Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide with high incidence and mortality (1,2). It
is estimated that the global burden of CRC will increase by 60% by
2030 (3). In addition, the
survival rates of patients with CRC with local and distant
metastases are 71% and 14%, respectively (4). Despite the major advances in
diagnosis and treatment, approximately 50% of patients with CRC
experience recurrence and succumb to this disease within 5 years
(5,6). Tumor invasion and metastasis in the
middle and late stages are the root causes of treatment failure and
poor therapeutic efficacy (7,8).
Hence, to improve the clinical outcome for patients with CRC, it is
imperative to identify safe and effective drugs for the inhibition
of tumor progression.
Resveratrol (3,5,4′-trihydroxystilbene), a
phytoalexin, is found in many plants, such as grapes, peanuts, and
berries. Evidence has confirmed that resveratrol offers protection
against several human diseases, such as metabolic diseases and
various cancers (9,10). In recent years, an increasing
number of studies have revealed that resveratrol can regulate tumor
cell migration and invasion through modulation of
epithelial-mesenchymal transition (EMT) in various cancers, such as
oral squamous cell carcinoma (11), glioma (12), and pancreatic cancer (13). In the malignant progression of
tumors, EMT is known to cause loss of mutual adhesion between tumor
cells, and therefore, lead to enhanced invasion and movement
ability (14). Serine/threonine
kinase (AKT) is closely related to the occurrence of EMT (15,16),
and is involved in many biological and pathological processes, such
as angiogenesis, invasion, and metastasis (17). In addition, the glycogen synthase
kinase (GSK)-3β pathway is the classical downstream pathway of AKT,
and often participates in the development of EMT with AKT (18). Furthermore, Snail expression can be
regulated via the AKT/GSK-3β signaling pathway (19), and activation of this pathway may
promote EMT in hepatoma cells (20) and ovarian clear cell carcinoma
cells (21). However, it is
unclear whether the AKT/GSK-3/Snail signaling pathway is a key
mechanism in the regulation of EMT by resveratrol.
In the present study, stable AKT1 knockdown was
successfully established in colon cancer cells and an animal model
of lung metastasis of colon cancer. Subsequently, the inhibitory
effects and possible mechanism of action of resveratrol against
invasion and metastasis of colon cancer both in vitro and
in vivo were explored. These findings may provide new
strategies for colon cancer treatment.
Materials and methods
Cell culture
The adherent human colon cancer cell lines SW480 and
SW620 were obtained from the Cell Bank of the Chinese Academy of
Sciences. SW480 cells were cultured in RPMI-1640 medium (Kino
Biological and Pharmaceutical Technology Co., Ltd.) supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.)
and maintained at 37°C in a humidified atmosphere with 5%
CO2. SW620 cells were grown in L-15 medium (Kino
Biological and Pharmaceutical Technology Co., Ltd.) supplemented
with 10% FBS in an incubator with the same culture conditions.
Construction of lentivirus vectors for
AKT1 knockdown and generation of stably infected SW480 and SW620
cell lines
Lentivirus vectors for small interfering RNA (siRNA)
were constructed to explore the function of AKT1 with assistance
from Shanghai GeneChem Co., Ltd. Three AKT1-siRNAs were designed
and used, with the following sequences: Sequence 1,
gaTCCTCAAGAAGGAAGTCAT; sequence 2, gcATCGCTTCTTTGCCGGTAT; and
sequence 3, ggACAAGGACGGGCACATTAA. After annealing, the double
oligonucleotides obtained were inserted into the
AgeI/EcoRI sites of the GV248 lentiviral vector with
green fluorescent protein (GFP; Shanghai GeneChem Co., Ltd.).
Moreover, one scrambled RNA (sequence, TTCTCCGAACGTGTCACGT) was
used as the negative control (NC) and cloned into the GV248
lentiviral vector (Shanghai GeneChem Co., Ltd.). After
identification and lentivirus packaging, SW480 and SW620 cells were
infected with the acquired lentiviruses [multiplicity of infection
(MOI)=10] for 16 h. After infection for 72 h, SW480 and SW620 cells
were harvested, and the knockdown efficiency of AKT1 was detected
by reverse transcription polymerase chain reaction (RT-PCR) and
western blotting.
Epidermal growth factor (EGF)-induced
EMT model
Treatment with various concentrations (10, 25, 50,
and 100 ng/ml) of EGF (R&D Systems, Inc.) has been revealed to
induce EMT in SW480 cells (22,23).
Therefore, SW480 and SW620 cells seeded at a density of
1×106 were treated with 50 ng/ml EGF for 48 h to
establish an EMT model. Expression of E-cadherin, N-cadherin, and
vimentin were then detected to confirm the success of the
EGF-induced EMT model. Subsequent experiments were then conducted
using this model.
Cell viability assay
The viability of SW480 and SW620 cells was estimated
using a Cell Counting Kit-8 (CCK-8) (Nanjing KeyGen Biotech Co.,
Ltd.). Briefly, 1×106 cells/well were seeded in 96-well
plates and incubated until the cells reached 60% confluence.
Resveratrol (Sigma-Aldrich; Merck KGaA) was then added at different
concentrations (0, 7.5, 15, 30, 60, 120, and 240 µmol/l) to the
cells and incubated for 48 h. Cells were further incubated with
CCK-8 reagent for 2 h, followed by optical density (OD) measurement
at a wavelength of 450 nm using a microplate reader (BioRad
Laboratories, Inc.). In addition, inhibition of SW480 and SW620
cell proliferation at various treatment concentrations was
calculated using the following formula: Proliferation inhibition
rate (%)=(average OD in all control group duplicates-average OD in
the treatment group)/average OD in the blank control group
×100%.
Scratch wound healing experiment
SW480 and SW620 cells were plated at densities of
2×105 and 3.5×105, respectively, into 6-well
plates. After treatment, cells were cultured until the well bottoms
were fully covered. Subsequently, a cell-free gap was created by
scratching the well bottom using a 200-µl pipette tip. The debris
produced after scratching was removed by rinsing with
phosphate-buffered saline (PBS). Subsequently, 3 ml serum-free
medium was added to the culture plates and the cells were incubated
for 24 h at 37°C in an incubator with 5% CO2. Finally,
cell migration was observed in 3–5 randomly selected fields using
an optical microscope (IX71; ×100, magnification; Olympus
Corporation) and the migration distance over 24 h (in pixels) was
evaluated using Image-Pro Plus software version 6 (Media
Cybernetics, Inc.).
Transwell migration and invasion
(Matrigel) experiment
The migration and invasion of SW480 and SW620 cells
were evaluated using Transwell assays. For cell invasion, 40 µl
diluted Matrigel glue (Corning Incorporated) was evenly spread in
the upper chamber (24-well) and allowed to solidify. Briefly, after
the different treatments were completed, 2×106 SW480 and
SW620 cells were resuspended in 200 µl serum-free medium and plated
in the upper chamber of each Transwell (6.5 mm insert; 8.0 µm
polycarbonate membrane; Corning Incorporated). Subsequently, 600 µl
complete medium (containing 10% FBS) was placed into the lower
chamber. After incubation for 48 h at 37°C in a 5% CO2
incubator, migrated or invaded cells that passed through the
membranes were fixed in 4% paraformaldehyde (Wuhan Boster
Biological Technology, Ltd.) for 20 min, washed once with PBS, and
stained with 0.1% crystal violet (Sangon Biotech Co., Ltd.) for 30
min. Finally, images of the migrated and invaded cells were
photographed and counted using a microscope (ix71; Olympus
Corporation, ×200, magnification).
Real-time quantitative PCR (qPCR)
Following treatment, total RNA was extracted from
the cells using TRIzol reagent (Shanghai Pufei Biotech Co., Ltd.).
Reverse transcription into cDNA was then performed using the M-MLV
Reverse Transcriptase kit (Promega Corporation). Real-time qPCR was
used to detect the expression of each target gene and the internal
reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each
reaction was performed in 12 µl reaction mixture, containing 0.6 µl
template cDNA, 0.3 µl primer mix (5 µM), 6 µl SYBR Premix Ex Taq
(Takara Bio, Inc.), and 5.1 µl RNase-Free H2O. The
reaction conditions of RT-qPCR were as follows: 95°C, 3 min,
pre-modification; 95°C, 3 sec, annealing; 60°C, 30 sec, extension;
intermediate cycle 40 times. The forward and reverse sequences of
primers used for gene amplification were as follows: GAPDH (121
bp): 5′-TGACTTCAACAGCGACACCCA-3′ and 5′-CACCCTGTTGCTGTAGCCAAA-3′;
AKT1 (287 bp): 5′-GTGCTGGAGGACAATGACTAC-3′ and
5′-TGCTGCCACACGATACCG-3′, respectively. The relative expression of
each gene was calculated using the 2−ΔΔCq method
(24).
Western blotting
Following different treatments, total protein was
extracted from cells or tissues using radioimmunoprecipitation
(RIPA) lysis buffer (Beyotime Institute of Biotechnology). Equal
amounts of protein extracts (~20 µg) were subjected to 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
the protein bands were transferred to a polyvinylidene fluoride
(PVDF) membrane (EMD Millipore). Following non-specific binding the
membrane was blocked by incubation with Tris-buffered saline with
Tween-20® (TBST) containing 5% skimmed milk powder, and
the membranes were then probed with appropriate primary antibodies
at 4°C overnight and incubated again with a corresponding secondary
horseradish peroxidase (HRP)-conjugated antibody for 1 h. AKT1
(cat. no. 4691S; 1:1,000), p-AKT (Ser473) (cat. no. 4060S;
1:1,000), GSK-3β (cat. no. 9315S; 1:1,000), p-GSK-3β (Ser9) (cat.
no. 5558P; 1:1,000) and Snai1 (cat. no. 3879S; 1:500) were obtained
from Cell Signaling Technology, Inc. N-cadherin (cat. no. 76011;
1:500) and E-cadherin (cat. no. 76319; 1:1,000) were purchased from
Abcam, and β-actin (cat. no. 20536-1-AP; 1:1,000) was purchased
from ProteinTech Group, Inc. Peroxidase Conjugated Goat anti-Mouse
IgG (H+L) (cat. no. DW0990; 1:1,000), and Peroxidase Conjugated
Goat anti-Rabbit IgG (H+L) (cat. no. DW-GAR007; 1:1,000) were
purchased from Hangzhou Dawen Biology Co., Ltd. To visualize the
bands, enhanced chemiluminescence solution (ECL; Thermo Fisher
Scientific, Inc.) was added, and the integral OD of each protein
band was analyzed using Image-Pro Plus version 6 software. β-actin
was used as the internal control.
Establishment of an in vivo tumor
xenograft model
In accordance with the protocols for experimentation
with animals (National Institutes of Health Publication; no. 85-23,
revised 1996), animal experiments conducted were approved by the
Institutional Animal Care and Use Committee of Zhejiang Chinese
Medical University [Laboratory Animals Production License No. SCXK
(Shanghai) 2013-0006, Shanghai Xipuer-Bikai Experimental Animal Co.
Ltd.; Laboratory Animals Usage License No. SYXK (Zhejiang)
2013-0184, Animal Experimental Research Center, Zhejiang University
of Traditional Chinese Medicine. In total, 32 male, 4-week-old
BALB/c (nu/nu) mice weighing 15±1 g were obtained from Shanghai
Xipuer-Bikai Experimental Animal Co., Ltd. The mice were fed a
normal diet and water and were housed in a specific pathogen-free
(SPF) barrier center under constant conditions (temperature,
25±2°C; humidity, 50±5%; 12-h light/dark cycles).
SW480 cells transfected with si-AKT1 or NC were
collected, and the density was adjusted to 3×107
cells/ml with normal saline. Each nude mouse was inoculated with
0.2 ml cell suspension into the tail vein to establish a lung
metastasis model of colon cancer (25,26).
Two weeks after inoculation, disease progression was assessed using
live fluorescence imaging. Nude mice were further divided into
control groups (NC-control and si-AKT1-control) and resveratrol
groups (NC-resveratrol, si-AKT1-resveratrol) according to the total
flux in in vivo imaging. During the experiment, the activity
of the nude mice was observed, they were weighed daily, and the
mice underwent intragastric administration according to their body
weight. Nude mice in the resveratrol groups received 150 mg/kg
resveratrol via gavage once daily for 2 weeks. Mice in the control
groups received an equal volume of normal saline once daily for 2
weeks. Subsequently, after the last intragastric administration,
and fasting and water prohibition for 4 h, lung metastasis was
detected using live cell fluorescence imaging technique. The nude
mice were then sacrificed by cervical dislocation and the tumor
foci and lung tissue were collected for subsequent experiments.
In vivo bioluminescence imaging
For in vivo bioluminescence imaging, the mice
were intraperitoneally injected with 150 mg/kg body weight
D-luciferin (cat. no. 40901ES03; 15 mg/ml, Yeasen Corp.) in normal
saline. In vivo imaging was performed half an hour after
intraperitoneal injection of D-luciferin. Luminescence image
acquisition was conducted using a cooled charge-coupled device
(CCD) camera system (IVIS Imaging System; PerkinElmer, Inc.) with
an integration time of 1–60 sec and a binning factor of 4. During
imaging, the nude mice were anesthetized by continuously inhaling
2% isoflurane (cat. no. O21400; Huazhong Haiwei Gene Technology
Co., Ltd.). The flux of all detected photon counts within a region
of interest prescribed over the tumor area was collected to
calculate the signal intensity using the Living Image software
package (Xenogen Corporation).
Hematoxylin and eosin (H&E)
staining and immunohistochemistry
The tumor tissues obtained were fixed in
paraformaldehyde at 4°C for 24 h, dehydrated in ethanol, treated
with xylene, embedded in paraffin, and sliced. Tissue slices of
4-µm thickness were subjected to H&E staining (cat. no.
ZLI-9609; ZSGB-BIO; OriGene Technologies, Inc.) and histological
changes in the tumor tissues were observed using a microscope
(ix71; Olympus Corporation; ×200, magnification).
For immunohistochemistry staining, 4 µm-thick tissue
slices were immersed in 1 mM ethylenediaminetetraacetic acid (EDTA)
buffer (pH=9.0) for antigen retrieval. After removal of endogenous
peroxidase activity with 3% H2O2,
non-specific protein binding was blocked with 5% normal goat serum
buffer at 37°C for 30 min. Samples were incubated at 4°C overnight
with primary antibodies, including N-cadherin antibody (cat. no.
76011; 1:300), E-cadherin rabbit monoclonal antibody (mAb; cat. no.
76319; 1:300), AKT1 rabbit mAb (cat. no. 4691S; 1:300), p-AKT
(Ser473; cat. no. 4060S; 1:300), GSK-3β polyclonal antibody (cat.
no. 9315S; 1:300), p-GSK-3β (Ser9; cat. no. 5558P; 1:300), and
Snai1 polyclonal antibody (cat. no. 3879S; 1:300). Next, the slides
were incubated first with biotin-labeled goat-rabbit immunoglobulin
G (IgG), followed by HRP-conjugated streptavidin for 1 h each. The
reaction products were visualized after staining with
diaminobenzidine (DAB; cat. no. ZLI-9065, ZSGB-BIO; OriGene
Technologies, Inc.) with a concentration of 2 mg/ml at room
temperature for 10 min and counterstaining with hematoxylin. The
results were observed and images were captured using an inverted
microscope (×200, magnification), followed by analysis of the total
integral optical density (IOD) using Image-Pro Plus version 6
software.
Statistical analysis
The data are presented as the means ± standard
deviation (SD) and the distribution of the data was assessed. All
statistical analyses were computed using SPSS version 22.0 software
(IBM Corp.). For data that were normally distributed, significant
differences between the groups were evaluated via one-way analysis
of variance (ANOVA) followed by Tukey's multiple comparison test.
For data that did not conform to a normal distribution, the Kruskal
Wallis H test was used. A value of P<0.05 indicated statistical
significance. All experiments were repeated three times.
Results
AKT1 is successfully knocked down in
SW480 and SW620 cells, and EGF successfully induces EMT in both
cell lines
For AKT1 knockdown, lentivirus vectors containing
si-ATK1 and NC were injected into SW480 and SW620 cells. As
revealed in Fig. 1A, the
transfection of any of the three si-ATK1 sequences significantly
inhibited the expression of AKT1 (P<0.01 and P<0.001). The
knockdown efficiency of sequence 1 was the highest and this was,
therefore, selected for subsequent experiments. In addition, the
expression of AKT1 mRNA in SW480 and SW620 cells transfected with
si-AKT1 was significantly decreased compared with that in the two
cell lines transfected with NC (P<0.001, Fig. 1B). Furthermore, western blotting
confirmed consistent changes in AKT1 protein expression after
knockdown (P<0.01, Fig. 1C and
D). Thus, stable AKT1-knockdown cell lines were successfully
established in SW480 and SW620 colon cancer cells.
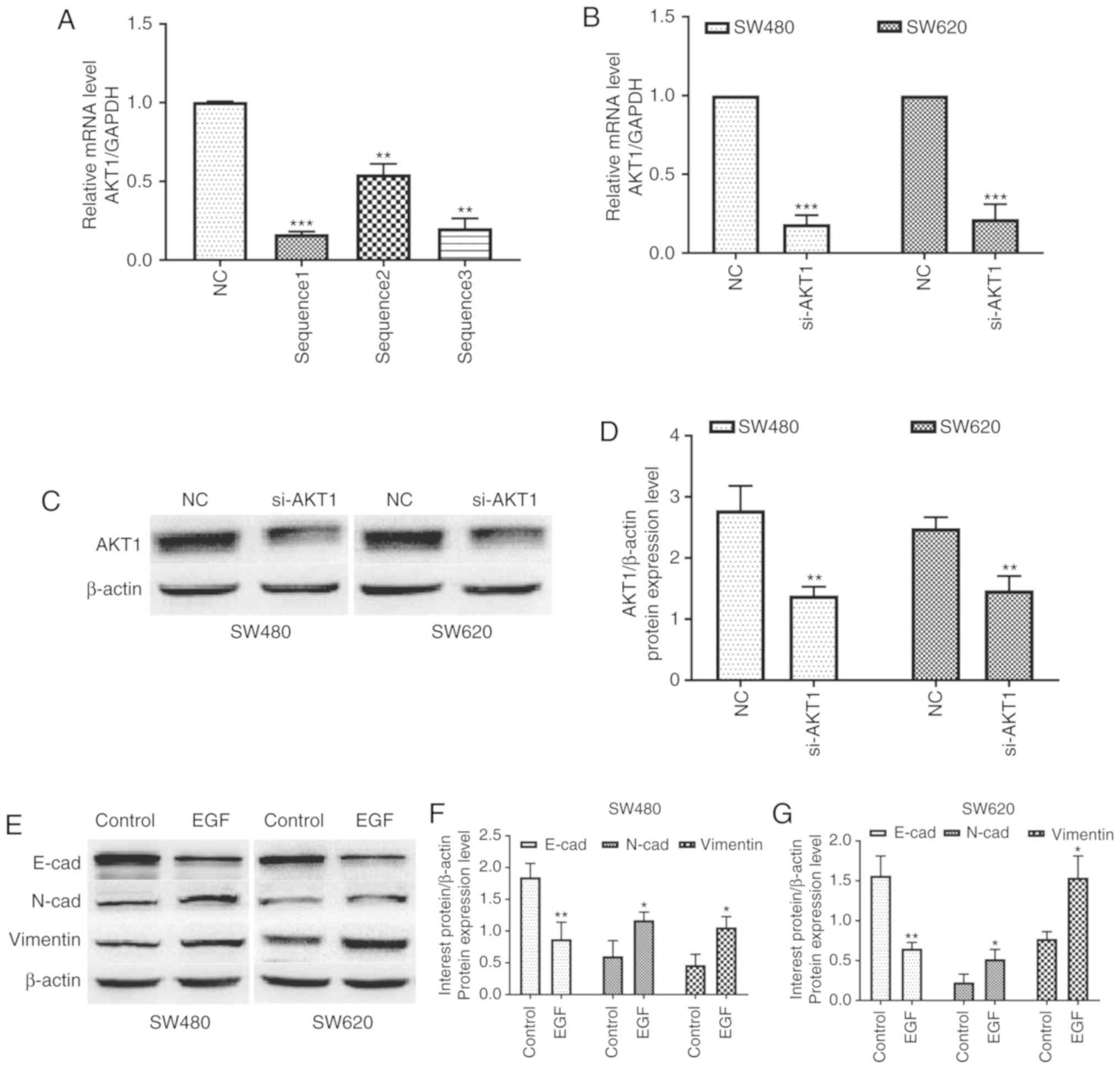 | Figure 1.AKT1 is successfully knocked down in
SW480 and SW620 cells, and EGF treatment induces EMT in both cell
lines. (A) The knockdown efficiency of si-AKT1 sequence 1 was the
highest and this was therefore selected for subsequent experiments.
(B-D) Real-time quantitative polymerase chain reaction (qPCR) and
western blotting revealed that the mRNA and protein expression of
AKT1 in SW480 and SW620 cells transfected with si-AKT1 was
significantly decreased compared with that in the two cell lines
transfected with NC. (E-G) Western blotting revealed that
E-cadherin expression in both cell lines was markedly downregulated
after treatment with 50 ng/ml EGF for 48 h, whereas the expression
of N-cadherin and vimentin was significantly upregulated.
*P<0.05, **P<0.01, ***P<0.001, and NS (P>0.05) compared
to the respective control; n=3. These data were evaluated by
one-way ANOVA. AKT1, serine/threonine kinase 1; EGF, epidermal
growth factor; EMT, epithelial-mesenchymal transition; si-, small
interfering-; NC, negative control; NS, not significant. |
In addition, EGF was used to induce EMT in both cell
lines. Western blotting revealed that E-cadherin expression in both
cell lines was markedly downregulated after treatment with 50 ng/ml
EGF, whereas the expression of N-cadherin and vimentin was
significantly upregulated (P<0.05 and P<0.01, Fig. 1E-G). These data indicated that the
EMT model was successfully established by EGF in both cell
lines.
Cytotoxic effects of resveratrol on
colon cancer cells
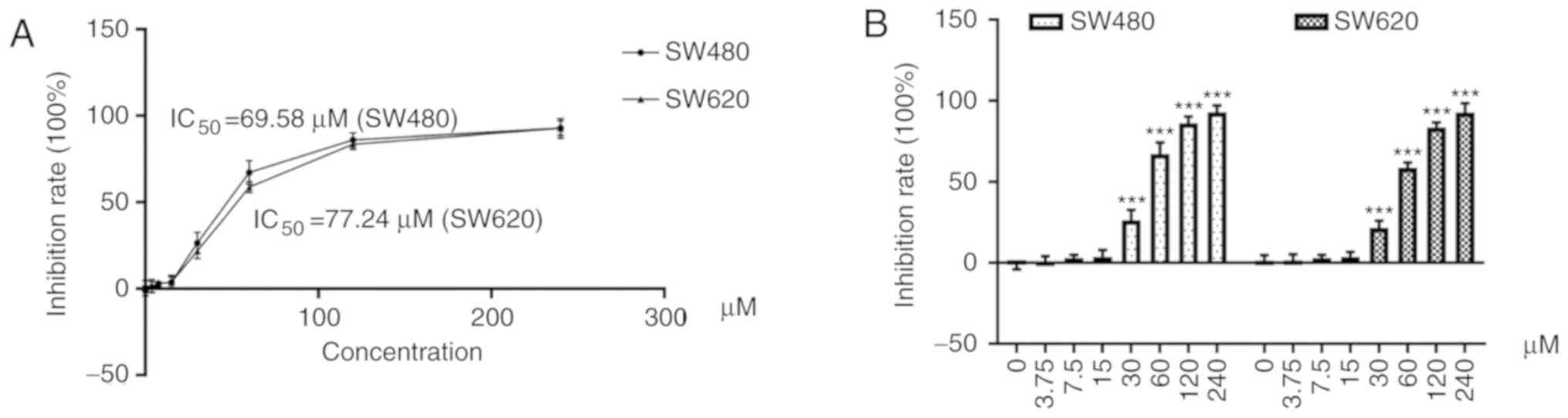
As revealed in Fig.
2A, cells were treated with resveratrol at concentrations of
3.75, 7.5, 15, 30, 60, 120, and 240 µM. The half maximal inhibition
concentration (IC50) of resveratrol was 69.58 µM in
SW480 cells and 77.24 µM in SW620 cells (Table I).
 | Table I.IR of SW480 and SW620 cell lines
treated with various concentrations of resveratrol for 48 h (X±S,
n=9). |
Table I.
IR of SW480 and SW620 cell lines
treated with various concentrations of resveratrol for 48 h (X±S,
n=9).
| Concentration
(µM) | 0 | 3.75 | 7.5 | 15 | 30 | 60 | 120 | 240 |
|---|
| IR (%) in SW480
cells | −1.21±2.96 | 0.93±3.18 | 3.04±1.95 | 4.39±3.32 | 26.43±6.23 | 67.32±6.91 | 86.11±4.07 | 92.88±4.24 |
| IR (%) in SW620
cells | 1.39±3.37 | 1.51±3.69 | 3.06±1.91 | 3.83±2.99 | 21.77±4.29 | 58.73±3.16 | 83.57±3.09 | 92.69±5.66 |
As revealed in Fig.
2B, resveratrol significantly decreased the cell survival rate
of SW480 and SW620 cells doses >30 µM (P<0.001). In addition,
the inhibitory effects increased in a dose-dependent manner.
Accordingly, the maximum nontoxic concentration of resveratrol was
defined as 15 µM and was used in subsequent experiments.
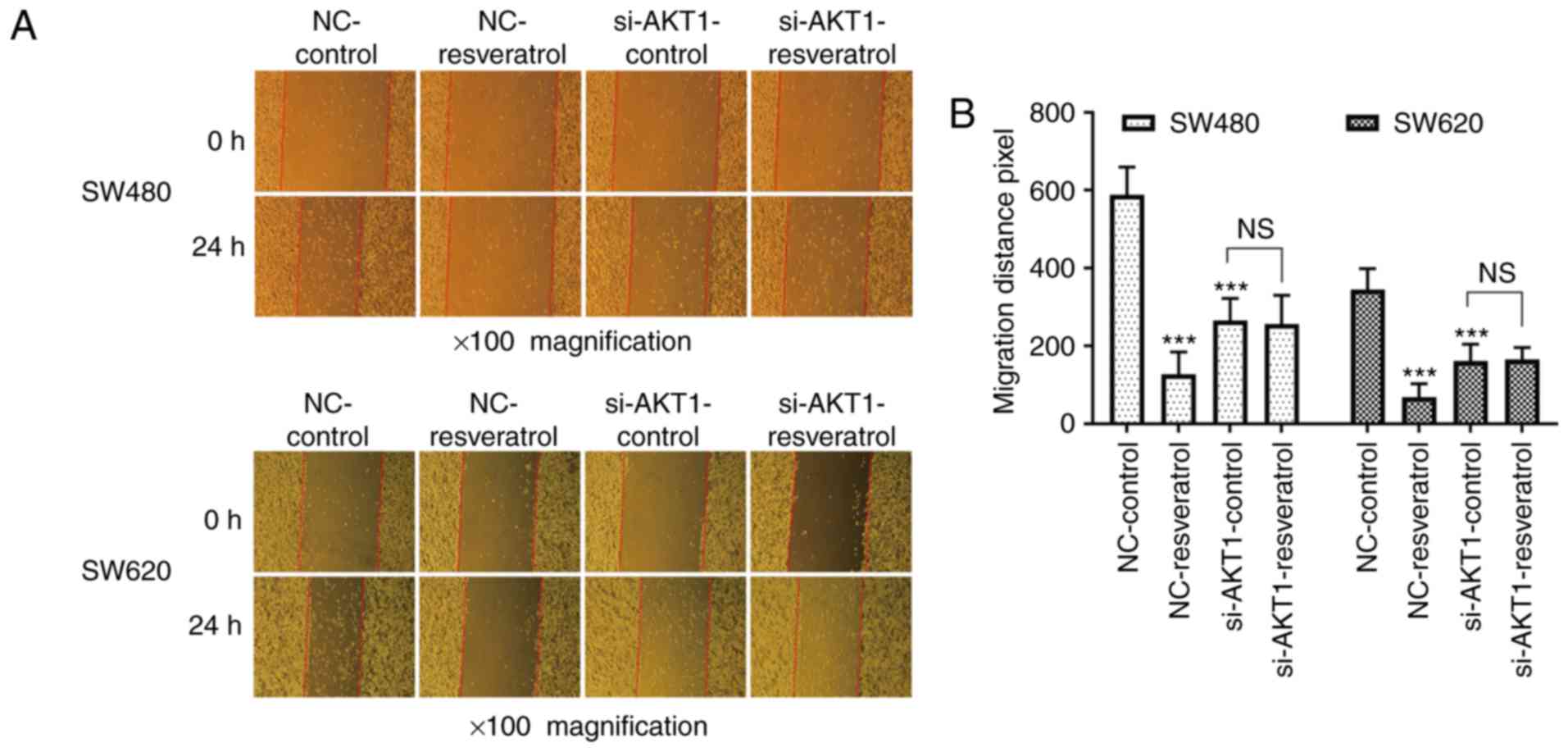
Resveratrol inhibits the migration and
invasion of colon cancer cells in vitro, and knockdown of AKT1
exhibits similar effects
Whether the effects of resveratrol on cell migration
and invasion were achieved via AKT1 regulation was then assessed.
The scratch wound healing assay revealed that the 24-h migration
distance of two cell lines in the NC-resveratrol group was
significantly shorter than that in NC-control group (P<0.001,
Fig. 3), indicating that
resveratrol inhibited the healing ability of colon cancer cells.
The 24-h migration distance of cells in the si-AKT1-control group
was also significantly shorter than that in NC-control group
(P<0.001, Fig. 3), indicating
that AKT1 knockdown also inhibited the healing ability of colon
cancer cells. However, no significant difference was observed
between the 24-h migration distance in the si-AKT1-control and
si-AKT1-resveratrol groups (P>0.05, Fig. 3), indicating that the ability of
resveratrol to inhibit the healing ability of colon cancer cells
was weakened or even nullified in AKT1-knockdown cells.
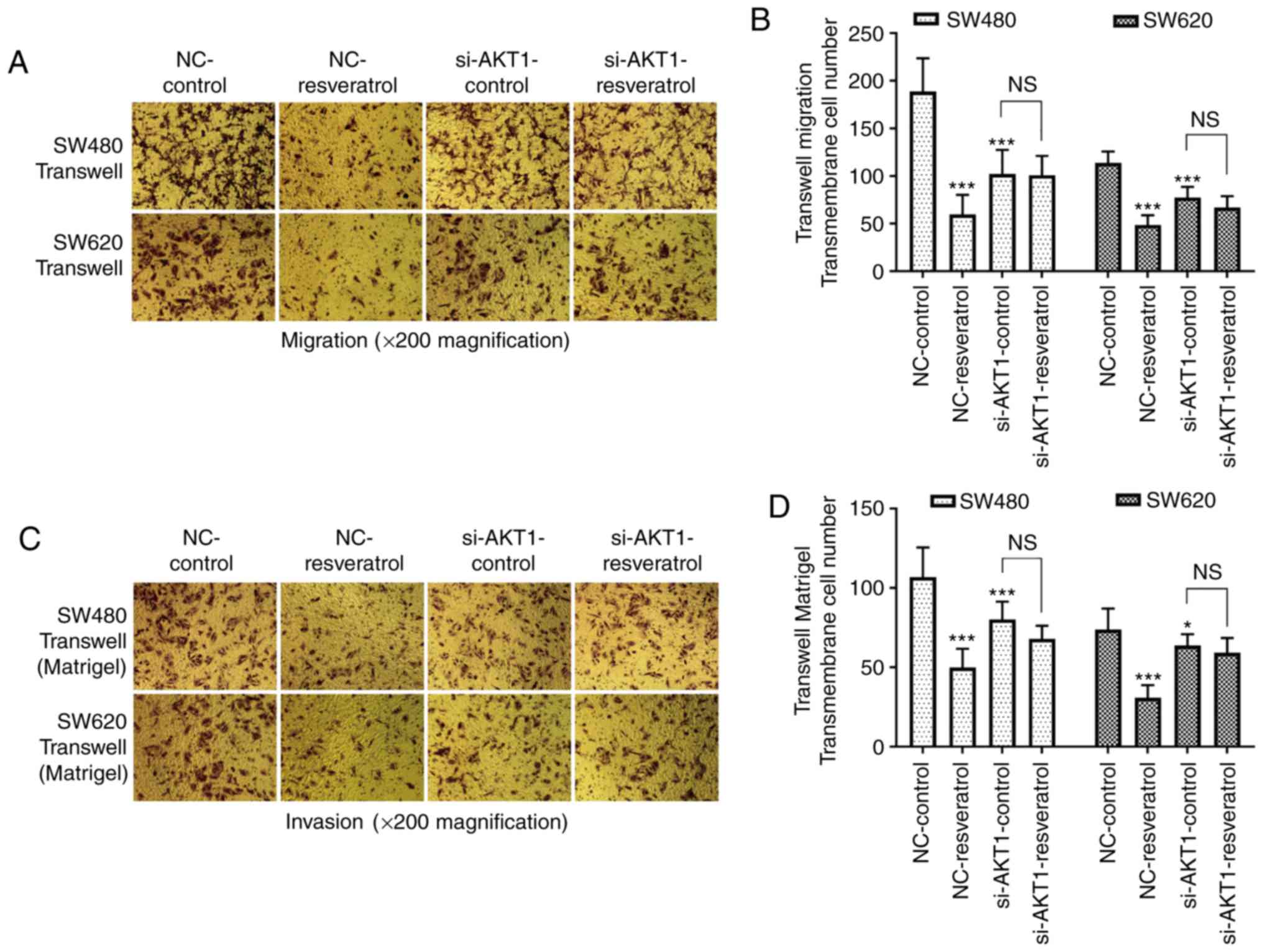
Transwell assays were also performed to detect the
migration and invasion of both cell lines (Fig. 4). The number of migrated and
invaded cells passing through the membrane in the NC-resveratrol
group was significantly lower than that in the NC-control group
(P<0.001), indicating that resveratrol markedly inhibited the
migration and invasion of colon cancer cells. In addition, the
number of migrated and invaded cells passing through the membrane
in the si-AKT1-control group was also significantly lower than that
in the NC-control group (P<0.05 and P<0.001), indicating that
AKT1 knockdown also inhibited the migration and invasion of colon
cancer cells. However, there was no significant difference between
the number of migrated and invaded cells passing through the
membrane in the si-AKT1-control and si-AKT1-resveratrol groups
(P>0.05), indicating that the inhibitory effects of resveratrol
on the migration and invasion of colon cancer cells were mitigated
by AKT1 knockdown.
Resveratrol reverses EMT in colon
cancer cells via regulation of the AKT/GSK-3β/Snail signaling
pathway in vitro
For further investigation of the effects of
resveratrol on EMT in colon cancer cells as well as its downstream
mechanisms, the expression of EMT markers and AKT/GSK-3β/Snail
signaling pathway-related molecules were evaluated in SW480 and
SW620 cells after treatment. As revealed by western blotting in
Fig. 5A and D, the expression of
E-cadherin in both cell lines was markedly upregulated in the
NC-resveratrol and si-AKT1-control groups compared to the
NC-control group, whereas N-cadherin, p-AKT1, p-GSK-3β, and Snail
expression was markedly downregulated. Densitometric analysis
revealed consistent changes in the protein expression of the
aforementioned genes after resveratrol treatment or AKT1 knockdown
(P<0.05, P<0.01 and P<0.001, Fig. 5B, C, E and F). However, protein
expression in SW480 and SW620 cells was not significantly different
in the si-AKT1-resveratrol and si-AKT1-control groups, indicating
that the effects of resveratrol on the reversal of EMT in colon
cancer cells was markedly weakened or even nullified by AKT1
knockdown (P>0.05). These data indicated that resveratrol
reversed EMT in colon cancer cells by regulating the
AKT/GSK-3β/Snail signaling pathway.
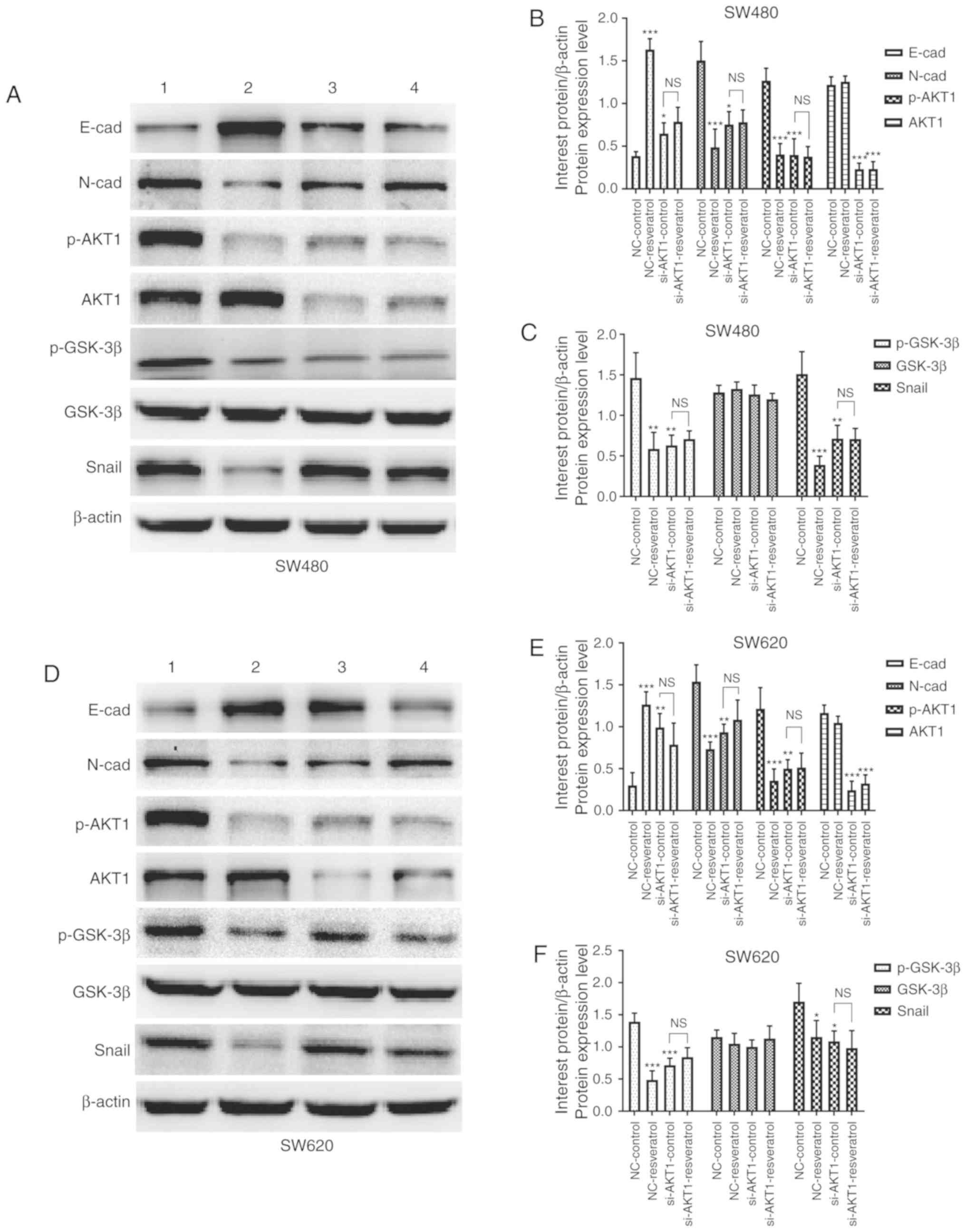 | Figure 5.Resveratrol reverses EMT in colon
cancer cells via regulation of the AKT/GSK-3β/Snail signaling
pathway in vitro (1=NC-control, 2=NC-resveratrol,
3=si-AKT1-control, 4=si-AKT1-resveratrol group). Cells were
subjected to the same drug treatments as those used in the scratch
wound healing assay. Expression of E-cadherin, N-cadherin, p-AKT1,
AKT1, p-GSK-3β, GSK-3β, and Snail was evaluated in (A-C) SW480
cells and (D-F) SW620 cells subjected to various treatments via
western blotting. In comparison with that in the NC-control group,
E-cadherin expression was upregulated in the NC-resveratrol and
si-AKT1-control groups, whereas the expression of N-cadherin,
p-AKT, p-GSK-3β, and Snail was downregulated. However, there was no
difference in the expression of these proteins between the
si-AKT1-resveratrol and si-AKT1-control groups. *P<0.05,
**P<0.01, ***P<0.001, and NS (P>0.05) compared with the
respective control; n=3. These data were evaluated by one-way
ANOVA. EMT, epithelial-mesenchymal transition; GSK, glycogen
synthase kinase; p-, phosphor-; NC, negative control; si-, small
interfering-; NS, not significant. |
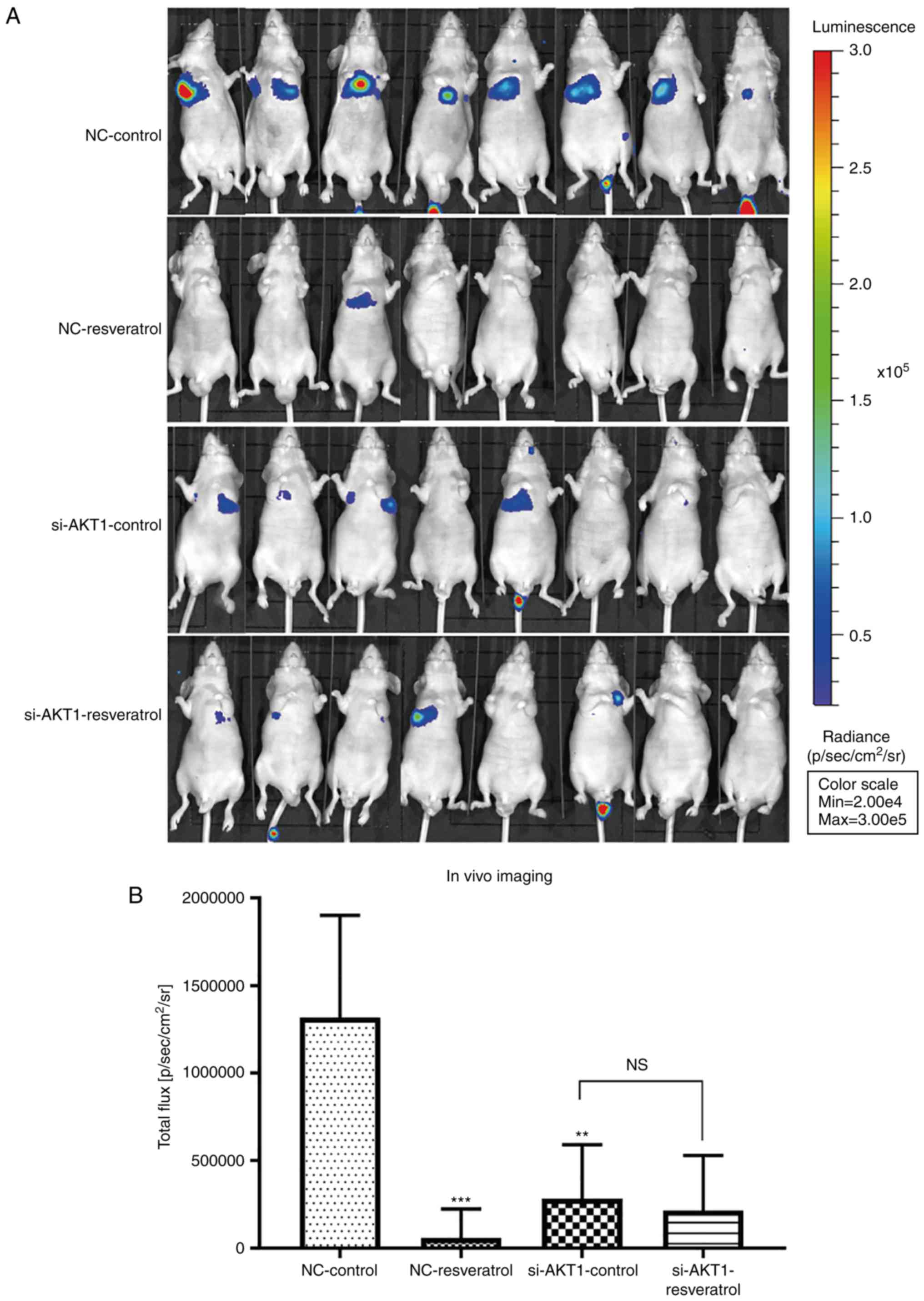
Resveratrol treatment and AKT1
knockdown inhibit lung metastasis of colon cancer in vivo
Lung metastasis in nude mice was evaluated using
live fluorescence imaging. The total flux in mice in the
NC-resveratrol or si-AKT1-control groups was significantly lower
than that in the NC-control group (P<0.01 and P<0.001,
Fig. 6A and B), indicating that
resveratrol treatment or AKT1 knockdown could inhibit lung
metastasis of colon cancer. Consistent with the in vitro
findings, no significant differences in total flux in the
si-AKT1-control and si-AKT1-resveratrol groups were observed
(Fig. 6A and B), indicating that
resveratrol did not significantly inhibit lung metastasis of colon
cancer after AKT1 knockdown. In addition, our animal experiments
revealed that some nude mice did not have lung metastasis, while
other nude mice had small or diffuse lung metastases, which could
not be counted and measured. Since we could not separate the lung
tumors alone, whole lung tissue was used for subsequent
experiments. H&E and immunohistochemical images of tumors were
observed under a 200-fold magnifying microscope. The total protein
in the animal western blotting experiment was extracted from lung
tissue.
Furthermore, H&E staining revealed
histopathological changes in tumor foci after treatment (Fig. 6C). The nuclei of tumor cells in the
NC-control group were hypertrophic, deformed, intensely stained,
and evidently heterogeneous and had several new tumor blood
vessels. Tumor cell density, pathological mitotic phase,
heteromorphic cells, and new tumor blood vessels were lower in the
NC-resveratrol group than in the NC-control group. There was no
difference between pathological mitosis and the number of
heterotypic cells in the si-AKT1-control and si-AKT1-resveratrol
groups. However, the si-AKT groups exhibited markedly lower
pathological mitosis, number of heteromorphic cells, and new tumor
blood vessels than those in the NC-control group.
Resveratrol reverses EMT in the tumor
tissues of nude mice via AKT/GSK-3β/Snail signaling regulation
To further confirm whether resveratrol inhibited
lung metastasis of colon cancer in vivo via the
AKT/GSK-3β/Snail signaling pathway, western blot and
immunohistochemical staining assays were performed to detect the
expression of EMT-markers as well as that of AKT/GSK-3β/Snail
signaling pathway-related molecules in the tumor tissues of nude
mice. Western blotting (Fig. 7)
and immunohistochemical staining (Fig.
8) assays revealed consistent results. E-cadherin expression
was upregulated in the NC-resveratrol and si-AKT1-control groups
compared to the NC-control group, whereas the expression of
N-cadherin, p-AKT, p-GSK-3β, and Snail was downregulated (P<0.05
and P<0.01, Figs. 7 and
8), indicating that resveratrol
and AKT1 knockdown could reverse EMT in colon cancer cells in tumor
tissues and promote the transformation of colon cancer cells from
mesenchymal to epithelial phenotypes. However, there was no
difference in the expression of these proteins in the
si-AKT1-resveratrol and si-AKT1-control groups (P>0.05, Figs. 7 and 8), further confirming that the ability of
resveratrol to reverse EMT in colon cancer cells in tumor tissues
almost disappeared after AKT1 knockdown.
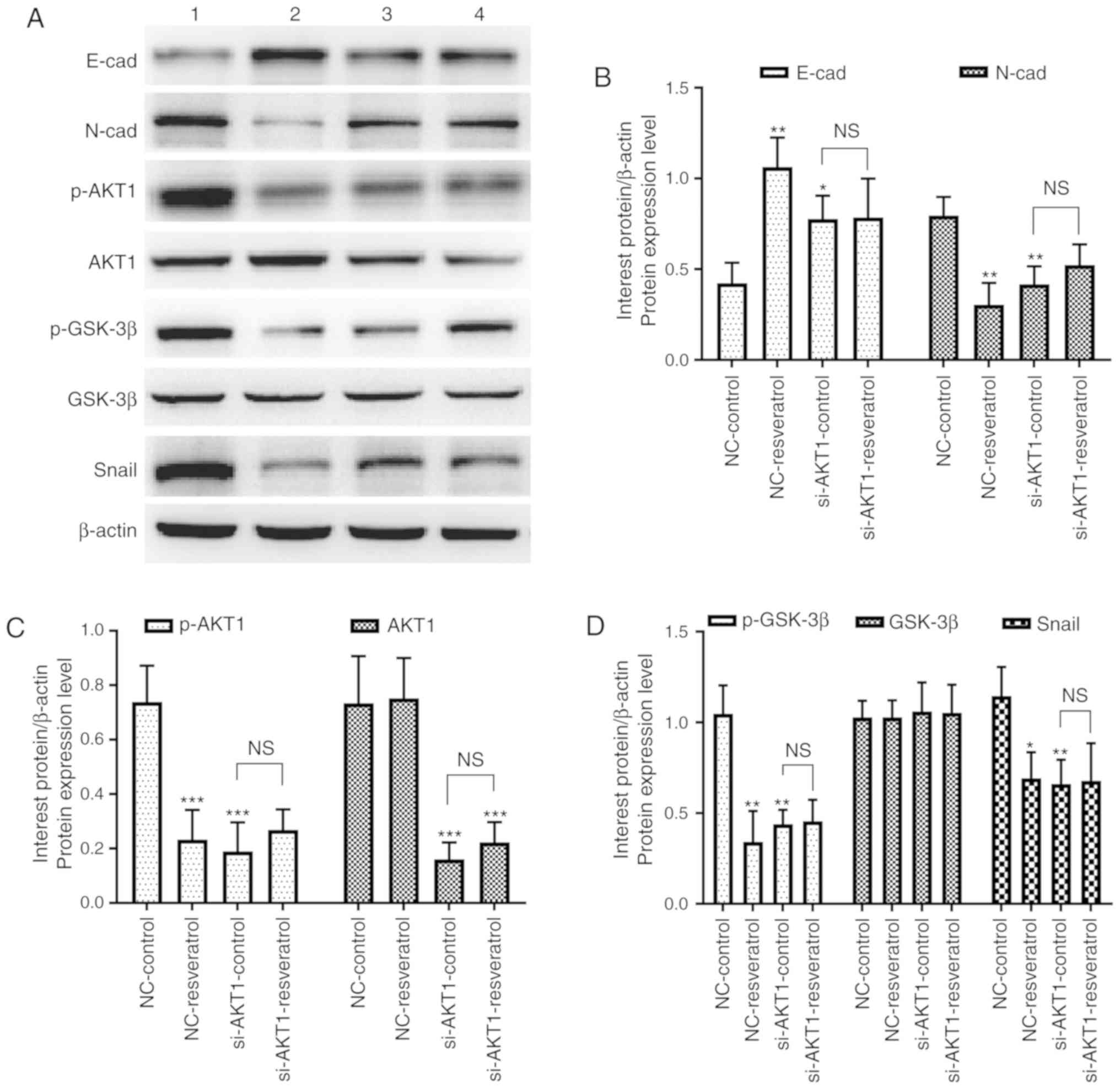 | Figure 7.Resveratrol reverses EMT in colon
cancer cells via regulation of the AKT/GSK-3β/Snail signaling
pathway in vivo (1=NC-control, 2=NC-resveratrol,
3=si-AKT1-control, 4=si-AKT1-resveratrol group). (A-D) Western
blotting was performed to assess the expression of E-cadherin,
N-cadherin, p-AKT1, AKT1, p-GSK-3β, GSK-3β, and Snail in mice
administered various treatments. In comparison with that in the
NC-control group, E-cadherin expression was upregulated in the
NC-resveratrol and si-AKT1-control groups, whereas the expression
of N-cadherin, p-AKT, p-GSK-3β, and Snail was downregulated.
However, there was no difference between the expression of these
proteins in the si-AKT1-resveratrol and si-AKT1-control groups.
*P<0.05, **P<0.01, ***P<0.001, and NS (P>0.05) compared
with the respective control; n=3. These data were evaluated by
one-way ANOVA. EMT, epithelial-mesenchymal transition; GSK,
glycogen synthase kinase; NC, negative control; si-, small
interfering-; p-, phosphor-; NS, not significant. |
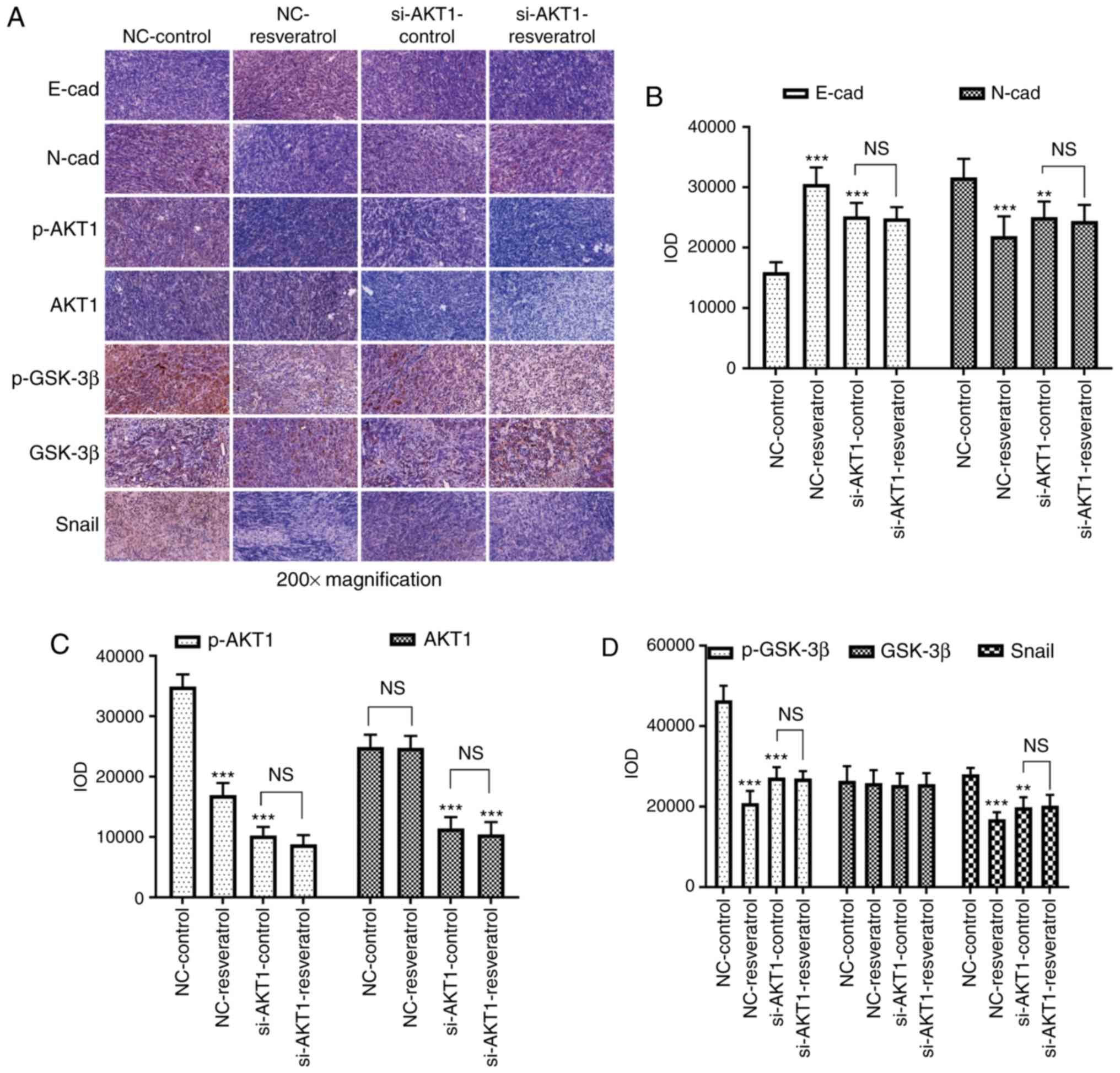 | Figure 8.Resveratrol reverses EMT in colon
cancer cells via regulation of the AKT/GSK-3β/Snail signaling
pathway in vivo. (A-D) Immunohistochemical staining assays
were conducted to evaluate the expression of E-cadherin,
N-cadherin, p-AKT1, AKT1, p-GSK-3β, GSK-3β, and Snail in mice
administered different treatments. In comparison with that in the
NC-control group, E-cadherin expression was upregulated in the
NC-resveratrol and si-AKT1-control groups, whereas the expression
of N-cadherin, p-AKT, p-GSK-3β, and Snail was downregulated.
However, there was no difference between the expression of these
proteins in the si-AKT1-resveratrol and si-AKT1-control groups.
**P<0.01, ***P<0.001, and NS (P>0.05) compared with the
respective control; n=6. These data were evaluated by one-way
ANOVA. EMT, epithelial-mesenchymal transition; GSK, glycogen
synthase kinase; p-, phosphor-; NC, negative control; si-, small
interfering-; NS, not significant. |
Discussion
At present, CRC is a malignant tumor that occurs
with a high incidence rate. Following the application of
comprehensive treatments, such as surgery, radiotherapy,
chemotherapy, and targeted therapies, the clinical cure rate of CRC
has clearly improved, although the 5-year survival rate of patients
with CRC with distant metastases remains low (4). Therefore, inhibition of invasion and
metastasis is a key step in the treatment of CRC. Traditional
Chinese medicine (TCM) compounds, which have been systematically
developed through continuous refinement and practice over thousands
of years offer the unique advantage of low toxicity and side
effects and can suppress the invasion and metastasis of CRC
(27,28). The identification of effective TCM
compounds for the treatment of CRC is of great importance.
Resveratrol is a natural polyphenol compound that
has several therapeutic effects (29). Several studies have confirmed that
resveratrol inhibits tumor invasion and metastasis (30–33).
Consistent with these findings, it was confirmed that resveratrol
suppressed the migration and invasion of colon cancer cells both
in vitro and in vivo. It is well established that EMT
leads to the loss of adhesion between tumor cells and is therefore
a regulator of tumor invasion and metastasis (34). Several studies have revealed that
resveratrol can also regulate the occurrence and development of
EMT. Resveratrol inhibited EMT by increasing miR-200c expression in
colon cancer cells, subsequently inhibiting the invasion and
metastasis of colon cancer cells (35). Resveratrol may also inhibit EMT in
colon cancer cells through increased E-cadherin expression and
reduced vimentin expression, thus, enhancing the sensitivity of
colon cancer cells to chemotherapeutic drugs (36). The present study revealed that
E-cadherin was upregulated and N-cadherin was downregulated by
resveratrol treatment. Since the dysregulation of E-cadherin and
N-cadherin is an important characteristic of EMT (37), it was confirmed that resveratrol
regulated EMT in colon cancer cells to inhibit the invasion and
metastasis of colon cancer.
Furthermore, the AKT/GSK-3β/Snail signaling pathway
is a key mechanism that modulates tumor invasion and metastasis.
Jiang et al demonstrated that phosphoinositide 3-kinase
(PI3K)/AKT/GSK-3β/Snail signaling was involved in the invasion and
metastasis of hepatocellular carcinoma cells (20). Zhang et al (38) revealed that cytosolic THUMP
domain-containing 1 (THUMPD1) facilitated the invasion and
metastasis of breast cancer cells though regulation of the
AKT/GSK-3β/Snail signaling pathway. Notably, tumor-derived C-X-C
motif chemokine ligand 5 (CXCL5) was revealed to promote the
metastasis of CRC via activation of the AKT/GSK3β/β-catenin pathway
(39). In the present study, AKT1
knockdown markedly inhibited cell migration and invasion, reversed
EMT, and activated AKT1/GSK-3β/Snail signaling in colon cancer
cells. Moreover, the effects of resveratrol were weakened or even
nullified after AKT1 knockdown. Therefore, resveratrol may regulate
EMT in colon cancer cells and inhibit the invasion and metastasis
of colon cancer through the AKT1/GSK-3β/Snail signaling
pathway.
In conclusion, resveratrol may inhibit the invasion
and metastasis of colon cancer cells through reversal of EMT via
the AKT/GSK-3β/Snail signaling pathway. AKT1 may be a key regulator
of EMT in colon cancer cells and serve as a potential therapeutic
target for this disease.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the Natural
Science Foundation of Zhejiang Province (LQ17H290002, LY17H270007),
the Zhejiang Provincial TCM Scientific Research Fund project
(2019ZQ015, 2017ZA048), the National Natural Science Foundation of
China (81573902), the Zhejiang Provincial Medical and Health
Science and Technology project (2017KY119), the China Postdoctoral
Science Foundation (2017M612040, 2018T110610), the Program for the
Cultivation of Youth Talents in China Association of Chinese
Medicine (QNRC2-C08), the Zhejiang Provincial Program for the
Cultivation of High-Level Innovative Health Talents (2015–43) and
the Zhejiang Provincial Project for the Key Discipline of
Traditional Chinese Medicine (2017-XK-A09).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SR conceived the study and acquired the funding. LY
and MZ conceived and designed the experiments. LY, MZ, KZ, LS and
DH collected samples and conducted the experiments. MS, SM, HH and
HSW performed the genome assembly and analysis of the data. All
authors have read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Zhejiang Chinese
Medical University. The procedures were conducted according to the
protocol for experimentation with animals (NIH Publication no.
85-23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
CRC
|
colorectal cancer
|
|
E-cad
|
E-cadherin
|
|
N-cad
|
N-cadherin
|
|
AKT
|
serine/threonine kinase
|
|
EGF
|
epidermal growth factor
|
|
HCC
|
human colon cancer
|
|
FBS
|
fetal bovine serum
|
|
CCK-8
|
Cell Counting Kit-8
|
|
IR
|
inhibition rate
|
|
H&E
|
hematoxylin and eosin
|
|
TCM
|
Traditional Chinese Medicine
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vences-Catalan F and Levy S: Immune
targeting of tetraspanins involved in cell invasion and metastasis.
Front Immunol. 9:12772018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borriello A: Resveratrol in cancer
prevention and treatment: Focusing on molecular targets and
mechanism of action. Proceedings. 1:9762017. View Article : Google Scholar
|
|
11
|
Kim SE, Shin SH, Lee JY, Kim CH, Chung IK,
Kang HM, Park HR, Park BS and Kim IR: Resveratrol induces
mitochondrial apoptosis and inhibits epithelial-mesenchymal
transition in oral squamous cell carcinoma cells. Nutr Cancer.
70:125–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cilibrasi C, Riva G, Romano G, Cadamuro M,
Bazzoni R, Butta V, Paoletta L, Dalprà L, Strazzabosco M, Lavitrano
M, et al: Resveratrol impairs glioma stem cells proliferation and
motility by modulating the wnt signaling pathway. PLoS One.
12:e01698542017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F and Wu E: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aiello NM, Maddipati R, Norgard RJ, Balli
D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al:
EMT subtype influences epithelial plasticity and mode of cell
migration. Dev Cell. 45:681–695.e684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Sun JD, Yan LJ and Zhao XP:
PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC)
progression via activating p38/AKT/ERK/EMT signal pathway. Biochem
Biophys Res Commun. 478:845–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saegusa M, Hashimura M, Kuwata T and
Okayasu I: Requirement of the Akt/beta-catenin pathway for uterine
carcinosarcoma genesis, modulating E-cadherin expression through
the transactivation of slug. Am J Pathol. 174:2107–2115. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
Morita M, Kakeji Y and Maehara Y: Deregulation of the Akt pathway
in human cancer. Curr Cancer Drug Targets. 8:27–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dou Y, Lei JQ, Guo SL, Zhao D, Yue HM and
Yu Q: The CNPY2 enhances epithelial-mesenchymal transition via
activating the AKT/GSK3β pathway in non-small cell lung cancer.
Cell Biol Int. 42:959–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu LL, Chen XH, Zhang G, Liu ZC, Wu N,
Wang H, Qi YF, Wang HS, Cai SH and Du J: CCL21 facilitates
chemoresistance and cancer stem cell-like properties of colorectal
cancer cells through AKT/GSK-3β/snail signals. Oxid Med Cell
Longev. 2016:58741272016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang H, Zhou Z, Jin S, Xu K, Zhang H and
Xu J, Sun Q, Wang J and Xu J: PRMT9 promotes hepatocellular
carcinoma invasion and metastasis via activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Sci. 109:1414–1427. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto T, Yokoi A, Hashimura M, Oguri
Y, Akiya M and Saegusa M: TGF-β-mediated LEFTY/Akt/GSK-3β/Snail
axis modulates epithelial-mesenchymal transition and cancer stem
cell properties in ovarian clear cell carcinomas. Mol Carcinog.
57:957–967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Lin Z, Chen B, Chen S, Jiang Z, Zhou
T, Hou Z and Wang Y: Ezrin/NF-kB activation regulates epithelial-
mesenchymal transition induced by EGF and promotes metastasis of
colorectal cancer. Biomed Pharmacother. 92:140–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZC, Chen XH, Song HX, Wang HS, Zhang
G, Wang H, Chen DY, Fang R, Liu H, Cai SH and Du J: Snail regulated
by PKC/GSK-3β pathway is crucial for EGF-induced
epithelial-mesenchymal transition (EMT) of cancer cells. Cell
Tissue Res. 358:491–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimaoka H, Takeno S, Maki K, Sasaki T,
Hasegawa S and Yamashita Y: A cytokine signal inhibitor for
rheumatoid arthritis enhances cancer metastasis via depletion of NK
cells in an experimental lung metastasis mouse model of colon
cancer. Oncol Lett. 14:3019–3027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mateo-Lozano S, Bazzocco S, Rodrigues P,
Mazzolini R, Andretta E, Dopeso H, Fernández Y, Del Llano E, Bilic
J, Suárez-López L, et al: Loss of the EPH receptor B6 contributes
to colorectal cancer metastasis. Sci Rep. 7:437022017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Ji Q, Deng W, Chai N, Feng Y, Zhou
L, Sui H, Li C, Sun X and Li Q: JianPi JieDu recipe inhibits
epithelial-to-mesenchymal transition in colorectal cancer through
TGF-β/Smad mediated Snail/E-cadherin expression. Biomed Res Int.
2017:26131982017.PubMed/NCBI
|
|
28
|
Zhang Z, Chen H, Xu C, Song L, Huang L,
Lai Y, Wang Y, Chen H, Gu D, Ren L and Yao Q: Curcumin inhibits
tumor epithelialmesenchymal transition by downregulating the Wnt
signaling pathway and upregulating NKD2 expression in colon cancer
cells. Oncol Rep. 35:2615–2623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berman AY, Motechin RA, Wiesenfeld MY and
Holz MK: The therapeutic potential of resveratrol: A review of
clinical trials. NPJ Precis Oncol. 1:352017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Q, Yuan Y, Gan HZ and Peng Q:
Resveratrol inhibits the hedgehog signaling pathway and
epithelial-mesenchymal transition and suppresses gastric cancer
invasion and metastasis. Oncol Lett. 9:2381–2387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X,
Wang S, Zhao J, Li Y and Cao Y: Resveratrol inhibits the invasion
of glioblastoma-initiating cells via down-regulation of the
PI3K/Akt/NF-κB signaling pathway. Nutrients. 7:4383–4402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YS, Sull JW and Sung HJ: Suppressing
effect of resveratrol on the migration and invasion of human
metastatic lung and cervical cancer cells. Mol Biol Rep.
39:8709–8716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J and Li Q: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. Plos One. 8:e787002013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karimi Dermani F, Saidijam M, Amini R,
Mahdavinezhad A, Heydari K and Najafi R: Resveratrol inhibits
proliferation, invasion, and epithelial-mesenchymal transition by
increasing miR-200c expression in HCT-116 colorectal cancer cells.
J Cell Biochem. 118:1547–1555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buhrmann C, Shayan P, Kraehe P, Popper B,
Goel A and Shakibaei M: Resveratrol induces chemosensitization to
5-fluorouracil through up-regulation of intercellular junctions,
Epithelial-to-mesenchymal transition and apoptosis in colorectal
cancer. Biochem Pharmacol. 98:51–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye X and Weinberg RA:
Epithelial-mesenchymal plasticity: A central regulator of cancer
progression. Trends Cell Biol. 25:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Jiang G, Sun M, Zhou H, Miao Y,
Liang M, Wang E and Zhang Y: Cytosolic THUMPD1 promotes breast
cancer cells invasion and metastasis via the AKT-GSK3-Snail
pathway. Oncotarget. 8:13357–13366. 2017.PubMed/NCBI
|
|
39
|
Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu
C, Liu D, Zheng M, Sun J, Feng H and Lu A: Tumor-derived CXCL5
promotes human colorectal cancer metastasis through activation of
the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer.
16:702017. View Article : Google Scholar : PubMed/NCBI
|