Introduction
Metformin is a first-line drug used in the treatment
of type 2 diabetes, and is positively correlated with increased
risk of liver cancer, pancreatic cancer and colorectal cancer
(1). A previous study showed that
metformin decreased the risk and mortality rate of colorectal
cancer (2), however, the detailed
mechanisms underlying the effect of metformin remain to be fully
elucidated.
The stemness of cancer cells is involved in tumor
progression, recurrence and chemoresistance (3). Non-migratory tumorigenic intrinsic
cancer stem cells (CSCs) ensure breast cancer metastasis by the
generation of C-X-C chemokine receptor 4 migrating CSCs (4). Aspirin can attenuate chemoresistance
in breast cancer by disrupting the nuclear factor-κB-interleukin-6
signaling axis, which is responsible for CSC generation (5). Previous studies have indicated that
nemo-like kinase maintains the proliferation and stemness of
non-small cell lung cancer (NSCLC) (6) and is a target of metformin, in which
metformin inhibits the stemness of glioma cells and
epithelial-mesenchymal transition (EMT) via suppressing the
activity of yes-associated protein (7). However, whether metformin can
attenuate the stemness of colorectal cancer cells remains to be
elucidated.
A previous study demonstrated that activation of the
WNT/β-catenin pathway is involved in vascular endothelial growth
factor-A/neuropilin 1 axis-induced breast cancer stemness (8). The WNT/β-catenin pathway has been
shown to direct the self-renewal symmetric cell division of human
telomerase reverse transcriptase-high prostate CSCs (9). In addition, the downregulation of
BORIS/CTCFL efficiently regulates cancer cells stemness and
metastasis in the MYCN-amplified neuroblastoma cell line by
modulating the Wnt/β-catenin signaling pathway (10). However, whether Wnt3a/β-catenin
pathway is also engaged in colorectal cancer cell stemness remains
to be elucidated.
In the present study, the results indicated that
metformin attenuated the stemness and EMT process in colorectal
cancer cells. Additionally, the Wnt3a/β-catenin signaling pathway
was suppressed by metformin in colorectal cancer cells. Notably,
re-activation of the Wnt3a/β-catenin pathway via its agonist or the
overexpression of Wnt3a attenuated the metformin-mediated
inhibition of stemness and EMT in colorectal cancer cells.
Metformin enhanced the sensitivity of the colorectal cancer cells
to 5-fluorouracil (5-FU). Therefore, it was suggested that
metformin suppresses the stemness and enhances the chemotherapeutic
sensitivity of colorectal cancer cells.
Materials and methods
Cell culture and reagents
The HCT116 human colorectal cell line was purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The HCT116 colorectal cancer cells were cultured in 1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) under a humidified atmosphere at 37°C with 5% CO2.
The Wnt3a/β-catenin agonist (SKL2001, cat. no. S8320) and 5-FU
(cat. no. S1209) were purchased from Selleck Chemicals (Shanghai,
China). For cell spheroid formation assay, the concentration of
SKL2001 was 10 nM, cell density was 1,000 cells/ml, duration was 10
days at 37°C with 5% CO2; and for other experiments the
concentration of SKL2001 was 5 µM at cell density of 50%
confluence, duration was 48 h at 37°C with 5% CO2.
Cell spheroid formation assay
The HCT116 colorectal cancer cells were cultured on
ultra-low attachment 24-well plates (Corning Incorporated, Union
City, CA, USA) at 500 cells/well, and maintained in MammoCult™
Human Medium (cat. no. 05620; StemCell Technologies, Inc.,
Vancouver, BC, Canada), and followed by treatment of various
concentrations of metformin (1, 2 or 4 mM) or solvent (control) for
10 days at 37°C with 5% CO2. Cell spheroid formation
efficiency was determined by counting the number of mammospheres
and identifying those with a diameter >50 µm under a light
microscope. All experiments were performed at least in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the HCT116 colorectal
cancer cells treated with or without metformin using TRIzol (cat.
no. R0016; Beyotime Institute of Biotechnology, Beijing, China).
cDNA was synthesized using a BeyoRT™ First Strand cDNA Synthesis
kit (RNaseH minus; cat. no. D7166; Beyotime Institute of
Biotechnology) following the manufacturer's protocols. The RT-qPCR
analysis was performed on the StepOne Plus PCR system with
BeyoFast™ SYBR-Green qPCR mix (2X, Low ROX; cat no. D7262; Beyotime
Institute of Biotechnology) containing 1 µl BeyoFast™ TaqDNA
Polymerase, 1 µl PCR Buffer, 0.5 µl dNTPs, 6.5 µl SYBR-Green I and
1 µl cDNA was used as templates in a 10 µl reaction volume. The
denaturing process was 95°C for 5 min, the annealing process was
58°C for 30 sec and the elongation process was 72°C for 30 sec. A
total of 35 cycles of this RT-qPCR was performed. All samples were
analyzed in triplicate and repeated at least three times. The
relative expression of genes was normalized to GAPDH. The
2−∆∆Cq method was used to determine the expression of
each transcript (11). Primers
sequences were: ALDH1, forward, 5′-CACGCCAGACTTACCTGTCCTACT-3′,
reverse, 5′-TGTCAACATCCTCCTTATCTCCTT-3′; Nanog, forward,
5′-CACGCCAGACTTACCTGTCCTACT-3′, reverse,
5′-TGTCAACATCCTCCTTATCTCCTT-3′; EpCAM, forward,
5′-TCCAGAAAGAAGAGAATGGCAAAG-3′, reverse,
5′-ACAAGACTCAAGTAAATAGAAAGG-3′; CD44, forward,
5′-GCAGGTATGGGTTCATAGAAGGGC-3′, reverse,
5′-TGTGAGTGTCTGGTAGCAGGGATT-3′; E-cadherin, forward,
5′-ATGGCTTCCCTCTTTCATCTCCTG-3′, reverse,
5′-CATAGTTCCGCTCTGTCTTTGGCT-3′; Vimentin, forward,
5′-GCCAGATGCGTGAAATGGAAGAGA-3′, reverse,
5′-TCAGGGAGGAAAAGTTTGGAAGAG-3′; Wnt3a, forward,
5′-GCAGGAGGGCCCAGCGACGCCGCCG-3′, reverse,
5′-CGGCGGCGTCGCTGGGCCCTCCTGC-3′; β-catenin, forward,
5′-TTCGGGCTGGTGACAGGGAAGACA-3′, reverse,
5′-TTTGCGGGACAAAGGGCAAGATTT-3′.
Western blot analysis
The HCT166 cells exposed to the different treatments
were harvested by ice-cold scraping and lysed with RIPA lysis
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology), and
protein concentration was measured using a Bradford protein
concentration assay kit (cat. no. P0009; Beyotime Institute of
Biotechnology). Subsequently, 30 µg of total protein was uploaded
and separated by 10% SDS-PAGE, and transferred onto a PVDF membrane
(cat. no. 1620177; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membranes were blocked with 5% non-fat milk in Tris-buffered
saline with 0.1% Tween-20 (TBST) for 1.5 h at room temperature and
then incubated with primary antibodies overnight at 4°C. The
primary antibodies were purchased from Abcam (Cambridge, MA, USA)
and were: ALDH1 (cat. no. ab52492), Nanog (cat. no. ab109250),
EpCAM (cat. no. ab32392), CD44 (cat. no. ab189524), E-cadherin
(cat. no. ab1416), Vimentin (cat. no. ab8978), Wnt3a (cat. no.
ab219412), β-catenin (cat. no. ab16051) and β-actin (cat. no.
ab8227). The dilution was 1:3,000 for all primary antibodies. The
membranes were then incubated with the following secondary
antibodies: HRP-labeled goat anti-rabbit IgG(H+L) from Beyotime
Institute of Biotechnology (cat. no. A0208) and HRP-labeled goat
anti-mouse IgG(H+L) from Beyotime Institute of Biotechnology (cat.
no. A0216) for 1 h at room temperature at a dilution of 1:500. The
membranes were washed with TBST for 15 min three times. An enhanced
chemiluminescence kit (BeyoECL Star, cat. no. P0018A; Beyotime
Institute of Biotechnology) was used to develop images in the Tanon
5200 machine (Tanon Science and Technology Co., Ltd., Shanghai,
China).
Plasmid and transfection
The Human Wnt3a expression plasmid (pcDNA-Wnt3a,
cat. no. 35908) was purchased from Addgene, Inc. (Cambridge, MA,
USA), which was transfected into the HCT116 colorectal cancer cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol. A total of 2
µg plasmid was used for transfection.
Cell viability assay
To determine whether metformin enhances the
sensitivity of HCT116 colorectal cancer cells to 5-FU. The HCT116
colorectal cancer cells were treated with 5-FU (25 µg/ml), with or
without metformin. The density of cells was 30–50% at 37°C with 5%
CO2. Cells that were left untreated served as the
control group. After 24, 48 and 72 h, the HCT116 colorectal cancer
cell viability was evaluated using a Cell Counting Kit-8 (cat no.
C0037; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The experiments were repeated at least
three times.
Statistical analysis
GraphPad Prism (version 5.01; GraphPad Software,
Inc., La Jolla, CA, USA) was used for data analysis. All data were
obtained from at least three independent experiments, and presented
as the mean ± standard deviation. Datasets with only two groups
were analyzed using Student's t-test. Differences between multiple
groups were analyzed using one-way analysis of variance with the
Tukey-Kramer post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Metformin attenuates stemness of
HCT116 colorectal cancer cells in a concentration-dependent
manner
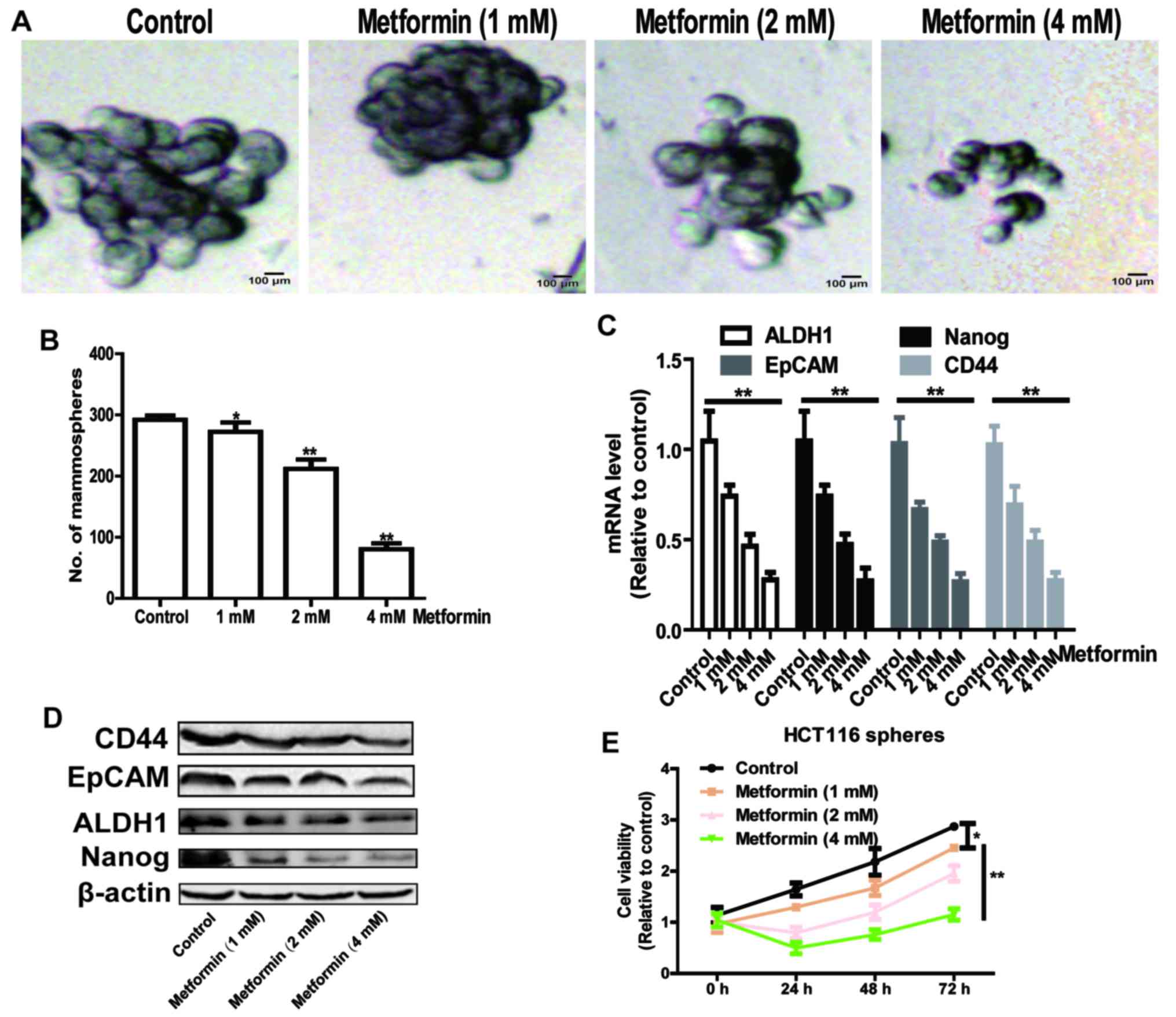
As CSCs are efficiently enriched in spheroid cells,
and they have the capacity of self-renewal and
multi-differentiation (12), the
present study initially identified whether metformin was able to
decrease the formation of cell spheroids in HCT116 colorectal
cancer cells. As shown in Fig. 1A and
B, the formation of cell spheroids was attenuated by metformin
treatment in a concentration-dependent manner, which was
characterized by a decrease in spheroid size and number.
Additionally, the expression levels of stemness markers epithelial
cell adhesion molecule (EpCAM), CD44, Nanog and aldehyde
dehydrogenase 1 (ALDH1) were detected, and it was found that
metformin also decreased the expression levels of these stemness
markers in a concentration-dependent manner (Fig. 1C and D). Furthermore, cells
digested from the spheres formed by HCT116 colorectal cancer cells
were subjected to a cell viability assay following metformin
treatment. As shown in Fig. 1E,
metformin significantly decreased cell viability in a
concentration-dependent manner. These results suggest that
metformin attenuated the stemness of HCT116 colorectal cancer cells
in a concentration-dependent manner.
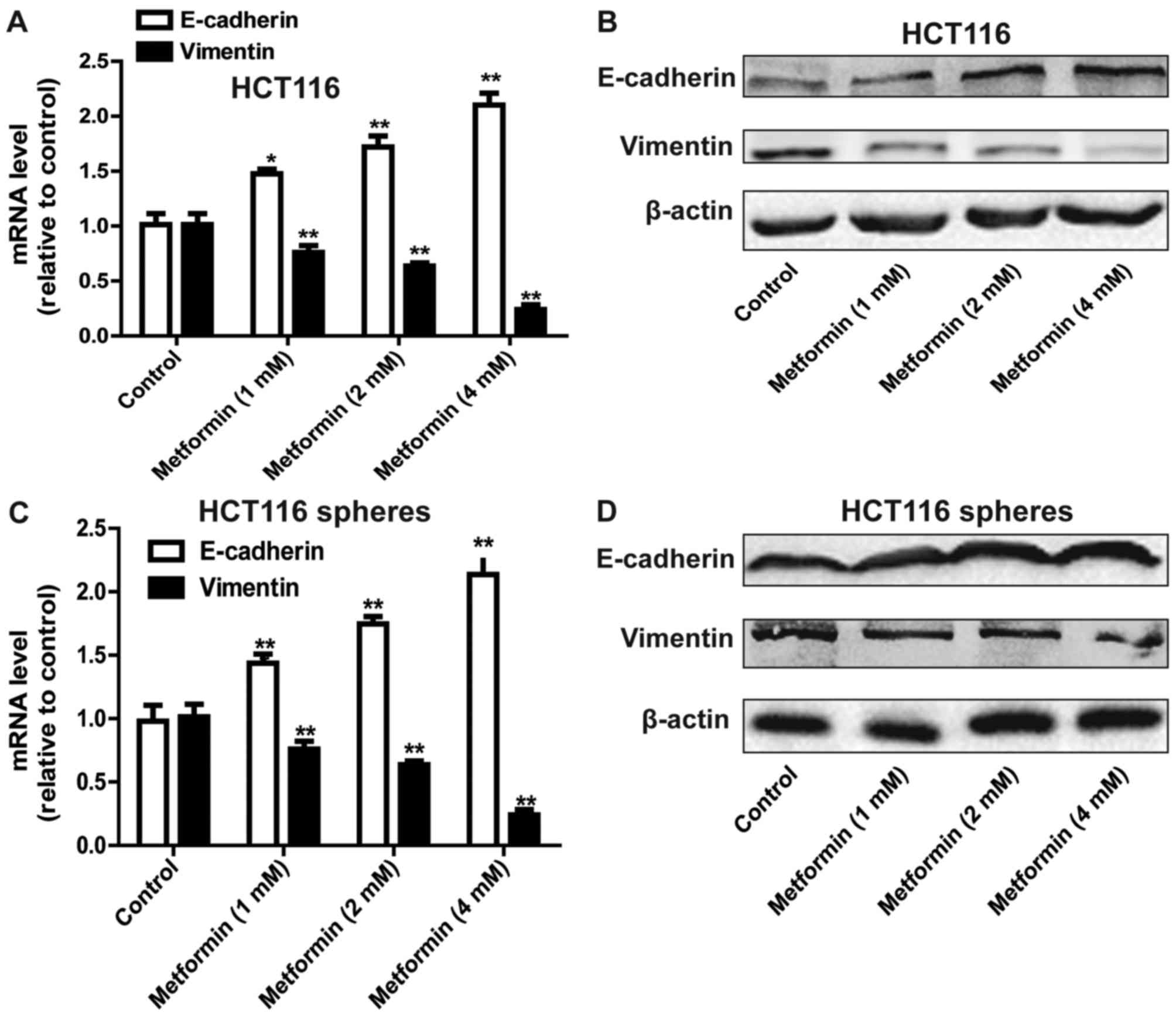
Metformin suppresses the EMT process
in HCT116 colorectal cancer cells
As the stemness of cells can confer mesenchymal
traits (13), it was hypothesized
that metformin can inhibit the EMT process in HCT116 colorectal
cancer cells. As expected, metformin attenuated the EMT process in
HCT116 colorectal cancer cells, characterized by a decrease in the
expression of mesenchymal marker Vimentin, and an increase in the
expression of epithelial marker E-cadherin (Fig. 2A and B). The EMT process in HCT116
colorectal cancer sphere cells was also suppressed by metformin
treatment (Fig. 2C and D).
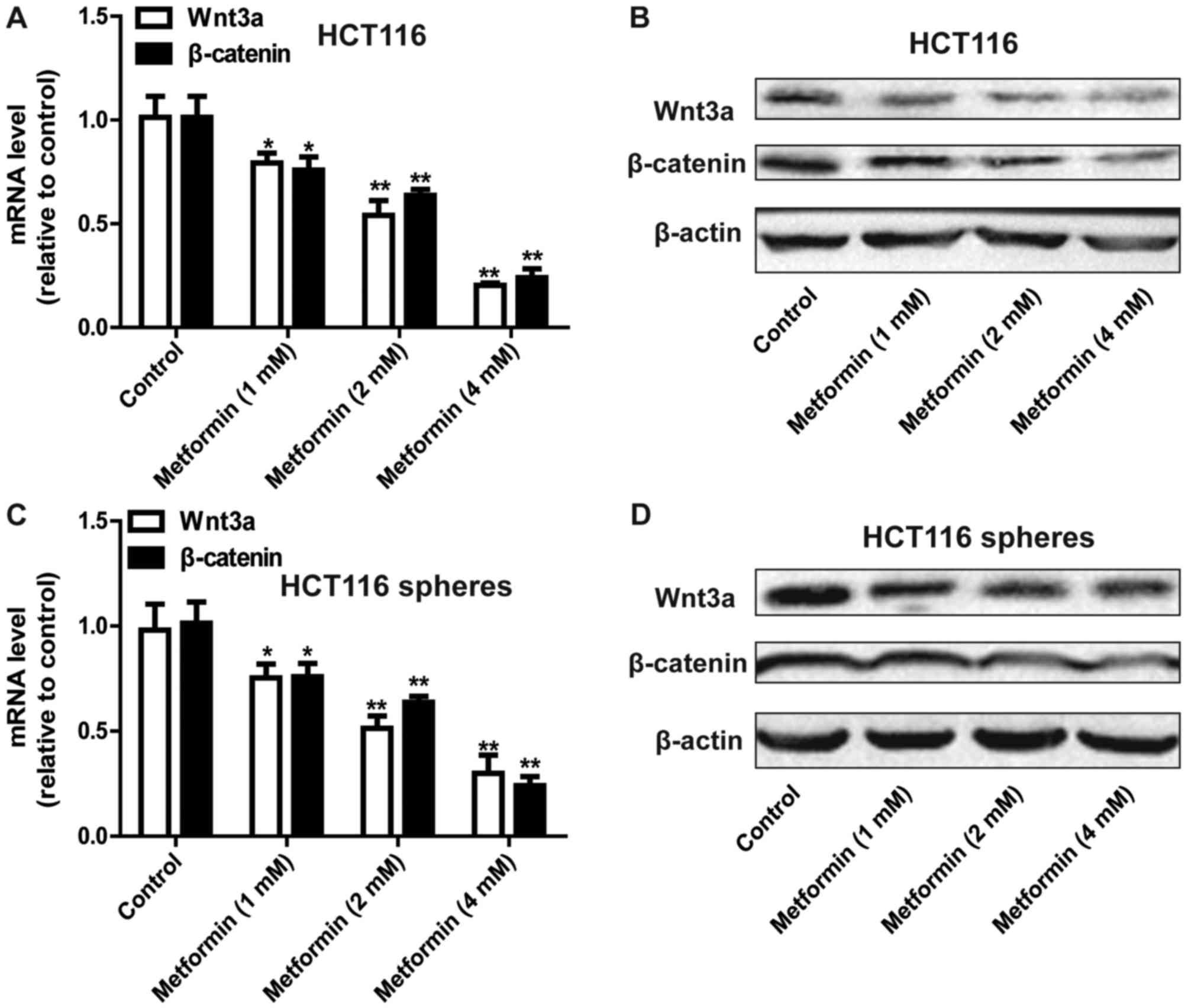
Metformin inhibits the Wnt3a/β-catenin
pathway in HCT116 colorectal cancer cells
The present study further examined the mechanisms by
which metformin inhibited the stemness of HCT116 colorectal cancer
cells. The focus of this investigation was on the Wnt3a/β-catenin
pathway owing to its critical promotive roles in cancer cell
stemness (8,9). As shown in Fig. 3A and B, the expression levels of
Wnt3a and β-catenin were decreased by metformin treatment in a
concentration-dependent manner in the HCT116 colorectal cancer
cells. Consistently, metformin decreased the expression of Wnt3a
and β-catenin in the sphere cells formed by HCT116 colorectal
cancer cells (Fig. 3C and D).
Therefore, metformin inhibited the Wnt3a/β-catenin signaling
pathway in HCT116 colorectal cancer cells.
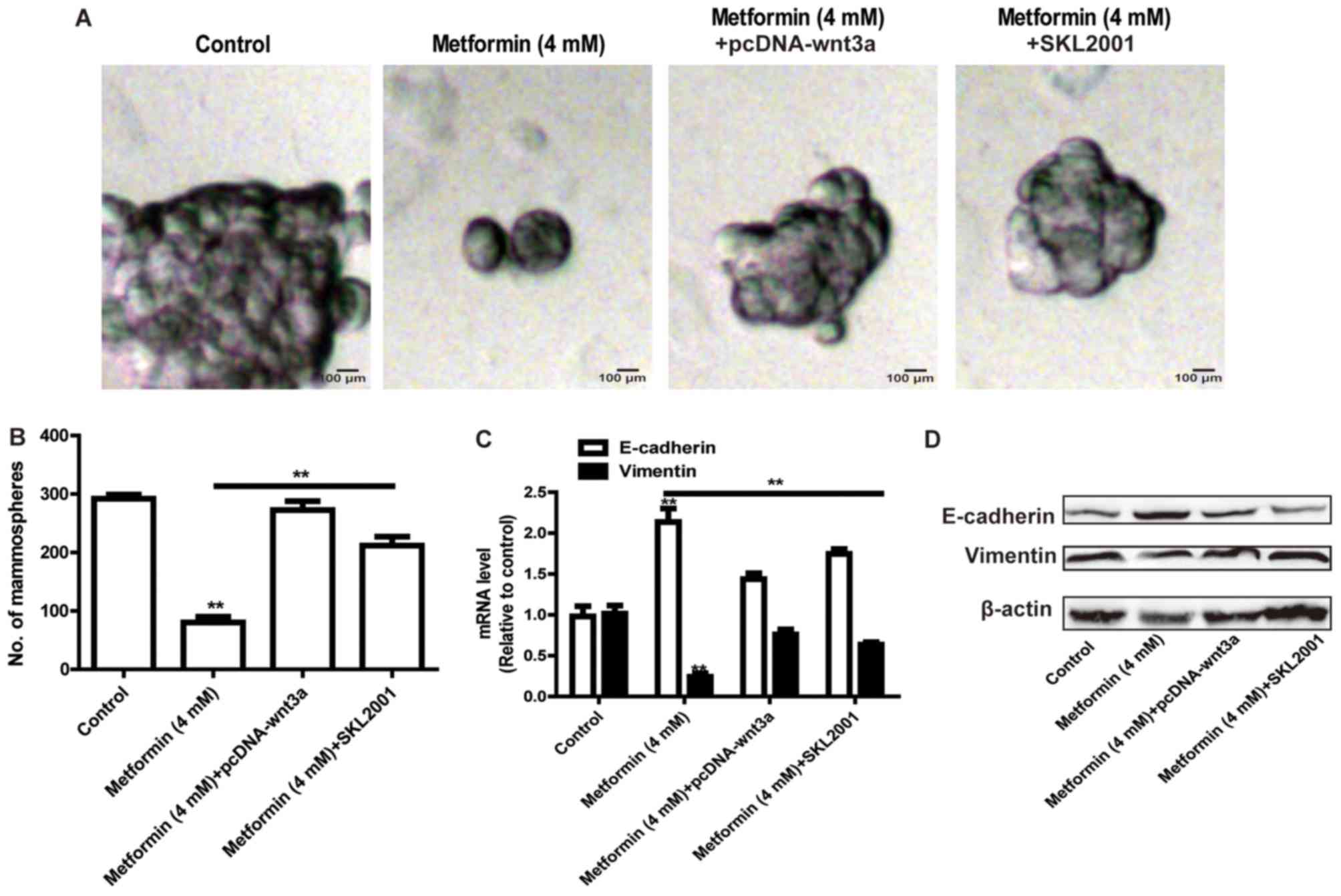
Reactivation of the Wnt3a/β-catenin
signaling pathway rescues metformin-mediated inhibition on stemness
and EMT of HCT116 colorectal cancer cells
The present study further assessed whether metformin
inhibited the stemness of HCT116 colorectal cancer cells and the
EMT process via the Wnt3a/β-catenin signaling pathway. The HCT116
colorectal cancer cells were transfected with the pcDNA-Wnt3a
plasmid or treated with the Wnt3a/β-catenin agonist (SKL2001),
followed by metformin treatment. As shown in Fig. 4A and B, the overexpression of Wnt3a
or treatment with SKL2001 attenuated the metformin-mediated
inhibition of the size and number of sphere cells. Additionally,
the metformin-induced inhibition of EMT was rescued by the
overexpression of Wnt3a or treatment with SKL2001 (Fig. 4C and D).
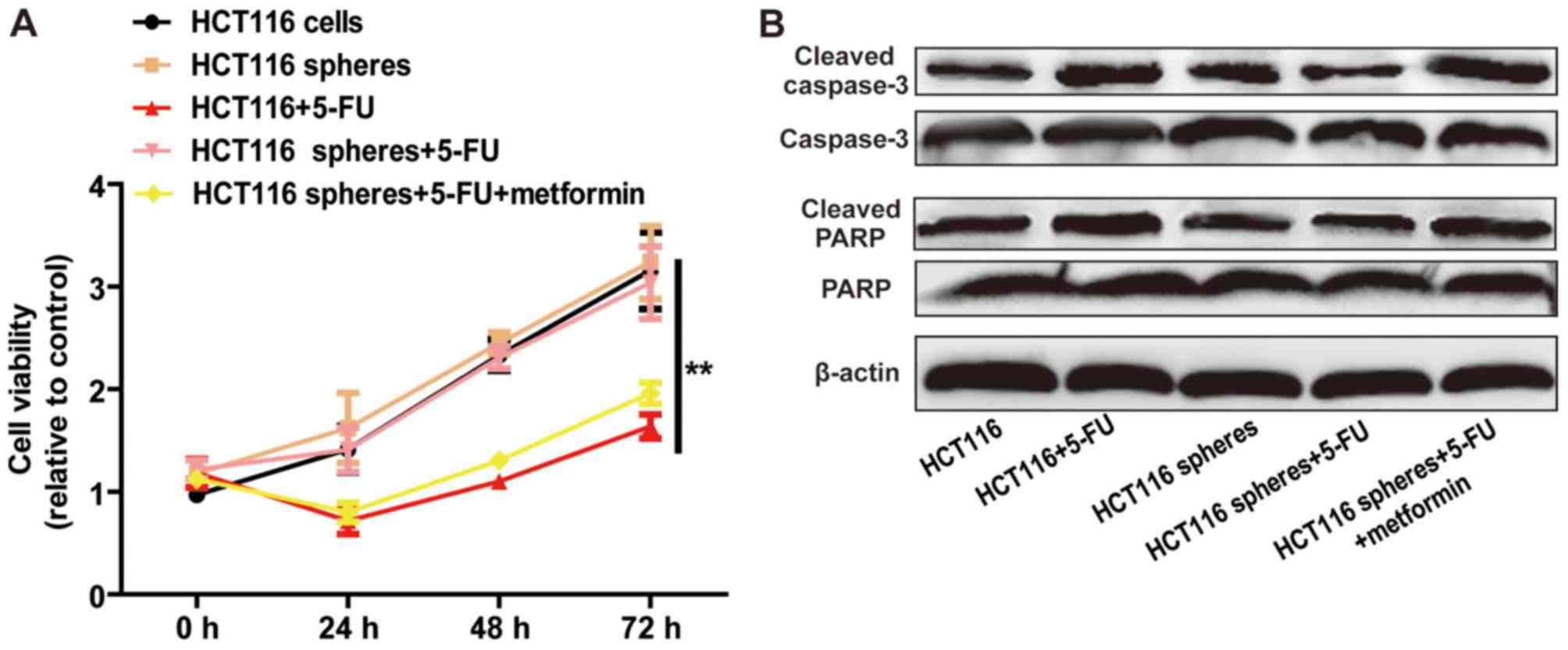
Metformin attenuates 5-FU resistance
of HCT116 sphere cells
As CSCs contribute to chemoresistance, it was
hypothesized that metformin can attenuate 5-FU resistance in HCT116
cell spheres. The results of the cell viability assay indicated
that metformin enhanced 5-FU sensitivity, characterized by a
decrease in cell viability (Fig.
5A) and increase in cell apoptosis (Fig. 5B). These results demonstrated that
metformin attenuated 5-FU resistance in HCT116 sphere cells.
Discussion
Metformin, a first-line drug for treating type 2
diabetes, has suppressive effects in various tumors (14,15).
However, the application of metformin in clinical treatments for
colorectal cancer has not been approved based on the fact its
functions and mechanisms remain to be fully elucidated. Therefore,
elucidation of the mechanisms underlying the role of metformin in
colorectal cancer progression is urgently required. The present
study focused on the roles and mechanisms of metformin on the
stemness of colorectal cancer cells. To the best of our knowledge,
this is the first study revealing the metformin-mediated inhibition
of the stemness of colorectal cancer cells, which may facilitate
the clinical evaluation of metformin in the treatment of colorectal
cancer.
In the present study, it was shown that metformin
inhibited the Wnt3a/β-catenin pathway in HCT116 colorectal cancer
cells. The Wnt3a/β-catenin pathway promotes tumor progression by
facilitating tumor metastasis, angiogenesis, EMT and CSC formation
(8,9). Notably, reactivation of the
Wnt3a/β-catenin pathway rescued the metformin-mediated inhibition
of HCT116 colorectal cancer cell stemness and the EMT process,
indicating that metformin exerted its effects at least partially
via the Wnt3a/β-catenin pathway. The present study also confirmed
that metformin enhanced 5-FU sensitivity in HCT116 sphere cells,
which is consistent with previous studies showing that metformin
mediated 5-FU sensitivity in hepatocellular carcinoma (16), pancreatic cancer cells (17) and NSCLC (18). However, it is noteworthy that the
results presented here are based on in vitro experiments,
and further in vivo experiments are required to confirm the
inhibitory effects of metformin in colorectal cancer.
In conclusion, the results of the present study
suggest that the interaction between metformin and the
Wnt3a/β-catenin may be therapeutically targetable providing novel
approaches to treat colorectal cancer and potentially other
diseases in which Wnt3a/β-catenin signaling transactivation is
aberrant.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CZ and YW designed the study, analyzed the data,
performed the experiments and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: A representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH
and Kim WH: The effects of metformin on the survival of colorectal
cancer patients with diabetes mellitus. Int J Cancer. 131:752–759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang N, Docherty F, Brown HK, Reeves K,
Fowles A, Lawson M, Ottewell PD, Holen I, Croucher PI and Eaton CL:
Mitotic quiescence, but not unique ‘stemness,’ marks the phenotype
of bone metastasis-initiating cells in prostate cancer. FASEB J.
29:3141–3150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukherjee S, Manna A, Bhattacharjee P,
Mazumdar M, Saha S, Chakraborty S, Guha D, Adhikary A, Jana D,
Gorain M, et al: Non-migratory tumorigenic intrinsic cancer stem
cells ensure breast cancer metastasis by generation of CXCR4(+)
migrating cancer stem cells. Oncogene. 35:4937–4948. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saha S, Mukherjee S, Khan P, Kajal K,
Mazumdar M, Manna A, Mukherjee S, De S, Jana D, Sarkar DK and Das
T: Aspirin suppresses the acquisition of chemoresistance in breast
cancer by disrupting an NFκB-IL6 signaling axis responsible for the
generation of cancer stem cells. Cancer Res. 76:2000–2012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suwei D, Liang Z, Zhimin L, Ruilei L,
Yingying Z, Zhen L, Chunlei G, Zhangchao L, Yuanbo X, Jinyan Y, et
al: NLK functions to maintain proliferation and stemness of NSCLC
and is a target of metformin. J Hematol Oncol. 8:1202015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan X, Wei W, Bao Q, Chen H, Jin P and
Jiang W: Metformin inhibits glioma cells stemness and
epithelial-mesenchymal transition via regulating YAP activity.
Biomed Pharmacother. 102:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Wang H, Li C, Zhao Y, Wu L, Du X
and Han Z: VEGF-A/neuropilin 1 pathway confers cancer stemness via
activating Wnt/β-catenin axis in breast cancer cells. Cell Physiol
Biochem. 44:1251–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang K, Guo Y, Wang X, Zhao H, Ji Z,
Cheng C, Li L, Fang Y, Xu D, Zhu HH and Gao WQ: WNT/β-catenin
directs self-renewal symmetric cell division of
hTERThigh prostate cancer stem cells. Cancer Res.
77:2534–2547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garikapati KR, Patel N, Makani VKK,
Cilamkoti P, Bhadra U and Bhadra MP: Down-regulation of BORIS/CTCFL
efficiently regulates cancer stemness and metastasis in MYCN
amplified neuroblastoma cell line by modulating Wnt/β-catenin
signaling pathway. Biochem Biophys Res Commun. 484:93–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kida K, Ishikawa T, Yamada A, Shimada K,
Narui K, Sugae S, Shimizu D, Tanabe M, Sasaki T, Ichikawa Y and
Endo I: Effect of ALDH1 on prognosis and chemoresistance by breast
cancer subtype. Breast Cancer Res Treat. 156:261–269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren D, Zhu X, Kong R, Zhao Z, Sheng J,
Wang J, Xu X, Liu J, Cui K, Zhang XH, et al: Targeting
brain-adaptive cancer stem cells prohibits brain metastatic
colonization of triple-negative breast cancer. Cancer Res.
78:2052–2064. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu S, Yang Z, Jin P, Yang X, Li X, Wei X,
Wang Y, Long S, Zhang T, Chen G, et al: Metformin suppresses tumor
progression by inactivating stromal fibroblasts in ovarian cancer.
Mol Cancer Ther. 17:1291–1302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JE, Lim JH, Hong YK and Yang SH:
High-dose metformin plus temozolomide shows increased anti-tumor
effects in glioblastoma in vitro and in vivo compared with
monotherapy. Cancer Res Treat. 50:1331–1342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian Y, Tang B, Wang C, Sun D, Zhang R,
Luo N, Han Z, Liang R, Gao Z and Wang L: Metformin mediates
resensitivity to 5-fluorouracil in hepatocellular carcinoma via the
suppression of YAP. Oncotarget. 7:46230–46241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng G and Lanza-Jacoby S: Metformin
decreases growth of pancreatic cancer cells by decreasing reactive
oxygen species: Role of NOX4. Biochem Biophys Res Commun.
465:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, He C and Huang X: Metformin
partially reverses the carboplatin-resistance in NSCLC by
inhibiting glucose metabolism. Oncotarget. 8:75206–75216.
2017.PubMed/NCBI
|