Introduction
The hippocampus, a part of the limbic system, has
been known to be one of the most vulnerable regions in the brain
following transient global cerebral ischemia (tgCI) (1). Especially, tgCI leads to selective
neuronal death at a few days, which is called ‘delayed neuronal
death, after a brief tgCI in the CA1 subfield among the four
subfields (CA1-CA4) of the hippocampus proper, while the CA3
subfield is known as the most tolerant hippocampal subregion
against tgCI (2,3). Until now, many studies have made an
effort to explain the pathophysiology of tgCI-induced neuronal
death/damage in the CA1 subfield (4–6);
however, the precise underlying mechanisms of tgCI-induced
selective and delayed neuronal death in the CA1 subfield have not
been fully understood.
Nuclear receptor related 1 protein (Nurr1), also
known as NR4A2, is a member of nuclear receptor 4 family of orphan
nuclear receptors, and Nurr1 has been studied to be expressed in
various types of neurons and glia in the central nervous system
(7–11). The Nurr1 function has been known as
a transcriptional activator of endogenous tyrosine hydroxylase in
neural progenitor cells and participates in the development and
maintenance of dopaminergic neurons in the mesencephalon (12–15).
In the hippocampus, Nurr1 is expressed in pyramidal neurons in the
stratum pyramidale of the hippocampus proper (CA1-CA3 subfields)
(16).
Recent studies have suggested that Nurr1 in the
hippocampus proper plays a major role in cognitive function and
stress (10,17,18).
Additionally, it has been demonstrated that Nurr1 can display
neuroprotective and anti-inflammatory properties (9,19–22).
Also, some studies have reported that Nurr1 expression is altered
in rodent brains following cerebral ischemia (23–27).
However, the expressional changes of Nurr1 in the hippocampus
proper after tgCI have not been fully elucidated. In the present
study, thus, we investigated the time-dependent changes of Nurr1
expression in the hippocampus proper after 5 min of tgCI in
gerbils, which are an excellent animal model of tgCI (5,28).
Materials and methods
Experimental animals
Six-month old male Mongolian gerbils (body weight,
70–80 g) were obtained from the Experimental Animal Center, Kangwon
National University, Chuncheon, Republic of Korea. Animal care and
all experimental procedures were approved by Institutional Animal
Care and Use Committee at Kangwon National University (approval no.
KW-180124-1). We minimized numbers of the gerbils used in this
study and the suffering caused by the procedures used in this
experiment.
Induction of tgCI
As described in our published papers (29–32),
the surgical procedure for tgCI was as follows. Briefly, gerbils
(total n=103; 63 gerbils for histological analysis and 40 gerbils
for western blot analysis) were anesthetized with a mixture of 2.5%
isoflurane in 34% oxygen (O2) and 66% nitrous oxide
(N2O). Blood flow to the brain was completely
interrupted by bilateral common carotid artery occlusion (BCCAO)
for 5 min by using aneurysm clips in order to confirm stop of blood
flow in the central artery in retinae under an ophthalmoscope. Body
temperature was controlled under normothermic (37±0.5°C) condition
with a rectal temperature probe: Normothermia was controlled with a
thermometric blanket before and during the surgery. Sham operated
gerbils were subjected to the same procedure without BCCAO.
Preparation of histological
sections
Brain sections containing the hippocampus were
prepared from sham and ischemia operated gerbils (n=7 at each point
in time) at designated times (6, 12 h, 1, 2, 3, 4, 7 and 10 days
after tgCI). Brain sections from the sham operated gerbils (n=7)
were prepared at 4 days after tgCI. As described in our published
papers (29–32), the gerbils were intraperitoneally
anaesthetized with sodium pentobarbital (60 mg/kg) (JW Pharm. Co.,
Ltd.) and perfused transcardially with 0.1 M phosphate-buffered
saline (PBS) followed by 4% paraformaldehyde. The brains were
removed, postfixed with the same fixative for 8 h and cryoprotected
by soaking in a 30% sucrose solution for 12 h. The brain tissues
were serially sectioned into 30-µm thickness of coronal sections in
a cryostat (Leica).
Examination of delayed neuronal
death
To examine tgCI-induced delayed neuronal death in
the hippocampus proper, CV staining and Fluoro-Jade B (FJB)
histofluorescence staining were performed by methods as we
described previously (29,30). In brief, the sections were stained
with solution of CV acetate (Sigma), which was dissolved at 1.0%
(w/v) and contained 0.28% glacial acetic acid, for 2 min. And the
stained sections were washed and dehydrated in ethanol series. For
FJB histofluorescence, the sections were serially stained with a 1%
sodium hydroxide solution, a 0.06% potassium permanganate solution
and a 0.0004% FJB (Histochem) solution. The reacted sections were
examined using an epifluorescent microscope (Carl Zeiss) equipped
with a blue excitation light (450–490 nm) and a barrier filter.
Immunohistochemistry
As we described in our published papers (29–32),
immunohistochemical staining for Nurr1 was performed as follows. In
brief, the brain sections were incubated with mouse anti-Nurr1
(1:100, Thermo Fisher Scientific, Inc.) as primary antibody and
exposed to biotinylated goat anti-mouse IgG and streptavidin
peroxidase complex (Vector) as secondary antibody. Finally, the
reacted sections were visualized with 3,3′-diaminobenzidine (in 0.1
M Tris-HCl buffer, pH 7.4).
In order to establish the specificity of Nurr1
immunoreaction, negative control test was done by using pre-immune
serum instead of the primary antibody. The negative control showed
no immunoreactivity in the immunostained tissues.
For the quantitative analysis of Nurr1
immunoreactivity, 6 sections were selected with 120-µm interval per
animal, and digital images of Nurr1 immunoreactivity were captured
with Axio Imager 2 microscope (Carl Zeiss) equipped with a digital
camera (Axiocam; Carl Zeiss). According to our published method
(31), the images were calibrated
into an array of 512×512 pixels corresponding to a tissue area of
140×140 µm including the stratum pyramidale of the sham and
ischemia operated gerbils. The density of all Nurr1-immunoreactive
structures was evaluated as relative optical density (ROD) as
follows. Optical density (RO) of all the Nurr1-immunoreactive
structures were obtained after the transformation of the mean gray
level using the formula: OD=log (256/mean gray level), and the OD
of background was taken from the areas adjacent to the measured
area. Finally, a ratio of the OD was calibrated as % (ROD) by using
NIH Image 1.59 software.
Double immunofluorescence
staining
To examine the cell type containing Nurr1
immunoreactivity, double immunofluorescence staining was performed
after tgCI according to our published protocol (30,31).
In brief, we used mouse anti-Nurr1 (1:40, Thermo Fisher Scientific,
Inc.)/rabbit anti-NeuN (a marker for neurons; 1:800, Millipore),
rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1, a
marker for microglia; 1:100, Wako) or rabbit anti-glial fibrillary
acidic protein (GFAP, a marker for astrocytes; 1:200, Millipore).
The sections were incubated in the mixture of antisera and reacted
in a mixture of Cy3-conjugated goat anti-mouse IgG (1:200; Jackson
ImmunoResearch, West Grove, PA, USA) and FITC-conjugated goat
anti-rabbit IgG (1:200; Jackson ImmunoResearch). The
immunoreactions were observed under a confocal MS (LSM510 META NLO,
Carl Zeiss).
Western blot analysis
Nurr1 protein level in the CA1 subfield were
analyzed at designated times (6, 12 h, 1, 2, 4, 7 and 10 days after
tgCI) using western blot method according to our published
procedure (29,30). In brief, 5 animals at each point in
time were intraperitoneally anaesthetized with sodium pentobarbital
(60 mg/kg), and their brains were removed. The brains were serially
and transversely cut into 400-µm thickness using a vibratome
(Leica). Hippocampal CA1 fields were cut by using a surgical blade
and homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene
glycol bis (2-aminoethyl ether)-N,N,N0,N0 tetraacetic acid (pH
8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH
8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM
NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM
phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT). The
homogenized tissues were centrifugated at 16,000 × g for 25 min,
and protein levels were determined using a Micro BCA protein assay
kit (Pierce Chemical). Aliquot containing total protein (20 mg) was
boiled in loading buffer containing 150 mM Tris-HCI (pH 6.8), 6%
SDS, 3 mM DTT, 0.3% bromophenol blue and 30% glycerol and loaded
onto 10% polyacrylamide gel. The gel was transferred to
nitrocellulose transfer membranes (Pall Crop.) after
electrophoresis. The background of the membrane was reduced with 5%
nonfat dry milk in PBS containing 0.1% Tween 20 for 40 min, and the
membrane was reacted with mouse anti-Nurr1 (1:200; Thermo Fisher
Scientific, Inc.), peroxidase-conjugated goat anti-mouse IgG and
ECL kit. The loading control was carried out using mouse
anti-β-actin (1:5,000) antibody. Western blot analysis using
homogenates at all experimental time-points was performed
simultaneously. Results of the western blotting were scanned, and
the quantification of the bands was densitometrically analyzed
using NIH Image 1.59 software. The quantification was represented
by relative optical density (ROD). A ratio of the ROD was
calibrated as %: The sham operated group was designated as
100%.
Statistical analysis
The data shown here represent the means ± SEM.
Differences of the means among the groups were statistically
analyzed by analysis of variance (ANOVA) with Duncan's post hoc
test in order to elucidate ischemia-related differences among
experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
tgCI-induced delayed neuronal
death
tgCI-induced delayed neuronal death in the
hippocampus proper (CA1-3 subfields) was examined at 2 days and 4
days after tgCI using CV staining and FJB histofluorescence
staining. In the sham group, cells in the CA1-3 subfields were
easily stained with CV. In particular, pyramidal neurons of the
stratum pyramidale of the CA1-3 subfields were large and pyramidal
in shape (Fig. 1A, D and J). In
the ischemia group, no difference in CV staining in the hippocampus
proper was found at 2 days after tgCI, compared to the sham group
(Fig. 1B, E and K). On the other
hand, most of CV-positive pyramidal neurons in the stratum
pyramidale of the CA1 subfield were dead at 4 days after tgCI
(Fig. 1C and F). At this point of
time, many FJB positive cells were observed in the stratum
pyramidale of the CA1 subfield (Fig.
1I). However, CV-positive pyramidal neurons in the CA2/3
subfield were not damaged at 4 days after tgCI (Fig. 1C and L). In addition, any FJB
positive cells were not found in the CA2/3 subfield (data not
shown). This result is consistent with the results of our previous
studies (29–31).
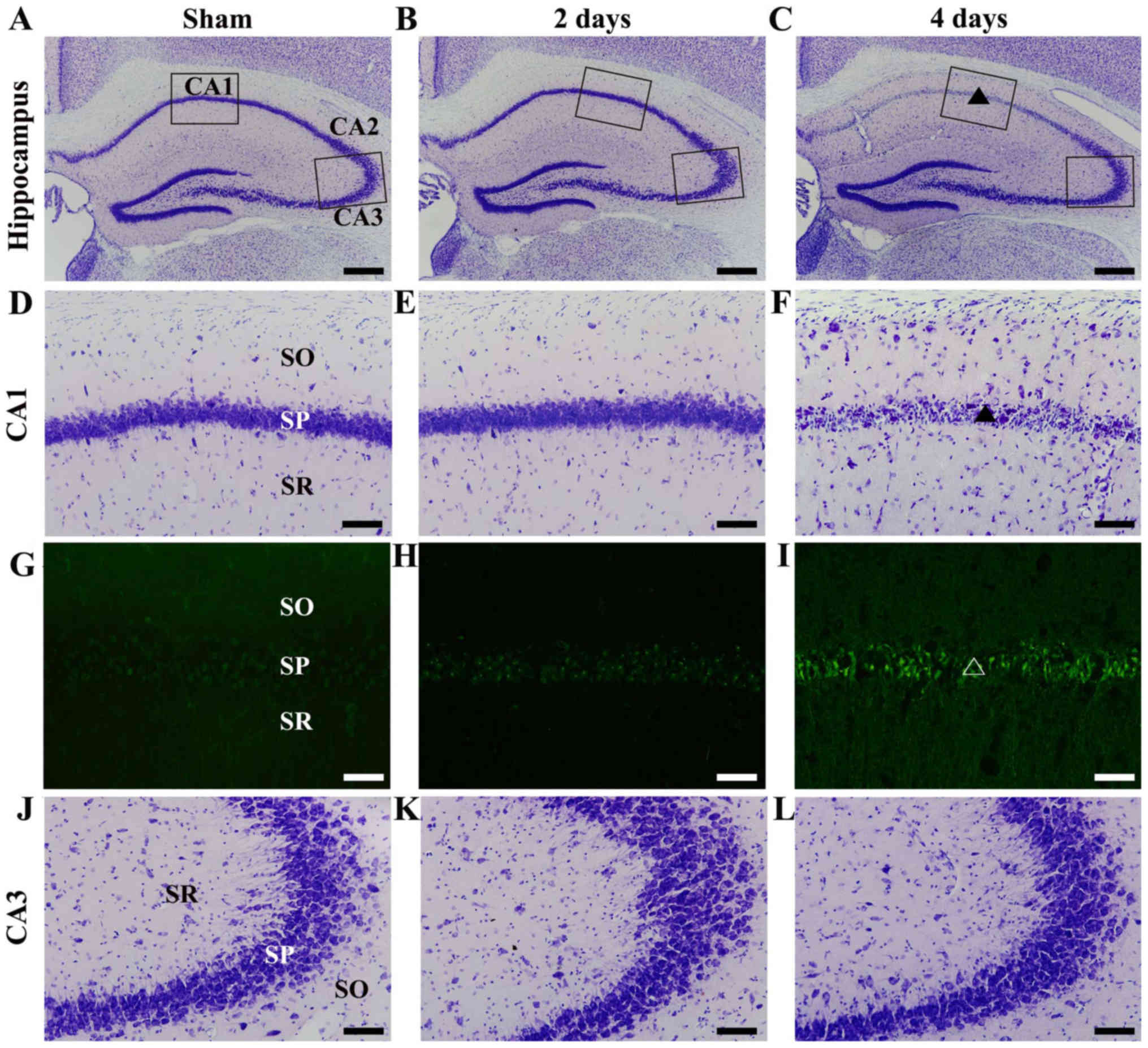 | Figure 1.CV and FJB histofluorescence staining
in the gerbil hippocampus. (A-C) Low magnification image of CV
staining in the gerbil hippocampus of the (A) sham and (B and C)
ischemia groups. The squares in the CA1 and CA3 areas indicate the
positions of the high magnification images in (D-F and J-L). (D-F)
High magnification images of CV staining in the CA1 subfield of the
(D) sham and (E and F) ischemia groups. CV-positive cells disappear
(triangle) in the SP of the CA1 subfield at 4 days after tgCI.
(G-I) High magnification images of FJB histofluorescence staining
in the CA1 subfield of the (G) sham and (H and I) ischemia groups.
Numerous FJB-positive cells were detected (triangle) in the SP of
the CA1 subfield at 4 days after tgCI. (J-L) High magnification
images of CV staining in the CA3 subfield of the (J) sham and (K
and L) ischemia groups. CV-positive cells were intact in the CA3
subfield until 4 days after tgCI. SO, stratum oriens; SR, stratum
radiatum. Scale bar, 400 µm (A-C) or 50 µm (D-L). CV, Cresyle
violet; FJB, Fluoro-Jade B; tgCI, transient global cerebral
ischemia; SO, stratum oriens; SR, stratum radiatum; SP, stratum
pyramidale; CA, cornu ammonis. |
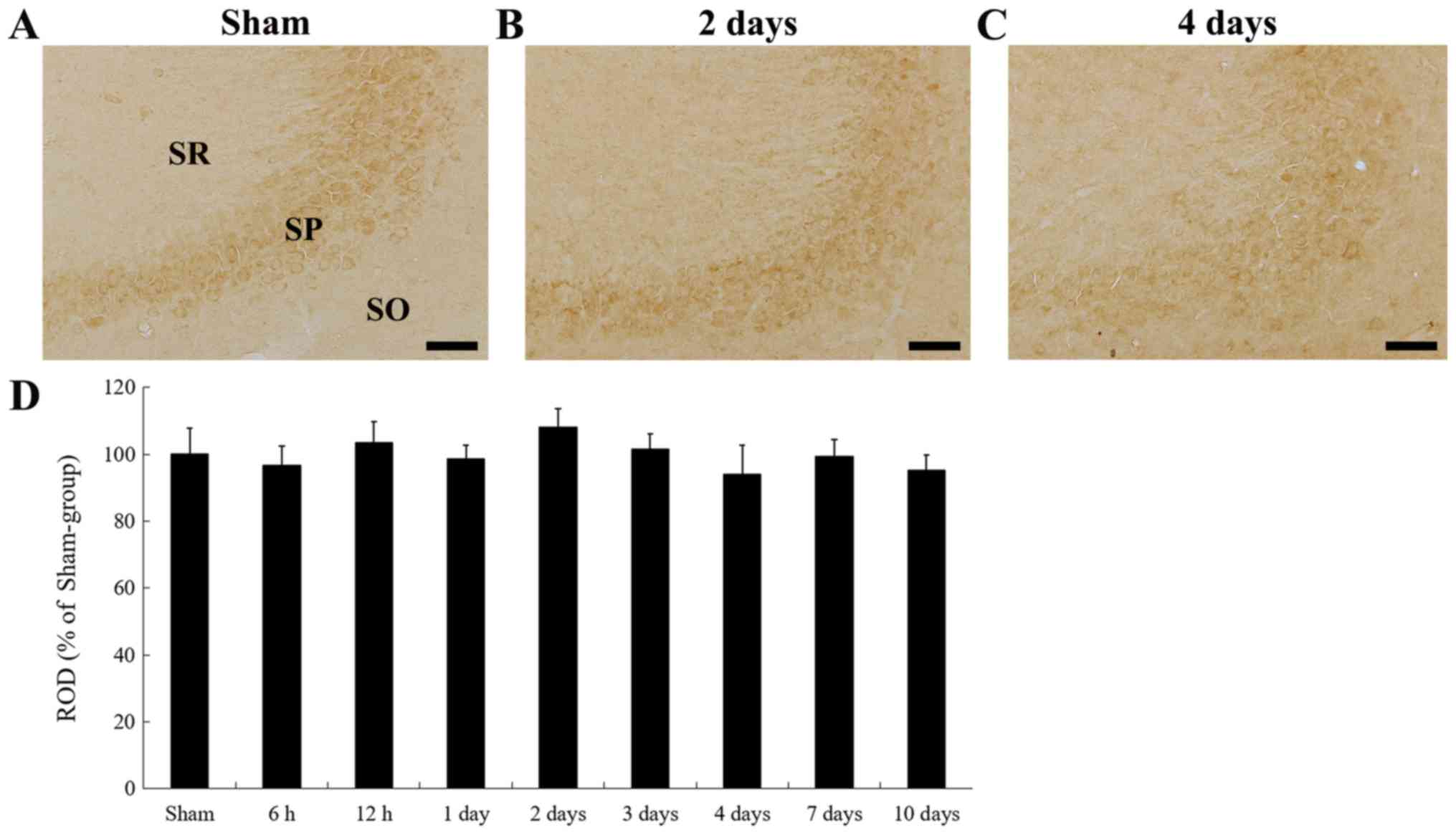
tgCI-induced change of Nurr1
immunoreactivity in CA1 subfield
In the sham group, Nurr1 immunoreactivity was
primarily shown in pyramidal neurons of the stratum pyramidale of
the hippocampus proper (CA1-3 subfields) (Fig. 2A). In the CA1 subfield of the
ischemia group, Nurr1 immunoreactivity in the CA1 pyramidal neurons
began to decrease from 6 h after tgCI, and the immunoreactivity was
gradually decreased with a time-dependent manner until 3 days after
tgCI (Fig. 2B-F and J).
Especially, at 2 and 3 days after tgCI, Nurr1 immunoreactivity in
the CA1 pyramidal neurons was hardly detected (Fig. 2E and F). However, Nurr1
immunoreactivity was increased in the CA1 subfield at 4 and 7 days
after tgCI (Fig. 2G, H and J). At
these points of time, Nurr1-immunoreactive cells were observed in
the stratum pyramidale of the CA1 subfield, and Nurr1
immunoreactivity was detected in non-pyramidal cells of the stratum
oriens and radiatum in the CA1 subfield (Fig. 2G and H). And then, Nurr1
immunoreactivity was decreased in the CA1 subfield at 10 days after
tgCI (Fig. 2I and J).
 | Figure 2.Immunoreactivity of Nurr1 in the CA1
subfield of the (A) sham and (B-I) ischemia groups. Nurr1
immunoreactivity in the sham group was observed in CA1 pyramidal
neurons (triangle). In the ischemia group, Nurr1 immunoreactivity
in CA1 pyramidal neurons gradually decreased until 2 days and 3
days post-ischemia. At 4 days and 7 days post-ischemia, Nurr1
immunoreactivity was increased in non-pyramidal cells (arrows) of
the CA1 subfield. Subsequently, Nurr1 immunoreactivity in the CA1
subfield was decreased again at 10 days after tgCI. Scale bar, 50
µm. (J) ROD as % of Nurr1-immunoreactive structures in the CA1
subfield after tgCI (n=7 at each time point). Bars indicate the
means ± SEM. *P<0.05 vs. sham group; †P<0.05 vs.
previous time point. tgCI, transient global cerebral ischemia; SO,
stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; CA,
cornu ammonis; Nurr1, nuclear receptor related 1 protein. |
Nurr1 expression in neurons or
microglia
As shown in Fig.
2A, Nurr1 immunoreactivity was observed in the stratum
pyramidale of the CA1 subfield of the sham operated group. To
confirm whether Nurr1 is expressed in pyramidal neurons, double
immunofluorescence staining was performed for Nurr1/NeuN (neurons).
We found that Nurr1-immunoreactive cells were colocalized with
NeuN-immunoreactive neurons in the CA1 subfield of the sham
operated group (Fig. 3A-C).
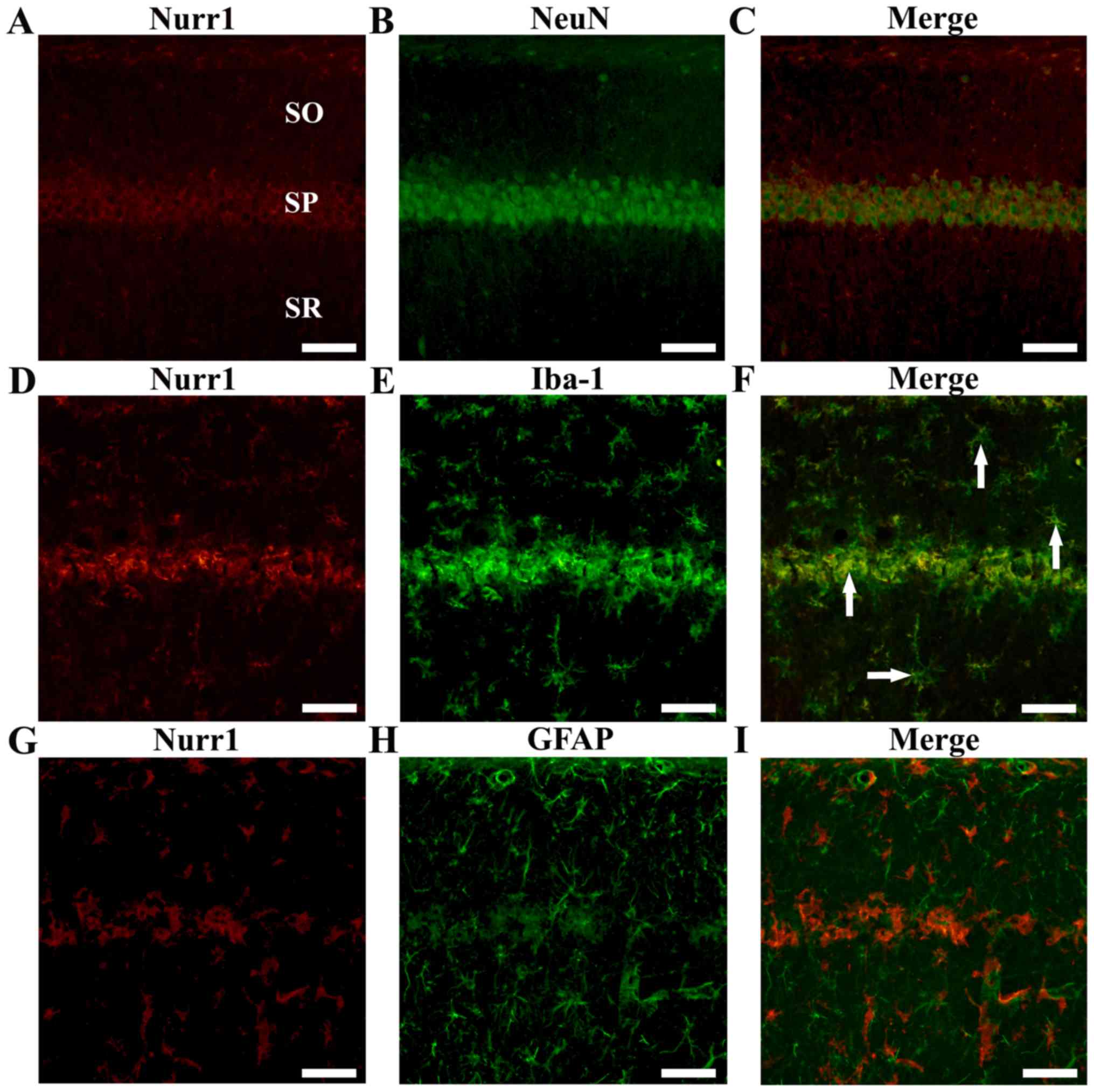 | Figure 3.Double immunofluorescence staining
for (A, D and G) Nurr1 (red), (B) NeuN (green) for neurons, (E)
Iba-1 (green) for microglia or (H) GFAP (green) for astrocytes, and
(C, F and I) merged images in the CA1 subfield.
Nurr1-immunoreactive cells were colocalized with
NeuN-immunoreactive neurons in the sham operated group. At 4 days
after tgCI, Nurr1-immunoreactive cells expressed Iba-1
immunoreactivity (arrows) in the CA1 subfield of the ischemia
operated group. Scale bar, 50 µm. tgCI, transient global cerebral
ischemia; SO, stratum oriens; SP, stratum pyramidale; SR, stratum
radiatum; CA, cornu ammonis; Nurr1, nuclear receptor related 1
protein; NeuN, neuronal nuclei; Iba-1, allograft inflammatory
factor 1. |
In addition, as shown in Fig. 2F and G, Nurr1 immunoreactivity was
newly expressed in non-pyramidal cells in the CA1 subfield at 4
days after tgCI. To identify the cell type, double
immunofluorescence staining was performed for Nurr1/Iba-1
(microglia) and Nurr1/GFAP (astrocytes). We found that
Nurr1-immunoreactive cells were colocalized with
Iba-1-immunoreactive microglia, not with GFAP-immunoreactive
astrocytes, in the CA1 subfield at 4 days after tgCI (Fig. 3D-I).
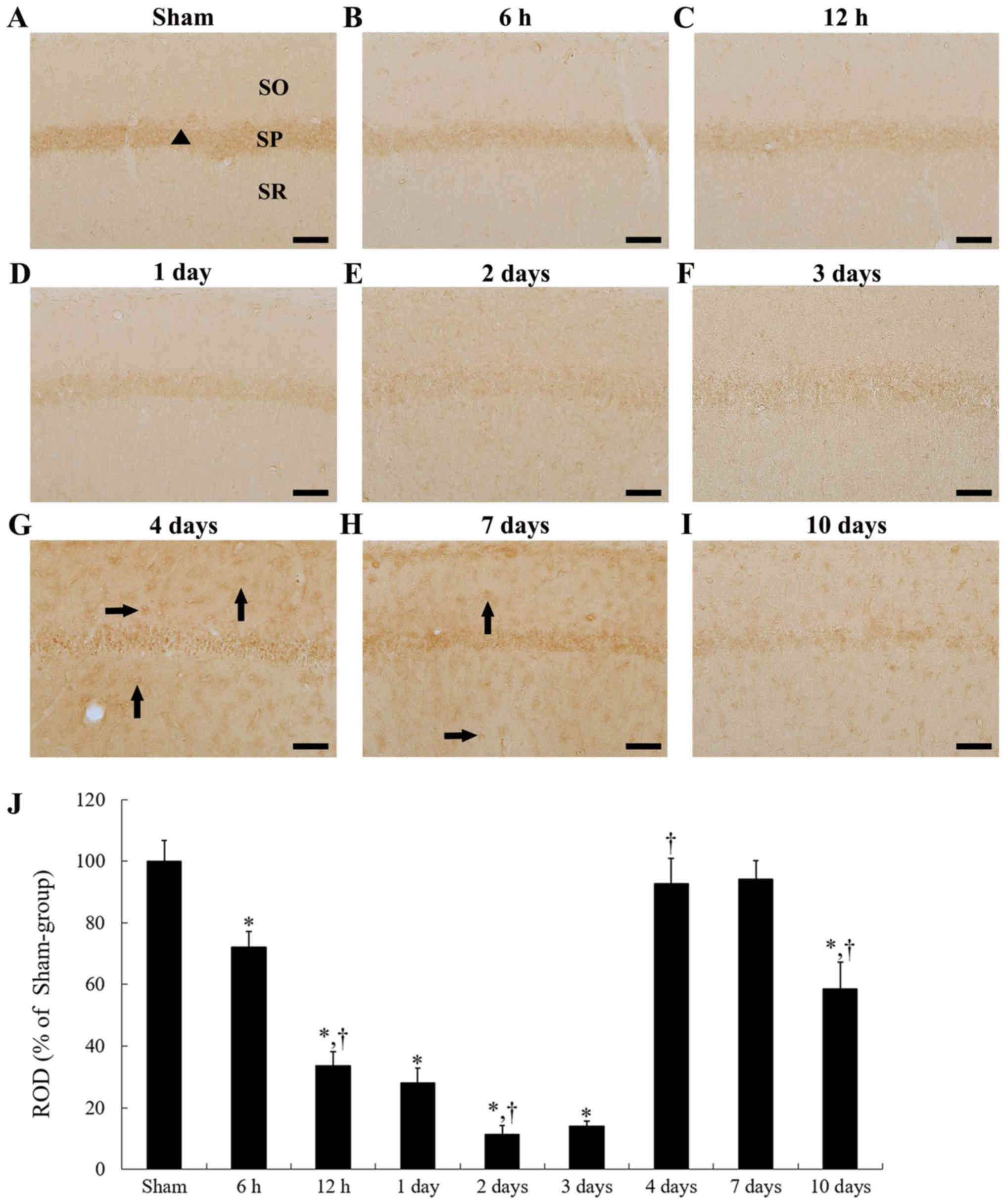
tgCI-induced change of Nurr1
immunoreactivity in CA2/3 subfield
Unlike the tgCI-induced change of Nurr1
immunoreactivity in the CA1 subfield, Nurr1 immunoreactivity in the
CA2/3 subfield was not significantly changed at any times after
tgCI (Fig. 4A-D).
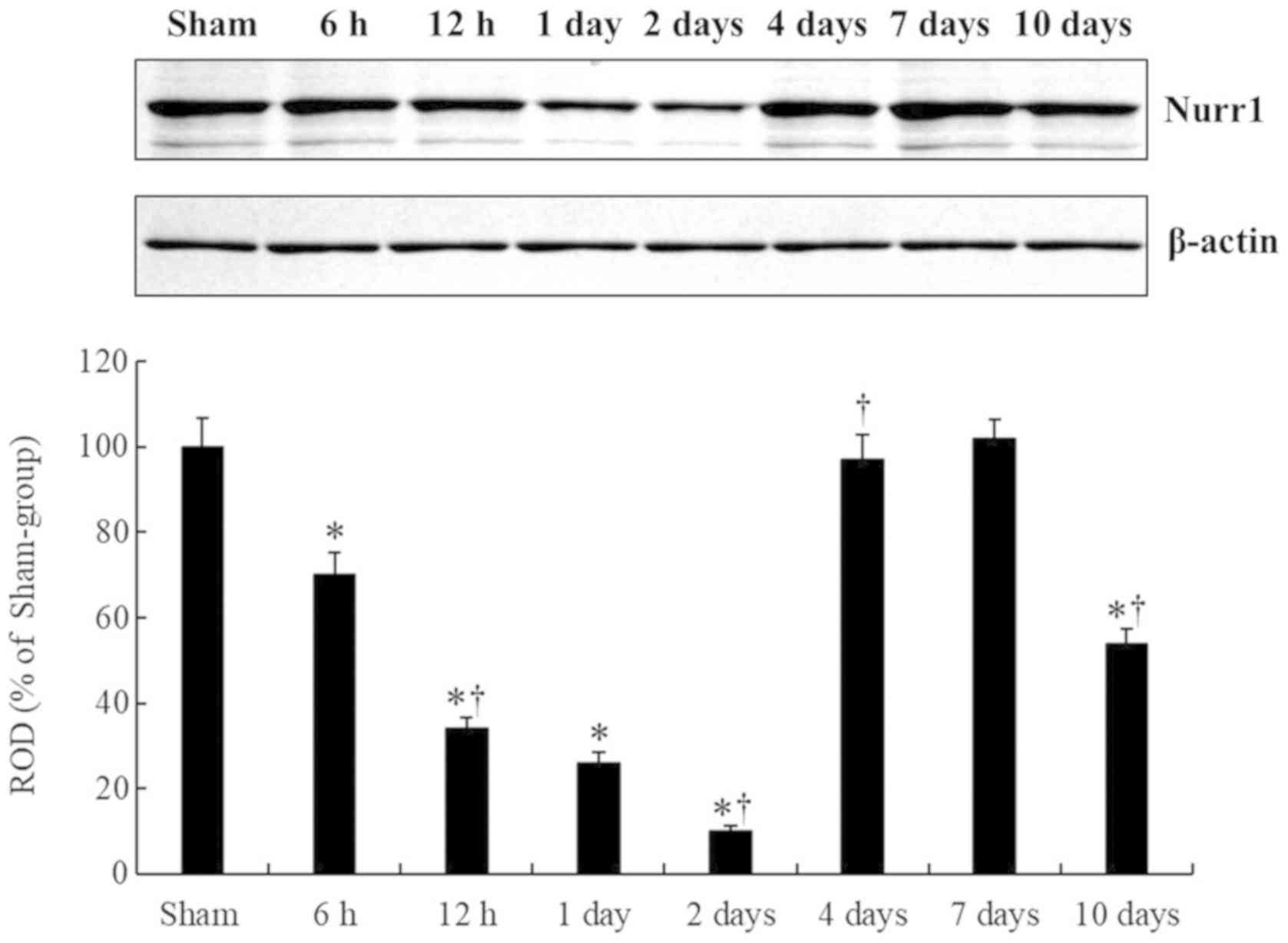
tgCI-induced change of Nurr1 protein
level
Nurr1 protein level in the CA1 field was
significantly changed with time after tgCI (Fig. 5), showing that the pattern of
change of Nurr1 protein level was similar to that observed in the
immunohistochemical data. Nurr1 protein level was significantly
decreased at 6 h after tgCI compared to that of the sham operated
group, and it was gradually decreased until 2 days after tgCI. At 4
days after tgCI, Nurr1 protein level was significantly increased,
compared to that at 2 days after tgCI. At 7 days after tgCI, Nurr1
protein level was not different from that at 4 days after tgCI. And
then, Nurr1 protein level was significantly decreased at 10 days
after tgCI, compared to that at 7 days after tgCI.
Discussion
In this study, we examined neuronal death in the CA1
subfield after tgCI and found that CA1 pyramidal neurons did not
die until 2 days after tgCI; however, CA1 pyramidal neurons died at
4 days post-tgCI. On the other hand, pyramidal neurons in the CA3
subfield did not die at any time after tgCI. These findings are
consistent with results of previous studies (30,32).
Some previous studies have reported that brain
ischemic insults cause changes of Nurr1 expression in various
rodent models of cerebral ischemia (23,25,27).
However, it is unclear if Nurr1 has a neuroprotective or
detrimental effect against cerebral ischemia injury. It has been
reported that a level of Nurr1 mRNA increased in the cerebral
cortex following permanent middle cerebral artery occlusion (MCAO)
in rats (25). They have also
shown that Nurr1 mRNA is induced in pyramidal neurons of the rat
hippocampal CA1 and CA3 subfields at 1 h after ischemic insult
induced by MCAO (25). Recently,
Zhang and Yu (27) have reported
that protein expression and mRNA transcription of Nurr1 are
significantly enhanced in the mouse brain following transient focal
ischemia by MCAO, showing that the infarct volume is significantly
reduced in Nurr1 knockout mice, compared with wild-type mice, after
transient focal ischemia. They have urged that an increase of Nurr1
is associated with the progression of cerebral ischemia-reperfusion
injury and that upregulated Nurr1 is involved in neuronal apoptosis
and mitochondrial injury (27).
Conversely, other previous studies have reported that cerebral
ischemic insults decrease Nurr1 expression in brains (23,24,26).
Erdo et al (23) have
demonstrated that Nurr1 protein expression is down-regulated in the
ipsilateral basal ganglia after transient focal ischemia induced by
MCAO in mice. Additionally, it has been reported that Nurr1 mRNA
expression is not changed in the pyramidal cell layer in the CA1
subfield of the gerbil hippocampus until 24 h after 5 min of tgCI,
and then decreased at 72 h after tgCI (24). Recently, Xie et al (26) have reported that mRNA and protein
levels of Nurr1 begin to decrease immediately after ischemic
damage, reaching a minimum at 12 h and increase at 48 h in the rat
brain including the hippocampus following transient MCAO. They have
also shown that increased expression of Nurr1 can inhibit TNF-α
expression in microglia, reduce ischemia-induced neuroinflammatory
and cytotoxic response in neurons, and decrease infarct volume at
an early stage after transient MCAO (26). In this study, we found that Nurr1
immunoreactivity, which was shown in pyramidal neurons of the CA1
subfield, gradually decreased with a time-dependent manner after
tgCI, and that Nurr1 immunoreactivity in the CA1 pyramidal neurons
was markedly weak at 2 and 3 days after tgCI. In addition, Nurr1
protein expression in the CA1 subfield was gradually decreased
until 2 days after tgCI. However, we found that tgCI-induced change
of Nurr1 immunoreactivity in the CA3 subfield was not shown at any
time after tgCI. It has been reported that CA3 subfield is known to
be a tolerant subfield following tgCI (3,29).
Thus, it can be postulated that tgCI-induced reduction of Nurr1
expression in pyramidal neurons of the CA1 subfield may be closely
involved with a process of the tgCI-induced death of CA1 pyramidal
neurons.
In the present study, we first found that Nurr1
immunoreactivity was detected in non-pyramidal cells in the strata
oriens and radiatum of the CA1 subfield at 4 days after tgCI. We
examined the type of these non-pyramidal cells and found that they
were microglia. It has been reported that Nurr1 expression in
microglia is regulated in response to inflammation (33). Additionally, it has well been known
that Nurr1 can attenuate expressions of proinflammatory and
neurotoxic mediators in microglia through its ability to decrease
NFκB activation (8,9,20).
Additionally, some studies have suggested that beneficial effects
of microglia, such as removal of cell debris and production of
neurotrophic factors, are effectively played in the ischemic brain
regions following ischemic insults (34,35).
Thus, it is likely that newly expressed Nurr1 in microglia in
strata oriens and radiatum of the CA1 subfield may be related with
a beneficial effect of microglia following tgCI.
In summary, Nurr1 immunoreactivity was significantly
changed in the CA1 subfield, not the CA3 subfield, following tgCI,
and Nurr1 immunoreactivity was newly expressed in microglia of
ischemic CA1 subfield at 4 days after tgCI. These results indicate
that tgCI-induced alteration of Nurr1 expression may be closely
related with tgCI-induced neuronal death in the hippocampal CA1
subfield.
Acknowledgements
Not applicable.
Funding
The present study was carried out with the support
of ‘Cooperative Research Program for Agriculture Science and
Technology Development, Rural Development Administration, Republic
of Korea (project no. PJ01321101), and was supported by the Bio
& Medical Technology Development Program of the NRF funded by
the Korean government, MSIP (grant no. NRF-2015M3A9B6066835), and
by the National Research Foundation of Korea funded by the Ministry
of Education (grant no. NRF-2017R1D1A1B03029311).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TKL, CWP and YEP performed the measurements. DWK,
JCL, HAL and GEY analyzed and interpreted data. JHP, JHA, MHW and
CHL made substantial contributions to conception and design, and
were involved in drafting and revising the manuscript, and
interpreting all data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol of this experiment was approved by
Institutional Animal Care and Use Committee (IACUC) at Kangwon
National University (approval no. KW-180124-1). This protocol
adhered to guidelines from the current international laws and
policies in the ‘Guide for the Care and Use of Laboratory
Animals’.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Globus MY, Busto R, Martinez E, Valdes I,
Dietrich WD and Ginsberg MD: Comparative effect of transient global
ischemia on extracellular levels of glutamate, glycine, and
gamma-aminobutyric acid in vulnerable and nonvulnerable brain
regions in the rat. J Neurochem. 57:470–478. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim H, Ahn JH, Song M, Kim DW, Lee TK, Lee
JC, Kim YM, Kim JD, Cho JH, Hwang IK, et al: Pretreated fucoidan
confers neuroprotection against transient global cerebral ischemic
injury in the gerbil hippocampal CA1 area via reducing of glial
cell activation and oxidative stress. Biomed Pharmacother.
109:1718–1727. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JC, Park JH, Kim IH, Cho GS, Ahn JH,
Tae HJ, Choi SY, Cho JH, Kim DW, Kwon YG, et al: Neuroprotection of
ischemic preconditioning is mediated by thioredoxin 2 in the
hippocampal CA1 region following a subsequent transient cerebral
ischemia. Brain Pathol. 27:276–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JH, Kim YH, Ahn JH, Choi SY, Hong S,
Kim SK, Kang IJ, Kim YM, Lee TK, Won MH and Lee CH: Atomoxetine
protects against NMDA Receptor-mediated hippocampal neuronal death
following transient global cerebral ischemia. Curr Neurovasc Res.
14:158–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arimatsu Y, Ishida M, Kaneko T, Ichinose S
and Omori A: Organization and development of corticocortical
associative neurons expressing the orphan nuclear receptor Nurr1. J
Comp Neurol. 466:180–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim CH, Han BS, Moon J, Kim DJ, Shin J,
Rajan S, Nguyen QT, Sohn M, Kim WG, Han M, et al: Nuclear receptor
Nurr1 agonists enhance its dual functions and improve behavioral
deficits in an animal model of Parkinson's disease. Proc Natl Acad
Sci USA. 112:8756–8761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saijo K, Winner B, Carson CT, Collier JG,
Boyer L, Rosenfeld MG, Gage FH and Glass CK: A Nurr1/CoREST pathway
in microglia and astrocytes protects dopaminergic neurons from
inflammation-induced death. Cell. 137:47–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Q, Castillo SO and Nikodem VM:
Distribution of messenger RNAs for the orphan nuclear receptors
Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ
hybridization. Neuroscience. 75:221–230. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou J, Chen Z, Wei X, Chen Z, Fu Y, Yang
X, Chen D, Wang R, Jenner P, Lu JH, et al: Cystatin C as a
potential therapeutic mediator against Parkinson's disease via
VEGF-induced angiogenesis and enhanced neuronal autophagy in
neurovascular units. Cell Death Dis. 8:e28542017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu Y, Kompoliti K, Cochran EJ, Mufson EJ
and Kordower JH: Age-related decreases in Nurr1 immunoreactivity in
the human substantia nigra. J Comp Neurol. 450:203–214. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Decressac M, Volakakis N, Bjorklund A and
Perlmann T: NURR1 in Parkinson disease-from pathogenesis to
therapeutic potential. Nat Rev Neurol. 9:629–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakurada K, Ohshima-Sakurada M, Palmer TD
and Gage FH: Nurr1, an orphan nuclear receptor, is a
transcriptional activator of endogenous tyrosine hydroxylase in
neural progenitor cells derived from the adult brain. Development.
126:4017–4026. 1999.PubMed/NCBI
|
|
15
|
Yan W, Chen ZY, Chen JQ and Chen HM: BMP2
promotes the differentiation of neural stem cells into dopaminergic
neurons in vitro via miR-145-mediated upregulation of Nurr1
expression. Am J Transl Res. 8:3689–3699. 2016.PubMed/NCBI
|
|
16
|
Ahn JH, Lee JS, Cho JH, Park JH, Lee TK,
Song M, Kim H, Kang SH, Won MH and Lee CH: Age-dependent decrease
of Nurr1 protein expression in the gerbil hippocampus. Biomed Rep.
8:517–522. 2018.PubMed/NCBI
|
|
17
|
Kim JI, Jeon SG, Kim KA, Kim YJ, Song EJ,
Choi J, Ahn KJ, Kim CJ, Chung HY, Moon M and Chung H: The
pharmacological stimulation of Nurr1 improves cognitive functions
via enhancement of adult hippocampal neurogenesis. Stem Cell Res.
17:534–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montes P, Ruiz-Sanchez E, Calvillo M and
Rojas P: Active coping of prenatally stressed rats in the forced
swimming test: Involvement of the Nurr1 gene. Stress. 19:506–515.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barneda-Zahonero B, Servitja JM, Badiola
N, Miñano-Molina AJ, Fadó R, Saura CA and Rodríguez-Alvarez J:
Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA)
receptor-mediated neuronal survival. J Biol Chem. 287:11351–11362.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Miranda BR, Popichak KA, Hammond SL,
Jorgensen BA, Phillips AT, Safe S and Tjalkens RB: The Nurr1
Activator 1,1-Bis(3′-Indolyl)-1-(p-Chlorophenyl)Methane blocks
inflammatory gene expression in BV-2 microglial cells by inhibiting
nuclear factor κB. Mol Pharmacol. 87:1021–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hammond SL, Safe S and Tjalkens RB: A
novel synthetic activator of Nurr1 induces dopaminergic gene
expression and protects against 6-hydroxydopamine neurotoxicity in
vitro. Neurosci Lett. 607:83–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loppi S, Kolosowska N, Kärkkäinen O,
Korhonen P, Huuskonen M, Grubman A, Dhungana H, Wojciechowski S,
Pomeshchik Y, Giordano M, et al: HX600, a synthetic agonist for
RXR-Nurr1 heterodimer complex, prevents ischemia-induced neuronal
damage. Brain Behav Immun. 73:670–681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erdo F, Trapp T, Mies G and Hossmann KA:
Immunohistochemical analysis of protein expression after middle
cerebral artery occlusion in mice. Acta Neuropathol. 107:127–136.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honkaniemi J and Sharp FR: Global ischemia
induces immediate-early genes encoding zinc finger transcription
factors. J Cereb Blood Flow Metab. 16:557–565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honkaniemi J, States BA, Weinstein PR,
Espinoza J and Sharp FR: Expression of zinc finger immediate early
genes in rat brain after permanent middle cerebral artery
occlusion. J Cereb Blood Flow Metab. 17:636–646. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie X, Peng L, Zhu J, Zhou Y, Li L, Chen
Y, Yu S and Zhao Y: miR-145-5p/Nurr1/TNF-a Signaling-induced
microglia activation regulates neuron injury of acute cerebral
Ischemic/reperfusion in rats. Front Mol Neurosci. 10:3832017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z and Yu J: Nurr1 exacerbates
cerebral ischemia-reperfusion injury via modulating
YAP-INF2-mitochondrial fission pathways. Int J Biochem Cell Biol.
104:149–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ginsberg MD and Busto R: Rodent models of
cerebral ischemia. Stroke. 20:1627–1642. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn JH, Shin MC, Kim DW, Kim H, Song M,
Lee TK, Lee JC, Kim H, Cho JH, Kim YM, et al: Antioxidant
properties of fucoidan alleviate acceleration and exacerbation of
hippocampal neuronal death following transient global cerebral
ischemia in high-fat diet-induced obese gerbils. Int J Mol Sci.
20(pii): E5542019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JH, Noh Y, Kim SS, Ahn JH, Ohk TG,
Cho JH, Lee TK, Kim H, Song M, Lee JC, et al: Time-course changes
and new expressions of MIP-3a and its receptor, CCR6, in the gerbil
hippocampal CA1 area following transient global cerebral ischemia.
Neurochem Res. 43:2102–2110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JH, Shin BN, Ahn JH, Cho JH, Kim IH,
Kim DW, Won MH, Hong S, Cho JH and Lee CH: Ischemia-induced changes
of PRAS40 and p-PRAS40 immunoreactivities in the gerbil hippocampal
CA1 region after transient cerebral ischemia. Cell Mol Neurobiol.
36:821–828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JH, Park CW, Ahn JH, Choi SY, Shin
MC, Cho JH, Lee TK, Kim IH, Cho JH, Lee JC, et al: Neuroprotection
and reduced gliosis by pre- and post-treatments of hydroquinone in
a gerbil model of transient cerebral ischemia. Chem Biol Interact.
278:230–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lallier SW, Graf AE, Waidyarante GR and
Rogers LK: Nurr1 expression is modified by inflammation in
microglia. Neuroreport. 27:1120–1127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu YZ, Lin CH, Cheng FC and Hsueh CM:
Molecular mechanisms responsible for microglia-derived protection
of Sprague-Dawley rat brain cells during in vitro ischemia.
Neurosci Lett. 373:159–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stoll G and Jander S: The role of
microglia and macrophages in the pathophysiology of the CNS. Prog
Neurobiol. 58:233–247. 1999. View Article : Google Scholar : PubMed/NCBI
|