Introduction
Bone mass regulation is under the control of
continuous bone remodeling, which is a balance of osteoclastic bone
resorption and osteoblastic bone formation. Disorders of bone
remodeling cause a variety of bone-associated diseases, including
osteoporosis (1). Osteoporosis
occurs when the body makes too little bone, loses too much bone, or
a combination of these two factors. Osteoporotic bones have lost
density or mass, and contain abnormal tissue structure. As a
result, bones become weak and may easily break following a fall.
Current therapies include anti-catabolic drugs, anti-resorptives
and anabolic agents (2,3). Unfortunately, the treatments
available for individuals with osteoporosis are ineffective due to
adverse effects (4). Therefore, it
is important to identify novel therapeutic strategies.
As the main bone-forming cells, osteoblasts produce
alkaline phosphatase (ALP), and bone matrix proteins such as
osteocalcin (OCN) and osteopontin (OPN), which are associated with
osteoblastic mineralization (5).
Osteoblast differentiation is regulated by bone morphogenetic
proteins (BMPs) and WNT family member (WNT) pathways (6,7).
BMPs are critically involved in bone formation processes for the
maintenance of bone homeostasis and skeletal development (8). The WNT pathway is also essential for
bone development (9). In addition,
these two pathways cooperate to regulate osteoblast differentiation
and bone formation.
Lonicera japonica has been used in
traditional Chinese medicine to treat various diseases, including
osteoporosis (10,11). As an active component obtained from
the flower buds of Lonicera japonica,
(R)-dehydroxyabscisic alcohol
β-D-apiofuranosyl-(1ˮ→6’)-β-D-glucopyranoside (DAG; Fig. 1) (12), was identified to have potent
activity in improving osteoblastic differentiation in our
preliminary compound screening. The present study aimed to further
investigate the effects of DAG on osteoblastic differentiation and
its underlying mechanisms, in order to support the potential
development of DAG as a therapeutic agent against bone-associated
diseases, such as osteoporosis.
Materials and methods
Materials
The ST2 bone marrow stromal cell line was purchased
from Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Fetal bovine serum (FBS) and RPMI-1640 medium
were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). MTT, Alizarin Red S and silver nitride were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Recombinant
Noggin was purchased from Sigma-Aldrich (Merck KGaA) and
Dickkopf-related protein 1 (Dkk-1) was purchased from PeproTech,
Inc. (Rocky Hill, USA). The ALP assay kit was purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Polymerase chain reaction (PCR) reagents were purchased from Thermo
Fisher Scientific, Inc. DAG was provided by Professor Han (Tongji
University, Shanghai, China) and was isolated and identified using
spectroscopic and HPLC methods with purity >98% (12). All other reagents including DMSO,
paraformaldehyde and Triton X-100 were of analytical grade and
purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China). DAG was dissolved in dimethyl sulfoxide (DMSO) for the
in vitro experiments.
Cell culture and treatment
ST2 cells were cultured in RPMI-1640 medium
supplemented with 15% FBS, 1% streptomycin-penicillin and
osteogenic differentiation medium (cat. no. A1007201; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a 37°C and 5% CO2
incubator. In the cell viability assays, ST2 cells were treated
with DAG at 0.1, 0.5 or 2.5 µM for 72 h at 37°C; in other
treatments, ST2 cells were treated with DAG at 0.1, 0.5 or 2.5 µM
at 37°C for different time periods according to the assays (14 days
for von Kossa staining; 5 days for ALP activity; 14 days for ARS
staining; 7 days for gene expression); in the mechanistic studies,
ST2 cells were pretreated with Noggin (10 µg/ml) or Dkk-1 (0.5
µg/ml) for 1 h at 37°C, and then treated with DAG at 2.5 µM for
different time periods according to the assays at 37°C (14 days for
von Kossa staining; 5 days for ALP activity; 14 days for ARS
staining; 7 days for gene expression; 10 days for protein
expression); cells were treated with DMSO as the negative
control.
Cell viability measurement
An MTT assay was performed to determine cell
viability. Treated ST2 cells were seeded into 96-well plates at a
density of 3×104/100 µl. MTT solution (10 µl) was added
to each well, and incubated for 4 h at 37°C. DMSO (200 µl) was
subsequently added into each well to dissolve the formazan. The
absorbance was measured by a plate reader at 570 nm.
ALP activity measurement
Following treatment, ST2 cells were collected and
lysed using 0.1% Triton X-100 (Sinopharm Chemical Reagent Co.,
Ltd.). ALP activity was measured using the cALP stain kit (cat. no.
D001-1; Nanjing Jiancheng BioEngineering Institute Co., Ltd.)
according to the manufacturer's instructions.
Von Kossa staining
Treated ST2 cells were washed twice with PBS and
fixed with 4% paraformaldehyde for 15 min at 4°C. Following washing
with PBS, cells were stained with 5% silver nitride under UV
radiation for 30 min at room temperature. The stained cells were
washed twice and observed using an Olympus BX60 light microscope
(magnification, ×40; Olympus Corporation, Tokyo, Japan).
Alizarin Red S staining
Treated ST2 cells were washed twice with PBS and
fixed in 70% ice-cold ethanol for 1 h. Following another wash,
cells were stained with 40 mM Alizarin Red S (pH 4.2) for 10 min at
room temperature. The stains were eluted with DMSO to quantify the
amount of Alizarin Red S staining, by measuring the absorbance at
570 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following treatment, ST2 cells were collected and
total RNA was extracted with TRIzol® reagent. RNA (0.4
µg) was used to generate cDNA using an iScript™ Reverse
Transcription Supermix kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at 25°C for 5 min, 46°C for 20 min and 95°C for 1 min.
qPCR was run with the SYBR-Green ER™ qPCR SuperMix
Universal (cat. no. 11762100; Thermo Fisher Scientific, Inc.) on a
MX3000p system (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA) (95°C for 10 min, 95°C for 15 sec and 60°C for 60
sec; 40 cycles), using the comparative Cq value method to quantify
the target gene expression in different samples (13). The gene expression was normalized
using the housekeeping gene β-actin. The gene-specific
primer sequences were as follows: Alp forward,
5′-GCTGATCATTCCCACGTTTT-3′ and reverse, 3′-CTGGGCCTGGTAGTTGTTGT-5′
(GenBank reference, X13409.1); Ocn forward,
5′-CTTGGGTTCTGACTGGGTGT-3′ and reverse, 3′-GCCCTCTGCAGGTCATAGAG-5′
(GenBank reference, L24431.1); Opn forward,
5′-TGCACCCAGATCCTATAGCC-3′ and reverse, 3′-CTCCATCGTCATCATCATCG-5′
(GenBank reference, AF515708.1); Bmp2 forward,
5′-CCCCAAGACACAGTTCCCTA-3′ and reverse, 3′-GAGACCGCAGTCCGTCTAAG-5′
(NCBI reference: NM_007553.3); Bmp4 forward,
5′-TCTAGAGGTCCCCAGAAGCA-3′ and reverse, 3′-CTTCCCGGTCTCAGGTATCA-5′
(GenBank reference, BC034053.1); Wnt1 forward,
5′-ACAGCAACCACAGTCGTCAG-3′ and reverse, 3′-GAATCCGTCAACAGGTTCGT-5′
(NCBI reference, NM_021279.4); Wnt3 forward,
5′-GCGACTTCCTCAAGGACAAG-3′ and reverse, 3′-AAAGTTGGGGGAGTTCTCGT-5′
(NCBI reference, NM_009521.2); runt related transcription factor 2
(Runx2) forward, 5′-CCCAGCCACCTTTACCTACA-3′ and reverse,
3′-TATGGAGTGCTGCTGGTCTG-5′ (NCBI reference, NM_001145920.2);
β-actin forward, 5′-AGCCATGTACGTAGCCATCC-3′ and reverse,
3′-CTCTCAGCTGTGGTGGTGAA-5′ (NCBI reference, NM_007393.5).
Western blot analysis
Treated ST2 cells were collected to extract protein
with RIPA lysis buffer (cat. no. 89900; Thermo Fisher Scientific,
Inc.). The protein concentration was determined by a bicinchoninic
acid protein assay kit. Protein samples of equal quantity (40
µg/lane) were subjected to 4–12% (v/v) SDS-PAGE. Proteins were
subsequently transferred onto polyvinylidene fluoride membranes.
Following blocking with 5% dried skimmed milk for 1 h at room
temperature, membranes were washed three times with PBS/T (PBS
containing 0.1%/Tween-20) and incubated with the following primary
antibodies: Anti-mothers against decapentaplegic homolog
(Smad)1/5/8 (cat. no. sc-6031-R; 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-phosphorylated (p)-Smad1/5/8 (cat. no.
AB3848-I; 1:500; EMD Millipore, Billerica, MA, USA), anti-β-catenin
(cat. no. sc-7199; 1:500, Santa Cruz Biotechnology, Inc.),
anti-Runx2 (cat. no. sc-10758; 1:500; Santa Cruz Biotechnology,
Inc.) and anti-β-actin (cat. no. ab8227; 1:5,000, Abcam, Cambridge,
UK) at 4°C overnight. Following washing with PBS, the membranes
were further incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G secondary antibody (cat. no. ab6721;
1:8,000; Abcam) for 1 h at room temperature. Following washing,
membranes were exposed to Pierce™ enhanced chemiluminescence
substrate (Thermo Fisher Scientific, Inc.) followed by X-ray film
development.
Statistical analysis
Data was analyzed by one-way analysis of variance
with SAS version 9.1 software (SAS Institute, Inc., Cary, NC, USA)
followed by Dunnett's t-test. Values were expressed as the mean ±
standard deviation. P<0.05 was considered to indicate as
statistically significant difference.
Results
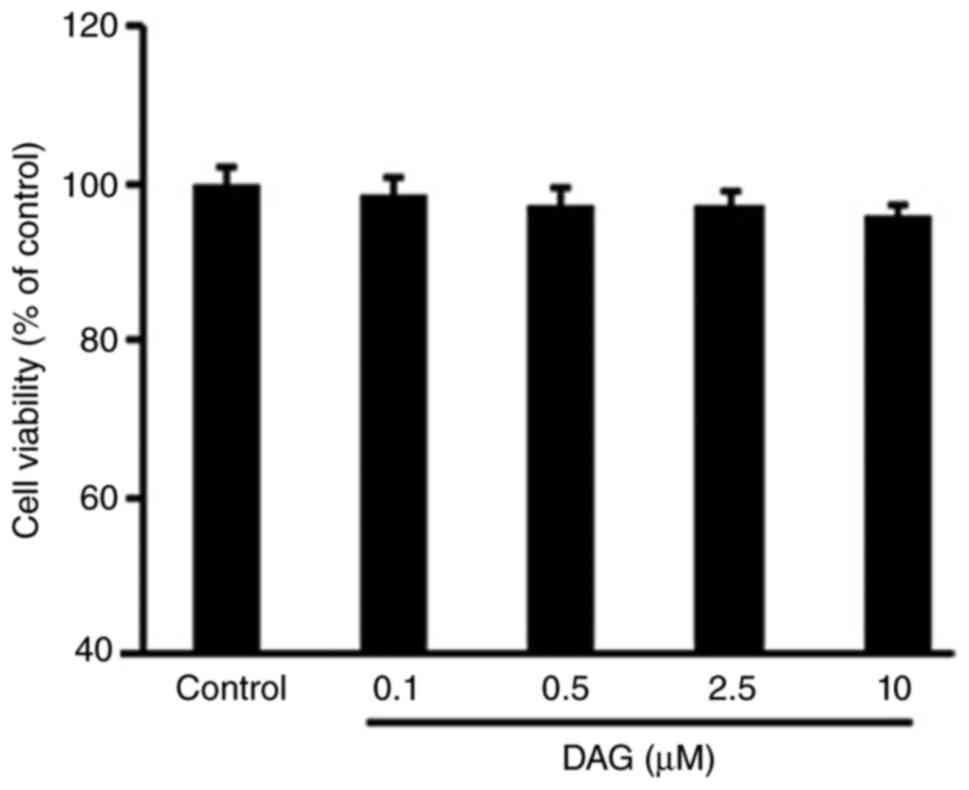
DAG does not affect ST2 cell
viability
ST2 cells were treated with DAG at different
concentrations for 72 h. DAG treatment did not result in any
significant cytotoxic effects when compared with the negative
control, as presented in Fig.
1.
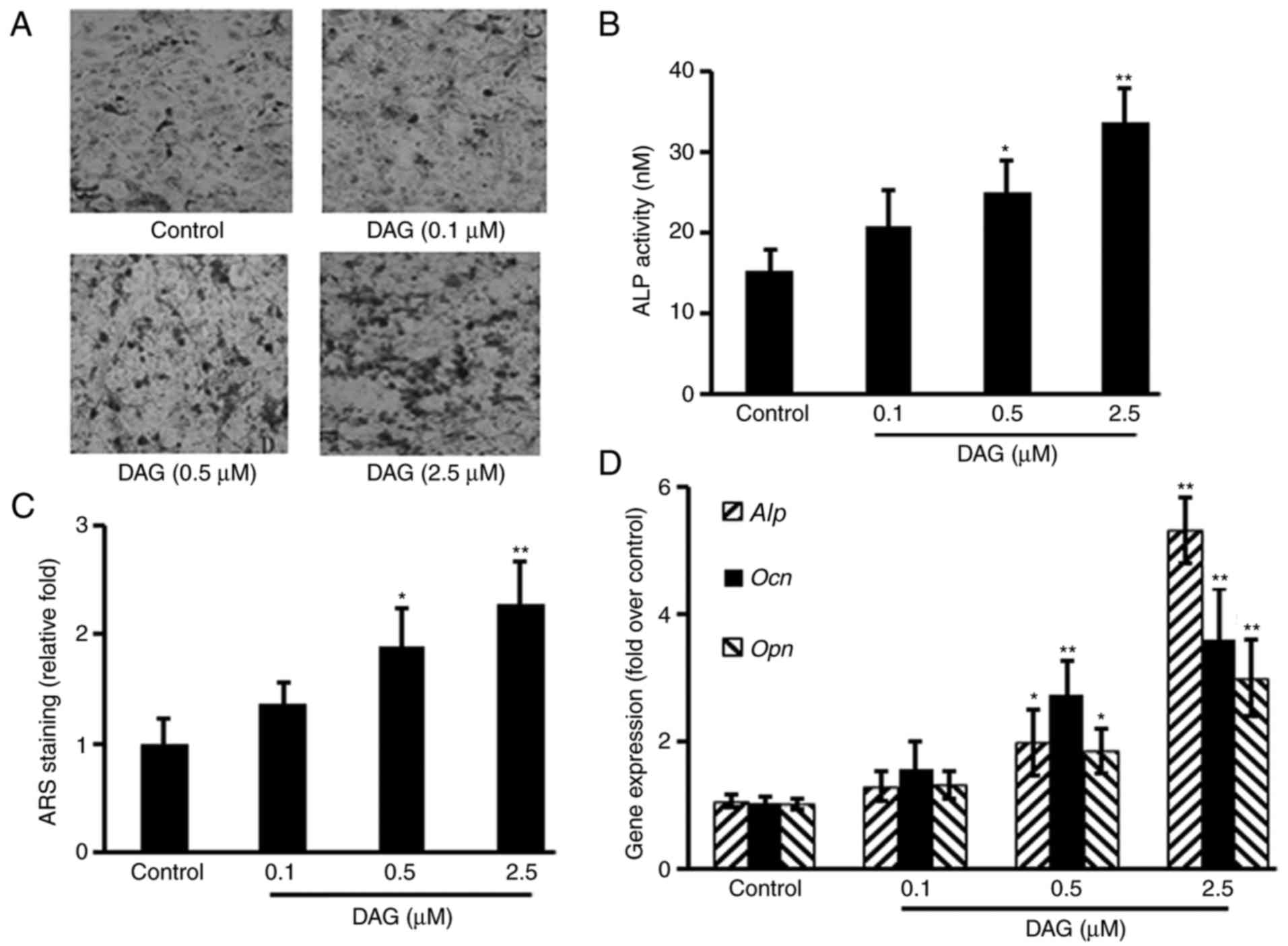
DAG treatment induces ST2 cell
osteoblastic differentiation
To study the effects of DAG on osteoblastic
differentiation, ST2 cells were treated with DAG at various
concentrations. The results demonstrated that DAG increased the
osteoblastic differentiation of ST2 cells, as evidenced by
increased mineralized nodule formation measured by von Kossa
staining (Fig. 2A), ALP activity
(Fig. 2B) and calcium deposits
measured by ARS staining (Fig. 2C)
in a dose-dependent manner, as well as by the upregulation of
Alp, Ocn and Opn gene expression (Fig. 2D).
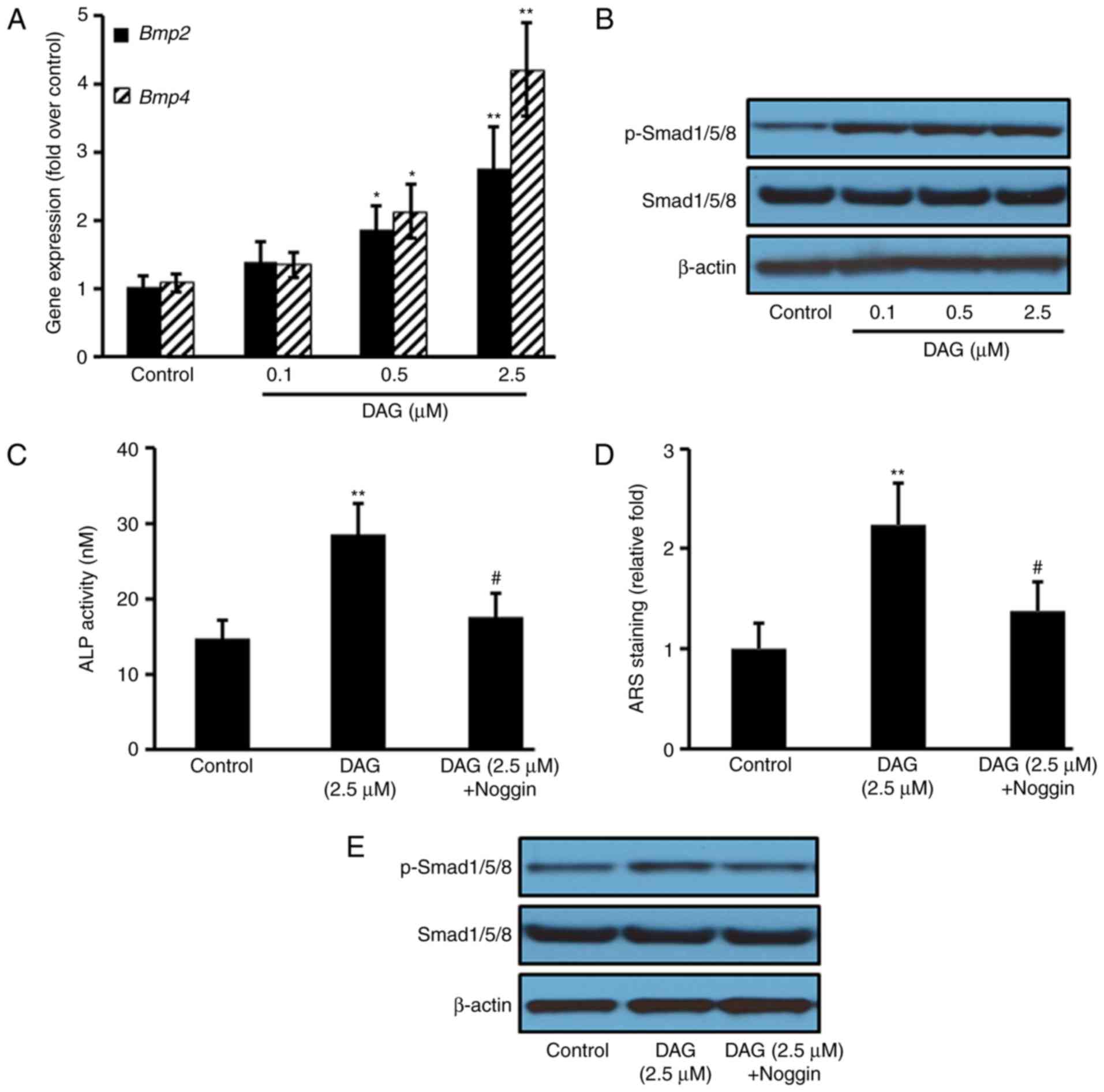
DAG treatment activates the BMP
signaling pathway
To investigate the signaling pathways involved in
the osteoblastic differentiation of ST2 cells induced by DAG, the
BMP pathway was analyzed by RT-qPCR and western blotting. The
results demonstrated that compared with the control group, DAG
treatment increased the gene expression of Bmp2 and
Bmp4 (Fig. 3A), as well as
the protein expression of p-Smad1, 5 and 8 (Fig. 3B). Pretreating the ST1 cells with
the BMP antagonist Noggin for 1 h prior to DAG treatment
significantly reduced the DAG-mediated increase in ALP levels
(Fig. 4C) and mineralization
(Fig. 4D), as well as the protein
expression of p-Smad1, 5 and 8 (Fig.
4E).
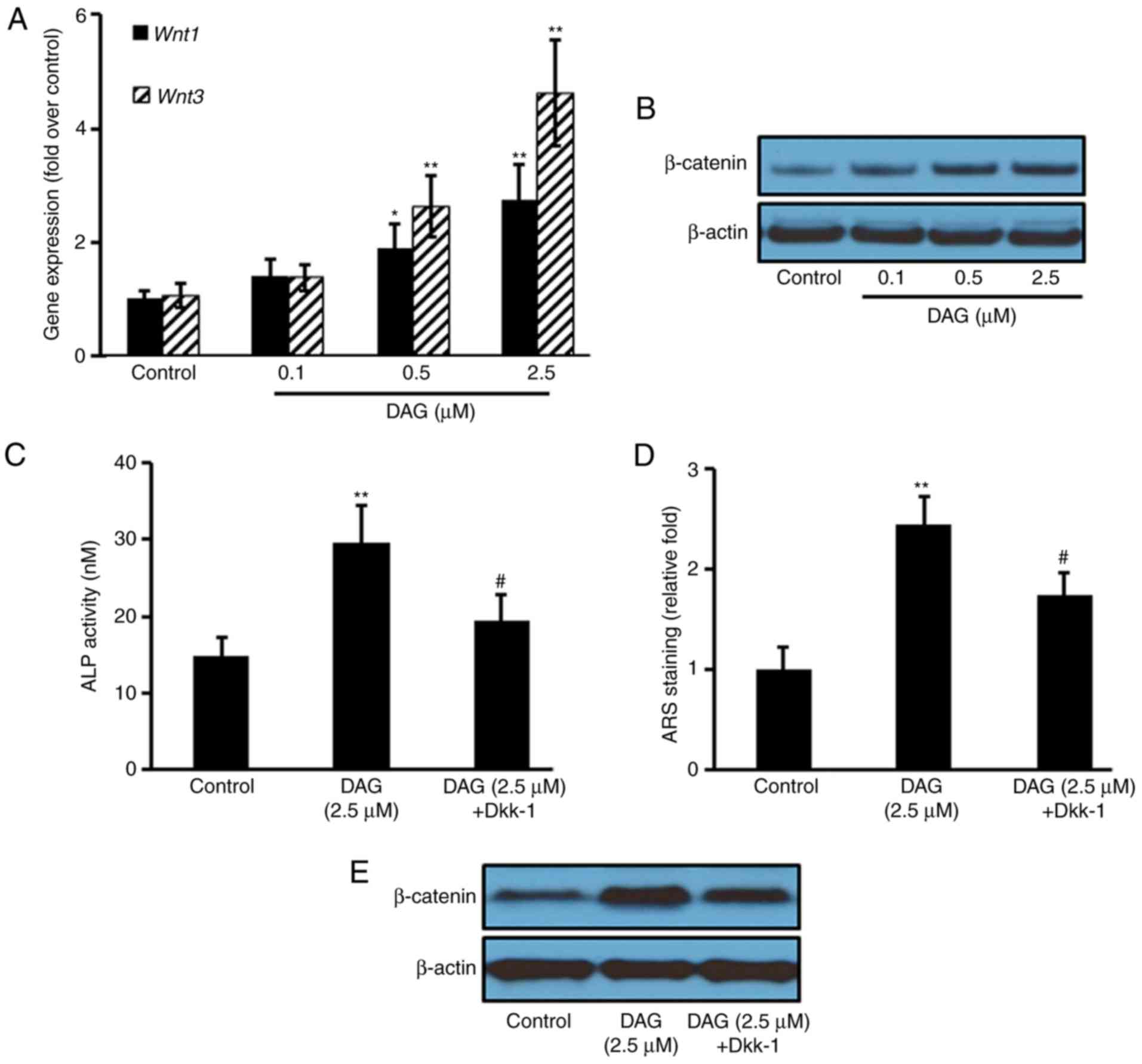
DAG treatment activates the WNT
signaling pathway
To further investigate the signaling pathways
involved in the osteoblastic differentiation of ST2 cells induced
by DAG, WNT pathway gene and protein expression was analyzed. The
results revealed that DAG treatment increased the gene expression
of Wnt1 and 3 (Fig.
4A), as well as the protein expression of β-catenin (Fig. 4B). Pretreating the ST1 cells with
the WNT inhibitor Dkk-1 for 1 h prior to DAG treatment
significantly attenuated the DAG-mediated increase in ALP levels
(Fig. 4C) and mineralization
(Fig. 4D), as well as the protein
expression of β-catenin (Fig.
4E).
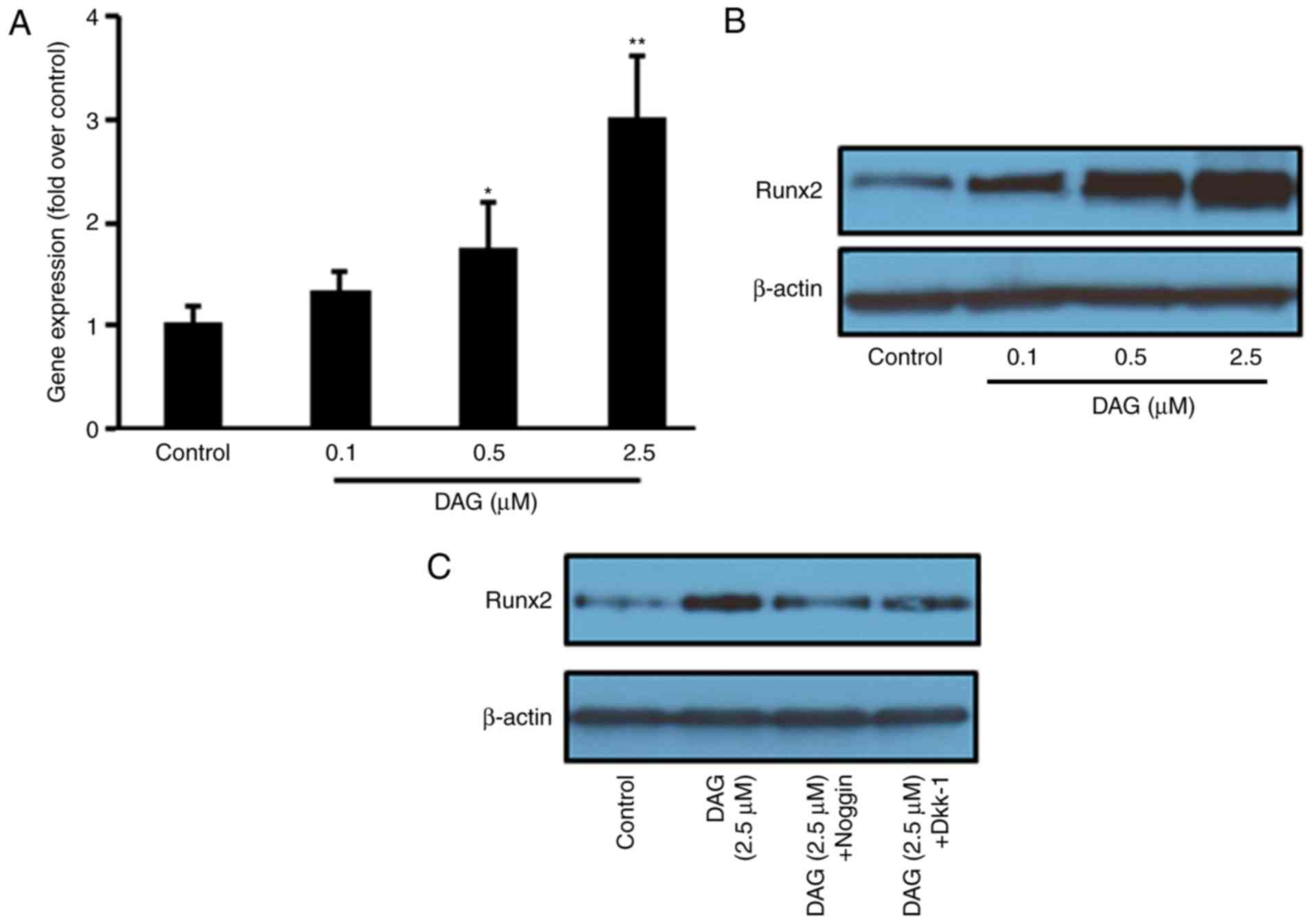
DAG treatment increases Runx2
expression
To further confirm that the DAG-induced osteoblastic
differentiation of ST2 cells occurred through the BMP and WNT
signaling pathways, the expression of Runx2, a key transcription
factor in osteoblastic differentiation, was measured following DAG
treatment. The results demonstrated that DAG treatment increased
the gene (Fig. 5A) and protein
(Fig. 5B) expression of Runx2.
Noggin and Dkk-1 pretreatment significantly inhibited the
DAG-stimulated increase in Runx2 protein expression (Fig. 5C).
Discussion
Osteoporosis is the most common bone-associated
disease, characterized by increased bone fragility and decreased
bone mass (14). As an important
process of bone formation, osteoblastic differentiation is severely
compromised in osteoporosis. Therefore, promoting osteoblastic
differentiation is an effective strategy to prevent pathological
progression. In the present study, it was demonstrated that DAG, a
novel compound from the flower buds of Lonicera japonica,
had no toxicity on bone marrow stromal cells and increased
osteoblastic differentiation. DAG differs from WIN-34B, another
compound isolated from Lonicera japonica, which has been
demonstrated to increase osteogenesis and decrease
osteoclastogenesis via promoting ALP activity and mineralization of
human mesenchymal stem cells through inhibition of the NF-κB, JNK
and p38 MAPK pathways (10). The
present study demonstrated that DAG exerted its effects by
activating the BMP/WNT signaling pathways.
The ST2 cell line has been widely used for the study
of osteoblastic differentiation, as these differentiate into
osteoblast-like cells and further mature into osteoblasts (15,16).
The process of new bone formation involves osteoblast
differentiation and proliferation, and finally extracellular matrix
mineralization. ALP activity is a well-recognized marker of early
osteoblast differentiation, and the mineralization may be detected
by von Kossa staining and Alizarin Red S staining, as a biological
marker of the terminal differentiation (17,18).
In the present study, ST2 cells treated with DAG significantly
increased ALP activity and mineralization, and upregulated the gene
expression of Alp, Ocn and Opn, suggesting that DAG
stimulated early and late osteoblastic differentiation, as well as
maturation.
BMP family proteins serve an important role in the
regulation of bone remodeling and formation, as one of the main
signaling cascades (19). Inactive
BMP resides in the cytoplasm, and upon activation by p-Smad1/5/8,
it translocates to the nucleus to regulate the transcription of
target genes (20). Several
compounds have been reported to promote osteoblastic
differentiation through activating the BMP signaling pathway
(21,22). In the present study, DAG treatment
increased the gene expression of Bmp, as well as the protein
expression of phosphorylated Smad1/5/8. Furthermore, the BMP
antagonist Noggin significantly inhibited DAG-induced ALP activity
and mineralization, further confirming that DAG induced
osteoblastic differentiation through the BMP signaling pathway.
WNT/β-catenin signaling contributes to osteoblastic
differentiation, as another critical pathway that regulates bone
remodeling and formation (23).
WNT ligands bind with Frizzled, low density lipoprotein
receptor-related protein (LRP) 5 and LRP6 receptors to stabilize
β-catenin in the cytoplasm, resulting in the translocation of
β-catenin into the nucleus to regulate osteoblastic
differentiation-associated gene expression (24,25).
The results of the present study revealed that treating ST2 cells
with DAG increased the gene expression of Wnt ligands and
the protein expression of β-catenin. Furthermore, the DAG-induced
osteogenic effects were attenuated by the WNT inhibitor Dkk-1,
indicating that WNT/β-catenin signaling was involved in DAG-induced
osteoblastic differentiation.
Runx2 is a downstream regulator of the WNT/BMP
signaling pathways, which is essential in the process of
osteoblastic differentiation (26). The present results indicated that
DAG treatment increased Runx2 gene and protein expression in ST2
cells, implying that functional cross-talk existed between the
WNT/BMP pathways.
In conclusion, the present study demonstrated that
DAG isolated from the flower buds of Lonicera japonica
stimulated the osteoblastic differentiation of ST2 cells through
the WNT/BMP signaling pathways. This provides scientific rationale
for the development of DAG as a therapeutic agent against bone
diseases, such as osteoporosis.
Acknowledgements
The authors thank Professor Han from Tongji
University (Shanghai, China) for providing DAG in this study.
Funding
Not applicable.
Availability of data and materials
The data and materials are available from the
corresponding author upon reasonable request.
Authors' contributions
Study concept and design was by WZ; experimental
studies by YL, TY, TC and JH; data analysis was by YL, TC and YG;
manuscript preparation by YL, TY, TC, JH, YG and WZ. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riggs BL and Parfitt AM: Drugs used to
treat osteoporosis: The critical need for a uniform nomenclature
based on their action on bone remodeling. J Bone Miner Res.
20:177–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Augustine M and Horwitz MJ: Parathyroid
hormone and parathyroid hormone-related protein analogs as
therapies for osteoporosis. Curr Osteoporos Rep. 11:400–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skjødt MK, Frost M and Abrahamsen B: Side
effects of drugs for osteoporosis and metastatic bone disease. Br J
Clin Pharmacol. 2018.(Epub ahead of print). doi: 10.1111/bcp.13759.
View Article : Google Scholar
|
|
5
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang W, Yang S, Shao J and Li YP:
Signaling and transcriptional regulation in osteoblast commitment
and differentiation. Front Biosci. 12:3068–3092. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rawadi G and Roman-Roman S: Wnt signaling
pathway: A new target for the treatment of osteoporosis. Expert
Opin Ther Targets. 9:1063–1077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamiya N: The role of BMPs in bone
anabolism and their potential targets SOST and DKK1. Curr Mol
Pharmacol. 5:153–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinzone JJ, Hall BM, Thudi NK, Vonau M,
Qiang YW, Rosol TJ and Shaughnessy JD Jr: The role of Dickkopf-1 in
bone development, homeostasis, and disease. Blood. 113:517–525.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo BK, Ryu HK, Park YC, Huh JE and Baek
YH: Dual effect of WIN-34B on osteogenesis and osteoclastogenesis
in cytokine-induced mesenchymal stem cells and bone marrow cells. J
Ethnopharmacol. 193:227–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Cai W, Weng X, Li Q, Wang Y, Chen Y,
Zhang W, Yang Q, Guo Y, Zhu X, et al: Lonicerae Japonicae flos and
lonicerae flos: A systematic pharmacology review. Evid Based
Complement Alternat Med. 2015:9050632015.PubMed/NCBI
|
|
12
|
Xu JR, Li GF, Wang JY, Zhou JR and Han J:
Gout prophylactic constituents from the flower buds of Lonicera
japonica. Phytochem Lett. 15:98–102. 2016. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marie PJ and Kassem M: Osteoblasts in
osteoporosis: Past, emerging, and future anabolic targets. Eur J
Endocrinol. 165:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsuka E, Yamaguchi A, Hirose S and
Hagiwara H: Characterization of osteoblastic differentiation of
stromal cell line ST2 that is induced by ascorbic acid. Am J
Physiol. 277:C132–C138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi A, Ishizuya T, Kintou N, Wada Y,
Katagiri T, Wozney JM, Rosen V and Yoshiki S: Effects of BMP-2,
BMP-4, and BMP-6 on osteoblastic differentiation of bone
marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem
Biophys Res Commun. 220:366–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MB, Song Y and Hwang JK: Kirenol
stimulates osteoblast differentiation through activation of the BMP
and Wnt/β-catenin signaling pathways in MC3T3-E1 cells.
Fitoterapia. 98:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aubin JE: Bone stem cells. J Cell Biochem
Suppl. 30-31:73–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan M and Cao X: BMP signaling in skeletal
development. Biochem Biophys Res Commun. 328:651–657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao M, Harris SE, Horn D, Geng Z,
Nishimura R, Mundy GR and Chen D: Bone morphogenetic protein
receptor signaling is necessary for normal murine postnatal bone
formation. J Cell Biol. 157:1049–1060. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia TL, Wang HZ, Xie LP, Wang XY and Zhang
RQ: Daidzein enhances osteoblast growth that may be mediated by
increased bone morphogenetic protein (BMP) production. Biochem
Pharmacol. 65:709–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo YC, Chang YH, Wei BL, Huang YL and
Chiou WF: Betulinic acid stimulates the differentiation and
mineralization of osteoblastic MC3T3-E1 cells: Involvement of
BMP/Runx2 and beta-catenin signals. J Agric Food Chem.
58:6643–6649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krishnan V, Bryant HU and Macdougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4(pii): a0078802012.PubMed/NCBI
|
|
25
|
Kikuchi A: Regulation of beta-catenin
signaling in the Wnt pathway. Biochem Biophys Res Commun.
268:243–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR,
Kyung HM, Sung JH, Wozney JM, Kim HJ and Ryoo HM: BMP-2-induced
Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the
BMP-2-induced osteoblast differentiation by suppression of Dlx5
expression. J Biol Chem. 278:34387–34394. 2003. View Article : Google Scholar : PubMed/NCBI
|