Introduction
Proliferative vitreoretinopathy (PVR) most commonly
occurs as a complication in rhegmatogenous retinal detachment and
is often the reason for the failure of this type of surgery
(1,2). Retinal pigment epithelial (RPE)
cells, macrophages, glial cells, fibroblasts and various
extracellular matrix molecules are the main components of the
fibrotic membrane in PVR (3). This
fibrotic membrane can contract, which can cause either a new
retinal detachment or the failure of surgery. Moreover, the
inflammation response and the proliferation and differentiation of
RPE cells might be aggravated by the high levels of inflammatory
factors in the vitreous of patients with vitreoretinal disorders
(4–7). PVR is triggered by many cytokines,
including interleukin-6 (IL-6), monocyte chemotactic protein-1
(MCP-1), tissue inhibitor of metalloproteinase-1 (TIMP-1) and
intercellular cell adhesion molecule-1 (ICAM-1) (8–10).
Macrophage migration inhibitory factor (MIF) plays a
vital role in immune and inflammatory responses. MIF is produced by
various types of cells, such as endothelial cells, keratinocytes,
anterior pituitary cells, monocytes and osteoblasts (11–16).
MIF has been reported to be involved in fibrosis diseases (17,18).
Furthermore, MIF has been shown to contribute to the inflammatory
phase of the wound healing process (19), and its expression has been
immunohistochemically detected in the iris, cornea, retina, and
ciliary body (20,21).
Previous studies have illustrated that the
expression of MIF in the vitreous is higher in PVR patients
compared to that noted in control groups (22). In addition, MIF activates the
mitogen activated protein kinase (MAPK) signaling pathway in human
ectopic endometrial stromal cells (23). However, little is known concerning
the correlation between MIF and the expression of IL-6, MCP-1 and
collagen I in human RPE cells. In the present study, the role of
MIF was investigated in regards to the regulation of the expression
of IL-6, MCP-1 and collagen I as well as the potential signaling
mechanism.
Materials and methods
Reagents
Recombinant human MIF was purchased from EMD
Millipore. Anti-p-p38, p38, anti-p-ERK and ERK were obtained from
Cell Signaling Technology (Danvers, MA). MIF inhibitor ISO-1 was
obtained from Abcam (Cambridge, MA, USA). P38 inhibitor SB203580
and ERK inhibitor PD98059 were obtained from Sigma-Aldrich/Merck
KGaA. Collagen I, IL-6, and an MCP-1 enzyme-linked immunosorbent
assay kit (ELISA) were obtained from Abcam (Cambridge, MA,
USA).
Cell culture
Human RPE cells (ARPE-19; CRL-2302) were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's Medium
(DMEM; Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) in
a humidified incubator at 37°C in 5% CO2.
Cell proliferation assay
MTT [3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide] was used to measure cell
proliferation. Cells (1×104), grown in a 96-well plate
for 24 h, were cultured in DMEM supplemented with 1% FBS (12 h) and
then stimulated with recombinant MIF for an additional 48 h. MTT
reagent was added to the culture medium, and the cells were
incubated for an additional 4 h in a 37°C atmosphere. Next, 100 µl
of dimethyl sulfoxide (DMSO) was added to each well to dissolve the
formed formazan crystals. Absorbance (540 nm) was measured using a
microplate reader.
Cell migration assay
A Boyden chamber was used to measure migration of
the RPE cells. The trypsinised RPE cells were placed in the upper
chamber. FBS (10%), with or without a concentration of 50 ng/ml
MIF, was then placed in the bottom chamber. The cells were fixed in
4% paraformaldehyde and stained with crystal violet after 6 h of
incubation. A cotton swab was used to swab away the non-migrated
cells in the upper chamber. Migratory cells that moved through the
filter towards the lower surface were counted with a cell counter
in three fields per filter under a fluorescence microscope
(magnification, ×200).
Reverse transcription-quantitative PCR
(RT-qPCR)
In the presence or absence of pretreatment with
PD98059, SB203580 and ISO-1 for 30 min, the cells
(1×106) were collected after 24 h of incubation with
MIF. Total RNA was extracted using a TRIzol kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The cDNA was synthesized using a RevertAid First Strand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.). RT-qPCR
was measured using a detector (Bio-Rad Laboratories, Inc.). The PCR
solution contained 12.5 µl Maxima SYBR Green qPCR Master Mix
(Fermentas; Thermo Fisher Scientific, Inc.), specific primers (0.3
µM each), and 2.5 µl cDNA with a final volume of 25 µl. The PCR
primers were as follows: Human collagen I forward,
5′-TGGTGGTTATGACTTTGGTTACGAT-3 and reverse,
5′-TGTGCGAGCTGGGTTCTTTCTA-3′; human IL-6 forward,
5′-TGGCTGAAAAAGATGGATGCT-3′ and reverse 5′-TCTGCACAGCTCTGGCTTGT-3′;
human MCP-1 forward, 55-CTCATAGCAGCCACCTTCATTC-3 and reverse,
5′-TCACAGCTTCTTTGGGACACTT-3′; human GAPDH forward,
5′-TGTTCGACAGTCAGCCGCAT-3′ and reverse, 5′-ACTCCGACCTTCACCTTCCC-3′.
The reaction conditions for amplifying DNA were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec, 61°C for 30 sec, and 72°C
for 30 sec. The mRNA expression was normalized to the expression of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and it was
calculated using the following formula: Fold
change=2−ΔΔCq (24).
Western blot analysis
Western blot was performed for analysis as
previously described (25). In
brief, protein samples were separated by electrophoresis on 10%
SDS-PAGE gels, transferred to PVDF membranes and then processed for
analysis using an enhanced chemiluminescence detection system
(Amersham). The primary antibodies were used at the following
dilutions: Anti-p-p38 (Cell Signaling Technology; 9216; 1:2,000),
anti-p38 (Cell Signaling Technology; 9212; 1:1,000), anti-p-ERK
(Cell Signaling Technology; 9106; 1:2,000) and anti-ERK (Cell
Signaling Technology; 4696; 1:1,000).
Enzyme-linked immunosorbent assay
(ELISA)
After the human RPE cells were treated, the
conditioned medium was collected. IL-6 (ab46027), MCP-1(ab100586)
and collagen I (ab210966) ELISA kits (Abcam), utilizing a sandwich
two-site immunoassay, were applied to test the IL-6, MCP-1 and
collagen I content in the medium.
Statistical analysis
All data are expressed as means ± standard deviation
(SD) and analysed with SPSS 17.0 (SPSS, Inc.). One-way analysis of
variance followed by Tukey's test was used for statistical
analysis, and P<0.05 was considered to be indicative of a
statistically significant result.
Results
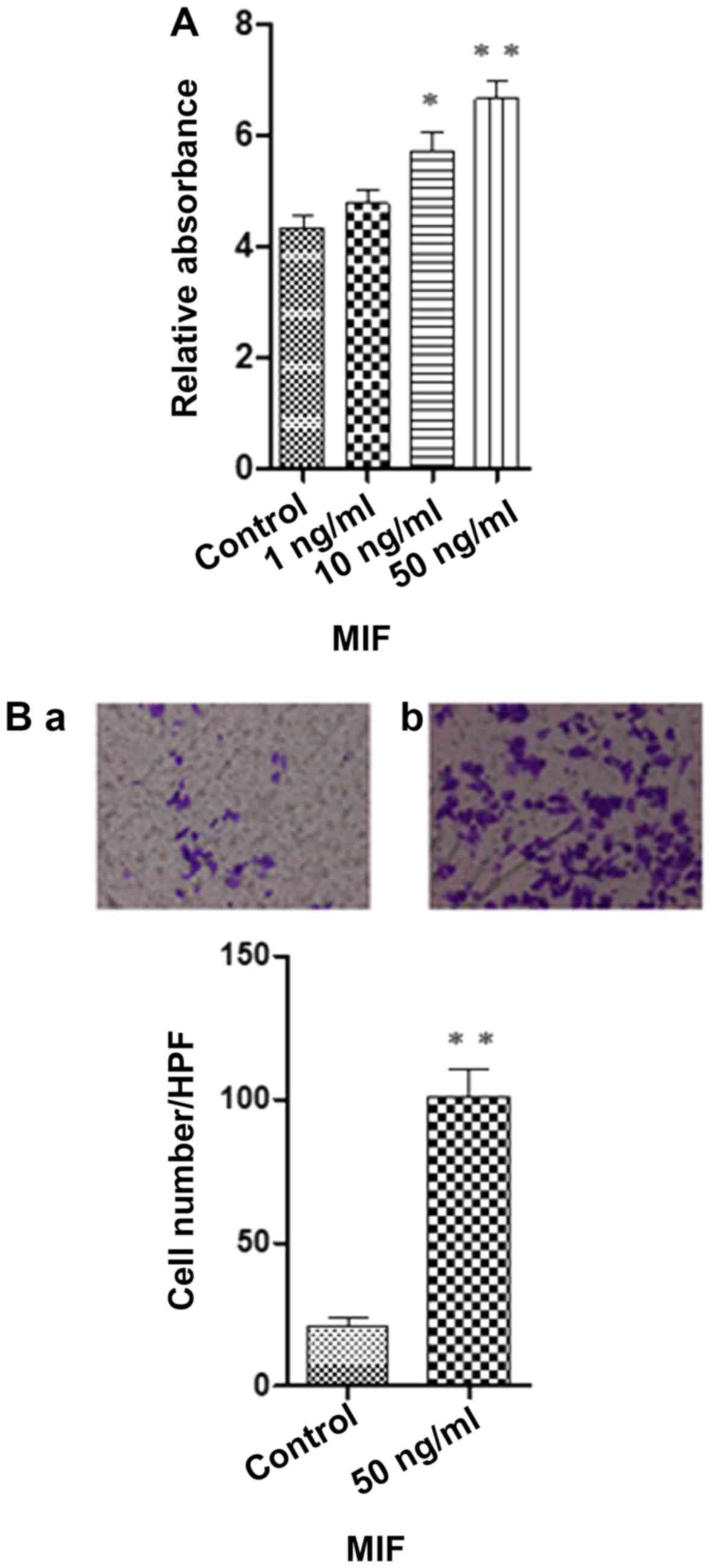
MIF enhances human RPE cell
proliferation and migration
The effects of MIF on the proliferation of RPE cells
were examined using an MTT assay. In the cell proliferation assay,
the cells were incubated with various concentrations of MIF [0
(control), 1, 10 and 50 ng/ml] for 48 h. MIF significantly
increased RPE cell proliferation compared to that noted in the
control group (Fig. 1A). In the
cell migration assay, the RPE cells were placed in a modified
Boyden chamber. The mean number of migrated cells in the
MIF-treated (50 ng/ml) RPE cells was significantly higher than that
of the control group (Fig.
1B).
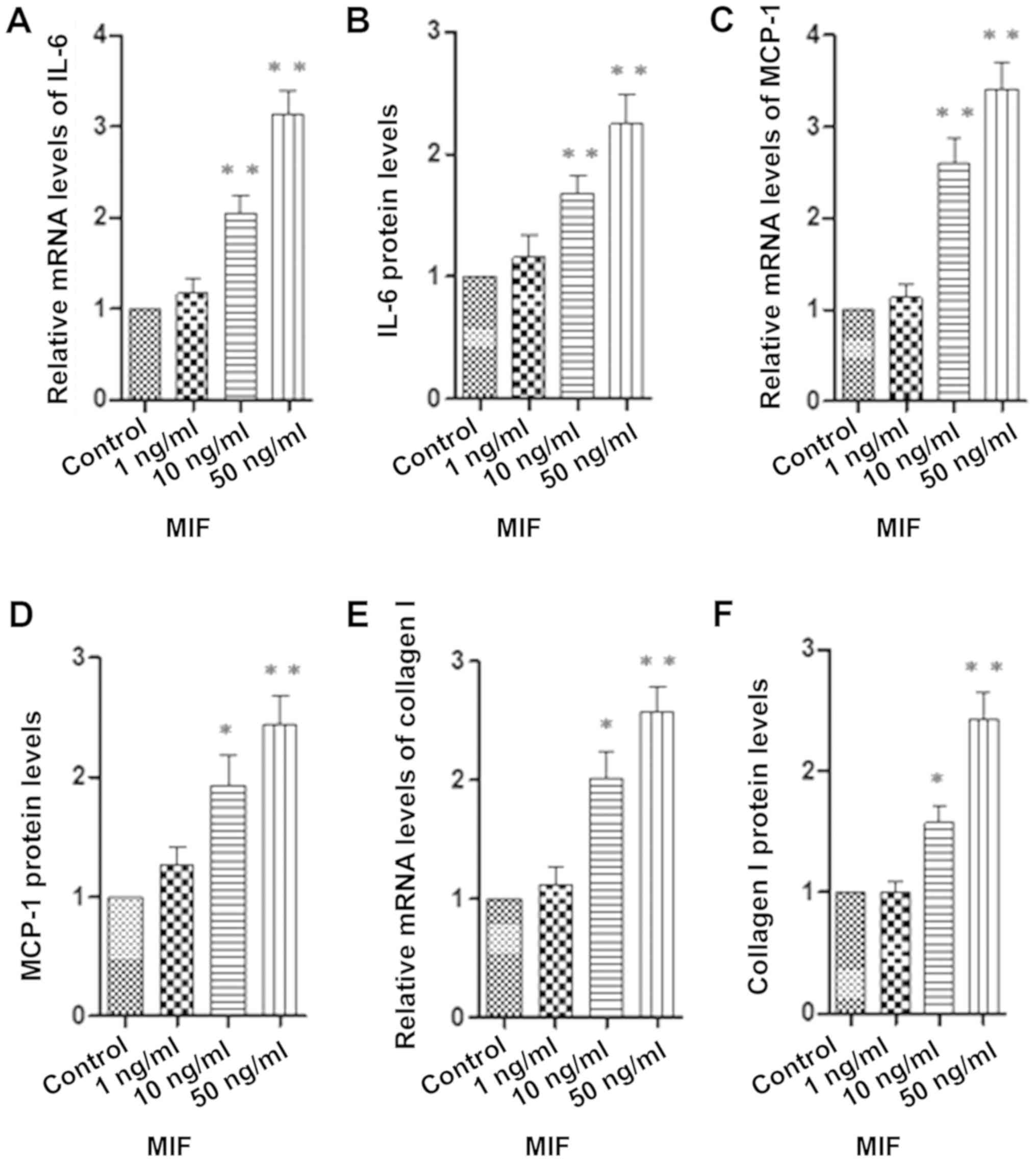
MIF-induced expression of IL-6, MCP-1
and collagen I
The RPE cells were stimulated with MIF (0, 1, 10 and
50 ng/ml). The expression of IL-6, MCP-1 and collagen I was
measured using real-time PCR and the relevant ELISA kits. The
levels of IL-6, MCP-1 and collagen I in human RPE cells were
measured after 24 h of incubation with various concentrations of
MIF. Our results showed that MIF (10 and 50 ng/ml) induced
significant increases in the mRNA and protein levels of IL-6
(Fig. 2A and B), MCP-1 (Fig. 2C and D) and collagen I (Fig. 2E and F) in the RPE cells compared
to the control group.
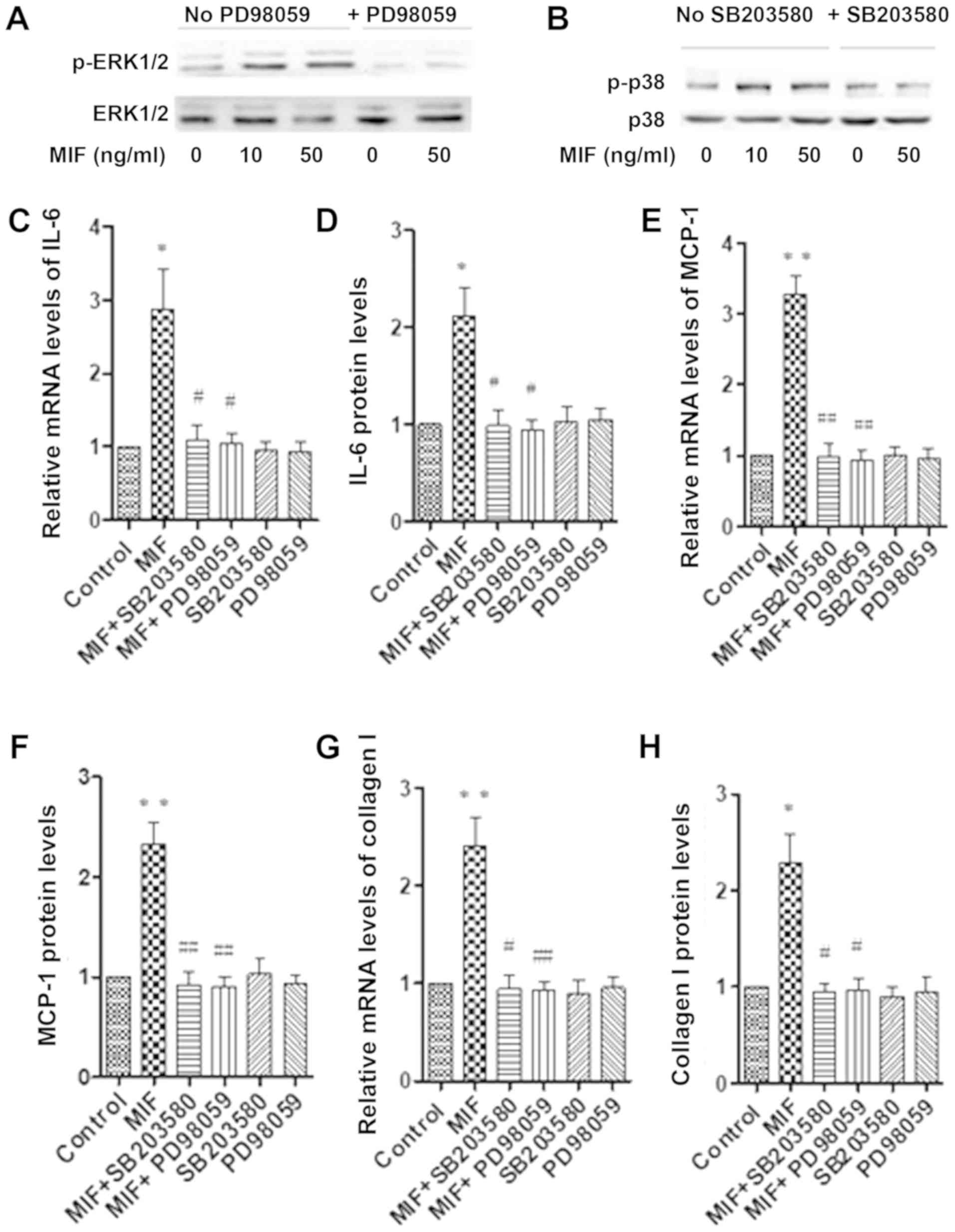
MIF stimulates the RPE cells via ERK
and p-38 pathways
The RPE cells were stimulated with 0, 10 and 50
ng/ml MIF, and the phosphorylation levels of ERK and p38 were
detected using western blot analysis. MIF (10 and 50 ng/ml)
significantly activated the phosphorylation of ERK and p38. The
phosphorylation of ERK and p38 was blocked by pretreatment with
PD98059 and SB203580 (Fig. 3A and
B). The levels of IL-6, MCP-1 and collagen I were determined
using real-time PCR and the relevant ELISA kits.
Having found that MIF treatment activated the ERK
and p38 signalling pathways in RPE cells, we then examined whether
the activation of the ERK and p38 signalling pathways plays an
important role in MIF-induced IL-6, MCP-1 and collagen I expression
in RPE cells. The data showed that pretreatment with PD98059 and
SB203580 for 30 min significantly blocked the MIF-induced
expression of IL-6 (Fig. 3C and
D), MCP-1 (Fig. 3E and F) and
collagen I (Fig. 3G and H). In
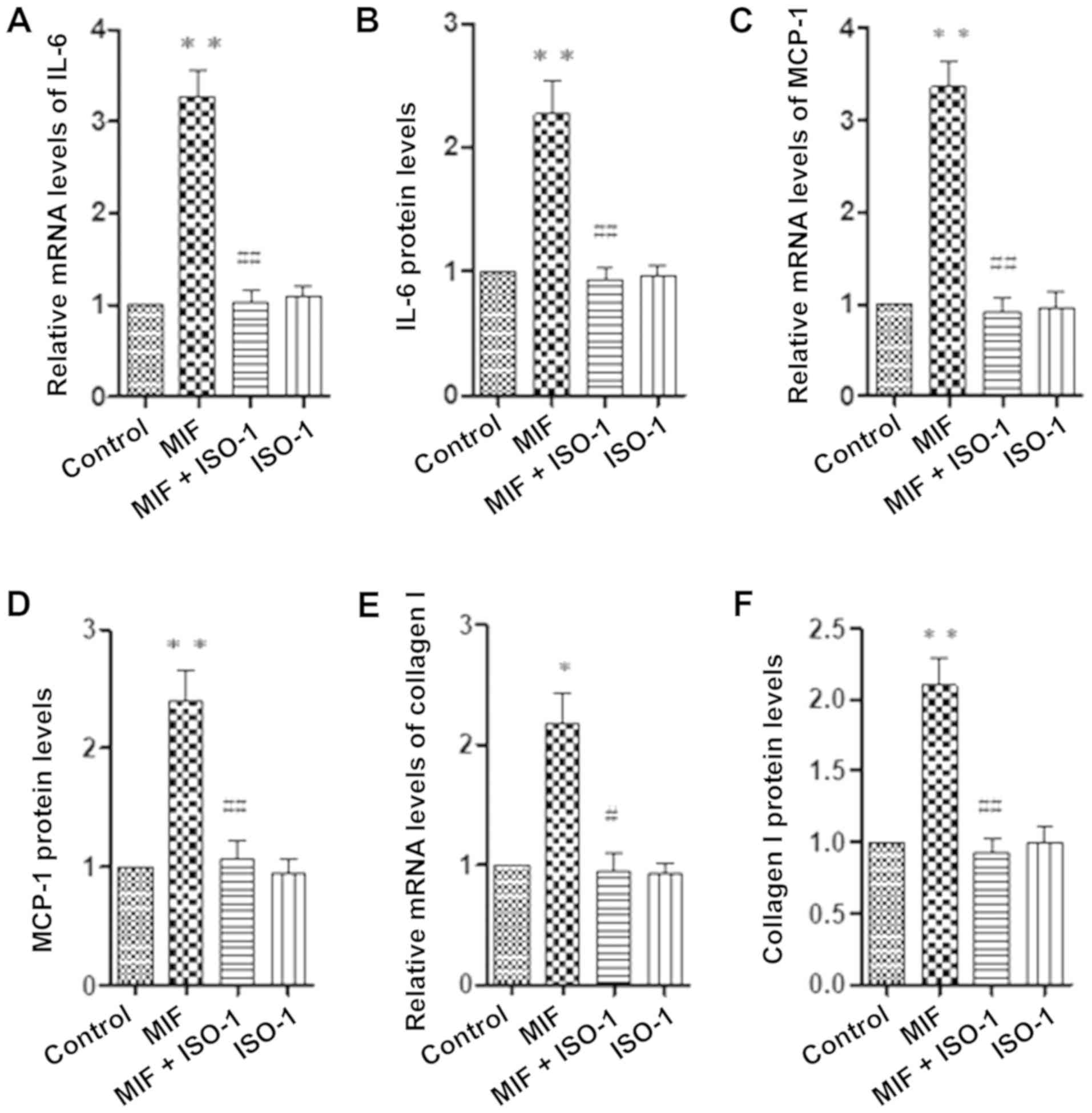
addition, pretreatment with MIF inhibitor ISO-1 for 30 min also
significantly blocked the MIF-induced expression of IL-6 (Fig. 4A and B), MCP-1 (Fig. 4C and D) and collagen I (Fig. 4E and F) in RPE cells.
Discussion
In patients with retinal detachment due to PVR, the
cell proliferation, migration and secretion of extracellular matrix
molecules, such as collagen I and fibronectin, are the main
components of the fibrotic membrane. These membranes are formed
during a fibrotic, inflammatory process involving cytokines, growth
factors and cells, such as RPE cells. To prevent or treat PVR,
anti-inflammatory (steroids) and cell-cycle inhibitors, such as
daunorubicin, retinoic acid and 5-fluorouracil (5-FU), have been
used, the latter particularly to decrease RPE cell proliferation
(26–31). In the present study, the data
showed that recombinant MIF induced cell proliferation, migration
and secretion of collagen I, which suggests that MIF plays a
profibrotic role in the development of PVR.
IL-6, a pleiotropic cytokine, is involved in immune
and inflammatory reactions (32).
Studies have shown IL-6 gene secretion and expression by
cytokine-stimulated human RPE cells (33,34).
Furthermore, IL-6 was observed to induce chemokines and to enhance
the recruitment of leukocytes in an animal model in vivo
(35). The IL-6 levels in the
vitreous were also found to be higher in a PVR group than that in a
control group (9). MCP-1, as a
representative chemokine, is the major determinant of macrophage
recruitment to the site of tissue injury (36). In addition, MCP-1 stimulates human
RPE cell migration, suggesting its role in PVR (37). The MCP-1 levels in the vitreous in
PVR patients were found to be significantly higher compared to the
control group (38). The above
evidence suggests that inflammatory cytokines, such as IL-6 and
MCP-1, play a vital role in the development of PVR. MIF regulates
MCP-1 expression in the hepatocytes of injured liver tissue
(39). MIF inhibitor decreases the
expression of IL-6 in lipopolysaccharide (LPS)-activated microglial
cells (40).
Furthermore, the level of MIF has been found to be
upregulated in the vitreous in PVR patients (22). Recent results implicate MIF in the
induction of epithelial- mesenchymal transition (EMT) and its
related processes by oxidative stress in human RPE cells, and MIF
was reported to regulate expression of EMT markers (41). In the present study, our data
revealed that recombinant MIF increased the expression of IL-6 and
MCP-1 in human RPE cells, which suggests that MIF plays a
proinflammatory role in the development of PVR.
MIF activates the ERK and p38 MAPK signaling
pathways in human ectopic endometrial stromal cells (23). However, the signaling mechanism of
MIF in RPE cells remains unclear. The data of the present study
indicated that MIF induced the activation of p38 and ERK in RPE
cells, and pretreatment with p38 inhibitor SB203580, ERK inhibitor
PD98059 and MIF inhibitor ISO-1 significantly decreased the
MIF-induced expression of IL-6, MCP-1 and collagen I in human RPE
cells.
In future research, the role of MIF should be
investigated in animal models in vivo and knockdown of MIF
receptor in RPE cells should be conducted. In addition, additional
inflammatory factors should be investigated. These are the
limitation of the present study. In summary, this study is the
first to demonstrate that MIF induced the expression of IL-6, MCP-1
and collagen I through the p38 and ERK signaling pathways in RPE
cells in vitro. Therefore, MIF may play a proinflammatory
and profibrotic role in the development of PVR and may be a
treatment option for PVR patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Department of
Science and Technology of Henan Province (grant no.
162300410112).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DQ performed the experiments and wrote the paper. XJ
designed the experiments. YJ analysed the data and revised the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ishikawa K, He S, Terasaki H, Nazari H,
Zhang H, Spee C, Kannan R and Hinton DR: Resveratrol inhibits
epithelial mesenchymal transition of retinal pigment epithelium and
development of proliferative vitreoretinopathy. Sci Rep.
5:163862015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan SJ: The pathophysiology of
proliferative vitreoretinopathy in its management. Am J Ophthalmol.
100:188–193. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newsome DA, Rodrigues MM and Machemer R:
Human massive periretinal proliferation. In vitro characteristics
of cellular components. Arch Ophthalmol. 99:873–880. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricker LJ, Kessels AG, de Jager W,
Hendrikse F, Kijlstra A and La Heij EC: Prediction of proliferative
vitreoretinopathy after retinal detachment surgery: Potential of
biomarker profiling. Am J Ophthalmol. 154:347–354.e2. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricker LJ, Altara R, Goezinne F, Hendrikse
F, Kijlstra A and La Heij EC: Soluble apoptotic factors and
adhesion molecules in rhegmatogenous retinal detachment. Invest
Ophthalmol Vis Sci. 52:4256–4262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ricker LJ, Kijlstra A, de Jager W, Liem
AT, Hendrikse F and La Heij EC: Chemokine levels in subretinal
fluid obtained during scleral buckling surgery after rhegmatogenous
retinal detachment. Invest Ophthalmol Vis Sci. 51:4143–4150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bromberg-White JL, Glazer L, Downer R,
Furge K, Boguslawski E and Duesbery NS: Identification of
VEGF-independent cytokines in proliferative diabetic retinopathy
vitreous. Invest Ophthalmol Vis Sci. 54:6472–6480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoerster R, Hermann MM, Rosentreter A,
Muether PS, Kirchhof B and Fauser S: Profibrotic cytokines in
aqueous humour correlate with aqueous flare in patients with
rhegmatogenous retinal detachment. Br J Ophthalmol. 97:450–453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricker LJ, Kijlstra A, Kessels AG, de
Jager W, Liem AT, Hendrikse F and La Heij EC: Interleukin and
growth factor levels in subretinal fluid in rhegmatogenous retinal
detachment: A case-control study. PLoS One. 6:e191412011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Symeonidis C, Papakonstantinou E, Androudi
S, Georgalas I, Rotsos T, Karakiulakis G, Diza E and Dimitrakos SA:
Comparison of interleukin-6 and matrix metalloproteinase expression
in the subretinal fluid and the vitreous during proliferative
vitreoretinopathy: Correlations with extent, duration of RRD and
PVR grade. Cytokine. 67:71–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
David J: Delayed hypersensitivity in
vitro: Its mediation by cell-free substances formed by lymphoid
cell-antigen interaction. Proc Natl Acad Sci USA. 56:72–77. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bloom B and Bennet B: Mechanism of a
reaction in vitro associated with delayed-type hypersensitivity.
Science. 153:80–82. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishihira J, Koyama Y and Mizue Y:
Identification of macrophage migration inhibitory factor (MIF) in
human vascular endothelial cells and its induction by
lipopolysaccharide. Cytokine. 10:199–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abe R, Shimizu T, Ohkawara A and Nishihira
J: Enhancement of macrophage migration inhibitory factor (MIF)
expression in injured epidermis and cultured fibroblasts.
Biochimica Biophysica Acta. 1500:1–9. 2000. View Article : Google Scholar
|
|
15
|
Onodera S, Suzuki K, Matsuno T, Kaneda K,
Kuriyama T and Nishihira J: Identification of macrophage migration
inhibitory factor in murine neonatal calvariae and osteoblasts.
Immunology. 89:430–435. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernhagen J, Calandra T, Mitchell RA,
Martin SB, Voelter W, Manogue KR, Cerami A and Bucala R: MIF is a
pituitary-derived cytokine that potentiates lethal endotoxaemia.
Nature. 365:756–759. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barnes MA, McMullen MR, Roychowdhury S,
Madhun NZ, Niese K, Olman MA, Stavitsky AB, Bucala R and Nagy LE:
Macrophage migration inhibitory factor is required for recruitment
of scar-associated macrophages during liver fibrosis. J Leukoc
Biol. 97:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olivieri C, Bargagli E, Inghilleri S,
Campo I, Cintorino M and Rottoli P: Macrophage migration inhibitory
factor in lung tissue of idiopathic pulmonary fibrosis patients.
Exp Lung Res. 42:263–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimizu T, Nishihira J, Watanabe H, Abe R,
Honda A, Ishibashi T and Shimizu H: Macrophage migration inhibitory
factor is induced by thrombin and factor Xa in endothelial cells. J
Biol Chem. 279:13729–12737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuda A, Tagawa Y, Matsuda H and
Nishihira J: Identification and immunohistochemical localization of
macrophage migration inhibitory factor in human cornea. FEBS Lett.
385:225–228. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuda A, Tagawa Y, Yoshida K, Matsuda H
and Nishihira J: Expression of macrophage migration inhibitory
factor in rat retina and its immunohistochemical localization. J
Neuroimmunol. 77:85–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitamura Y, Takeuchi S, Matsuda A, Tagawa
Y, Mizue Y and Nishihira J: Macrophage migration inhibitory factor
levels in the vitreous of patients with proliferative
vitreoretinopathy. Am J Ophthalmol. 128:763–765. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Veillat V, Carli C, Metz CN, Al-Abed Y,
Naccache PH and Akoum A: Macrophage migration inhibitory factor
elicits an angiogenic phenotype in human ectopic endometrial cells
and triggers the production of major angiogenic factors via CD44,
CD74, and MAPK signaling pathways. J Clin Endocrinol Metab.
95:E403–E412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung BH, Kim JD, Kim CK, Kim JH, Won MH,
Lee HS, Dong MS, Ha KS, Kwon YG and Kim YM: Icariin stimulates
angiogenesis by activating the MEK/ERK-and PI3K/Akt/eNOS-dependent
signal pathways in human endothelial cells. Biochem Biophys Res
Commun. 376:404–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fekrat S, de Juan E Jr and Campochiaro PA:
The effect of oral 13-cis-retinoic acid on retinal redetachment
after surgical repair in eyes with proliferative vitreoretinopathy.
Ophthalmology. 102:412–418. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiedemann P, Hilgers RD, Bauer P and
Heimann K: Adjunctive daunorubicin in the treatment of
proliferative vitreoretinopathy: Results of a multicenter clinical
trial. Daunomycin Study Group. Am J Ophthalmol. 126:550–559. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asaria RH, Kon CH, Bunce C, Charteris DG,
Wong D, Khaw PT and Aylward GW: Adjuvant 5-fluorouracil and heparin
prevents proliferative vitreoretinopathy: Results from a
randomized, double-blind, controlled clinical trial. Ophthalmol.
108:1179–1183. 2001. View Article : Google Scholar
|
|
29
|
Chang YC, Kao YH, Hu DN, Tsai LY and Wu
WC: All-trans retinoic acid remodels extracellular matrix and
suppresses laminin-enhanced contractility of cultured human retinal
pigment epithelial cells. Exp Eye Res. 8:900–909. 2009. View Article : Google Scholar
|
|
30
|
Sadaka A and Giuliari GP: Proliferative
vitreoretinopathy: Current and emerging treatments. Clin
Ophthalmol. 6:1325–1333. 2012.PubMed/NCBI
|
|
31
|
Chang YC, Hu DN and Wu WC: Effect of oral
13-cis-retinoic acid treatment on postoperative clinical outcome of
eyes with proliferative vitreoretinopathy. Am J Ophthalmol.
146:440–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kishimoto T: Interleukin-6: Discovery of a
pleiotropic cytokine. Arthritis Res Ther. 8 (Suppl 2):S22006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuppner MC, McKillop-Smith S and Forrester
JV: TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8
mRNA levels and IL-6 production by human retinal pigment epithelial
cells. Immunology. 84:265–271. 1995.PubMed/NCBI
|
|
34
|
Holtkamp GM, Van Rossem M, de Vos AF,
Willekens B, Peek R and Kijlstra A: Polarized secretion of IL-6 and
IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol.
112:34–43. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Romano M, Sironi M, Toniatti C,
Polentarutti N, Fruscella P, Faggioni R, Luini W, van Hinsbergh V,
Sozzani S, Bussolino F, et al: Role of IL-6 and its soluble
receptor in induction of chemokines and leukocyte recruitment.
Immunity. 6:315–325. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han QH, Hui YN, Du HJ, Zhang WJ, Ma JX and
Wang SY: Migration of retinal pigment epithelial cells in vitro
modulated by monocyte chemotactic protein-1: Enhancement and
inhibition. Graefes Arch Clin Exp Ophthalmol. 239:531–538. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mitamura Y, Takeuchi S, Yamamoto S,
Yamamoto T, Tsukahara I, Matsuda A, Tagawa Y, Mizue Y and Nishihira
J: Monocyte chemotactic protein-1 levels in the vitreous of
patients with proliferative vitreoretinopathy. Jpn J Ophthalmol.
46:218–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie J, Yang L, Tian L, Li W, Yang L and Li
L: Macrophage migration inhibitor factor upregulates MCP-1
expression in an autocrine manner in hepatocytes during acute mouse
liver injury. Sci Rep. 6:276652016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Gu R, Jia J, Hou T, Zheng LT and
Zhen X: Inhibition of macrophage migration inhibitory factor (MIF)
tautomerase activity suppresses microglia-mediated inflammatory
responses. Clin Exp Pharmacol Physiol. 43:1134–1144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ko JA, Sotani Y, Ibrahim DG and Kiuchi Y:
Role of macrophage migration inhibitory factor (MIF) in the effects
of oxidative stress on human retinal pigment epithelial cells. Cell
Biochem Funct. 35:426–432. 2017. View
Article : Google Scholar : PubMed/NCBI
|