Introduction
Vulvovaginal candidiasis (VVC) is a common disease
that is unique to women and is mainly caused by Candida albicans
(CA) (1,2). In recent years, the incidence of VVC
has increased significantly, particularly in younger women, due to
the intensified influence of environmental pollution, increased
pressure of competitiveness, accelerated work pace, lifestyle
changes, and many other factors (3). The extensive use of broad-spectrum
antibiotics, hormones, and immunosuppressive agents has also
contributed to the prevalence of VVC. Moreover, some patients fail
to follow advice from doctors and insist on taking drugs or
irregular treatments, resulting in repeated relapses of VVC
(4,5). CA herbal treatment based on syndrome
differentiation in traditional Chinese medicine directly inhibits
or eliminates the disease (6–8) and
its pathogens by enhancing the body's immunity. It also possesses
antipyretic, analgesic, and anti-inflammatory properties, with
minimal adverse reactions, thereby addressing the symptoms as well
as the root cause of the problem (9). Among the natural products that have
been traditionally used to treat CA are black and green teas. These
products have demonstrated appreciable inhibitory effects on the
growth of CA, possibly due to the arrest of CA biofilm formation
upon the impairment of proteasome activity (10). Antimicrobial experiments have shown
that propolis extracts derived from bees can also effectively
control the proliferation of CA (11). Lavender honey is another product
that has been reported to have a certain inhibitory effect on CA
and the growth of pathogenic yeasts (12). In vitro antimicrobial activity
assays reveal that the natural formula traditionally used in
Chinese herbal medicine for curing CA targets 23 strains of
Staphylococcus aureus, among others (13). The andrographolide derivative,
Omeprazole, inhibits CA proliferation and biofilm formation in rats
by interfering with the transcription and expression of CA
adhesion-related genes (14). In
addition, Pulsatilla decoction, another Chinese herbal medicine,
has been shown to effectively prevent the recurrence of CA
vaginitis in 49 patients (15).
Such valuable effects of Chinese herbal medicine in the treatment
of VVC have prompted great interest in this particular field of
research.
Dandelion is included in the 2015 edition of
‘Chinese Pharmacopoeia’ for the Asteraceae (Taraxacum spp.)
perennial herb dandelion (T. mongolicum Hand-Mazz.), Alkaline
dandelion (T. sinicum Kitag.) (16). Several studies have demonstrated
that dandelion has anti-viral (9),
anti-tumor, hypolipidemic (17)
and other functions, particularly anti-bacterial and
anti-inflammatory effects (18).
At present, the use of dandelion in the treatment of human and
animal diseases, especially mastitis is extremely extensive.
Studies have shown that dandelion active ingredients (DAIS) possess
notable bacteriostatic activity against the main pathogens of
recessive mastitis in dairy cows, including Staphylococcus aureus,
Escherichia coli, Streptococcus agalactiae, Streptococcus
dysgalactiae, and Streptococcus uberis (19). Moreover, dandelion water extract
has been reported to significantly reduce the expression of tumor
necrosis factor-α and intracellular adhesion molecule 1 in the
mastitic microvascular endothelium of rats (20). It may also be effective in treating
breast edema (21) and
triple-negative breast cancer by inducing endoplasmic reticulum (an
organelle with protein synthesis and folding as its major function)
stress-related cell apoptosis (22). Despite the abundance of studies
concerning the therapeutic effect of dandelion extract on mastitis
and cancer, its activity against VVC requires further
investigation.
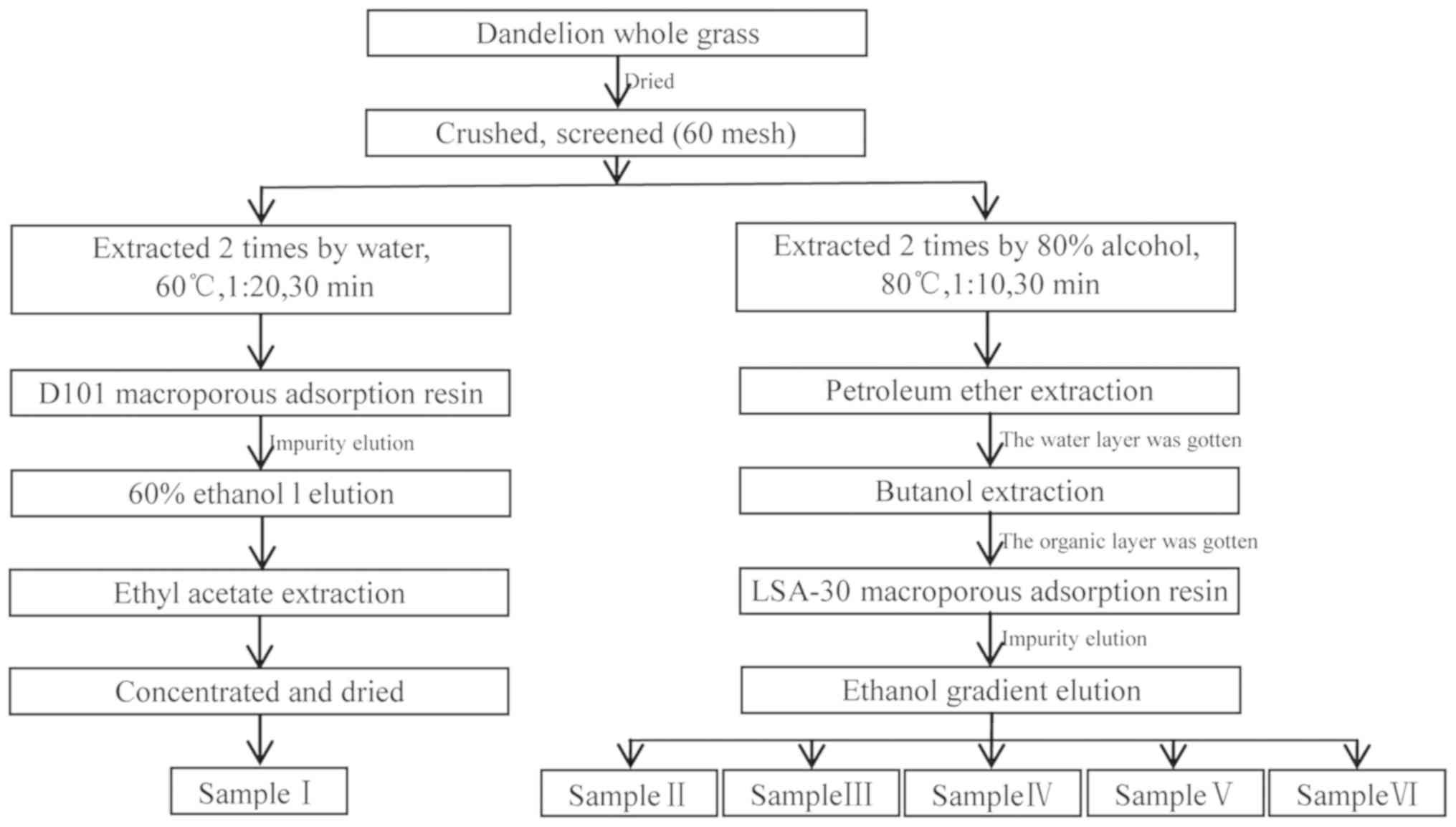
In this study, DAIS was obtained using D101 and
LSA-30 macroporous resins, screened using bacteriostatic
experiments, and detected by high-performance liquid
chromatography-diode-array detector-electrospray ionization-tandem
mass spectrometry (HPLC-DAD-ESI-MS/MS). In particular, minimal
inhibitory concentration, bacteriostatic kinetics, cell
ultrastructure, cell membrane permeability, and synthesis of cell
wall component β-(1–3)-D glucan were assessed in order to
elucidate the mechanism of DAIS antifungal activity against CA. The
results reported herein constitute the fundamental theoretical
background required to conduct further, more detailed research on
the development and utilization of dandelion resources.
Materials and methods
Chemicals and materials
Anhydrous ethanol, ethyl ether, petroleum ether,
n-butanol, concentrated hydrochloric acid (37%), sodium hydroxide,
concentrated sulfuric acid (98%), Tween 80, Tris (hydroxymethyl)
aminomethane, dimethyl sulfoxide, glutaraldehyde, sodium dihydrogen
phosphate, disodium hydrogen phosphate, KBr, phosphoric acid, and
ethyl acetate were of analytical grade and were purchased from
Tianjin Fuyu Fine Chemical Co. Ltd. Analytical grade glucose,
peptone, yeast extract, and agar powder were provided by AOBOX.
Spectrally pure potassium bromide was purchased from Beijing Inluck
Science and Technology Development Co. Ltd., whereas
chromatographically pure methanol and acetonitrile were purchased
from Honeywell (Burdick & Jackson). D101 and LSA-30 macroporous
resins were purchased from Sunresin.
Fresh dandelion whole-grass samples were collected
and identified as Taraxacum mongolicum by Professor Xinsheng Li of
the Shaanxi University of Technology. The samples were dried to
constant weight at 55°C, crushed, passed through a 60-mesh screen,
then dried again. Active ingredients of dandelion were extracted
using the method illustrated in Fig.
1.
Samples of lyophilized CA (ATCC10231) provided by
the China Food and Drug Administration were stored in a
low-temperature refrigerator (Thermo Fisher Scientific, Inc.) at
−80°C. Prior to use, the samples were rehydrated, passaged, then
cultured in Sabouraud agar medium (10 g of peptone, 40 g of
glucose, 15 g of agar and 0.1 g of chloramphenicol added to
distilled water with final volume 1L). No agar was added to the
liquid culture, and other components remained unchanged.
Antibacterial assays
CA (1 µl; OD600=0.4) was inoculated in liquid
Sabouraud medium then cultured in a shaker at 37°C and 200 rpm
until the optical density (OD)600nm reached 0.60 (Ultraviolet
Spectrophotometer, Shimadzu). Next, 100 µl of the CA suspension
were pipetted onto six Sabouraud agar plates and spread evenly
using an L-shaped glass rod. Later, 10 µl dandelion extract samples
I–VI were each pipetted onto sterile filter paper (6 mm in
diameter). Then sterile filter papers were each transferred to
Sabouraud agar medium (23,24).
The plates were incubated at 37°C for 36 h, and the antifungal
activity of DAIS was determined based on the inhibition zone of the
plate. The same active ingredients were used for subsequent
experimental studies. All of the experiments were repeated three
times for verification.
HPLC-DAD-ESI-MS/MS
An Agilent 6410B HPLC-ESI-MS/MS system (Agilent
Technologies, Inc.) was used to analyze DAIS. The ingredients were
separated on a DiKMA Diamonsil C18 column (250×4.6 mm, 5 cm) using
a mobile phase composed of acetonitrile (eluent A) and 1.0% formic
acid (v/v, eluent B). Gradient elution was performed at a flow rate
of 1 ml/min, starting with 8% A/92% B at 0 min and passing through
14% A/86% B at 24 min, then 23% A/77% B at 35 min, 24% A/76% B at
44 min, 32% A/68% B at 60 min, 37% A/63% B at 66 min, and finally
back to 8% A/92% B at 68 min (maintained for 6 min) (24,25).
The detection wavelength of the HPLC system was set to 280 nm and
the injection volume to 10 µl. The ESI source was kept at 110°C,
and the desolvation temperature was set to 400°C. The nebulizer
pressure, fragmentor voltage, and capillary voltage were maintained
at 35 psi, 135 V, and 3,000 V, respectively. Full spectra were
recorded in the negative ionization mode over the m/z range of
100–1,000 Da.
Determination of the minimum
inhibitory concentration (MIC)
A single activated CA colony was inoculated in
liquid sabourand medium, incubated at 37°C and shaken at 200 rpm
for 12 h, then adjusted to a CA concentration of 107 CFU/ml. A 400
mg/ml DAIS mother liquor solution was also prepared. Both solutions
were added to test tubes containing liquid Sabouraud medium. The
final DAIS concentrations in the tubes were adjusted to 4.0, 8.0,
16.0, 32.0, 48.0, 64.0, or 128 mg/ml, whereas the amount of added
CA suspension was set to 5% (v/v) in all of the tubes. A test tube
containing an equivalent volume of pure water was used as control.
Fluconazole was added to test tubes containing liquid Sabouraud
medium and 5% CA suspension at final concentrations of 0.20, 0.40,
0.60, 0.80, 1.00, 1.20, and 1.40 mg/ml, as positive control group.
Next, the mixtures were incubated at 37°C and shaken at 200 rpm for
24 h. The MIC was taken to be the lowest sample group concentration
beyond which changes in OD600 values compared with the next sample
group were ≤5% (26,27).
Bacteriostatic kinetic analysis
The CA culture and DAIS mother solution, prepared as
aforementioned, were added to liquid medium in three test tubes at
DAIS concentrations of 0, 1 and 3X MIC, and a CA concentration of
5% (v/v); 0X MIC served as the control group. The mixtures were
then incubated at 37°C and 200 rpm followed by obtaining the
OD600nm every 3 h for a total of 36 h (Ultraviolet
Spectrophotometer, Shimazu). The CA survival and antifungal rates
were calculated according to equations (1) and (2) (28–30):
Survivalrate(%)=OD600nmSamplegroupOD600nmControlgroup
Antifungalrate%=1-Survivalrate(%)
Morphological observation by scanning
electron microscopy (SEM)
Mixtures of 0, 0.5, 1.0, and 2.0X MIC DAIS and 5%
(v/v) CA were incubated at 37°C and shaken at 200 rpm for 5 h. 1 ml
aliquots of each sample were centrifuged at 7,104 × g for 6 min
(Eppendorf) at 4°C then washed three times with PBS (0.01 M, pH
5.8). Subsequently, the centrifuged aliquots were fixed overnight
at room temperature with 2.5% glutaraldehyde (prepared using 25%
glutaraldehyde, deionized water, and PBS at a ratio of 1:4:5 in a
dark, enclosed environment to prevent evaporation), dehydrated with
a graded alcohol solution, and lyophilized for 12 h using a vacuum
freeze dryer (Four-Ring). Finally, the samples were coated with
gold using an ion coater (31–33)
prior to SEM analysis at different magnifications (×2,000, ×5,000
and ×10,000 times (JEOL, Ltd.).
Cell membrane permeability
analysis
CA cultures prepared as aforementioned were
centrifuged at 3,996 × g for 10 min at 4°C then washed three times
with PBS (0.01 M, pH 5.8). The cultured cells were subsequently
suspended in 0.01% Tween 80/PBS (0.01 M, pH 5.8) and their
concentration was adjusted to 109 CFU/ml. DAIS mother solution was
added to four test tubes containing suspended CA cells to attain
final DAIS concentrations of 0, 0.5, 1.0 and 2.0X MIC. The 0X MIC
group served as the control group. The mixtures groups were then
incubated at 37°C and 200 rpm. Subsequently, 200 µl aliquots of
each sample were collected after 0, 1, 2, 4, and 6 h of incubation
at room temperature. The OD260nm values of supernatants were
measured after centrifugation at 3,996 × g for 10 min (34) at 4°C.
Effects on the synthesis of cell wall
β-(1–3)-D-glucan
The 5% (v/v) CA suspensions were added to and 0.5X
MIC DAIS in liquid Sabouraud medium. The 0 X MIC group was
considered as the control group. After incubation at 37°C and 200
rpm for 48 h, the CA cells were inactivated at 121°C for 20 min
then collected by centrifugation at 3,996 × g for 10 min at 4°C.
The collected cells were washed three times with 0.01% Tween 80/PBS
(0.01 M, pH 5.8) and once with sterile water, followed by
lyophilization and weighing.
Cell wall β-(1–3)-D-glucan was extracted according to the
method reported in the literature (35), and the extraction rate was
determined according to the dextran/cell dry weight. The extracted
β-(1–3)-D-glucan was analyzed using infrared
spectrometry (Nicolet; Thermo Fisher Scientific, Inc.) in the range
of 4,000–500 cm−1.
Statistical analysis
Data analysis was performed using SPSS 22.0 (IBM,
Corp.) and Origin 8.0 (OriginLab) software. ChemDraw v. 17
(CambridgeSoft) software was used to illustrate chemical
structures. Experimental data were analyzed using one-way analysis
of variance followed by a Tukey's post hoc test to determine the
significant differences between the groups. P<0.05 and P<0.01
were considered to indicate statistically significant and highly
significant differences, respectively. The results were expressed
as the mean ± standard deviation.
Results
Study of the antimicrobial activity of
DAIS
In vitro and in vivo assessments have
confirm the antifungal activity of dandelion, a plant that has been
commonly used in China to treat gynecopathy (20), such as mammitis and vaginitis.
Dandelion contains various phytochemicals, including flavonoids,
phenolic acids, alkaloids, and terpenes (25). In the present study, 6 samples (I,
II, III, IV, V, VI) of DAIS were extracted and purified using
hot-water (Fig. 1). The antifungal
activity of the dandelion extracts was determined by assessing
their effect on the growth of CA using the K-B paper disk method.
As presented in Fig. 2, dandelion
sample I is the only sample to exhibit an inhibition zone,
indicating that all of the other samples (II, III, IV, V and VI)
lack notable antifungal activity with respect to CA. The mean
diameter of the CA inhibition zone in sample I (concentration=53.2
mg/ml) was found to be 10.38±0.15 mm, signifying marked antifungal
effects against CA.
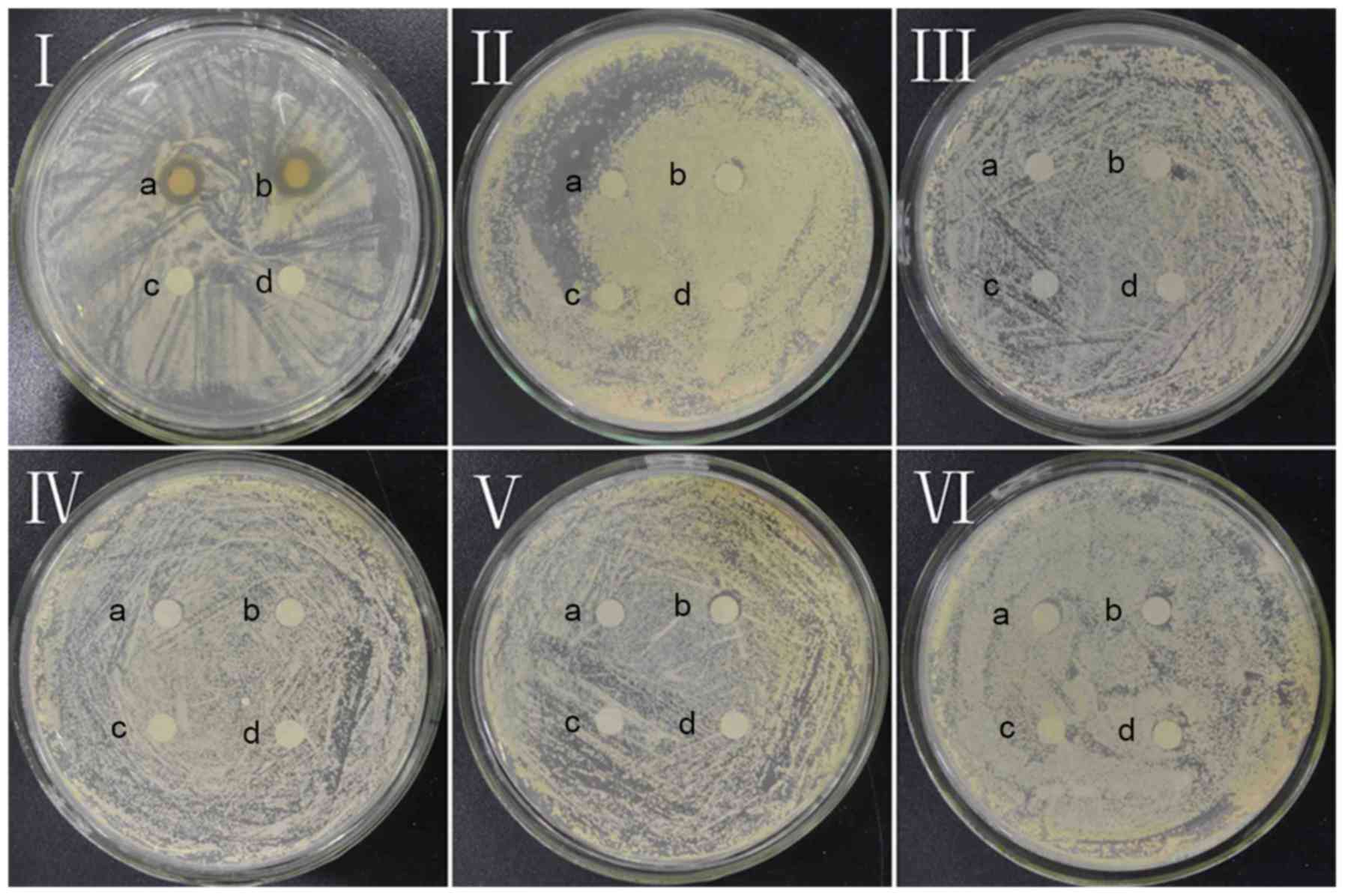 | Figure 2.Antifungal effect of dandelion active
ingredients in samples I, II, III, IV, V, and VI against Candida
albicans. a and b, experimental groups; c and d, control groups in
I, II, III, IV, V, VI. |
Qualitative and quantitative analyses
of dandelion sample I by LC-DAD-ESI-MS
Eight compounds in dandelion sample I were separated
within 78 min and identified at 280 nm using HPLC (Fig. 3). The compounds were further
identified by tandem mass spectrometry based on their [M-H]- values
and corresponding ion fragments (Table
I). Peak with retention times of 21.35 min in Fig. 3 was identified as 4-coumaric acid
based on the [M-H]- m/z value of 163. The ion fragment at m/z=119
corresponds to the loss of a CO2 moiety. With an [M-H]- m/z value
of 193 and ion fragment at m/z=134, a peak with a retention time of
28.3 min was identified as ferulic acid (36). Similarly, a peak with a retention
time of 30.05 min was identified as quercetin pentoside based on
the molecular ion peak at m/z=433 and the ion fragment at m/z=301
(formed upon the removal of pentose) (25). Peaks with retention times of 37.29
and 38.45 min both yielded an [M-H]- m/z value of 515, and ion
fragments at m/z=353 and 173. However, the former showed a unique
fragment at m/z=191, whereas the distinguished fragment in the
latter was detected at m/z=179. Based on these values, these two
peaks were identified as 3,5-di-O-caffeoylquinic acid and
4,5-di-O-caffeoylquinic acid, respectively (37). The detected fragments at 353, 191,
and 173 correspond to the loss of caffeoyl, two caffeoyl, and
caffeoyl + quinic groups, respectively (37). The peak with a retention time of
39.56 min was identified as luteolin due to the detection of an ion
fragment at m/z=133 (38).
Finally, the peaks with retention times of 44.61 and 65.22 min were
unidentified. Comparing with reports of Kenny et al
(36), 4-coumaric acid, ferulic
acid, Quercetin and luteolin had the same [M-H]- values and
corresponding ion fragments. 3,5-Di-O-caffeoylquinic acid and
4,5-di-O-caffeoylquinic acid had same [M-H]- values and similar
corresponding ion fragments compared with Dias et al
(37); quercetin pentoside had the
same [M-H]- value and corresponding ion fragment compare with Chen
et al (25). Thus, the
composition of sample I included 4-coumaric acid, ferulic acid,
quercetin pentoside, 3,5-di-O-caffeoylquinic acid,
4,5-di-O-caffeoylquinic acid, luteolin and two unknown compounds.
The relative percentages of peaks 1, 2, 3, 4, 5, 6, 7, and 8 were
found to be 11.45, 3.96, 10.48, 34.24, 3.91, 11.80, 3.65 and 4.21%,
respectively. Therefore, 3,5-di-O-caffeoylquinic acid was
recognized as the main ingredient in dandelion extract sample
I.
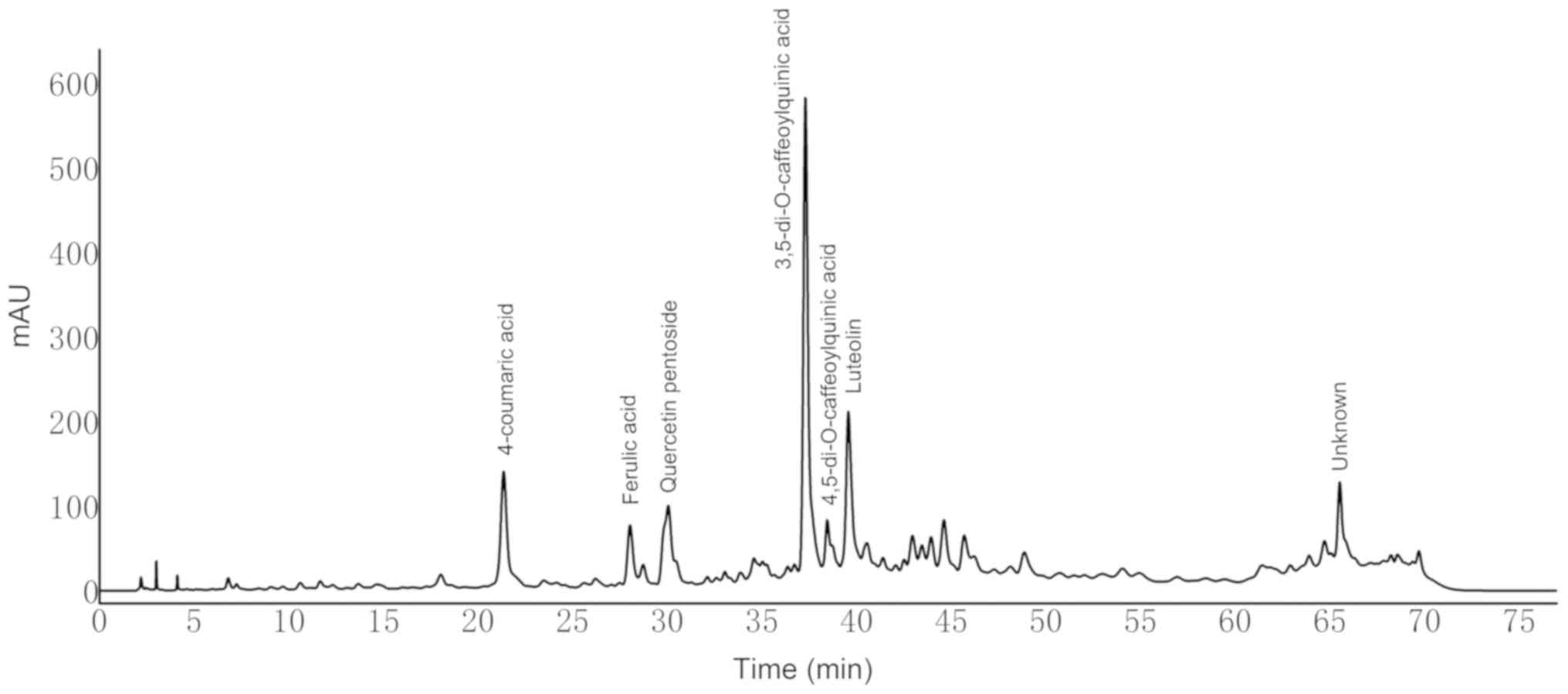 | Figure 3.High-performance liquid
chromatography-diode-array detector profiles of dandelion sample I
at 280 nm. Peak 1, 4-coumaric acid; 2, ferulic acid; 3, quercetin
pentoside; 4, 3,5-di-O-caffeoylquinic acid; 5,
4,5-di-O-caffeoylquinic acid; 6, luteolin; 7, unknown; 8,
unknown. |
 | Table I.Analysis of dandelion sample I by
high performance liquid chromatography-electrospray
ionization-tandem mass spectrometry. |
Table I.
Analysis of dandelion sample I by
high performance liquid chromatography-electrospray
ionization-tandem mass spectrometry.
| Author, year | Peak no. | Identity | Retention time
(min) | [M-H]−
(Online parent ion) | Fragment ions
(Online, MRM mode, daughter ion) | [M-H]−
(Reported parent ion) | Fragment ions
(Reported daughter ion) | Relative percentage
(%) | (Refs.) |
|---|
| Kenny et al,
2014 | 1 | 4-coumaric
acid | 21.35 | 163 | 119[M-H-CO2] | 163 | 119 | 11.45 | (36) |
| Kenny et al,
2014 | 2 | Ferulic acid | 28.03 | 193 | 134 | 193 | 134 | 3.96 | (36) |
| Chen et al,
2012 | 3 | Quercetin
pentoside | 30.05 | 433 |
301[M-H-pentose] | 433 | 301 | 10.48 | (25) |
| Dias et al,
2014 | 4 |
3,5-Di-O-caffeoylquinic acid | 37.29 | 515 | 353[M-H-caffeoyl],
191[M-H-2 caffeoyl], 173[M-H-caffeoyl- quinic], 163 | 515 | 353, 191, 179, 135,
173, 163 | 34.24 | (37) |
| Dias et al,
2014 | 5 |
4,5-Di-O-caffeoylquinic acid | 38.45 | 515 | 353[M-H-caffeoyl],
173[M-H-caffeoyl- quinic], 179 | 515 | 353, 173, 179, 191,
135 | 3.91 | (37) |
| Kenny et al,
2014 | 6 | Luteolin | 39.56 | 285 | 133 | 285 | 133 | 11.80 | (36) |
| Agregán et
al, 2017 | 7 | Unknown | 44.61 | 327 | – | 327 | 283 | 3.65 | (38) |
|
| 8 | Unknown | 65.22 | 297 | – | –a | –a | 4.21 |
|
MIC analysis
The results summarized in Table II revealed that the CA growth
inhibition capacity of dandelion sample I increases with increasing
concentration of the extract. The MIC was taken to be the lowest
sample group concentration beyond which changes in OD600 values
compared with the next sample group are less than or equal to 5%.
Therefore, the MIC of sample I was determined as 32.0 mg/ml. The
MIC of fluconazole was 0.60 mg/ml which was notably lower than the
MIC of sample I because sample I was a mixture and its purity low.
The low purity of samples I led to low activity.
 | Table II.Minimum inhibitory concentration of
sample I and fluconazole against CA. |
Table II.
Minimum inhibitory concentration of
sample I and fluconazole against CA.
|
| Negative control
group | Experimental
group | Positive control
group |
|---|
|
|
|
|
|
|---|
| Concentration
(mg/ml) | 0 | 4.00 | 8.00 | 16.00 | 32.00 | 64.00 | 128.00 | 0.60 |
|---|
| The growth of
CA |
| – | – | – | +a | + | + | + |
Kinetic analysis
Microbial growth curves include four phases
corresponding to a delay period, an exponential growth period, a
stable period, and a decay period. The CA mechanism of inhibition
of dandelion sample I was investigated by analyzing the exponential
phase. The kinetic equation of the CA exponential growth period is
expressed as (39):
r=dNdt=μN
N is the number of CA at time t, and µ is the growth
rate of CA.
Integration on both sides yields the following
equation:
∫N2N1dN/N=∫t2t1μdt
At constant specific growth rate of microorganisms,
the integrated form of kinetic equation given by equation (3), which can be rearranged to produce
equation (4), where Δt =
t2-t1.
In[N2N1]=μΔt
μ=In[N2N1]Δt
Antimicrobial kinetics refers to the kinetics of
antifungal activity as determined by plotting the inhibitory growth
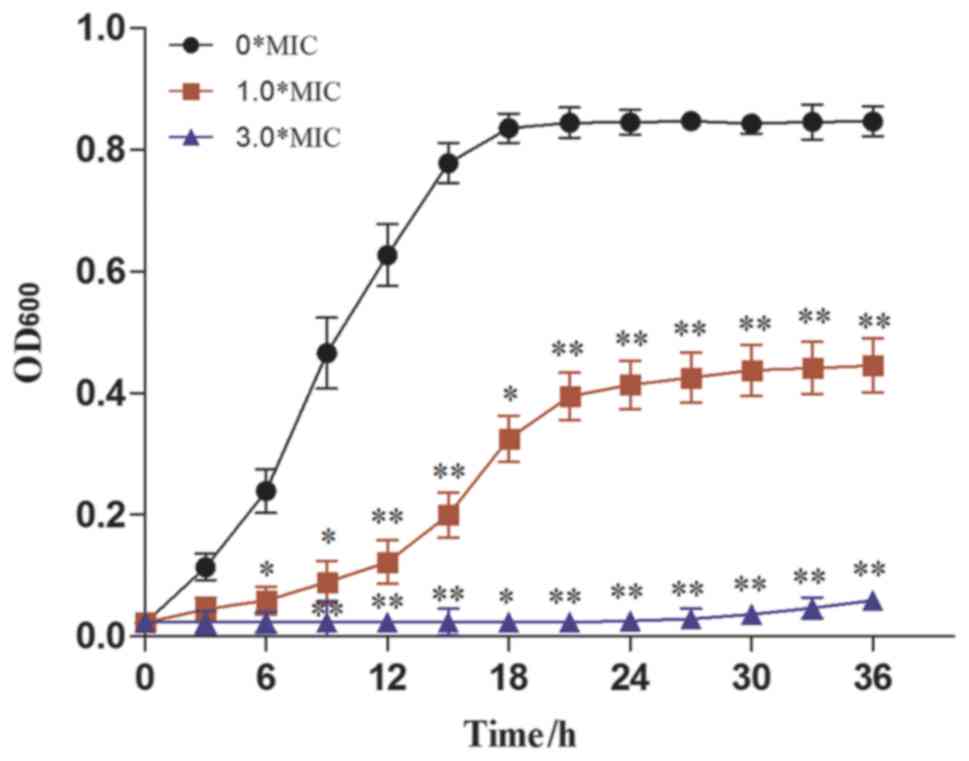
curve of CA. As presented in Fig.
4, the OD600nm of CA was significantly reduced upon the
addition of dandelion sample I at a concentration of 1.0X MIC. It
was further decreased when the concentration of the dandelion
extract was augmented to 3.0X MIC. This indicated that the
antifungal activity of sample I increases with increasing DAIS
concentration. In terms of values, an inhibitory rate of 97.13% was
recorded using a DAIS concentration of 3.0X MIC, with a CA growth
rate of only 0.00007 (Table
III). The obtained results indicate that dandelion sample I
inhibits CA proliferation mainly in the exponential phase,
resulting in greatly reduced CA growth rates. Such antifungal
activity depends on concentration and exposure time (31).
 | Table III.Inhibitory rate of sample I in
Candida albicans. |
Table III.
Inhibitory rate of sample I in
Candida albicans.
| Sample | Concentration MIC
(mg/ml) | OD600nm (3 h) | OD600nm (18 h) | Growth rate/µ | Antibacterial
rate/% (18 h) |
|---|
| Sample I | 0 (0) | 0.1143±0.0221 | 0.8352±0.0244 | 0.04806 | 0.00 |
|
| 1.0 (32.00) | 0.0451±0.0192 |
0.3252±0.0231a | 0.01867 | 61.06 |
|
| 3.0 (96.00) | 0.0228±0.0132 |
0.0239±0.0142a | 0.0007 | 97.13 |
Morphological observation by SEM
Presently, SEM has become necessary for the study of
cell morphology. The SEM micrographs of CA cells in the control
groups were shown in Fig. 5A-C. CA
cells exhibited an almost ellipsoid shape, with complete structure,
smooth surface, and notable refraction. The morphology of CA cells
was notably unchanged upon treatment with 0.5X MIC of sample I for
5 h; however, the surface became rough, and the refraction was
weakened (Fig. 5D-F). After
treatment with 1.0X MIC of sample I for 5 h, the CA cell structure
remained intact, but most cells were no longer ellipsoidal in
shape. In fact, the surfaces of many cells were found to be
wrinkled, and some of them appeared to be depressed (Fig. 5G-I). When the concentration of DAIS
was further increased to 2.0X MIC, all CA cells showed
non-ellipsoidal shape with relatively rough surfaces. The cells
also began to shrink, and their morphology was irregular. Some
cells showed marked compression, and damaged cell membranes of CA
appeared (Fig. 5J-L). These
results suggest that dandelion sample I is capable of destroying
the membrane of CA cells, changing their normal morphology,
shrinking their size, wrinkling their surface, and giving them an
asymmetrical structure (40). The
reason behind such changes may be attributed to significant leakage
in cell content.
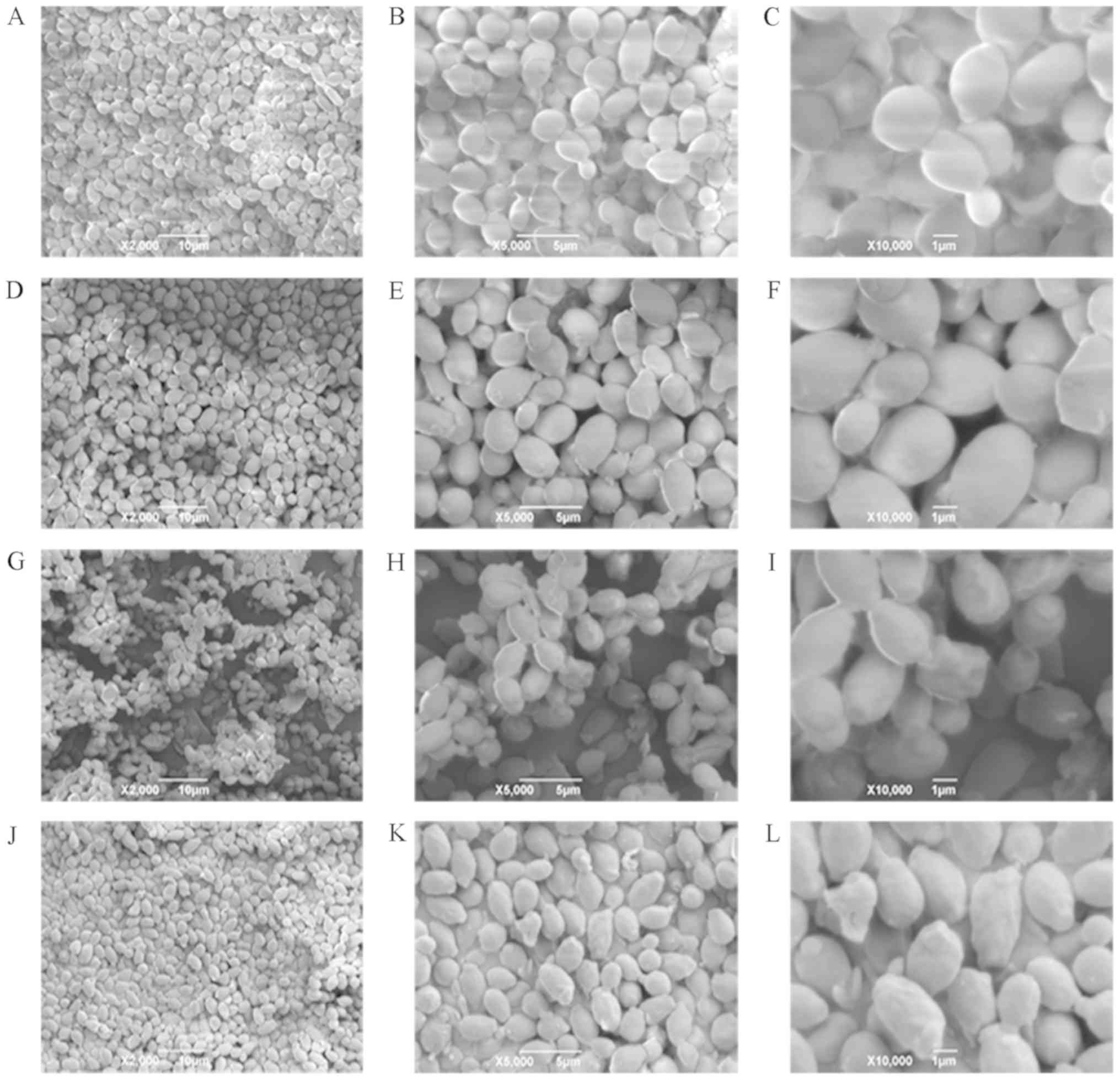 | Figure 5.Scanning electron microscopy images
of CA. (A-C) Images are of 2,000, 5,000, and 10,000 magnification;
CA cells were treated for 5 h with 0X MIC sample I, respectively.
(D-F) Images are of 2,000, 5,000, and 10,000 magnification; CA
cells were treated for 5 h with 0.5X MIC sample I, respectively.
(G-I) Images are of 2,000, 5,000, and 10,000 magnification; CA
cells were treated for 5 h with 1.0X MIC sample I, respectively.
(J-L) Images are of 2,000, 5,000, and 10,000 magnification; CA
cells were treated for 5 h with 2.0X MIC sample I, respectively.
CA, Candida albicans. |
Cell membrane permeability of CA
Under normal circumstances, intracellular
macromolecules cannot pass through the cell membrane. However, when
cells are adversely affected by unfavorable growth conditions or
antibacterial agents, their membranes become damaged, thereby
allowing UV-absorbing macromolecules, such as DNA and RNA, to leak
into the culture medium, leading to an increase in cell absorbance
at 260 nm (28). Thus, the
integrity of the cell membranes can be estimated based on cellular
absorbance at 260 nm.
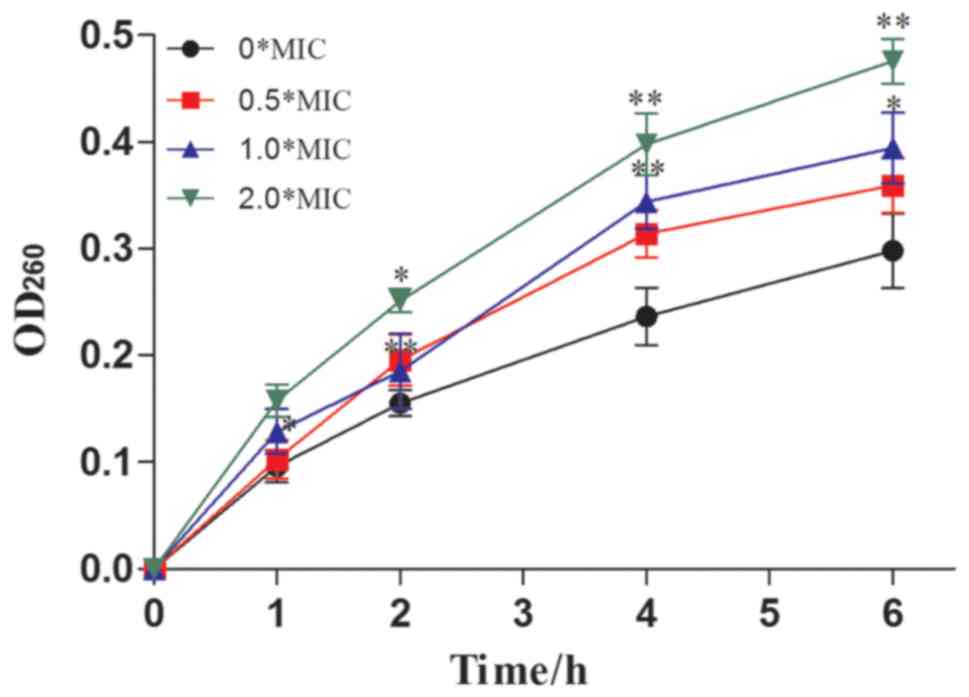
The UV absorbance values of the culture medium at a
wavelength of 260 nm were relatively low in the control group (0X
MIC) compared with the sample groups (0.5, 1.0, and 2.0X MIC sample
I-treated CA) (Fig. 6). After
incubation for 1 h, the OD260nm values of the 0, 0.5, 1.0 and 2.0 X
MIC supernatants were found to be 0.0926±0.0252, 0.1027±0.0183,
0.1286±0.0214, and 0.1579±0.0152, respectively. These values
increased to 0.2982±0.0333, 0.3594±0.0165, 0.3942±0.0231, and
0.4756±0.0216, respectively, after 6 h of incubation. With
increases in treatment time, the absorbance of the culture medium
at 260 nm was gradually increased. These results indicated that
dandelion sample I could be capable of destroying the cell
membranes of CA, resulting in extensive nucleic acid leakage and
ultimately, the inhibition of growth (41).
Effects of DAIS on CA cell wall
β-(1–3)-D glucan synthesis
β-(1–3)-D glucan is the main component of the
CA cell wall (42), and structural
abnormalities in this component may lead to cell wall rupture.
Therefore, the integrity of the CA cell wall was investigated by
assessing the cellular content of β-(1–3)-D-glucan using infrared spectroscopy.
The results indicated β-(1–3)-D-glucan
contents of 22.13 and 20.86% for the 0 and 0.5X MIC groups,
respectively (data not shown). The difference between the two
groups is insignificant, which implies that sample I has no
significant effect on the concentration of β-(1–3)-D-glucan in the CA wall.
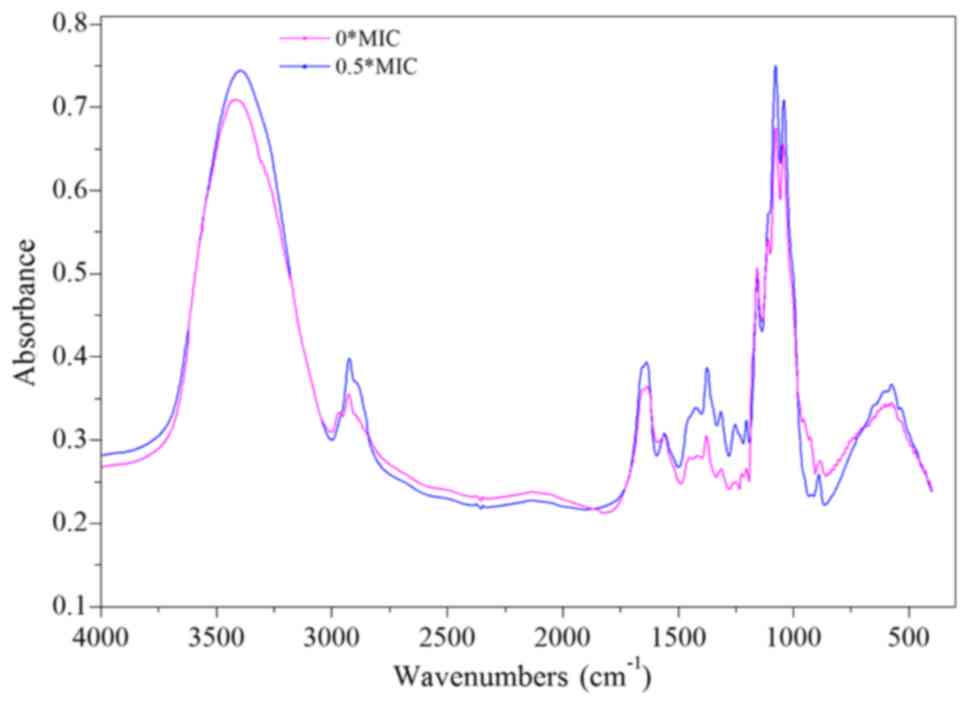
The infrared spectrum of β-(1–3)-D-glucan comprises a peak between 3,000
and 2,800 cm−1 corresponding to the ‘C-H’ stretching
vibration (Fig. 7). A wide peak
between 800 and 400 cm−1 corresponds to the ‘-CH2-’
moieties. The peaks at 2,920, 1,370, 1,260, and 1,250
cm−1 characterize the vibrations of polysaccharides,
whereas the peak at 1650 cm−1 peak is related to the
‘C=O’ vibration. Finally, the absorption peak at 890
cm−1 is indicative of the presence of a β-configuration
polysaccharide glycosidic bond.
Compared with the control group, the ‘C-H’, ‘C=O’,
and ‘-CH2-’ absorption peaks were all increased in the 0.5X MIC
group. Meanwhile, the absorption peak of the β-configuration
polysaccharide glycosidic bond is decreased. However, no
significant change was noted in the other absorption peaks. These
results indicate that dandelion sample I altered the structure of
β-(1–3)-D glucan in CA cell walls, possibly via
the partial breaking of the glycosidic bond to form more ‘C=O’ and
‘-CH2-’ groups (Fig. 7). Another
plausible explanation could be that after treatment with sample I,
glycosidic bond synthesis was inhibited (42), resulting in the formation of
dextran molecules with an incomplete structure. The reduced number
of glycosidic bonds inhibits the cross-linking of dextran,
ultimately affecting the synthesis of CA cell walls. Abnormal cell
wall structure leads to fragile cells that are easily ruptured
(43,44).
Discussion
According to the literature, dandelion crude extract
has notable bacteriostatic activity and is effective against a
variety of bacteria, including Bacillus subtilis, Staphylococcus
aureus (45), Escherichia coli,
Pseudomonas aeruginosa (46),
methicillin-resistant Staphylococcus aureus, Bacillus cereus
(47), Vibrio harveyi (48), Pseudomonas aeruginosa, Paracoccus
bacillus (49), and other
microorganisms.
Studies of the fungistatic activity in CA have
revealed that the mechanism involves alterations in the gene
encoding of the target enzyme lanosterol 14-α demethylase or
overexpression of the efflux pump genes containing cerebellar
degeneration related protein (CDR)1, CDR2, and multidrug resistance
mutation 1 (50). Ding et
al (51) reported that the
molecular mechanism underlying the ATB-induced apoptosis of CA
cells is based on the inhibition of tubulin polymerization, leading
to G2/M phase cell cycle arrest. CA also has a strong effect on
mycelial development and cell membrane morphology, properties that
are related to abnormal actin skeleton and subsequent translational
defects of hyphae-associated factors (52). The 20-polymer peptide Ib-AMP1
exhibits a specific fungistatic effect on CA by inhibiting
different cellular processes rather than cell membrane ion channels
or pores (53). Caspofungin
hinders the growth of CA by restricting the synthesis of CA cell
wall β-glucan (42).
Overall, existing research shows that the mechanism
of antifungal activity in CA is very complex, and more extensive
and comprehensive investigations are needed to properly elucidate
this mechanism. At present, to the best of our knowledge, no
studies have evaluated the antifungal activity of DAIS against
CA.
In this study, the active ingredients in dandelion
were extracted, isolated, and analyzed. The results demonstrated
that among six different dandelion extracts, only sample I
exhibited marked antifungal activity against CA, with an inhibition
zone diameter of 10.38 mm recorded at sample I concentration of
53.2 mg/ml. HPLC-ESI-MS/MS analysis revealed that the main chemical
components in sample I are 4-coumaric acid, ferulic acid, quercetin
pentoside, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic
acid, luteolin, and two unknown components, with relative
percentages of 11.45, 3.96, 10.48, 34.24, 3.91, 11.80, 3.65 and
4.21%, respectively. Therefore, 3,5-di-O-caffeoylquinic acid is the
most abundant component.
The results of preliminary antifungal activity
analyses show that the concentration of dandelion sample I is
significantly correlated with the growth inhibition effect of CA.
Comparing the absorbances of different concentrations of sample
group, the MIC of DAIS was identified as 32.0 mg/ml. Kinetically,
it was found that dandelion sample I mainly inhibits CA growth in
the exponential phase. Furthermore, SEM images showed that DAIS are
capable of damaging the membranes of CA cells, leading to cell
surface depression, wrinkling, increased cell membrane
permeability, macromolecule leakage, and ultimately, disordered
cell metabolism. This suggests that inhibition of CA growth by DAIS
may be related to the damaging effect of the latter on the cell
membranes of the former. In addition, infrared spectrometry
indicated that sample I destroy the glycosidic bond of β-(1–3)-D-glucan in CA cell walls, thereby
changing its structure. In summary, dandelion sample I was proposed
to increase the permeability of CA by destroying its cell wall and
membrane, ultimately delaying cellular growth or leading to cell
death. Sample I was reported to include eight chemical components;
comparing the antifungal activity of these identified constituents
with previous literature, these identified compounds did not have
antifungal activities against CA. Thus, these two unknown compounds
could exert antifungal activities against CA or there may be a
synergistic effect between these unknown compounds with these
identified compound; further investigation is required.
In the present study, we reported that dandelion
sample I, composed of 4-coumaric acid, ferulic acid, quercetin
pentoside, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic
acid, luteolin, and two unknown components, exhibits good
antifungal activity against CA. Our findings provide a basis for
novel approaches to screen for anti-VVC drugs. However, further
investigation is needed to determine the antifungal activities of
the individual components, as well as the synergistic effects.
Acknowledgements
The authors would like to thank Professor Zhang
Xiaoying (College of Veterinary Medicine, Northwest A&F
University, Yangling, Shaanxi, China) for assisting with the
preparation of this manuscript.
Funding
The present study was supported by the Project of
Shaanxi Province Key Laboratory of Bio-Resources at the Shaanxi
Provincial Department of Science and Technology (grant no.
SZS-15-03), the Qinba Mountain Biological Resources Comprehensive
Development Collaborative Innovation Center Project, China [grant
no. QBXT-Z (Y)-15-2], and the education department of Shaanxi
province, China (grant no. 18JS018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL was responsible for the design of this research.
KL, HD, PZ, HH, FG, YL and ZX acquired and analyzed the data. ZX
and HD drafted the manuscript. KL and HD wrote and revised the
paper. All authors agreed with the final revision.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CA
|
Candida albicans
|
|
VVC
|
vulvovaginal candidiasis
|
|
DAIS
|
dandelion active ingredients
|
|
LC-DAD-ESI-MS/MS
|
high performance liquid
chromatography-diode-array detector-tandem mass spectrometry
|
|
MIC
|
minimum inhibitory concentration
|
|
SEM
|
scanning electron microscopy
|
|
OD
|
optical density
|
References
|
1
|
Stockdale CK: Clinical spectrum of
desquamative inflammatory vaginitis. Curr Infect Dis Rep.
12:479–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theodoropoulos DS, Stockdale CK, Duquette
DR and Morris MS: Inhalant allergy compounding the chronic
vaginitis syndrome: Characterization of sensitization patterns,
comorbidities and responses to sublingual immunotherapy. Arch
Gynecol Obstet. 294:541–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guaschino S and Benvenuti C; SOPHY Study
Group, : SOPHY project: An observational study of vaginal pH,
lifestyle and correct intimate hygiene in women of different ages
and in different physiopathological conditions. Part II. Minerva
Ginecol. 60:353–362. 2008.(In English, Italian). PubMed/NCBI
|
|
4
|
Minooeianhaghighi MH, Sepehrian L and
Shokri H: Antifungal effects of Lavandula binaludensis and Cuminum
cyminum essential oils against Candida albicans strains isolated
from patients with recurrent vulvovaginal candidiasis. J Mycol Med.
27:65–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang S, Zhang Y, Liu Y, Wang J, Chen S and
Li S: Clinical significance and characteristic clinical differences
of cytolytic vaginosis in recurrent vulvovaginitis. Gynecol Obstet
Invest. 82:137–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YW, Li YL, Fang Q, Yang ZX and Bing
AY: Advances in the study of treatment of candidiasis with
traditional Chinese medicine. J Pathogen Biol. 29:620–622.
2009.
|
|
7
|
Wang PB, Peng C, Tang ZW, Wan F, Dai M and
Cao XY: A study on the effect of Patchouli essential oil against
mice model of candidal vaginitis. Lishizhen Med Materia Medica Res.
35:592–594. 2014.(In Chinese).
|
|
8
|
Zhang XF and Zhang CY: Treatment of
recurrent candidal vaginosis with Pulsatilla decoction. Chin J Exp
Traditional Med Formulae. 18:279–281. 2012.(In Chinese).
|
|
9
|
He W, Han H, Wang W and Gao B:
Anti-influenza virus effect of aqueous extracts rom Dandelion.
Virol J. 8:5382011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evensen NA and Braun PC: The effects of
tea polyphenols on Candida albicans: Inhibition of biofilm
formation and proteasome inactivation. Can J Microbiol.
55:1033–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gavanji S and Larki B: Comparative effect
of propolis of honey bee and some herbal extracts on Candida
albicans. Chin J Integr Med. 23:201–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Estevinho ML, Afonso SE and Feás X:
Antifungal effect of lavender honey against Candida albicans,
Candida krusei and Cryptococcus neoformans. J Food Sci Technol.
48:640–643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Li XD, Yang LX and Jiang H:
Experimental research of single herb medicine in vitro
antibacterial activity. Chin J Exp Traditional Med Formulae.
17:283–286. 2011.(In Chinese).
|
|
14
|
Shi GX, Yan YY, Shao J, Zhang MX, Lu KQ,
Wang TM and Wang CZ: Effect of andrographolide derivative Yanhuning
on in vivo Candida albicans biofilms in rats. Zhongguo Zhong Yao Za
Zhi. 39:2924–2929. 2014.(In Chinese). PubMed/NCBI
|
|
15
|
Zhang GQ, Feng WR and Mi S: Therapeutic
effects of ethanol extracts from Euphorbia humifusa on monilial
vaginitis in rats. Chin J Exp Traditional Med Formulae. 18:191–194.
2012.(In Chinese).
|
|
16
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China. China Medical
Science and Technology Press; I. pp. 3522015
|
|
17
|
Kim JJ, Park CM, Kim MJ, Cho CW and Song
YS: Hypolipidemic effect of dandelion (Taraxacum officinale)
extracts via fecal lipid excretion in C57BL/6 mice fed an
atherogenic diet. Food Sci Biotechnol. 23:841–847. 2014. View Article : Google Scholar
|
|
18
|
Tahir K, Nazir S, Ahmad A, Li B, Khan AU,
Khan ZU, Khan FU, Khan QU, Khan A and Rahman AU: Facile and green
synthesis of phytochemicals capped platinum nanoparticles and in
vitro their superior antibacterial activity. J Photochem Photobiol
B. 166:246–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song YM, Li DG, Zhang Y, Zhang WG, Jin YP,
Zhou L and Tang JN: The bacteriostasis of the extracts from herba
taraxaci on bacteria derived from bovine hidden mastitis. Acta
Agriculturae Boreali Occidentalis Sinica. 15:55–57. 2006.(In
Chinese).
|
|
20
|
Hu G, Wang J, Hong D, Zhang T, Duan H, Mu
X and Yang Z: Effects of aqueous extracts of taraxacum officinale
on expression of tumor necrosis factor-alpha and intracellsular
adhesion molecule 1 in LPS-stimulated RMMVECs. BMC Complement
Altern Med. 17:382017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lans C, Turner N, Khan T, Brauer G and
Boepple W: Ethnoveterinary medicines used for ruminants in British
Columbia, Canada. J Ethnobiol Ethnomed. 3:112007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li XH, He XR, Zhou YY, Zhao HY, Zheng WX,
Jiang ST, Zhou Q, Li PP and Han SY: Taraxacum mongolicum extract
induced endoplasmic reticulum stress associated-apoptosis in
triple-negative breast cancer cells. J Ethnopharmacol. 206:55–64.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan HB and Liang YK: Extraction and
antioxidant and antibacterial activity of dandelion sample I. China
Food Additives. 27:80–86. 2017.(In Chinese).
|
|
24
|
Andrade JC, Morais Braga MF, Guedes GM,
Tintino SR, Freitas MA, Quintans LJ Jr, Menezes IR and Coutinho HD:
Menadione (vitamin K) enhances the antibiotic activity of drugs by
cells membrane permeabilization mechanism. Saudi J Biol Sci.
24:59–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen HJ, Inbaraj BS and Chen BH:
Determination of phenolic acids and flavonoids in Taraxacum
formosanum Kitam by liquid chromatography-tandem mass spectrometry
coupled with a post-column derivatization technique. Int J Mol Sci.
13:260–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szczepaniak J, Cieślik W, Romanowicz A,
Musioł R and Krasowska A: Blocking and dislocation of Candida
albicans Cdr1p transporter by styrylquinolines. Int J Antimicrob
Agents. 50:171–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rad HI, Arzanlou M, Omid MR, Ravaji S and
Doghaheh HP: Effect of culture media on chemical stability and
antibacterial activity of allicin. J Functional Foods. 28:321–325.
2017. View Article : Google Scholar
|
|
28
|
Li JL, Yang XH, Wang TF, Hao ZC, Dai K and
Wang RM: Studies on antibacterial mechanism of 10-HDA against
Staphylococcus aureus. J Chin Institute Food Sci Technol. 14:73–79.
2014.
|
|
29
|
Wang HL, Hao LL, Wang P, Chen MM, Jiang SW
and Jiang ST: Release kinetics and antibacterial activity of
curcumin loaded zein fibers. Food Hydrocolloids. 63:437–446. 2017.
View Article : Google Scholar
|
|
30
|
Moghayedi M, Goharshadi EK, Ghazvini K,
Ahmadzadeh H, Ranjbaran L, Masoudi R and Ludwig R: Kinetics and
mechanism of antibacterial activity and cytotoxicity of Ag-RGO
nanocomposite. Colloids Surf B Biointerfaces. 159:366–374. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen B, Fan DQ, Zhu KX, Shan ZG, Chen FY,
Hou L, Cai L and Wang KJ: Mechanism study on a new antimicrobial
peptide Sphistin derived from the N-terminus of crab histone H2A
identified in haemolymphs of Scylla paramamosain. Fish Shellfish
Immunol. 47:833–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Memariani H, Shahbazzadeh D, Sabatier JM,
Memariani M, Karbalaeimahdi A and Bagheri KP: Mechanism of action
and in vitro activity of short hybrid antimicrobial peptide PV3
against Pseudomonas aeruginosa. Biochem Biophys Res Commun.
479:103–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yi L, Dang J, Zhang L, Wu Y, Liu B and Lü
X: Purification, characterization and bactericidal mechanism of a
broad spectrum bacteriocin with antimicrobial activity against
multidrug-resistant strains produced by Lactobacillus coryniformis
XN8. Food Control. 67:53–62. 2016. View Article : Google Scholar
|
|
34
|
Ma Q, Davidson PM, Critzer F and Zhong Q:
Antimicrobial activities of lauric arginate and cinnamon oil
combination against foodborne pathogens: Improvement by
ethylenediaminetetraacetate and possible mechanisms. LWT-Food Sci
Technol. 72:9–18. 2016. View Article : Google Scholar
|
|
35
|
Tang QL, Huang GL, Zhao FY, Zhou L, Huang
SQ and Li H: The antioxidant activities of six (1→3)-â-D-glucan
derivatives prepared from yeast cells wall. Int J Biol Macromol.
98:216–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kenny O, Smyth TJ, Walsh D, Kelleher CT,
Hewage CM and Brunton NP: Investigating the potential of
under-utilised plants from the Asteraceae family as a source of
natural antimicrobial and antioxidant extracts. Food Chem.
161:79–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dias MI, Barros L, Alves RC, Oliveira MB,
Santos-Buelga C and Ferreira IC: Nutritional composition,
antioxidant activity and phenolic compounds of wild Taraxacum sect.
Ruderalia. Food Res Int. 56:266–271. 2014. View Article : Google Scholar
|
|
38
|
Agregán R, Munekata PES, Franco D,
Dominguez R, Carballo JM and Lorenzo JM: Phenolic compounds from
three brown seaweed species using LC-DAD-ESI-MS/MS. Food Res Int.
99:979–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie CL, Tang HK, Song ZH, Qu SS, Liao YT
and Liu HS: Microcalorimetric study of bacterial growth.
Thermochimica Acta. 123:33–41. 1988. View Article : Google Scholar
|
|
40
|
Sun D, Zhang W, Lv M, Yang E, Zhao Q and
Wang W: Antibacterial activity of ruthenium(II) polypyridyl complex
manipulated by membrane permeability and cells morphology. Bioor
Med Chem Lett. 25:2068–2073. 2015. View Article : Google Scholar
|
|
41
|
Ajiboye TO, Mohammed AO, Bello SA, Yusuf
II, Ibitoye OB, Muritala HF and Onajobi IB: Antibacterial activity
of Syzygium aromaticum seed: Studies on oxidative stress biomarkers
and membrane permeability. Microb Pathog. 95:208–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deresinski SC and Stevens DA: Caspofungin.
Clin Infect Dis. 36:1445–1157. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Georgopapadakou NH: Update on antifungals
targeted to the cells wall: Focus on beta-1,3-glucan synthase
inhibitors. Expert Opin Investig Drugs. 10:269–280. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meng S, Hu SB, Xie WA, Ding XZ, Sun YJ and
Xia LQ: Antifungal effects and mechanism of bioactive components of
Allinm chinense on Candida albicans. Food Sci. 262005.(In
Chinese).
|
|
45
|
Qian L, Zhou Y, Teng Z, Du CL and Tian C:
Preparation and antibacterial activity of oligosaccharides derived
from dandelion. Int J Biol Macromol. 64:392–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao DA: Analysis of nutritional components
of taraxacum mongolicum and its antibacterial activity.
Pharmacognosy J. 2:502–505. 2010. View Article : Google Scholar
|
|
47
|
Kenny O, Brunton NP, Walsh D, Hewage CM,
McLoughlin P and Smyth TJ: Characterisation of antimicrobial
extracts from dandelion root (Taraxacum officinale) using
LC-SPE-NMR. Phytother Res. 29:526–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan X, Sun Z, Chen S, Chen S, Huang Z,
Zhou C, Zou C, Liu Q, Ye H, Lin H, et al: Effects of dietary
dandelion extracts on growth performance, body composition, plasma
biochemical parameters, immune responses and disease resistance of
juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol.
66:198–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li LS, Shi WJ, Guan Ming, Li XJ, Fang T
and Zheng JL: Study on active component and bacteriostasis of
tetraploid plant of herba Taraxaci. Chin J Exp Traditional Med
Formulae. 14:55–58. 2008.(In Chinese).
|
|
50
|
White TC, Marr KA and Bowden RA: Clinical,
cellsular, and molecular factors that contribute to antifungal drug
resistance. Clin Microbiol Rev. 11:382–402. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ding Y, Li Y, Li Z, Zhang J, Lu C, Wang H,
Shen Y and Du L: Alteramide B is a microtubule antagonist of
inhibiting Candida albicans. Biochim Biophys Acta. 1860:2097–2106.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu QL, Zhang B, Ma F, Jia C, Xiao C, Zhang
B and Li M: Novel mechanisms of surfactants against Candida
albicans growth and morphogenesis. Chem Biol Interact. 227:1–6.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee DG, Shin SY, Kim DH, Seo MY, Kang JH,
Lee Y and Hahm KS: Antifungal mechanism of a cysteine-rich
antimicrobial peptide, Ib-AMP1, from Impatiens balsamina against
Candida albicans. Biotechnol Lett. 21:1047–1050. 1999. View Article : Google Scholar
|