Introduction
Gastric cancer is a malignant tumor that accounts
for 7% of all human cancers. It is one of the leading causes of
cancer-related deaths, with about 1.4 million new cases diagnosed
worldwide (1). Since the rise and
development of chemotherapy in recent decades, it has become a
feasible therapeutic strategy for gastric cancer treatment, but
these chemotherapeutic drugs represented by 5-fluorouracil (5-FU)
and shikonin have limited clinical applications due to severe side
effects (2,3). Therefore, there is an urgent need to
identify novel chemotherapeutic drugs that improve the therapeutic
effects for gastric cancer.
Induction of apoptosis or cell cycle arrest is
considered to be an important means for exerting an anticancer
effect characteristic of chemotherapy drugs (4). Apoptosis can be activated through two
pathways: Mitochondria-dependent and mitochondria-independent
pathways (5). The
mitochondria-dependent pathway of apoptosis is regulated by the
B-cell lymphoma 2 (Bcl-2) family of proteins. The balance between
Bcl-2-associated death promoter (Bad) and Bcl-2 proteins regulates
the activation of caspase-3, ultimately leading to mitochondrial
apoptosis (6). The cell cycle is
the basic process underlying biological activity and cell cycle
arrest can lead to apoptosis. Many anticancer drugs block the cell
cycle at specific checkpoints through the Akt signaling pathway,
thereby inducing apoptosis in cancer cells. The cyclin-dependent
kinase/cyclin complex plays a significant role in cell cycle
regulation (7–9).
Growing evidence has shown that the generation of
reactive oxygen species (ROS) and relevant signaling pathways play
critical roles in the apoptosis of cancer cells (10). Recent research has demonstrated
ROS-mediated mitogen-activated protein kinase (MAPK) activation in
apoptosis triggered by various stimuli (11). The protein kinase B (Akt) signaling
pathway, an intracellular regulator of multiple pathways that play
fundamental roles in apoptosis and cell cycle arrest, can be
modulated by ROS (12). It has
also been suggested that ROS are important cellular mediators that
trigger activation of the signal transducer and activator of
transcription 3 (STAT3)-dependent pathway after chemotherapeutic
drug administration (13).
1,4-Naphthoquinone exhibits a variety of biological
activities, and its superior anticancer activity has attracted
widespread attention (14,15). Many 1,4-naphthoquinone derivatives
(plumbagin, mitomycin and shikonin) have been demonstrated to
strongly inhibit the proliferation and induce apoptosis in cancer
cells, but most of the derivatives also cause adverse side effects
in normal cells (16). Previous
studies have demonstrated that different substituent structures
enable naphthoquinone derivatives to have different biological
activities (17,18). Therefore, substitution of a
molecular group has been investigated as an effective strategy to
develop anticancer drugs. The biological activity and toxic side
effects of naphthoquinone derivatives are correlated with the
structure. The length of the alkane chain on the C2 position can
affect the biological activity of these compounds (19,20).
Here, we increased the fat solubility of the
compounds and studied the effects of carbon chain length on their
anticancer activity. Based on the principles of structural design,
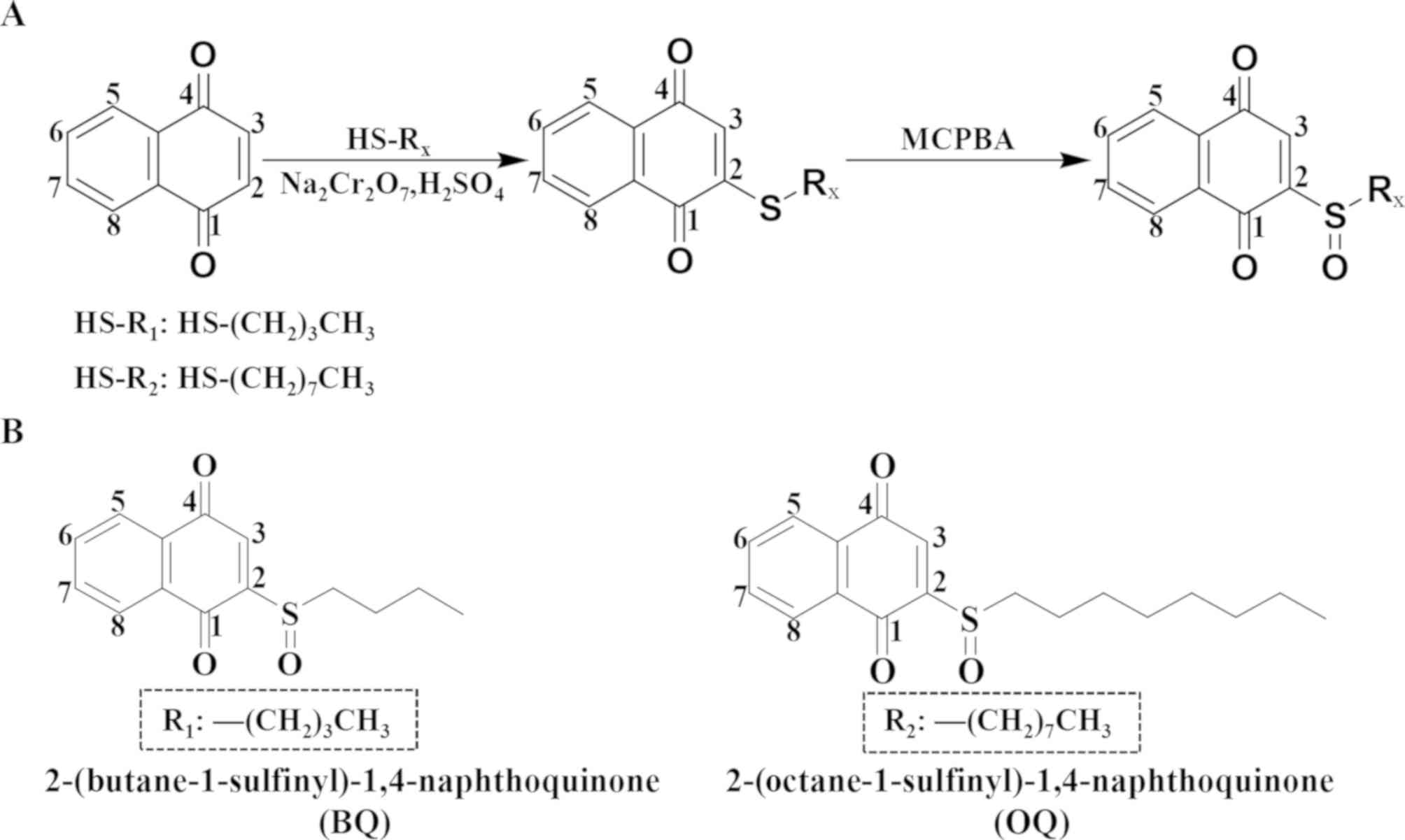
two 1,4-naphthoquinone derivatives named
2-(butane-1-sulfinyl)-1,4-naphthoquinone (BQ) and
2-(octane-1-sulfinyl)-1,4-naphthoquinone (OQ) were synthesized to
reduce the toxicity and optimize the efficacy of these agents. Then
their effects on anti-proliferation, apoptosis induction, cell
cycle arrest and ROS generation in gastric cancer cells were
evaluated. The molecular mechanisms of apoptosis induced by BQ and
OQ were then explored using AGS gastric cancer cells.
Materials and methods
Synthesis of the 1,4-naphthoquinone
derivatives BQ and OQ
1,4-Naphthoquinone (0.5 mM) was dissolved in MeOH
(15 ml), and alkyl thiol (0.75 mM) was added to this solution and
then stirred for 4 h at room temperature. Sodium dichromate (0.1
mM) and sulfuric acid (0.4 mM) were added to the mixture dropwise
and then stirred for 0.5 h at room temperature. The mixture was
extracted with dichloromethane and brine, dried over anhydrous
sodium sulfate, filtered, and concentrated under reduced pressure.
M-CPBA (0.6 mM) was added to this mixture in chloroform (10 ml) and
stirred at 0°C for 1 h. At the end of the reaction, 5%
NaHCO3 was added to this mixture. Then the solution was
extracted with dichloromethane and brine, dried over anhydrous
sodium sulfate, filtered, and concentrated under reduced pressure.
The residue underwent chromatography to obtain
2-(butane-1-sulfinyl)-1,4-naphthoquinone (BQ) and
2-(octane-1-sulfinyl)-1,4-naphthoquinone (OQ).
Chemicals and materials
Reagents were freshly diluted to the desired
concentration with the culture medium before use. Fetal bovine
serum (FBS), Roswell Park Memorial Institute 1640 (RPMI 1640),
Dulbecco's modified Eagle's medium (DMEM), penicillin (100 U/ml),
and streptomycin (100 µg/ml) were purchased from Gibco (Thermo
Fisher Scientific, Inc.). The Annexin V-FITC Apoptosis Detection
Kit, Cell Cycle Detection Kit, 2′,7′-dichlorofluorescein diacetate
(DCFH-DA), and N-acetyl-L-cysteine (NAC) were purchased from
Beyotime Institute of Biotechnology. DMSO was obtained from
Sigma-Aldrich (Merck KGaA). All primary antibodies used in the
present study were purchased from Santa Cruz Biotechnology, Inc.
and all secondary antibodies used were purchased from ZSGB-BIO.
Cell lines and cell culture
All eight gastric cancer cell lines (AGS, MKN-45,
NCI-N87, SUN-5, KATO-3, YCC-1, YCC-6, YCC-16 and SNU-5) were
purchased from the American Type Culture Collection. Normal stomach
GES-1 and normal liver L-02 cell lines and human lung fibroblasts
(IMR-90) were obtained from Saiqi Biotech Co., Ltd. This study
investigated the effects of BQ and OQ on gastric cancer cells, and
we selected a normal gastric cell line to evaluate toxicity and
side effects. The GES-1 cell line is a type of normal gastric
mucosal epithelial cell line that grows in the stomach, thus we
chose GES-1 as a control. The liver is the important target organ
for drug toxicity testing, and can directly reflect toxic effects
in toxicity studies. In addition, IMR-90 cells were extracted from
human lung tissue, and their primary properties were not
transformed, which can directly reflect the toxic effects in
toxicity studies. For this reason, GES-1, L-02 and IMR-90 cell
lines were chosen as controls. AGS, MKN-45, KATO-3 and SNU-5 cells
were cultured in RPMI-1640 medium, and the remaining cell lines
were cultured in DMEM. RPMI-1640 medium or DMEM contained 10%
heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin. All cells were maintained at 37°C in a humidified
atmosphere with 5% CO2.
MTT assay
Cytotoxicity was measured using an MTT assay.
Briefly, the eight gastric cancer cell lines and the three normal
cell lines were treated with BQ, OQ, 5-FU or shikonin at different
concentrations. At the end of the treatment, 15 µl MTT was added to
each well and 100 µl DMSO was added to each well after the cells
were incubated for another 15 min. Absorbance at 490 nm was
measured by microplate illuminometer (BioTek Instruments, Inc.),
and the results were used to calculate cell viability.
Cell apoptosis analysis
The effects of BQ and OQ on the apoptosis of AGS
cells were quantitated using the Annexin V-FITC Apoptosis Detection
Kit (Beyotime Institute of Biotechnology) and flow cytometry. AGS
cells were treated with BQ, OQ, or 5-FU at a concentration of 3 µM
for 3, 6, 12 or 24 h. A volume of 200 µl binding buffer was
incubated with AGS cells, followed by staining with 3 µl Annexin
V-FITC and 2 µl propidium iodide (PI) at the 4°C for 30 min. The
stained AGS cells were observed using the EVOS FL Auto Cell Imaging
System (Thermo Fisher Scientific, Inc.) at a magnification of ×400,
and analyzed using a flow cytometer (Beckman Coulter, Inc.). Data
were analyzed using CytExpert software 2.0 (Beckman Coulter,
Inc.).
Cell cycle analysis
The cell cycle was detected using a Cell Cycle
Detection kit (Beyotime Institute of Biotechnology, Shanghai,
China) and flow cytometry. AGS cells were treated with 3 µM BQ or
OQ for 3, 6, 12 or 24 h. The cells were harvested, incubated with
70% cold ethanol at −20°C for 12 h, washed with PBS, and stained
with PI (10 µg/ml)/RNase staining buffer (Beyotime Institute of
Biotechnology). The staining took place at 4°C for 30 min in the
dark. Samples were analyzed with a flow cytometer (Beckman Coulter,
Inc.). Data were analyzed using CytExpert software 2.0 (Beckman
Coulter, Inc.).
Measurement of ROS
To determine the intracellular levels of ROS,
DCFH-DA was utilized. AGS cells were plated in 6-well plates and
grown to 60% confluence. AGS cells were treated with 3 µM BQ or OQ
for 3, 6, 12 or 24 h. Cells were harvested, stained with 10 µM
DCFH-DA for 30 min at 37°C, washed twice with PBS, and then
immediately analyzed by flow cytometry (Beckman Coulter, Inc.). ROS
levels were analyzed using CytExpert software 2.0 (Beckman Coulter,
Inc.).
Western blot analysis
Western blot analysis was performed using standard
methods. AGS cells were normally cultured and treated with 3 µM BQ
or OQ for 3, 6, 12 or 24 h. Cells were lysed in lysis buffer
containing protease inhibitors (50 mmol/l Tris (pH 7.4), 150 mmol/l
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 20 mg/ml
AEBSF, 0.5 mg/ml pepstatin, 0.5 mg/ml leupeptin and 2 mg/ml
aprotinin), and the supernatant was collected followed by
centrifugation at 16,000 × g for 30 min at 4°C, and total protein
was quantified using coomassie blue staining. A total of 20 µl
protein samples were separated on 8–12% SDS-PAGE, and
electrophoretically transferred to nitrocellulose membranes. After
blocking in 5% skimmed milk at 37°C for 2 h, the membrane was
incubated overnight at 4°C with a primary antibody against mouse
monoclonal α-tubulin (1:2,500; cat. no. sc-8035), Bad (1:1,500;
cat. no. sc-8044), Bcl-2 (1:1,500; cat. no. sc-7382), pro-PARP
(1:1,500; cat. no. sc-74469), cleaved poly (ADP ribose) polymerase
1 (cle-PARP; 1:1,500; cat. no. sc-8007), pro-caspase-3 (pro-cas-3;
1:1,500; cat. no. sc-7272); cleaved-caspase-3 (cle-cas-3; 1:1,500;
cat. no. sc-373730), phosphorylated (p-) extracellular
signal-regulated kinase (ERK) (Tyr204, 1:1,500; cat. no.
sc-8059), p-JNK (Tyr183 and Tyr185; 1:1,500;
cat. no. sc-6254), JNK (1:1,500; cat. no. sc-7345), p-p38
(Tyr182, 1:1,500; cat. no. sc-7973), p-STAT3
(Tyr705, 1:1,500; cat. no. sc-8059), STAT3 (1:1,500;
cat. no. sc-8019), cyclin-dependent 1/2 (CDK1/2) (1:1,500; cat. no.
sc-53219), cyclin B1 (1:1,500; cat. no. sc-166210), rabbit
polyclonal ERK (1:1,500; cat. no. sc-154), p38 (1:1,500; cat. no.
sc-7972), Akt (1:1,500; cat. no. sc-271149), p-Akt (1:1,500; cat.
no. sc-135651) and p27 (1:1,500; cat. no. sc-1641). This was
followed by incubation with horseradish peroxidase-conjugated
anti-mouse (1:5,000; cat. no. ZB-2301) and anti-rabbit
immunoglobulin G (1:5,000; cat. no. ZB-2305) secondary antibodies
for 2–3 h at room temperature. The proteins were visualized using
ECL reagents and the AI600 Imager (GE Healthcare). The blots were
analyzed using ImageJ 1.46r software (National Institutes of
Health), and protein levels were normalized to the matching
densitometry value of α-tubulin as the internal control.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. The samples of each group were
compared by analysis of variance, and multiple comparisons between
groups were performed using one-way analysis of variance followed
by Tukey's post hoc tests using SPSS version 18.0 statistical
software (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Structure and synthetic route of the
two derivatives
To improve the anticancer activity and reduce the
adverse side effects, BQ and OQ were synthesized by performing a
series of steps to modify the 1,4-naphthoquinone chemical structure
(Fig. 1). By performing nuclear
magnetic resonance at a wavelength of 400 MHz, we analyzed the H
and C spectra in deuterated chloroform solvent.
Effects of BQ and OQ on the
proliferation of gastric cancer cells
The results of the MTT assays showed that BQ and OQ
significantly decreased the viability of human gastric cancer cells
in a dose-dependent manner, and the pro-apoptotic effects were
greater than those in the 5-FU and shikonin positive control groups
(Fig. 2A). The viability of human
normal cell lines (GES-1, L-02 and IMR-90) was minimally affected
after exposure to high concentrations (100 µM) of the two novel
1,4-naphthoquinone derivatives (BQ and OQ) compared with the 5-FU
and shikonin groups (Fig. 2B).
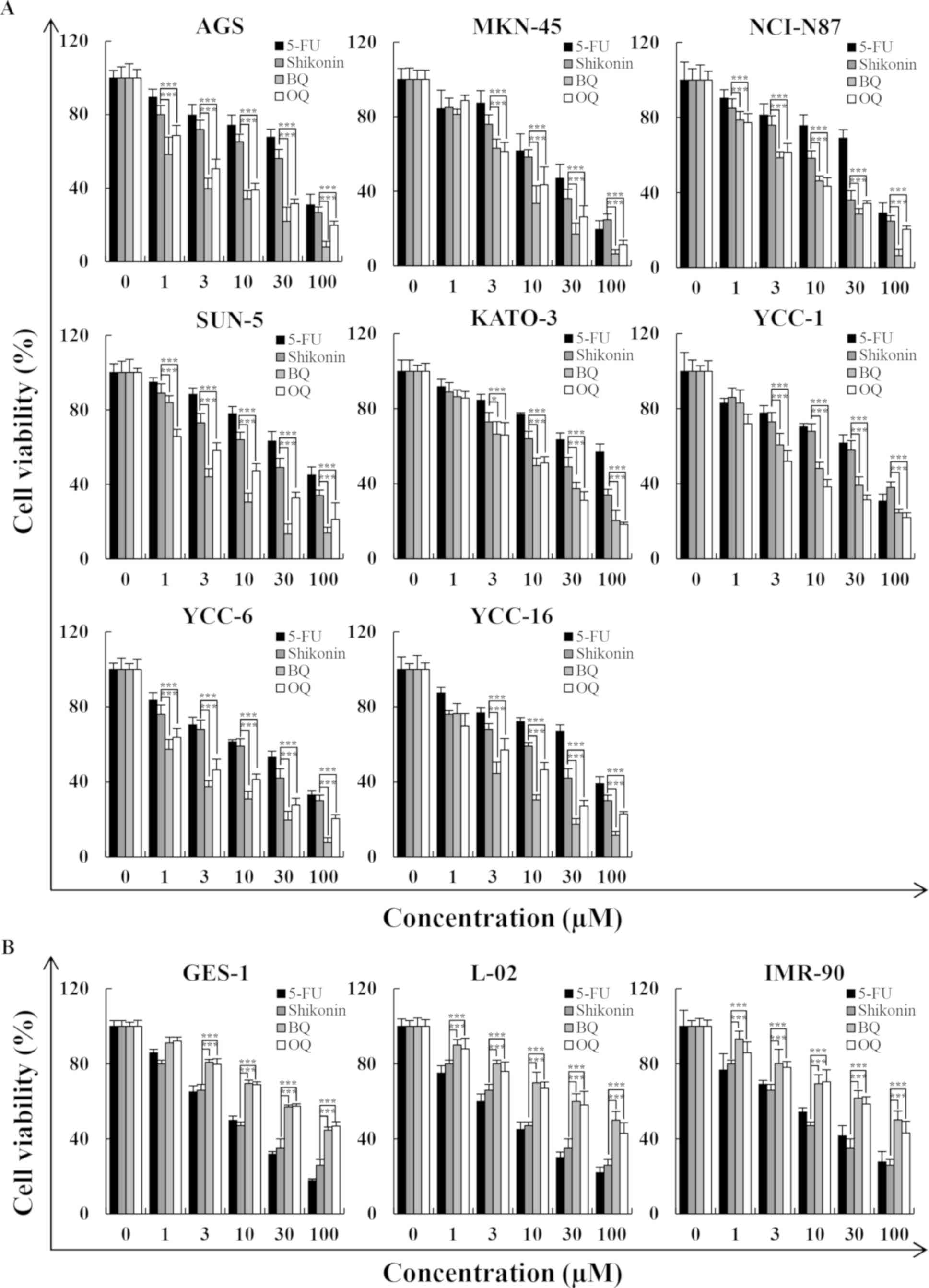 | Figure 2.Cytotoxic effects of 5-FU, shikonin,
BQ and OQ on eight gastric cancer cell lines, as determined by MTT
assay. (A) Eight gastric cancer cell lines were treated with
different concentrations of 5-FU, shikonin, BQ and OQ for 24 h. (B)
Three normal cell lines were treated with different concentrations
of 5-FU, shikonin, BQ and OQ for 24 h. Data are expressed as the
means ± standard deviation of the results from three independent
experiments. *P<0.05 and ***P<0.001. 5-FU, 5-fluorouracil;
BQ, 2-(butane-1-sulfinyl)-1,4-naphthoquinone; OQ,
2-(octane-1-sulfinyl)-1,4-naphthoquinone. |
Effects of BQ and OQ on the apoptosis
of AGS cells
The results of flow cytometry and western blotting
showed that BQ and OQ induced cell apoptosis in a time-dependent
manner (Fig. 3A). The flow
cytometry results showed that the population of apoptotic cells was
significantly increased after treatment with 5 µM BQ (from
approximately 10 to 54%) and OQ (from approximately 10 to 37%) in a
time-dependent manner (Fig. 3C and
D). In addition, BQ and OQ significantly increased the
expression levels of pro-apoptotic protein Bad, cle-cas-3 and
cle-PARP and decreased the expression levels of the anti-apoptotic
protein Bcl-2 in a time-dependent manner (Fig. 3E).
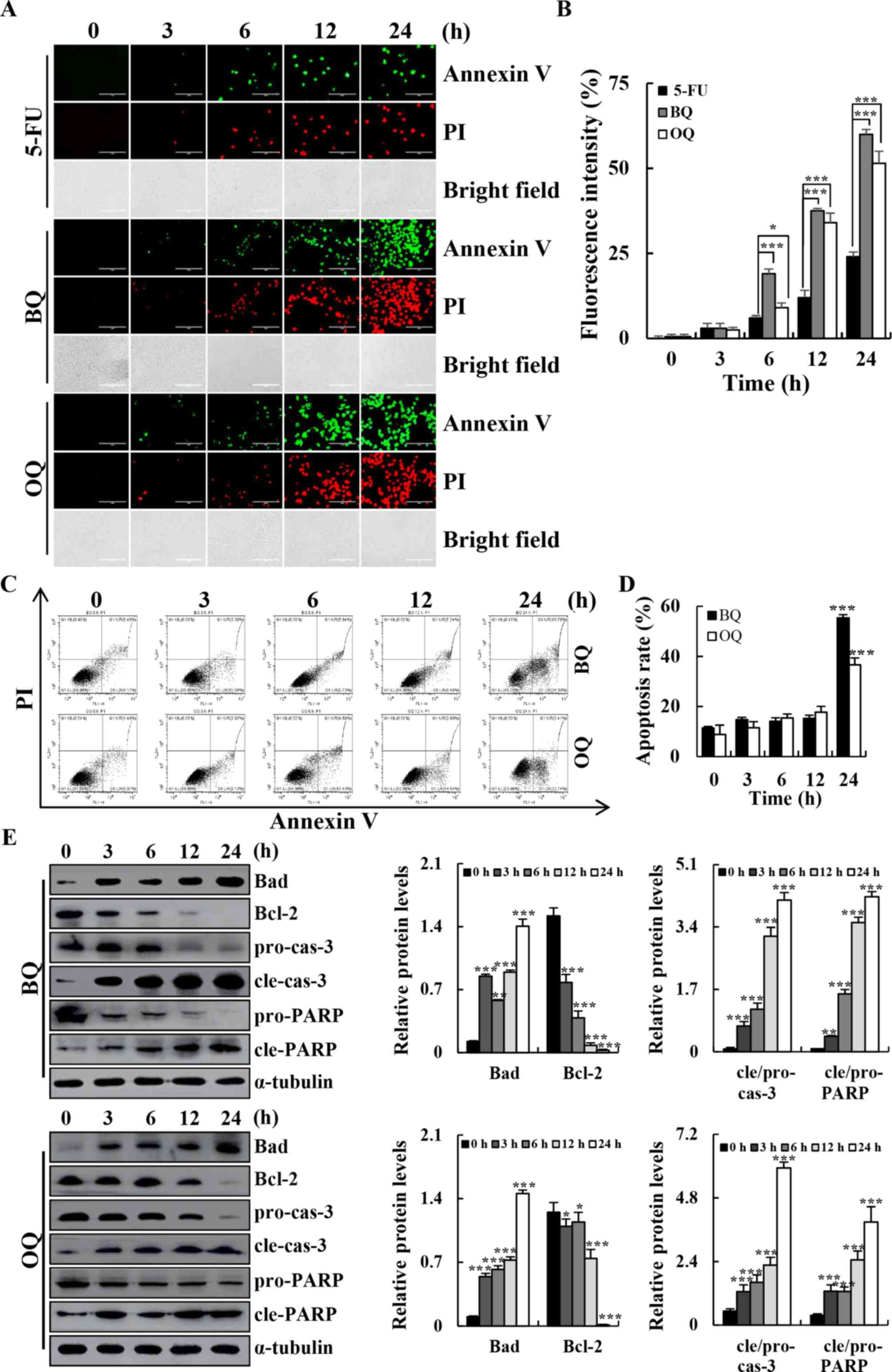 | Figure 3.Apoptotic effects of BQ and OQ in AGS
cells. (A) Cells were treated with 5-FU, BQ and OQ for different
time points (3, 6, 12 and 24 h), and stained with Annexin
V-FITC/PI. Data shown represent fluorescence microscopic images
(original magnifications, ×200). (B) Quantification of fluorescent
intensity. (C) Cells were incubated with Annexin V-FITC/PI and
analyzed by flow cytometry. (D) Quantification of the percentage of
apoptotic cells. (E) Western blotting with antibodies against Bad,
Bcl-2, cle-cas-3 and cle-PARP. *P<0.05, **P<0.01 and
***P<0.001. 5-FU, 5-fluorouracil; BQ,
2-(butane-1-sulfinyl)-1,4-naphthoquinone; OQ,
2-(octane-1-sulfinyl)-1,4-naphthoquinone; cle, cleaved; pro,
precursor; cas-3, caspase-3; PARP, Poly (ADP-ribose)
polymerase. |
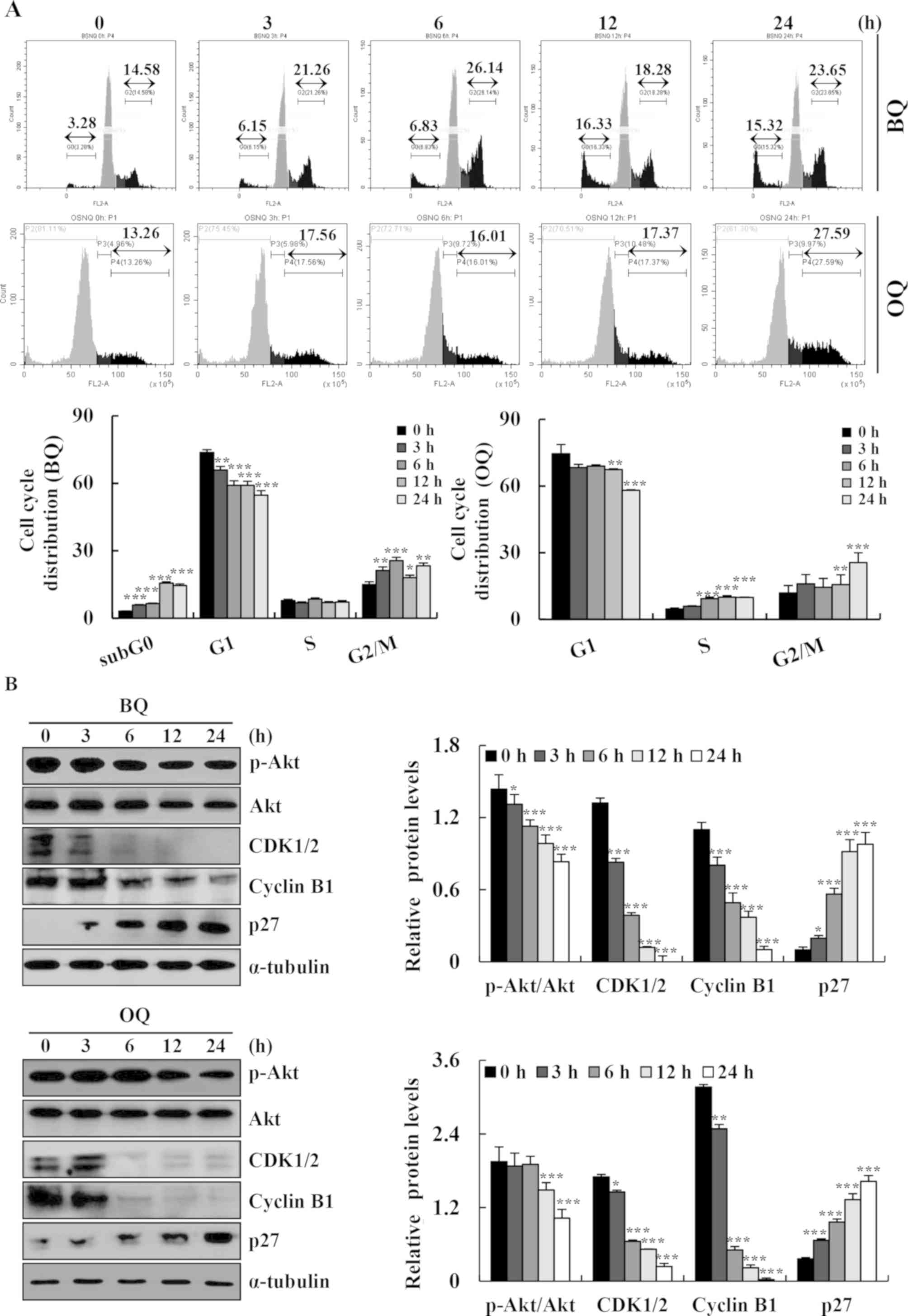
Effects of BQ and OQ on cell cycle
arrest in AGS cells
The cell cycle distribution was assessed by flow
cytometry and western blotting. BQ and OQ induced the accumulation
of cells in the G2/M phase and decreased the cell population in the
G1 phase (Fig. 4A). Meanwhile, BQ
and OQ increased the expression levels of p27 and the expression
levels of p-Akt, CDK1/2, and cyclin B1 were decreased in a
time-dependent manner (Fig. 4B).
These results indicated that BQ and OQ induced G2/M phase cell
cycle arrest and promoted apoptosis in gastric cancer cells through
the Akt signaling pathway.
Effects of BQ and OQ on apoptosis
through MAPK and STAT3 signaling pathways in AGS cells
We further determined the signaling pathways that
mediated BQ- and OQ-induced AGS cell apoptosis. The expression
levels of p38, JNK, ERK, and STAT3 signaling-related proteins were
assessed in AGS cells after treatment with BQ and OQ by western
blotting. BQ and OQ markedly upregulated the phosphorylation of p38
and JNK, and reduced the phosphorylation of ERK and STAT3 in AGS
cells in a time-dependent manner (Fig.
5A and B).
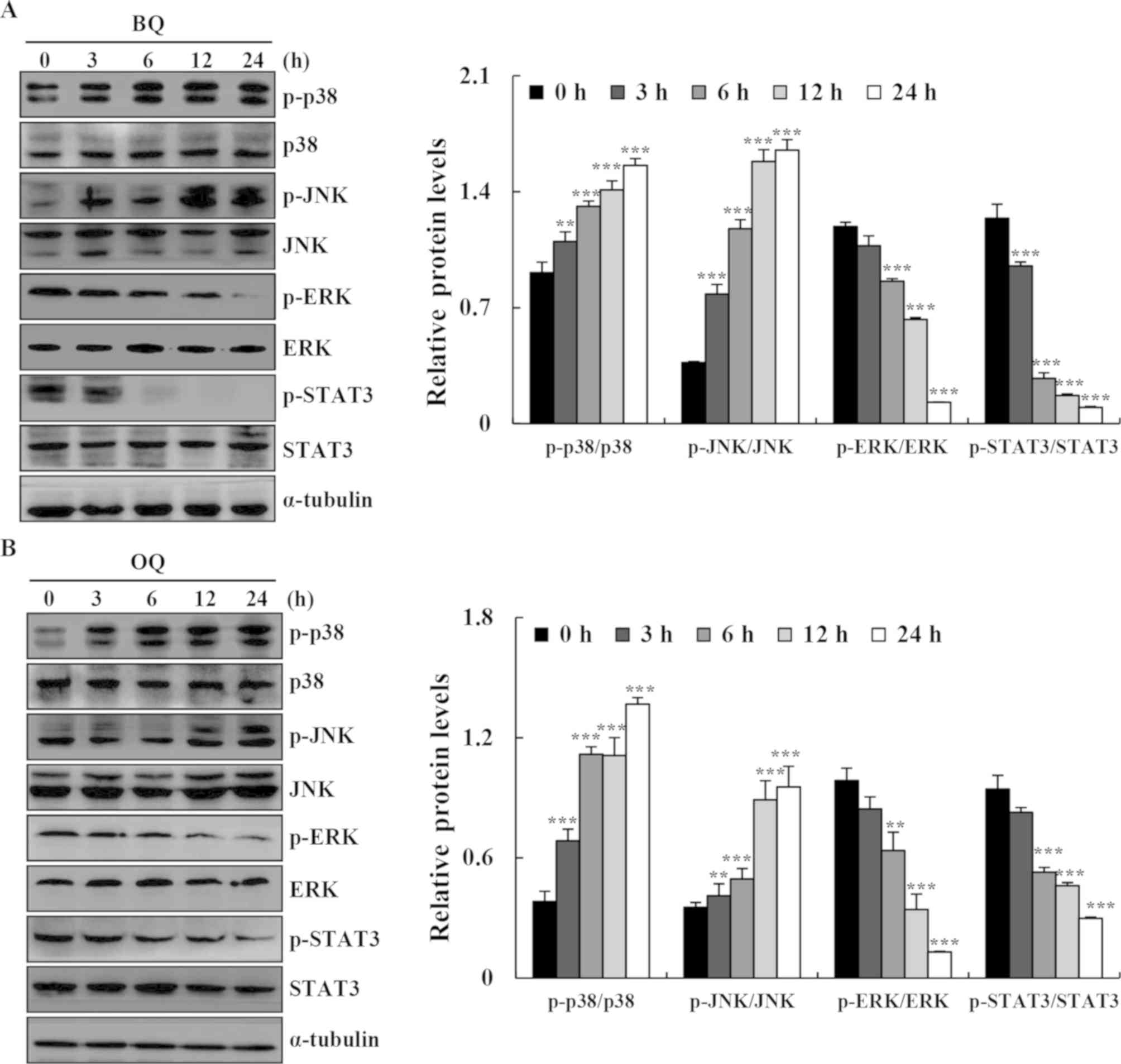 | Figure 5.Effects of BQ and OQ on the MAPK, AKT
and STAT3 signaling pathways in AGS cells. (A and B) Cells were
treated with BQ and OQ for various time points (3, 6, 12 and 24 h),
and the expression levels of p-p38, p-JNK, p-ERK, p-AKT, and
p-STAT3 were assessed by western blotting; α-tubulin was used as an
internal control. Data are expressed as the means ± standard
deviation of the results from three independent experiments.
**P<0.01 and ***P<0.001. BQ,
2-(butane-1-sulfinyl)-1,4-naphthoquinone; OQ,
2-(octane-1-sulfinyl)-1,4-naphthoquinone; p-, phosphorylated. |
Effects of BQ and OQ on ROS-mediated
apoptosis in AGS cells
To investigate the underlying relationship between
the two compounds and ROS generation, we performed flow cytometry
and western blotting. BQ and OQ significantly increased ROS
generation in a time-dependent manner up to almost 1.8-fold
compared to the untreated control (Fig. 6A), whereas pretreatment with NAC
strongly inhibited BQ- and OQ-induced apoptosis (Fig. 6B). Furthermore, the levels of
cle-cas-3 and cle-PARP and the phosphorylation levels of MAPK, Akt
and STAT3 signaling pathway-related proteins were almost unchanged
from the control condition following pre-treatment with NAC
(Fig. 7A and B). These results
suggest that ROS generation is required and plays an essential role
in BQ- and OQ-triggered apoptosis in AGS cells.
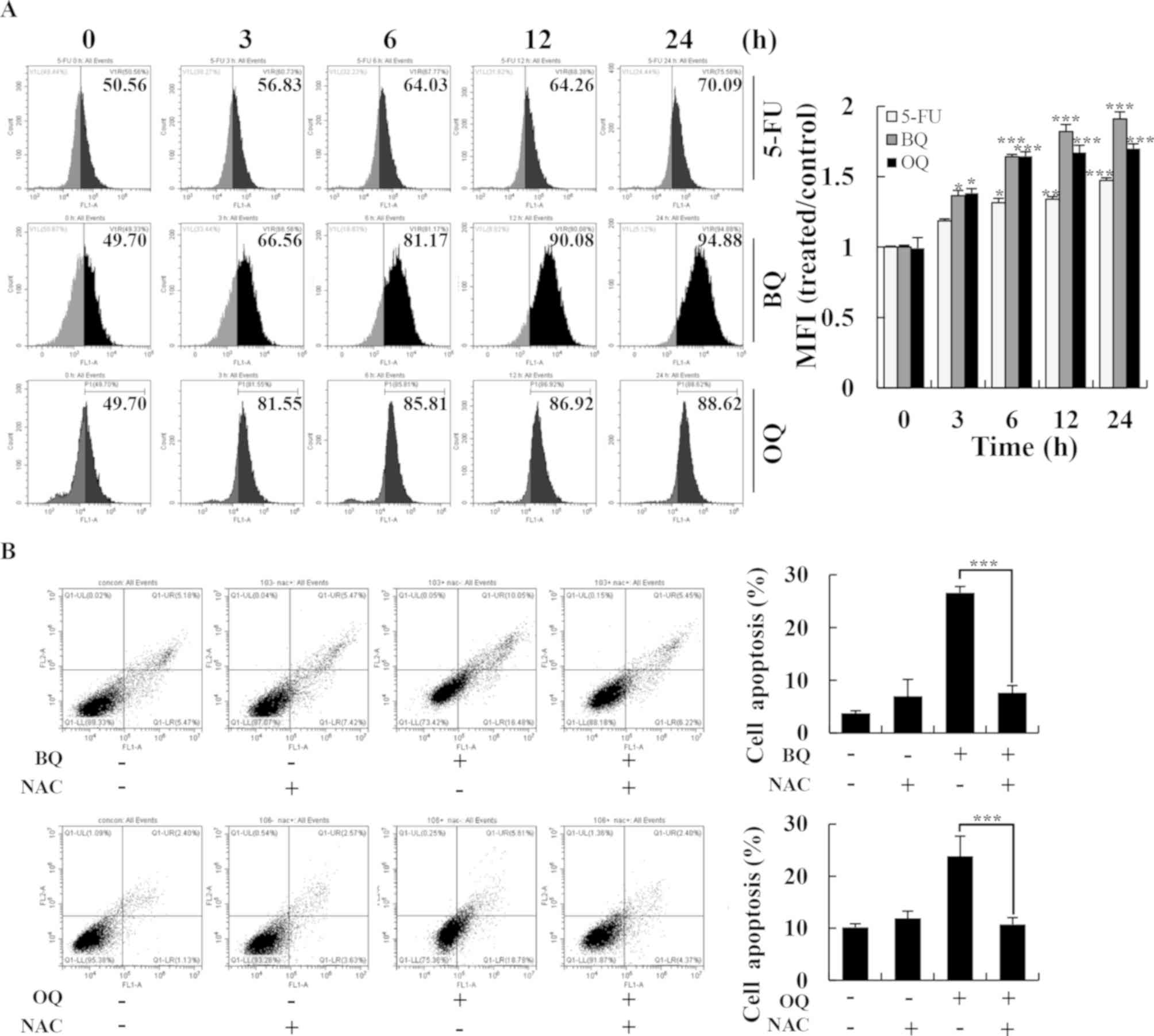 | Figure 6.Effects of BQ and OQ on induced
ROS-mediated apoptosis in AGS cells. (A) Cells were treated with
5-FU, BQ and OQ, and then the intracellular ROS levels were
measured by flow cytometry (left panels) and mean fluorescence
intensity (MFI) was quantitated (right panel). (B) Cells were
treated with NAC for 30 min and then incubated with BQ and OQ for
24 h. The intracellular apoptosis levels were measured by flow
cytometry. Data are expressed as the means ± standard deviation of
the results from three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001. 5-FU, 5-fluorouracil; BQ,
2-(butane-1-sulfinyl)-1,4-naphthoquinone; OQ,
2-(octane-1-sulfinyl)-1,4-naphthoquinone; ROS, reactive oxygen
species; NAC, N-acetyl-L-cysteine. |
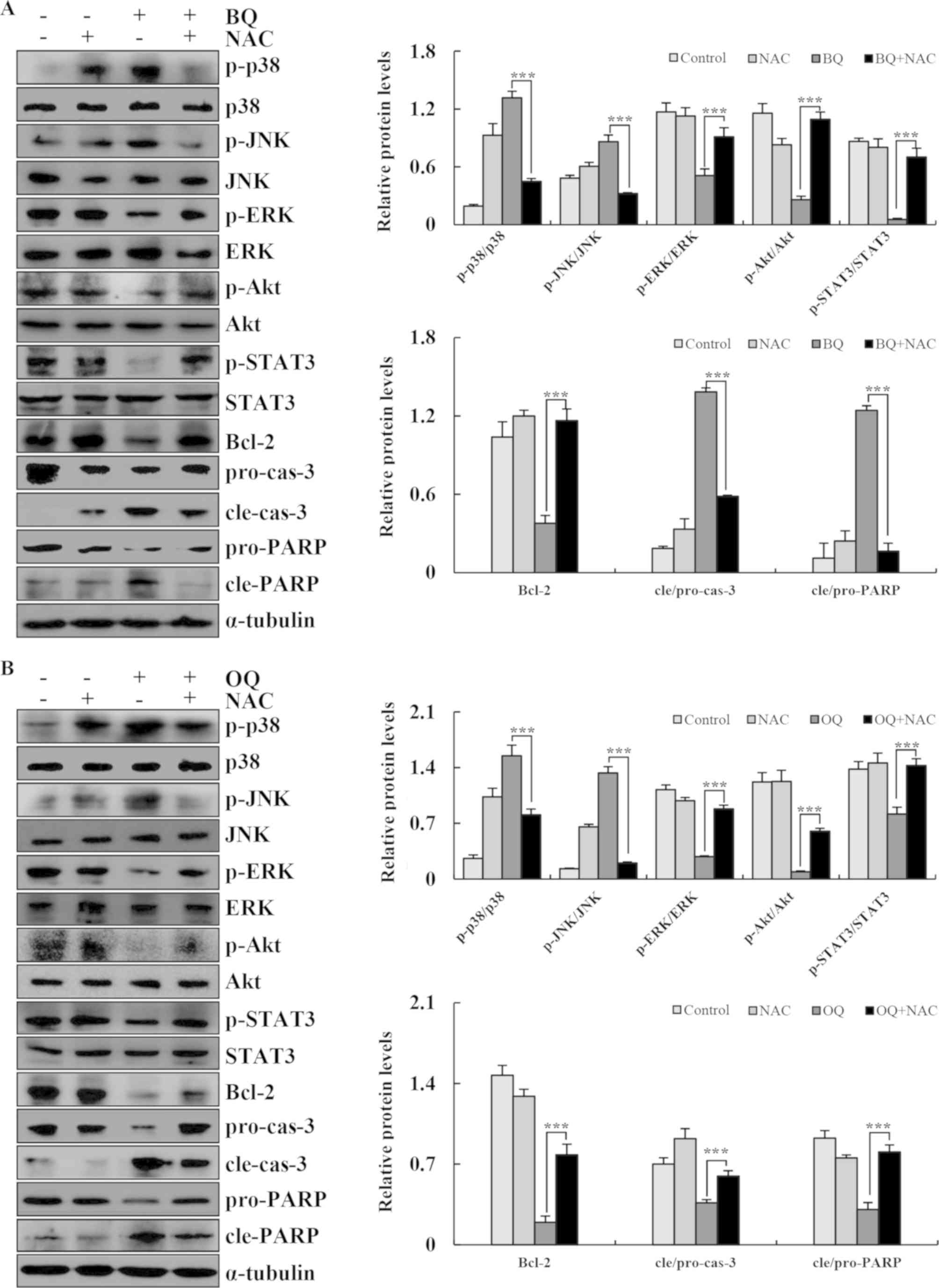 | Figure 7.BQ and OQ induce ROS-mediated
apoptosis via MAPK and STAT3 signaling pathways. (A and B) The
expression levels of phosphorylated (p)-p38, p-JNK, p-ERK, p-AKT,
p-STAT3, Bcl-2, cle-cas-3, pro-cas-3, pro-PARP and cle-PARP were
detected by western blotting; α-tubulin was used as an internal
control. Data are expressed as the means ± standard deviation of
the results from three independent experiments. ***P<0.001. BQ,
2-(butane-1-sulfinyl)-1,4-naphthoquinone; OQ,
2-(octane-1-sulfinyl)-1,4-naphthoquinone; ROS, reactive oxygen
species; NAC, N-acetyl-L-cysteine; p-, phosphorylated; cle,
cleaved; pro, precursor; cas-3, caspase-3; PARP, Poly (ADP-ribose)
polymerase. |
Discussion
1,4-Naphthoquinones are active quinone derivatives
that exhibit several different biological responses including
anti-inflammatory, anticancer, apoptosis and radical scavenging
activities (21); however, the
severe side effects have limited their utility as clinical agents.
To reduce their side effects and improve the effects of
1,4-naphthoquinone derivatives, BQ and OQ were synthesized and
their potential mechanisms were explored in human gastric cancer
cells. BQ and OQ significantly inhibited the viability of AGS and
another seven human gastric cancer cell lines. Despite the fact
that the two compounds share the same targets, the anticancer
ability of BQ was more effective than that of OQ in AGS cells. It
was speculated that this difference was caused by two reasons.
First, the two compounds have low polarity and high lipid
solubility, thus BQ with a lower molecular mass is more likely to
cross the cell membrane and enter the cell to perform biological
functions. Second, OQ with a longer carbon chain has good
film-forming properties, thus its own effector group is not easily
exposed for binding with the corresponding target molecule. To
further illustrate the mechanism of cytotoxicity, apoptosis
induction, cell cycle arrest, ROS generation and signaling-related
protein expression were observed after BQ and OQ treatment in AGS
cells.
As shown, compared with 5-FU, BQ and OQ had no
significant cytotoxic effects on normal cells. The reason that BQ
and OQ had low cytotoxicity in normal cells may be due to the fact
that these cells have a strong antioxidant defense system and can
resist oxidative stress damage. However, cancer cells have highly
impaired antioxidant defense mechanisms, which may lead to ROS
accumulation and oxidative stress (22). This is why BQ and OQ can inhibit
the proliferation of cancer cells but demonstrate less inhibitory
effects on normal cells.
The induction of apoptosis can effectively inhibit
the growth of tumor cells, and is considered a novel target for
cancer drug research and development (23). Some studies have reported that
1,4-naphthoquinone derivatives induce apoptosis in human cancer
cells including breast, lung and colon cancer cells through the
mitochondrial-dependent pathway (24–26).
The present results demonstrated that BQ and OQ activated Bcl-2,
Bad, cle-caspase-3 and cle-PARP in gastric cancer cells.
Mitochondrial apoptosis is partly dependent on the transition and
release of Bcl-2 family proteins such as Bcl-2 and Bad (27). These results clearly suggest that
BQ and OQ treatment leads to the induction of
mitochondrial-dependent apoptosis in human gastric cancer AGS
cells. Various naphthoquinone derivatives such as menadione and
plumbagin can undergo oxidative stress-induced mitochondrial
apoptosis by Fas-dependent pathways in cancer cells (28,29).
Therefore, it was speculated that the commonality of the compound
structure between BQ and OQ can also induce mitochondrial-dependent
apoptosis of cancer cells through the Fas ligand pathway, which
will be verified in future studies.
Increasing research has focused on cell cycle
regulation as a target for the development of anticancer drugs
(30). This study showed that BQ
and OQ treatment inhibited G2/M progression in AGS cancer cells,
concomitant with the increased expression of p27 and the decreased
expression of Akt, CDK1/2 and cyclin B1. However, the decrease in
Akt was reversed by co-treatment with NAC, which is an ROS
scavenger. These results suggest a strong link between cell cycle
arrest and ROS generation. Research has demonstrated that ROS
generation can inhibit the phosphorylation of Akt, thereby
activating formation of the CDK1/cyclin B1 kinase complex (31). These results indicate that BQ- and
OQ-induced cell cycle arrest is associated with ROS generation and
p-Akt, CDK1/2, and cyclin B1 reduction.
MAPKs including p38, JNK and ERK play crucial roles
in cell proliferation, survival and apoptosis. It has been reported
that shikonin increases p-p38 levels but downregulates c-Myc to
further inhibit DNA repair-induced apoptosis in NB4 cells (32). Decreased phosphorylation of ERK can
affect the expression of multiple genes such as AQP5, Vav3 and
PANS-4, which in turn induces the apoptosis of cancer cells
(33–35). STAT3 belongs to the family of
cytoplasmic latent transcription factors, which is associated with
tumor progression and poor survival in gastric cancer. Plumbagin, a
natural naphthoquinone, inhibits the growth of esophageal squamous
cell carcinoma cells by downregulating the expression of STAT3
(36). To elucidate the exact
mechanism involving BQ and OQ-induced apoptosis in human gastric
cancer cells, the effects of two novel 1,4-naphthoquinone
derivatives on activation of MAPKs and STAT3 were examined. The
results showed that BQ and OQ markedly upregulated the
phosphorylation of p38 and JNK, substantially reducing the
phosphorylation of ERK and STAT3 in AGS cells in a time-dependent
manner. Therefore, it appears that the MAPK and STAT3 signaling
pathways play important roles in BQ and OQ-induced apoptosis.
ROS mediate intracellular signal cascades and
excessive ROS production leads to cell apoptosis (37). It has been shown that half-redox
potentials significantly influence ROS formation and possibly
predict the cytotoxicity of quinone derivatives in cancer cells
(38). The cytotoxicity of
1,4-naphthoquinones is related to their electron-accepting
capability, which gives rise to ROS production leading to DNA
damage and cancer cell apoptosis (39). Naphthoquinone derivatives also
stimulate apoptosis via ROS-dependent mechanisms in various types
of cancer cells (14). In the
present study, it was illustrated that BQ and OQ could markedly
induce the production of ROS. However, pretreatment with the ROS
scavenger NAC prevented BQ- and OQ-induced apoptosis, showing that
ROS play a significant role in BQ- and OQ-induced apoptosis in AGS
cells (Fig. 6B). NAC significantly
reversed the decrease in p-ERK and p-STAT3, and the increase in
p-p38, p-JNK, cle-caspase-3 and cle-PARP. BQ and OQ induced
apoptosis involving the ROS-dependent MAPK and STAT3 pathways in
AGS cells (Fig. 7A and B). These
data suggest that the MAPK, STAT3 and AKT signaling pathways
induced by the two novel 1,4-naphthoquinone derivatives were
mediated by ROS.
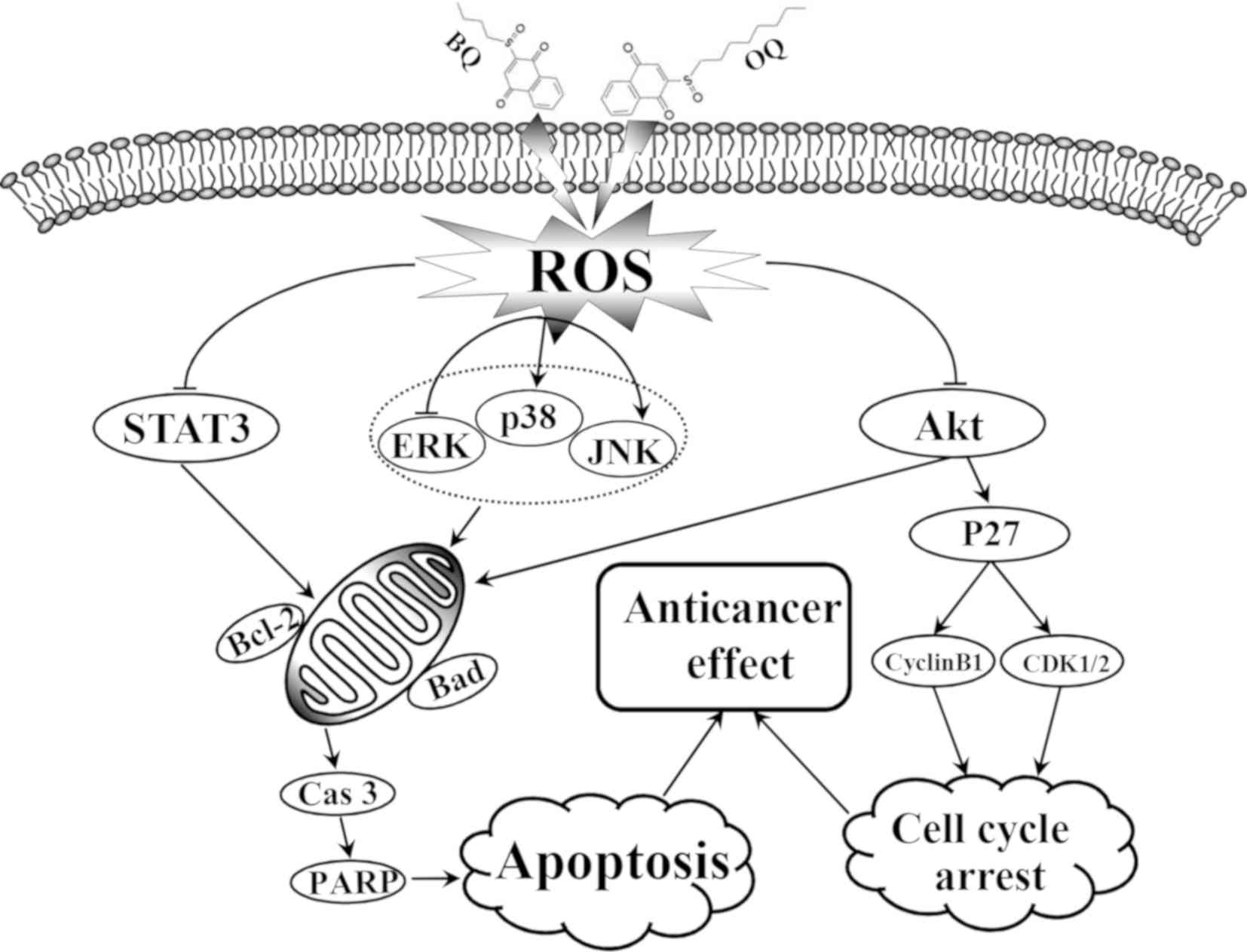
In conclusion, the present study demonstrated that
BQ and OQ induced G2/M phase cell cycle arrest and apoptosis in AGS
cells via the ROS-mediated activation of MAPK, Akt and STAT3
signaling pathways (Fig. 8). From
the different anticancer effects of the two compounds, substitution
of these functional groups at the meta position appears to be an
appropriate strategy for developing anticancer agents against AGS
cells. This possibility will be investigated in vivo in
further studies. In the present study, we examined the anticancer
effects of the two derivatives and further determined the mechanism
of apoptosis at the molecular level. In future research, the
effects of the two derivatives should be evaluated in
vivo.
Acknowledgements
Not applicable.
Funding
This study was funded by the Multigrain Production
and Processing Characteristic Discipline Construction Project
(grant no. 2042070010), and the Postdoctoral Scientific Research
Foundation of Heilongjiang Province of China (grant no.
LBH-Q13132).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHJ and CYW conceived and designed the study. HW,
YHL, GNS, XJP, WTX, YZ, JRW and YCF performed the experiments. GNS,
JQL, YZ, TZ, SNW, HX and HXW analyzed the data. HW and YHL wrote
the manuscript. All authors read, edited and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jou E and Rajdev L: Current and emerging
therapies in unresectable and recurrent gastric cancer. World J
Gastroenterol. 20:4812–4823. 2016. View Article : Google Scholar
|
|
2
|
Lee SY and Oh SC: Changing strategies for
target therapy in gastric cancer. World J Gastroenterol.
3:1179–1189. 2016. View Article : Google Scholar
|
|
3
|
Han G, Gong H, Wang Y, Guo S and Liu K:
AMPK/mTOR-mediated inhibition of surviving partly contributes to
metformin-induced apoptosis in human gastric cancer cell. Cancer
Biol Ther. 16:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Chen Z, Zhou X, Xu W, Wang G,
Tang X, Luo L, Tu J, Zhu Y, Hu W, et al: Cantharidin induces
G2/M phase arrest and apoptosis in human gastric cancer
SGC-7901 and BGC-823 cells. Oncol Lett. 6:2721–2726. 2014.
View Article : Google Scholar
|
|
5
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woo SM, Choi YK, Kim AJ, Cho SG and Ko SG:
p53 causes butein-mediated apoptosis of chronic myeloid leukemia
cells. Mol Med Rep. 13:1091–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lan Q, Li S, Lai W, Xu H, Zhang Y, Zeng Y,
Lan W and Chu Z: Methyl sartortuoate inhibits colon cancer cell
growth by inducing apoptosis and G2/M-phase arrest. Int J Mol Sci.
16:19401–194118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee H, Lee H, Chin H, Kim K and Lee D:
ERBB3 knockdown induces cell cycle arrest and activation of Bak and
Bax-dependent apoptosis in colon cancer cells. Oncotarget.
5:5138–5152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Zhou Y, Li Y, Zhou J, Wu Y, Cui Y,
Yang G and Hong Y: Mutations of p53 and KRAS activate NF-κB to
promote chemoresistance and tumorigenesis via dysregulation of cell
cycle and suppression of apoptosis in lung cancer cells. Cancer
Lett. 357:520–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar K, Sabarwal A and Singh RP: Mancozeb
selectively induces mitochondrial-mediated apoptosis in human
gastric carcinoma cells through ROS generation. Mitochondrion. Jun
11–2018.(Epub ahead of print). View Article : Google Scholar :
|
|
11
|
Duan F, Yu Y, Guan R, Xu Z, Liang H and
Hong L: Vitamin K2 induces mitochondria-related apoptosis in human
bladder cancer cells via ROS and JNK/p38 MAPK signal pathways. PLoS
One. 11:e01618862016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng HB, Bo Y, Shen WX, Ren XG, Tan JN,
Jia ZR and Xu CL: Longikaurin E induces apoptosis of pancreatic
cancer cells via modulation of the p38 and PI3K/AKT pathways by
ROS. Naunyn Schmiedebergs Arch Pharmacol. 388:623–634. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajamanickam V, Zhu H, Feng C, Chen X,
Zheng H, Xu X, Zhang Q, Zou P, He G, Dai X, et al: Novel allylated
monocarbonyl analogs of curcumin induce mitotic arrest and
apoptosis by reactive oxygen species-mediated endoplasmic reticulum
stress and inhibition of STAT3. Oncotarget. 8:101112–101129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Dong J, Cui J, Huang G, Meng Q
and Li S: Cytotoxicity of Synthesized 1,4-Naphthoquinone Oxime
derivatives on selected human cancer cell lines. Chem Pharm Bull
(Tokyo). 66:612–619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh SK, Ganta A and Spanjaard RA:
Discovery and cellular stress pathway analysis of
1,4-naphthoquinone derivatives with novel, highly potent
broad-spectrum anticancer activity. J Biomed Sci. 25:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farias MS, Pich CT, Kviecinski MR, Bucker
NC, Felipe KB, Da Silva FO, Günther TM, Correia JF, Ríos D, Benites
J, et al: Substituted 3-acyl-2-2-phenylamino-1,4-naphthoquinones
intercalate into DNA and cause genotoxicity through the increased
generation of reactive oxygen species culminating in cell death.
Mol Med Rep. 10:405–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ollinger K and Brunmark A: Effect of
hydroxy substituentposition on 1,4-naphthoquinone toxicity to rat
hepatocyt. J Biol Chem. 266:21496–22150. 1991.PubMed/NCBI
|
|
18
|
Ball MD, Bartlett MS, Shaw M, Smith JW,
Nasr M and Meshnick SR: Activities and conformational fitting of
1,4-Naphthoquinone Derivatives and other cyclic 1,4-Diones tested
in vitro against Pneumocystis carinii. Antimicrob Agents
Chemother. 45:1473–1479. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suhara Y, Watanabe M, Motoyoshi S,
Nakagawa K, Wada A, Takeda K, Takahashi K, Tokiwa H and Okano T:
Synthesis of new vitamin K analogues as steroid and xenobiotic
receptor (SXR) agonists: Insights into the biological role of the
side chain part of vitamin K. J Med Chem. 54:4918–4922. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abiko Y, Shinkai Y, Unoki T, Hirose R,
Uehara T and Kumagai Y: Polysulfide Na2S4
regulates the activation of PTEN/Akt/CREB signaling and
cytotoxicity mediated by 1,4-naphthoquinone through formation of
sulfur adducts. Sci Rep. 7:48142017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bezkorovaynyj AO, Zyn AR, Harasym NM, Len
JT, Figurka OM and Figurka DI: Loach embryos prooxidant-antioxidant
status under the influence of amide derivatives of
1,4-naphthoquinone. Ukr Biochem J. 88:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh B, Figtree G, Costa D, Eade T, Hruby G,
Lim S, Elfiky A, Martine N, Rosenthal D, Clarke S and Back M:
Oxidative stress in prostate cancer patients: A systematic review
of case control studies. Prostate Int. 4:71–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Zheng YX, Deng HZ, Liang L and
Peng J: Aloperine induces G2/M phase cell cycle arrest and
apoptosis in HCT116 human colon cancer cells. Int J Mol Med.
33:1613–1620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma WD, Zou YP, Wang P, Yao XH, Sun Y, Duan
MH, Fu YJ and Yu B: Chimaphilin induces apoptosis in human breast
cancer MCF-7 cells through a ROS-mediated mitochondrial pathway.
Food Chem Toxicol. 70:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ong JY, Yong PV, Lim YM and Ho AS:
2-Methoxy-1, 4-naphthoquinone (MNQ) induces apoptosis of A549 lung
adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK
signaling pathways. Life Sci. 135:158–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eldhose B, Gunawan M, Rahman M, Latha MS
and Notario V: Plumbagin reduces human colon cancer cell survival
by inducing cell cycle arrest and mitochondria-mediated apoptosis.
Int J Oncol. 45:1913–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YL, Zhang R, Xu HL, Yu XF, Qu SC and
Sui DY: 20(S)-protopanaxadiol triggers mitochondrial-mediated
apoptosis in human lung adenocarcinoma A549 cells via inhibiting
the PI3K/Akt signaling pathway. Am J Chin Med. 41:1137–1152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laux I and Nel A: Evidence that oxidative
stress-induced apoptosis by menadione involves Fas-dependent and
Fas-independent pathways. Clin Immunol. 101:335–344. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McKallip RJ, Lombard C, Sun J and
Ramakrishnan R: Plumbagin-induced apoptosis in lymphocytes is
mediated through increased reactive oxygen species production,
upregulation of Fas, and activation of the caspase cascade. Toxicol
Appl Pharmacol. 247:41–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian R, Li Y and Gao M: Shikonin causes
cell-cycle arrest and induces apoptosis by regulating the
EGFR-NF-κB signalling pathway in human epidermoid carcinoma A431
cells. Biosci Rep. 35(pii): e001892015.PubMed/NCBI
|
|
31
|
Xu N, Lao Y, Zhang Y and Gillespie DA:
Akt: A double-edged sword in cell proliferation and genome
stability. J Oncol. 2012:9517242012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan ZL, Zhong L, Xiao CL, Gan LG, Xu T,
Song H, Yang R, Li L and Liu BZ: Shikonin suppresses proliferation
and induces apoptosis in human leukemia NB4 cells through
modulation of MAPKs and c-Myc. Mol Med Rep. 16:3055–3060. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Zhang JN, Chen WL, Wang GS, Mao Q,
Li SQ, Xiong WH, Lin YY, Ge JW, Li XX, et al: Effects of AQP5 gene
silencing on proliferation, migration and apoptosis of human glioma
cells through regulating EGFR/ERK/p38 MAPK signaling pathway.
Oncotarget. 8:38444–38455. 2017.PubMed/NCBI
|
|
34
|
Tan BB, Zhang MM, Li Y, Zhao Q, Fan LQ,
Liu Y and Wang D: Inhibition of Vav3 gene can promote apoptosis of
human gastric cancer cell line MGC803 by regulating ERK pathway.
Tumour Biol. 37:7823–7833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan Z, Guo W, Yang J, Li L, Wang M, Lei
Y, Wan Y, Zhao X, Luo N, Cheng P, et al: PNAS-4, an Early DNA
damage response gene, induces S phase arrest and apoptosis by
activating checkpoint kinases in lung cancer cells. J Biol Chem.
290:14927–14944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao Y, Yin X, Jia Y, Liu B, Wu S and Shang
M: Plumbagin, a natural naphthoquinone, inhibits the growth of
esophageal squamous cell carcinoma cells through inactivation of
STAT3. Int J Mol Med. 42:1569–1576. 2018.PubMed/NCBI
|
|
37
|
Zhong WF, Wang XH, Pan B, Li F, Kuang L
and Su ZX: Eupatilin induces human renal cancer cell apoptosis via
ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol Lett.
12:2894–2899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Verrax J, Delvaux M, Beghein N, Taper H,
Gallez B and Buc Calderon P: Enhancement of quinone redox cycling
by ascorbate induces a caspase-3 independent cell death in human
leukaemia cells. An in vitro comparative study. Free Radic Res.
39:649–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prachayasittikul V, Pingaew R,
Worachartcheewan A, Nantasenamat C, Prachayasittikul S, Ruchirawat
S and Prachayasittikul V: Synthesis, anticancer activity and QSAR
study of 1,4-naphthoquinone derivatives. Eur J Med Chem.
84:247–263. 2014. View Article : Google Scholar : PubMed/NCBI
|