsequently maintains bone homeostasis.
In conclusion, these results demonstrated that LPS
enhances osteoclast differentiation from Raw264.7 cells induced by
RANKL. miR-146a is highly induced by LPS and RANKL stimulation. The
overexpression of miR-146a inhibits osteoclast transformation by
targeting the key regulators of NF-κβ signaling, TRAF6 and IRAK1.
Therefore, these results indicate that using an miR-146a mimic may
be a promising therapeutic strategy for the prevention and
treatment of inflammation mediated bone loss.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation Of China (grant no. 81773089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX conceived and designed the experiments. YG, BW
and CS performed the experiments. YG produced the manuscript. BW
and CS conducted the data analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mizoguchi T, Muto A, Udagawa N, Arai A,
Yamashita T, Hosoya A, Ninomiya T, Nakamura H, Yamamoto Y, Kinugawa
S, et al: Identification of cell cycle-arrested quiescent
osteoclast precursors in vivo. J Cell Biol. 184:541–554. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring SR and Gravallese EM: Mechanisms
of bone loss in inflammatory arthritis: Diagnosis and therapeutic
implications. Arthritis Res. 2:33–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayanagi H, Ogasawara K, Hida S, Chiba
T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al:
T-cell-mediated regulation of osteoclastogenesis by signalling
cross-talk between RANKL and IFN-gamma. Nature. 408:600–605. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oostlander AE, Everts V, Schoenmaker T,
Bravenboer N, van Vliet SJ, van Bodegraven AA, Lips P and de Vries
TJ: T cell-mediated increased osteoclast formation from peripheral
blood as a mechanism for Crohn's disease-associated bone loss. J
Cell Biochem. 113:260–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horowitz MC, Xi Y, Pflugh DL, Hesslein DG,
Schatz DG, Lorenzo JA and Bothwell AL: Pax5-deficient mice exhibit
early onset osteopenia with increased osteoclast progenitors. J
Immunol. 173:6583–6591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cappariello A, Maurizi A, Veeriah V and
Teti A: The great beauty of the osteoclast. Arch Biochem Biophys.
558:70–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gillespie MT: Impact of cytokines and T
lymphocytes upon osteoclast differentiation and function. Arthritis
Res Ther. 9:1032007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian J, Chen J, Gao J, Li L and Xie X:
Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human
rheumatoid arthritis fibroblast-like synoviocytes via modulation of
PI3kinase/Akt pathway. Rheumatol Int. 33:1829–1835. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tucci M, Stucci S, Savonarola A,
Ciavarella S, Cafforio P, Dammacco F and Silvestris F: Immature
dendritic cells in multiple myeloma are prone to osteoclast-like
differentiation through interleukin-17A stimulation. Br J Haematol.
161:821–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou GQ, Guo C, Song GH, Fang N, Fan WJ,
Chen XD, Yuan L and Wang ZQ: Lipopolysaccharide (LPS) promotes
osteoclast differentiation and activation by enhancing the MAPK
pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med.
32:503–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji JD, Park-Min KH, Shen Z, Fajardo RJ,
Goldring SR, Mchugh KP and Ivashkiv LB: Inhibition of RANK
expression and osteoclastogenesis by TLRs and IFN-gamma in human
osteoclast precursors. J Immunol. 183:7223–7233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai R and Ahmed SA: MicroRNA, a new
paradigm for understanding immunoregulation, inflammation, and
autoimmune diseases. Transl Res. 157:163–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mandal P, Mcmullen MR, Park PH, Roge T and
Nagy LE: Adiponectin decreases expression of TLR4 and MyD-88
independent signal transduction in RAW 264.7 macrophages. Cytokine.
48:1302009. View Article : Google Scholar
|
|
17
|
Tang P, Xiong Q, Wei G and Zhang L: The
role of microRNAs in osteoclasts and osteoporosis. RNA Biol.
11:1355–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim K, Kim JH, Kim I, Lee J, Seong S, Park
YW and Kim N: MicroRNA-26a regulates RANKL-induced osteoclast
formation. Mol Cells. 38:75–80. 2015.PubMed/NCBI
|
|
19
|
Li Z, Zhang W and Huang Y: MiRNA-133a is
involved in the regulation of postmenopausal osteoporosis through
promoting osteoclast differentiation. Acta Biochim Biophys Sin
(Shanghai). 50:273–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pauley KM, Satoh M, Chan AL, Bubb MR,
Reeves WH and Chan EK: Upregulated miR-146a expression in
peripheral blood mononuclear cells from rheumatoid arthritis.
Arthritis Res Ther. 10:R1012008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boldin MP, Taganov KD, Rao DS, Yang L,
Zhao JL, Kalwani M, Garciaflores Y, Luong M, Devrekanli A, Xu J, et
al: miR-146a is a significant brake on autoimmunity,
myeloproliferation, and cancer in mice. J Exp Med. 208:1189–1201.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang
Y, Zhang J, Zhang J, Fu X, Liu H, et al: Altered microRNA
expression profile with miR-146a upregulation in CD4+ T cells from
patients with rheumatoid arthritis. Arthritis Res Ther. 12:R812010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi K, Takahashi N, Jimi E, Udagawa
N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima
N, et al: Tumor necrosis factor α stimulates osteoclast
differentiation by a mechanism independent of the Odf/Rankl-Rank
interaction. J Exp Med. 191:275–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao Y, Grassi F, Ryan MR, Terauchi M, Page
K, Yang X, Weitzmann MN and Pacifici R: IFN-gamma stimulates
osteoclast formation and bone loss in vivo via antigen-driven T
cell activation. J Clin Invest. 117:122–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1alpha) induction of human osteoclast formation. J
Pathol. 198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Benedetto A, Gigante I, Colucci S and
Grano M: Periodontal disease: Linking the primary inflammation to
bone loss. Clin Dev Immunol. 2013:5037542013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romas E and Gillespie MT:
Inflammation-induced bone loss: Can it be prevented? Rheum Dis Clin
North Am. 32:759–773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Graves DT, Li J and Cochran DL:
Inflammation and uncoupling as mechanisms of periodontal bone loss.
J Dent Res. 90:143–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Yan M, Yu QF, Yang PF, Zhang HD,
Sun YH, Zhang ZF and Gao YF: Puerarin prevents LPS-induced
osteoclast formation and bone loss via inhibition of Akt
activation. Biol Pharm Bull. 39:2028–2035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim DY, Jun JH, Lee HL, Woo KM, Ryoo HM,
Kim GS, Baek JH and Han SB: N-acetylcysteine prevents LPS-induced
pro-inflammatory cytokines and MMP2 production in gingival
fibroblasts. Arch Pharm Res. 30:1283–1292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rossol M, Heine H, Meusch U, Quandt D,
Klein C, Sweet MJ and Hauschildt S: LPS-induced cytokine production
in human monocytes and macrophages. Crit Rev Immunol. 31:379–446.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Islam S, Hassan F, Tumurkhuu G, Dagvadorj
J, Koide N, Naiki Y, Mori I, Yoshida T and Yokochi T: Bacterial
lipopolysaccharide induces osteoclast formation in RAW 264.7
macrophage cells. Biochem Biophys Res Commun. 360:346–351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takami M, Kim N, Rho J and Choi Y:
Stimulation by toll-like receptors inhibits osteoclast
differentiation. J Immunol. 169:1516–1523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mörmann M, Thederan M, Nackchbandi I,
Giese T, Wagner C and Hänsch GM: Lipopolysaccharides (LPS) induce
the differentiation of human monocytes to osteoclasts in a tumour
necrosis factor (TNF) alpha-dependent manner: A link between
infection and pathological bone resorption. Mol Immunol.
45:3330–3337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baek JM, Kim JY, Yoon KH, Oh J and Lee MS:
Ebselen is a potential anti-osteoporosis agent by suppressing
receptor activator of nuclear factor Kappa-B ligand-induced
osteoclast differentiation in vitro and lipopolysaccharide-induced
Inflammatory bone destruction in vivo. Int J Biol Sci. 12:478–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zou W and Bar-Shavit Z: Dual modulation of
osteoclast differentiation by lipopolysaccharide. J Bone Miner Res.
17:1211–1218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Itoh K, Udagawa N, Kobayashi K, Suda K, Li
X, Takami M, Okahashi N, Nishihara T and Takahashi N:
Lipopolysaccharide promotes the survival of osteoclasts via
Toll-like receptor 4, but cytokine production of osteoclasts in
response to lipopolysaccharide is different from that of
macrophages. J Immunol. 170:3688–3695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kikuchi T, Matsuguchi T, Tsuboi N, Mitani
A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T and
Yoshikai Y: Gene expression of osteoclast differentiation factor is
induced by lipopolysaccharide in mouse osteoblasts via Toll-like
receptors. J Immunol. 166:3574–3579. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yousefzadeh N, Alipour MR and Soufi FG:
Deregulation of NF-кB-miR-146a negative feedback loop may be
involved in the pathogenesis of diabetic neuropathy. J Physiol
Biochem. 71:51–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakasa T, Shibuya H, Nagata Y, Niimoto T
and Ochi M: The inhibitory effect of microRNA-146a expression on
bone destruction in collagen-induced arthritis. Arthritis Rheum.
63:1582–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Wang C, Zhao J, Xu J, Geng Y, Dai
L, Huang Y, Fu SC, Dai K and Zhang X: miR-146a facilitates
osteoarthritis by regulating cartilage homeostasis via targeting
Camk2d and Ppp3r2. Cell Death Dis. 8:e27342017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang L, Boldin MP, Yu Y, Liu CS, Ea CK,
Ramakrishnan P, Taganov KD, Zhao JL and Baltimore D: miR-146a
controls the resolution of T cell responses in mice. J Exp Med.
209:1655–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng Y, Kuang W, Hao Y, Zhang D, Lei M,
Du L, Jiao H, Zhang X and Wang F: Downregulation of miR-27a* and
miR-532-5p and upregulation of miR-146a and miR-155 in LPS-induced
RAW264.7 macrophage cells. Inflammation. 35:1308–1313. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|