Introduction
Neuropathic pain following spinal surgery is a
problem that has the common characteristics of being severe and
chronic for patients (1–3). Although much progress has been made
in clinical treatment, a number of patients still suffer chronic
pain and psychological problems (4). At present, the molecular mechanism of
neuropathic pain remains unclear. To provide a new treatment for
neuropathic pain, it is urgent to understand its molecular
mechanism.
It has been reported that proinflammatory factors
may participate in regulating pain sensitivity and inducing chronic
pain by activating the secretion of inflammatory molecules such as
interleukin (IL)-1β, tumor necrosis factor (TNF)-α, cyclooxygenase
(COX)-2 and IL-6 (5). Therefore,
targeting inflammation signaling pathways may provide potential
therapeutic opportunities in neuropathic pain. It has been reported
that enhancer of zeste homolog 2 (EZH2) signaling pathway could
inhibit spinal neuroinflammation and alleviate occurrence and
development of neuropathic pain (6).
Long non-coding RNAs (lncRNAs) are a type of RNA
that are longer than 200 nucleotides but do not have capacity for
encoding protein; they participate in a number of biological
processes in various diseases (7,8). It
has been reported that lncRNAs can be involved in the regulation of
several neurological diseases including spinal cord injury (SCI)
(9). For example, lnc X-inactive
specific transcript can regulate the spinal cord injury in rats by
responding to micro (miR)-494 (9).
Metastasis associated lung adenocarcinoma transcript (MALAT)1 has
been identified in multiple types of physiological and pathological
processes, including organizing nuclear construction, modulating
gene expression, participating in diabetes complications and in
cancers (10–14). lnc-MALAT1 participates in the
post-ischemic perfusion neuroprotection by interacting with miR-204
(15). However, whether it
participate in neuropathic pain remains to be elucidated.
Several studies have reported that miRNAs are
involved in the development of neuropathic pain. For example,
miR-183 inhibits neuropathic pain by inhibiting the mTOR/vascular
endothelial growth factor signaling pathway (16). It can activate p38 through
downregulating miR-128 and then trigger neuropathic pain (17). The dysregulation of miR-154-5p is
common in a number of tumors. For example, miRNA-154-5p inhibits
the proliferation, migration and invasion of prostate cancer cells
by targeting E2F transcription factor 5 (18) and miRNA-154-5p inhibits cell
proliferation and metastasis by targeting Piwi like RNA-mediated
gene silencing 4 (19). However,
the role of miR-154-5p in neuropathic pain remains to be
elucidated.
Aquaporin (AQP) 9 is a protein encoded by the AQP9
gene in humans, which belongs to the aquaporin family and is a type
of water-selective membrane channel that allows the passage of
multiple non-charged solutes, including water, glycerol, purine,
pyrimidine and lactic acid. It serves a key role in leukocyte
immune response and bactericidal activity (20,21).
AQP9 also serves an important role in tumor cells. For example,
AQP9 can activate the Akt signaling pathway to promote astrocytoma
cell invasion (22). It can also
arrest cell cycle by inducing Ras to improve the efficacy of
colorectal cancer chemotherapy (23). However, the role of AQP9 in
neuropathic pain is unclear.
The present study attempted to investigate the role
of MALAT1 in the development of neuropathic pain. A significant
increase of MALAT1 expression in the chronic constriction injury
(CCI) rat model was observed, whereas inhibition of MALAT1
attenuated the development of neuropathic pain. In a rat model of
neuropathic pain, miR-154-5p was decreased, while AQP9 was
increased. Therefore, it was speculated that MALAT1 could serve an
important role in the development of neuropathic pain by regulating
miR-154-5p/AQP9.
Materials and methods
Animal studies
A total of 60 adult SD rats (female, 4 weeks,
180–200 g) were purchased from Shanghai Animal Laboratory Center
and randomly divided into sham control group and CCI groups. Rats
were housed in standard plastic cages at 24±1°C and 50–70% of
humidity. The rats were kept in the animal environment on a 12-h
light-dark cycle and could drink and eat freely. In the CCI rat
groups, the rats were divided into LV-NC, LV-shMALAT1,
LV-miR-154-5p and LV-shAQP9 groups (12 rats in each group). In
addition, miR-154-5p inhibitor or miR-NC was injected intrathecally
into rats treated with LV-shMALAT1 for 3 days (4 rats in each
group). Rats were group-fed in every cage at 25°C. An animal model
of neuropathic pain model was established by the CCI method
(24). Rats were intraperitoneally
injected with sodium pentobarbital (40 mg/kg) for anesthesia. In
the CCI rat groups, the bilateral sciatic nerves of two legs were
exposed and ligated, while nothing was ligated in the sham group.
Then, 3 rats in each group were randomly selected and sacrificed
following surgery for days 0, 3, 7 and 14, the spinal cord tissue
was collected and microglia were isolated for further study.
Following CCI surgery, different recombinant lentivirus was
respectively injected into the rats through intrathecal microneedle
injection for lentiviral infection. The present study was carried
out in strict accordance with the requirements in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (25). The experiments were
carried out strictly in line with the requirements of the
International Association for the Study of Pain (IASP) (26). The present study was approved by
the ethics committee of Lishui Municipal Central Hospital. In order
to minimize the time that rats suffered pain from experimental
treatment as much as possible, when the following pain
characteristics were observed in each rat, they were rapidly
euthanized by cervical dislocation during deep anesthesia at the
end of the experiment: Rapid weight loss, self-mutilation, muscle
stiffness, biting/aggression and skin dehydration.
Cell isolation and cell culture
Primary microglia were isolated according to the
method of Beltramo et al (27). The spinal cord tissue from rats was
collected and was digested by 0.25% trypsin at 4°C for 20 mins.
Following centrifugation at 4°C and 800 × g for 5 min, the mixed
glial cells were isolated and cultured in DMEM/F12 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
and 5% CO2 culture incubator. After two weeks, microglia
were isolated from the mixed glial cells and cultured in DMEM/F12
medium containing 10% FBS at 37°C and 5% CO2 culture
incubator.
Construction of lentivirus and cell
transfection
The lentivirus vectors of LV-NC (cat. no. D03003,
Shanghai GenePharma Co., Ltd.), LV-shMALAT1, LV-miR-154-5p and
LV-shAQP9 were synthesized by Shanghai GenePharma Co, Ltd.
Following CCI surgery, these lentivirus vectors
(1×107/0.1 ml) were respectively injected into the rats
through intrathecal microneedle injection for infection. Microglia
cells were seeded in 6-well plates (2×106/well) until
reached 70–80%, before transfection, the transfection reagent
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), serum-free DMEM and 100 nM miR-NC (cat. no.
miR0190513015853, Guangzhou RiboBio Co., Ltd.) or 100 nM miR-154-5p
mimics (cat. no. miR10000452-1-5, Guangzhou RiboBio Co., Ltd.) were
mixed and incubated for 30 min, and then added into microglia with
complete medium containing 15% FBS. At the indicated time point
following transfection, cells were harvested for further study.
Relative expression levels of miR-154-5p were significantly
increased in cells transfected with miR-154-5p mimics compared with
in cells transfected with miR-NC (data not shown).
Detection of Cox-2, IL-6 and TNF-α
levels by ELISA
The spinal cord tissues were collected and the
microglia isolated. Protein lysate was added to the spinal cord
tissue and microglia samples of each group to homogenize the
tissue, and the supernatant was collected following centrifugation
with 8,000 × g at 4°C. Levels of COX-2 (cat. no. kt22030, rat),
IL-6 (cat. no. kt22084, rat) and TNF-α (cat. no. kt30484, rat) were
detected by ELISA kits according to the manufacturer's protocol
(Wuhan MSK Biotechnology Co, Ltd.). The concentrations of the
standard wells were 0, 7.5, 15, 30, 60 and 120 pg/ml. In addition
to the blank wells, 100 µl horseradish peroxidase (HRP)-labeled
detection antibody (cat. no. ab181658, 1:1,000, Abcam, Cambridge,
USA) was added to each well of the standard wells and sample wells.
The wells were sealed with a sealing membrane and following
incubation at 37°C for 60 min, the liquid was removed, the plate
was dried with absorbent paper and the plate was repeatedly washed
with PBS 5 times. Then, 50 µl of each of the substrates A and B
were added to each well, and the mixture was incubated at 37°C for
15 min in the dark. Finally, 50 µl of the stop solution was added
to each well, the OD value of each well was measured by a
microplate reader at a wavelength of 450 nm within 15 min and the
protein concentration calculated according to the standard
curve.
RNA extraction and reverse
transcription-quantitative [(RT-q) PCR]
Spinal cord tissues and 106 microglia of
rats in each group were homogenized with Polytron PT100 (Kinematica
AG) and 1 ml RNAiso Plus (TaKaRa, Tokyo, Japan) was added to
extract total RNAs and then purified by using GeneJET RNA
Purification Kit (Thermo Fisher Scientific, Shanghai, China)
according to the manufacturer's protocols. RT-qPCR was performed by
using PrimeScript RT reagent kit (TaKaRa, Tokyo, Japan) according
to the manufacturer's protocol. RT reaction was conducted for 15
min at 42°C followed by 5 min at 98°C and the reaction volume was
20 µl. The qPCR thermocycling conditions were: 95°C for 30 sec
followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec and the
reaction volume was 25 µl. PCR primers were synthesized by Gene
Pharma (GenePharma, Co., Ltd, Shanghai, China) and sequences are
listed in Table I. The levels of
mRNA expression were detected by SYBR Premix Ex Taq II Takara Bio,
Inc.). The mRNA expressions of MALAT1 and AQP9 were normalized to
GAPDH and miR-154-5p was normalized to U6 and 2−ΔΔCq
method was used to calculate the relative gene expressions
(28).
 | Table I.RT-qPCR primer sequences. |
Table I.
RT-qPCR primer sequences.
| Gene names | Primer
sequences |
|---|
| MALAT1 | Forward: 5′-
AAAGCAAGGTCTCCCCACAAG-3′ |
|
| Reverse: 5′-
GGTCTGTGCTAGATCAAAAGGCA-3′ |
| miR-154-5p | Forward:
5′-CGCGAATTCGCATCTAGGACCTCCATCAC −3′ |
|
| Reverse:
5′-ACGGGATCCGAACCATCCCTTCACTTACC −3′ |
| AQP9 | Forward:
5′-TCTCGTCCTTGGATGTGGC-3′ |
|
| Reverse:
5′-CAAAAGACACCGCTGGGTTG-3′ |
| GAPDH | Forward:
5′-GGAGTCCACTGGTGTCTTCA-3′ |
|
| Reverse:
5′-GGGAACTGAGCAATTGGTGG-3′ |
| U6 | Forward:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse:
5′-AACGCTTCACGAATTTGCGT-3′ |
Protein extraction and western blot
analysis
Total protein was extracted from spinal cord tissue
and microglia of each group by using a RIPA lysis buffer (Beyotime
Institute of Biotechnology) and the protein concentration was
measured using a BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). Proteins (50 µg) were added to 10% SDS-PAGE and then
transferred onto PVDF membranes (GE Healthcare Life Sciences),
which were blocked with 5% non-fat milk in Tris-buffered saline at
25°C for 60 min. Then the membranes were incubated with primary
antibodies overnight at 4°C. AQP9 (1:1,000, cat. no. ab15129, 35
kDa, Abcam) and GAPDH (1:10,000, cat. no. ab181602, 36 kDa, Abcam).
Following washing with Tris Buffered saline Tween (20% Tween) TBST
for 4 times, the membrane was then incubated with the secondary
antibody, goat anti-Rat IgG (1:10,000, cat. no. ab150165, Abcam),
for 1 h at room temperature. Protein bands were detected by Pierce
ECL western blot substrate (Thermo Fisher Scientific, Inc.) with
ECL detection system (Thermo Fisher Scientific, Inc.). Quantity One
software (version 4.6.2, Bio-Rad Laboratories, Inc.) was used to
quantify relative protein expression.
Pain threshold assessment
Pain behavior indicators were detected following
lentivirus infection. The paw withdrawal threshold (PWT/g) and the
paw withdrawal latency (PWL/s) of the sham group, CCI-LV-NC and
CCI-LV-shMALAT1 groups were respectively detected at 0, 1, 3, 7 and
14 days. PWT was used to evaluate the mechanical allodynia. Rats of
each group were placed in a transparent plastic box with metal mesh
padding. The hind paw of the rats was placed in a calibrated Von
Frey fiber and stimulation was given to them, then the minimum
fiber strength was recorded. PWL was used to evaluate the thermal
hyperalgesia and rats in each group were housed in a thick
plexiglass box and tested by thermal radiation stimulation. The
initial threshold of the base leg was set to 10 sec, and the time
between the start of stimulation and the cessation of the action of
the paws was recorded (PWL/s).
Bioinformatics analysis
Target genes were predicted through different
bioinformatics databases, including starBase V2.0 (29) and TargetScan 7.1 (http://www.targetscan.org/vert_71/). First, the
miRNAs that may bind to MALAT1 were analyzed and the miRNA in the
two databases was taken for further verification; miR-154-5p was
identified. Second, the potential target genes of miR-154-5p were
predicted with the two bioinformatics databases; 104 target genes
had been reported, including transcription factors, kinases,
channel proteins and cancer-related genes. The top 5 genes were
E2F5, HDHD2, SOS2, AQP9 and HNRNPR. The most likely target genes
were selected for experimental validation.
Luciferase assays
The MALAT/AQP9 wild type (WT) sequences or mutant
(mut) sequences in 3′-UTR containing the miR-154-5p binding site
were constructed and subcloned into the pGL3 basic plasmid
(Addgene, Inc.). 293 cells were seeded in 48-well plates for 24 h.
Then miR-154-5p mimics and miR-NC were co-transfected into 293
cells with pGL3-WT/mut-MALAT1 for 24 h. Plasmids (200 ng) were
mixed with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and DMEM medium for 20 mins, then the
mixtures were added into 293 cells for 24 h. Following transfection
for 24 h, the cells were lysed and the activity of firefly
luciferase and Renilla luciferase was measured by using
dual-luciferase reporter assay (Promega Corporation). The ratio of
the two identified the relative activity of luciferase.
Statistical analysis
All the data were expressed as the mean ± standard
deviation, the significance between groups was analyzed by
Student's t-test and multiple comparison between the groups was
performed by the SNK method following one-way ANOVA. All
statistical analyses were performed using SPSS 19.0 (IBM Corp.) and
GraphPad Prism 6.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
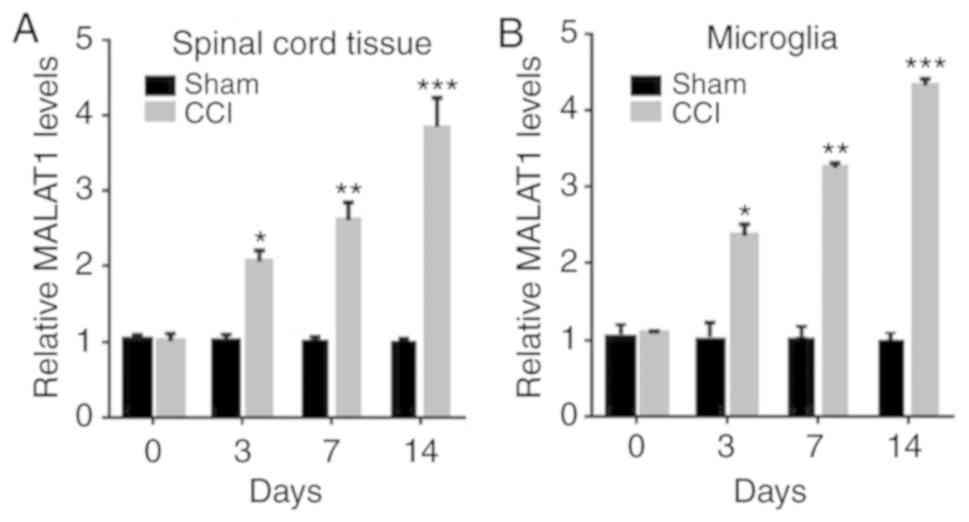
MALAT1 is increased in CCI rat
models
To explore the roles of MALAT1 in neuropathic pain,
the expressions of MALAT1 in spinal cord tissue and microglia of
CCI rats were detected for the first time. The expression of MALAT1
in spinal cord tissue and microglia of CCI rats was significantly
increased with time, compared with the sham group (Fig. 1A and B; P<0.05), which indicated
that MALAT1 was increased in CCI rat models.
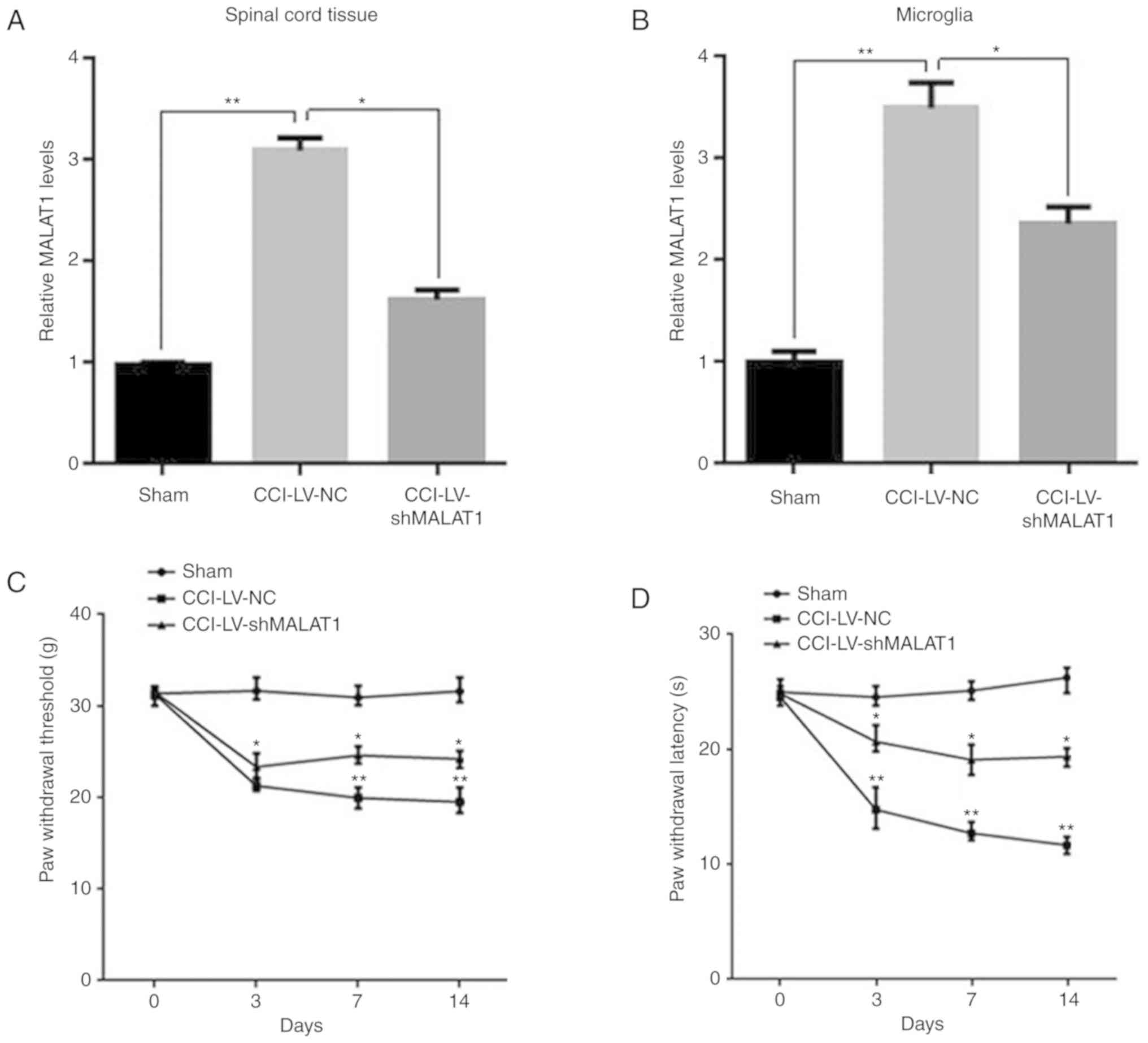
Inhibition of MALAT1 reduces the
incidence of neuropathic pain
To further investigate of the function of MALAT1 in
CCI model rats, recombinant lentiviral LV-shMALAT1 and LV-NC were
injected into CCI rats. After 14 days, spinal cord tissue and
microglia were isolated from rats and MALAT1 levels were detected
by RT-qPCR. The results demonstrated that the expression of MALAT1
was significantly increased in CCI LV-NC group, while it was
repressed following LV-shMALAT1 transfection in spinal cord tissue
and microglia (Fig. 2A and B;
P<0.05). The neuropathic pain in each group of rats was
evaluated using PWT and PWL. The results demonstrated that the PWT
and PWL of CCI rats were significantly decreased with time
dependence, compared with the sham group, while they were increased
following LV-shMALAT1 transfection (Fig. 2C and D; P<0.05). These results
indicated that downregulation of MALAT1 in the CCI model promoted
neuropathic pain in rats, whereas inhibition of MALAT1 reduced the
incidence of neuropathic pain.
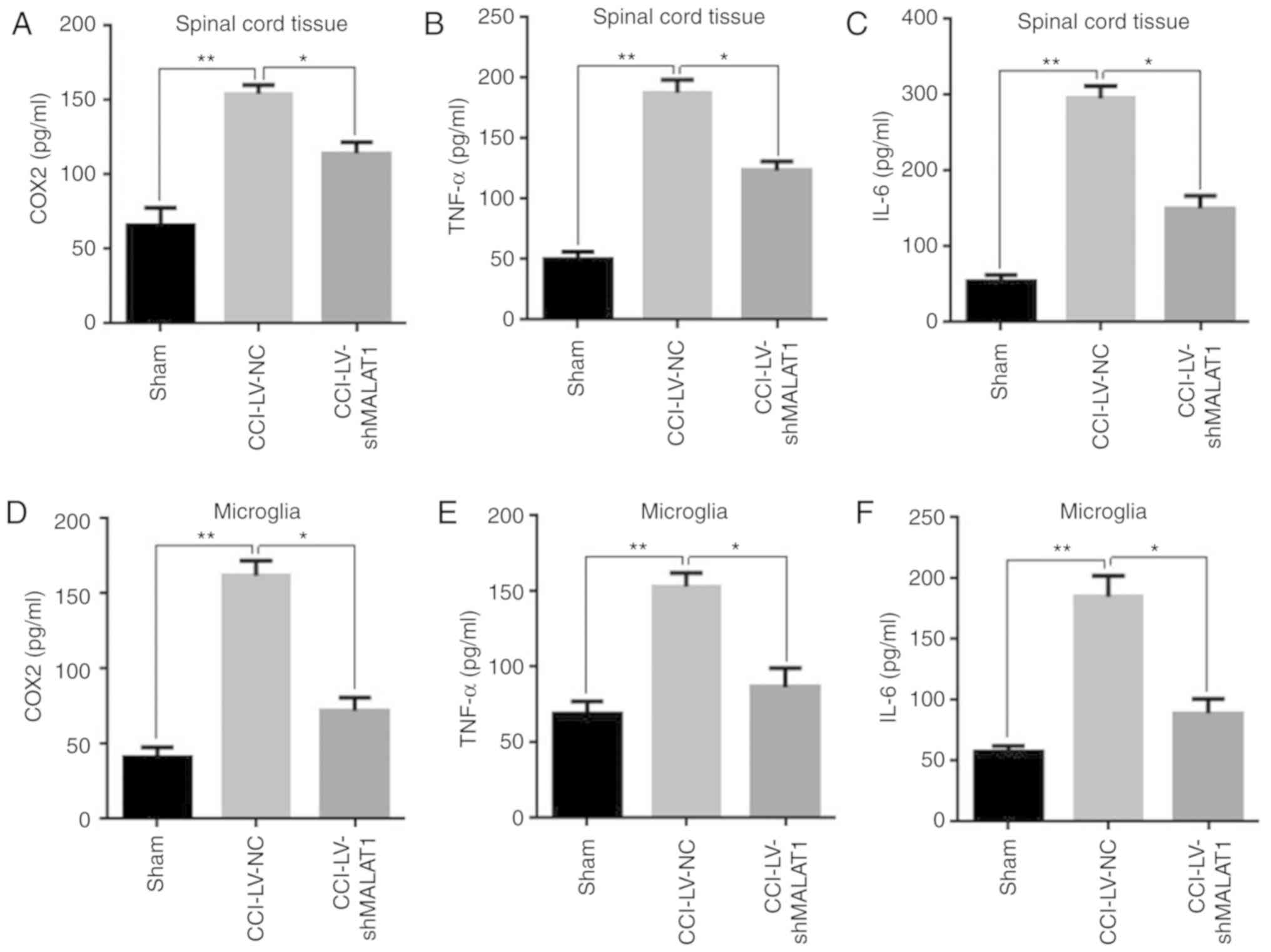
Inhibition of MALAT1 reduces the
levels of inflammatory cytokines
To further explore whether MALAT1 served some roles
in inflammation of CCI model rats, the expression levels of
inflammatory cytokines in spinal cord tissue and microglia were
detected using ELISA in each group. The results demonstrated that
the levels of the inflammatory cytokines COX2, TNF-α and IL-6 were
significantly increased in the CCI model group, compared with the
sham group, while these inflammatory factors were significantly
decreased in MALAT1 inhibition model in both spinal cord tissue and
microglia (Fig. 3A-F; P<0.05).
These results indicated that inhibition of MALAT1 reduced the
levels of inflammatory cytokines and mitigated inflammatory
responses in CCI rats.
MALAT1 can directly bind with
miR-154-5p
To investigate the molecular mechanism whereby
MALAT1 promoted neuropathic pain in CCI rats, miRNAs were predicted
by starBase v2.0 database and it was identified that miR-154-5p may
be a target of MALAT1; therefore, the levels of miR-154-5p were
detected by RT-qPCR. The results demonstrated that MALAT1 was
decreased in LV-shMALAT1 group, while miR-154-5p was respectively
increased, which might suggest that MALAT1 was negatively
correlated with miR-154-5p (Fig. 4A
and B; P<0.05). The potential binding site with miR-154-5p
was predicted by starBase v2.0 database and sequences of wt-MALAT1
and mut-MALAT1 were constructed into luciferase reporter vector
(Fig. 4C). The results
demonstrated that the luciferase activity in cells co-transfected
with miR-154-5p mimic and wt-MALAT1 was significantly decreased,
while it was increased in mut-MALAT1 group in 293 cells (Fig. 4D; P<0.05). In addition,
LV-miR-154-5p was injected into CCI rats and PWT and PWL detected.
The results showed that miR-154-5p was significantly increased
following LV-miR-154-5p infection, which suggested that it was
successfully infected into the rats and it was found that
overexpression of miR-154-5p could have a similar effect to the
inhibition of MALAT1 (Fig. 4E and
F; P<0.05). These results indicated that MALAT1 could
directly bind with miR-154-5p.
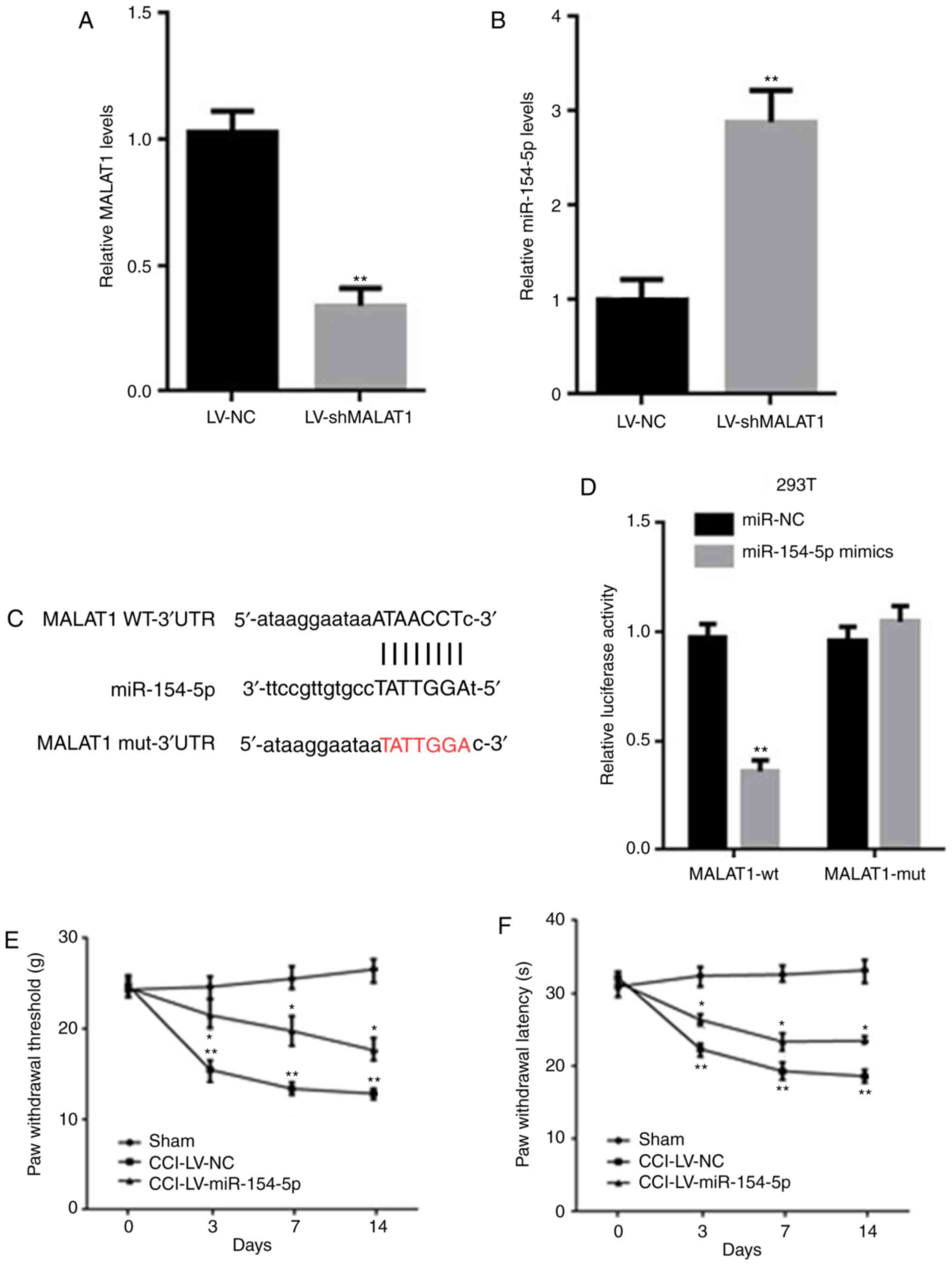 | Figure 4.MALAT1 could directly bind with
miR-154-5p. (A, B) The mRNA levels of (A) MALAT1 and (B) miR-154-5p
in microglia of CCI rats were detected by RT-qPCR. (C) Potential
binding sites between MALAT1 and miR-154-5p was predicted by
starBase v2.0 database. (D) The luciferase reporter assay was
performed to determine the binding site. (E) PWT and (F) PWL were
detected after LV-miR-154-5p injection into CCI rats. Data are
shown as mean ± standard deviation based on at least three
independent experiments. *P<0.05, **P<0.01 vs. LV-NC CCI
group. MALAT, metastasis associated lung adenocarcinoma transcript;
miR, microRNA; CCI, chronic constriction injury; RT-qPCR, reverse
transcription-quantitative PCR; PWT, paw withdrawal threshold; PWL,
paw withdrawal latency; LV, lentivirus; NC, negative control; sh,
short hairpin; wt, wild type; mut, mutation. |
miR-154-5p overexpression inhibits
inflammatory responses in CCI rats
MALAT1 could directly bind with miR-154-5p; however,
the roles of miR-154-5p in CCI rats remained to be elucidated, so
the expression of miR-154-5p was detected in CCI rats. The results
demonstrated that the miR-154-5p of CCI rats was decreased in
spinal cord tissue and microglia with time, compared with the sham
group (Fig. 5A and B; P<0.05).
To further explore the roles of miR-154-5p in CCI rats, lentiviral
vector LV-miR-154-5p was constructed and injected into the rats.
After 14 days, spinal cord tissue and microglia were separated and
the levels of miR-154-5p, COX2, TNF-α and IL-6 were detected by
ELISA kits. The results demonstrated that miR-154-5p was
significantly increased in the LV-miR-154-5p group, which indicated
that LV-miR-154-5p was successfully transfected into CCI rats
(Fig. 5C and D; P<0.05). The
levels of COX2, TNF-α and IL-6 were significantly decreased in the
LV-miR-154-5p group (Fig. 5E and
F; P<0.05). These results suggested that miR-154-5p
overexpression inhibited inflammatory responses in CCI rats.
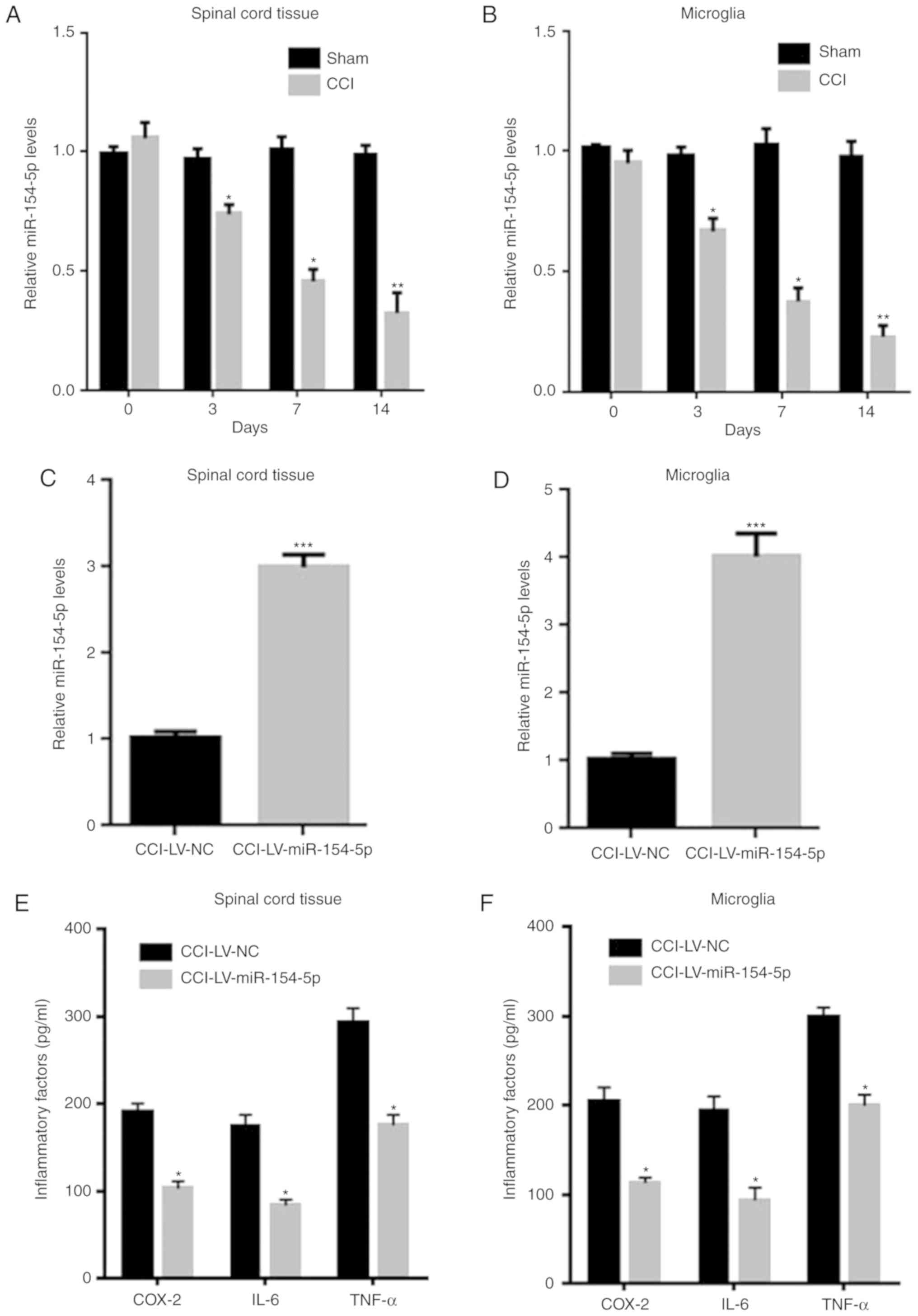 | Figure 5.miR-154-5p overexpression inhibited
inflammatory responses in CCI rats. The mRNA levels of miR-154-5p
in (A) spinal cord tissue and (B) microglia of sham and CCI rats
were detected by RT-qPCR. The mRNA levels of miR-154-5p in (C)
spinal cord tissue and (D) microglia were detected after
LV-miR-154-5p injection into CCI rats. Inflammatory cytokines in
(E) spinal cord tissue and (F) microglia were detected by ELISA.
Data are shown as mean ± standard deviation based on at least three
independent experiments, *P<0.05, **P<0.01, ***P<0.001 vs.
LV-NC CCI group. miR, microRNA; CCI, chro nic constriction injury;
RT-qPCR, reverse transcription-quantitative PCR; LV, lentivirus;
COX, cyclooxygenase; TNF, tumor necrosis factor; IL, interleukin;
NC, negative control. |
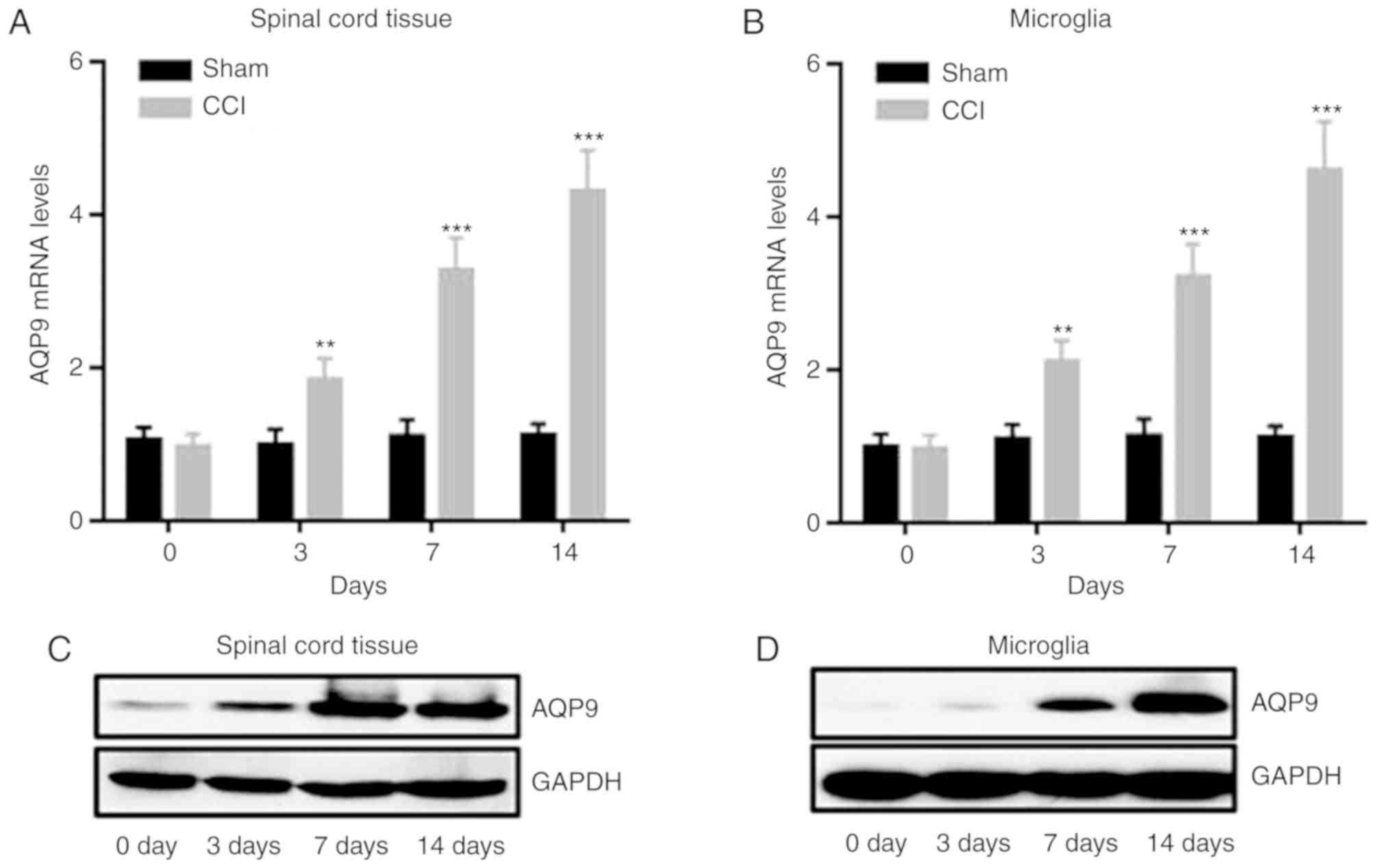
miR-154-5p can directly bind with
AQP9
To further explore the detailed mechanisms of
miR-154-5p in the inhibition of the inflammatory responses in CCI
rats, target genes were analyzed by the TargetScan database and
AQP9, a member of the aquaporins, was predicted to be the target
gene of miR-154-5p. Therefore, the expression of AQP9 was detected
in CCI rats. The results demonstrated that mRNA and protein levels
of AQP9 were significantly increased, while miR-154-5p was
decreased in CCI rats in spinal cord tissue and microglia with
time, compared with the sham group (Fig. 6A-F; P<0.05). To further explore
the binding site, the potential binding site was predicted. A
wt-AQP9 luciferase reporter vector (wt-AQP9) and a mut-AQP9 3′UTR
luciferase reporter vector (mut-AQP9) were constructed (Fig. 6G). Compared with control group, the
luciferase activity in co-transfection with miR-154-5p mimic and
wt-AQP9 was significantly decreased, while it was increased in
mut-AQP9 group in 293 cells (Fig.
6H; P<0.05). These data indicated that miR-154-5p could
directly bind with AQP9.
MALAT1 promotes neuropathic pain via
the miR-154-5p/AQP9 axis in CCI rats
From the above results, it was hypothesized that
MALAT1 might promote neuropathic pain through miR-154-5p/AQP9 axis
in CCI rats. To confirm this hypothesis, recombinant lentiviral
vector LV-shAQP9 was constructed and injected into CCI rats. AQP9
was detected by RT-qPCR and western blotting. The results
demonstrated that the expression of AQP9 was significantly
increased in spinal cord tissue and microglia of CCI rats compared
with the sham group, while it was significantly decreased in
LV-shAQP9 (Fig. 7A-F; P<0.05).
The mechanical pain threshold in each group of rats was evaluated
using PWT and PWL. The results demonstrated that the PWT and PWL of
CCI rats were significantly decreased with time, compared with the
sham group, but significantly increased in LV-shAQP9 group
(Fig. 7G and H; P<0.05), which
suggested that AQP9 could reduce the neuropathic pain. To further
explore whether MALAT1 interacted with AQP9 through miR-154-5p,
miR-154-5p inhibitor was added into LV-shMALAT1 rats and the
expressions of miR-154-5p and AQP9 were detected by RT-qPCR and
western blotting. The results demonstrated that miR-154-5p was
significantly upregulated following infection with shMALAT1, while
it was decreased in spinal cord tissue following the addition of
miR-154-5p inhibitor (Fig. 7I;
P<0.05). AQP9 expression was significantly decreased following
infecting with shMALAT1, while it was significantly increased
following the addition of miR-154-5p inhibitor (Fig. 7J and K; P<0.05) and the similar
results had been found in microglia (Fig. 7L-N; P<0.05). These results
indicated that MALAT1 promoted the occurrence of neuropathic pain
in CCI rats by targeting the miR-154-5p/AQP9 axis.
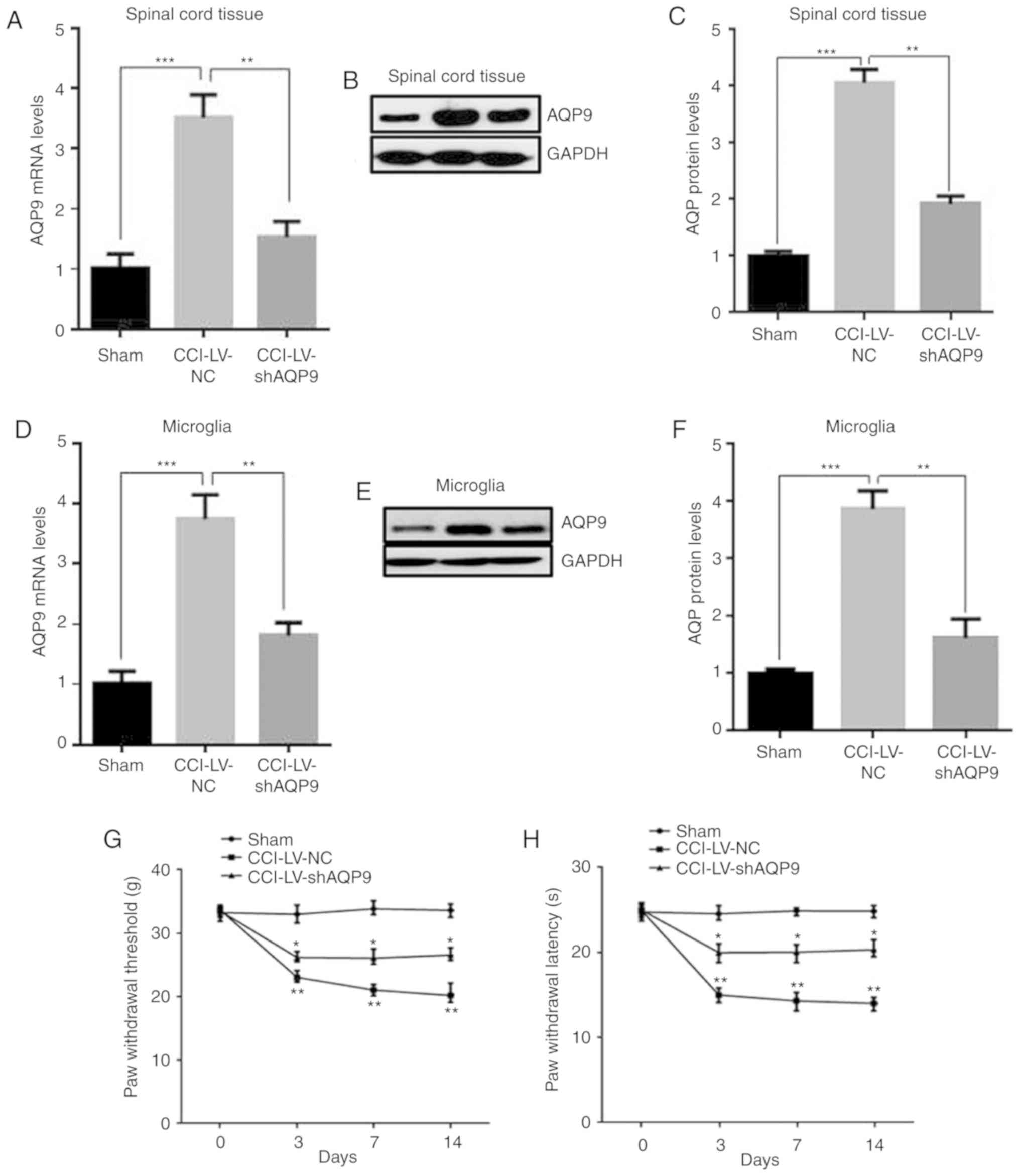 | Figure 7.MALAT1 promoted neuropathic pain
through miR-154-5p/AQP9 axis in CCI rats. mRNA levels of AQP9 in
(A) spinal cord tissue. Protein levels of (B) spinal cord tissue
with (C) subsequent quantification. (D) microglia detected after
LV-shAQP9 transfection using RT-qPCR. Protein levels of (E)
microglia with (F) subsequent quantification. (G) The mechanical
allodynia of PWT and (H) thermal hyperalgesia of PWL were detected.
Data are shown as mean ± standard deviation based on at least three
independent experiments. *P<0.05, **P<0.01 vs. LV-NC CCI
group; ***P<0.001 vs. sham group. MALAT1 promoted neuropathic
pain through miR-154-5p/AQP9 axis in CCI rats. The levels of
miR-154-5p were detected after adding LV-shAMALAT1 with miR-154-5p
inhibitor in (I) spinal cord tissue and (L) microglia. Protein
levels of AQP9 were detected in (J) spinal cord tissue and (M)
microglia of CCI rats using western blot analysis, with subsequent
quantification of levels in (K) spinal cord tissue and (N)
microglia. Data are shown as mean ± standard deviation based on at
least three independent experiments. **P<0.01 vs. LV-NC CCI
group; ***P<0.001 vs. sham group. MALAT, metastasis associated
lung adenocarcinoma transcript; miR, microRNA; AQP, aquaporin; CCI,
chronic constriction injury; LV, lentivirus; sh, short hairpin;
PWT, paw withdrawal threshold; PWL, paw withdrawal latency; NC,
negative control. |
Discussion
Neuropathic pain is a problem following spinal
surgery for patients and may be associated with abnormal sensations
called dysesthesia, or pain from normally non-painful stimuli,
called allodynia (1–3,30,31).
Although progress has been made in clinical treatment, a number of
patients still suffer chronic pain and psychological problems
(4). However, the molecular
mechanism of neuropathic pain remains to be elucidated.
In recent years, lncRNAs and miRNAs have attracted
much attentions due to their potential in the diagnosis and
treatment of neuropathic pain. Therefore, it is worth studying the
specific mechanism of non-coding RNAs in neuropathic pain. MALAT1
is widely considered to be involved in human cancer, vascular
disease and nervous system diseases (1–3,30,31).
However, the specific function of MALAT1 in neuropathic pain CCI
model rats is still unclear. The present study identified that
MALAT1 expression was upregulated in bone marrow tissue and
microglia in CCI rats, compared with the sham group. MALAT1 may
serve a role in a CCI model and in neuropathic pain.
To further investigate the function of MALAT1 in
neural system of CCI model rats, LV-shMALAT1 lentivirus was
administered by intrathecal injection and microglia were isolated
from the bone marrow tissue of each group. The results demonstrated
that upregulation of MALAT1 in the CCI model promoted neuropathic
pain in rats, whereas inhibition of MALAT1 reduced the incidence of
neuropathic pain. The levels of inflammatory cytokines COX2, TNF-α,
and IL-6 were significantly increased in the CCI model group, while
these inflammatory factors were significantly decreased in the
MALAT1 inhibition model. These results indicated that inhibition of
MALAT1 reduced the levels of inflammatory cytokines and mitigated
inflammatory responses in CCI rats.
To explore the molecular mechanism by which MALAT1
regulated the neuropathic pain in rats and using starBase v2.0, it
was hypothesized that there was a potential binding site between
MALAT1 and miR-154-5p. Then the levels of miR-154-5p and MALAT1
were detected by RT-qPCR. The results demonstrated that MALAT1 was
decreased in the LV-shMALAT1 group, while miR-154-5p was
respectively increased, which may suggest that MALAT1 was
negatively correlated with miR-154-5p. To further explore whether
MALAT1 bound with miR-154-5p, the wt-MALAT1 luciferase reporter
vector and a mut-MALAT1 3′UTR luciferase reporter vector were
constructed and the luciferase reporter gene assay was performed.
The results demonstrated that MALAT1 could directly bind with
miR-154-5p. However, the roles of miR-154-5p in CCI rats remained
unclear.
It has been identified that the dysregulation of
miR-154-5p is common in a number of cancers, including colorectal
cancer, hepatocellular carcinoma and prostate cancer (18,32–34).
Studies have reported that miRNA-154-5p can inhibit the
proliferation, migration and invasion of prostate cancer cells by
targeting E2F5 (18), and
miRNA-154-5p inhibits cell proliferation and metastasis through
targeting PIWIL1 (19). However,
the role of miR-154-5p in neuropathic pain remains to be
elucidated. Therefore, the expression of miR-154-5p was detected in
CCI rats. The results demonstrated that miR-154-5p in CCI rats was
decreased in spinal cord tissue and microglia time-dependently. To
further explore the roles of miR-154-5p in CCI rats, lentiviral
vector LV-miR-154-5p was constructed and injected into rats. The
levels of miR-154-5p, COX2, TNF-α and IL-6 were detected. The
results demonstrated that miR-154-5p was significantly increased in
LV-miR-154-5p and the levels of COX2, TNF-α and IL-6 were
significantly decreased in LV-miR-154-5p group, suggesting that
miR-154-5p overexpression inhibited inflammatory responses in CCI
rats.
To further explore the detailed mechanism of
miR-154-5p in inhibiting the inflammatory responses in CCI rats,
target genes were analyzed by the TargetScan database and AQP9 was
predicted to be a target gene of miR-154-5p. It was identified that
the mRNA and protein levels of AQP9 in CCI rats were significantly
increased in spinal cord tissue and microglia time-dependently. A
wt-AQP9 luciferase reporter vector and a mut-AQP9 3′UTR luciferase
reporter vector were constructed and the luciferase reporter gene
assay was performed. The results demonstrated that miR-154-5p could
directly bind with AQP9.
SCI is one of the most debilitating neurological
conditions and most SCI patients remain in a chronic phase. As the
specific molecular mechanism of neuropathic pain is unclear, there
is still no effective intervention and treatment. A previous study
demonstrated that MALAT1 has a protective neuroprotective effect
through regulating with miR-204 (15). The present study demonstrated that
the inhibition of MALAT1 in CCI rats could reduce neuropathic pain
and neuroinflammation, suggesting that MALAT1 might be used as a
new target for relieving neuropathic pain in humans and that it
might provide a new intervention strategy for patients with SCI;
however, this should be verified in human SCI patients.
Previous studies have demonstrated that the
aquaporin family serve an important role in SCI (35,36).
AQP1/4/9 is expressed in a variety of cells such as astrocytes,
neurons, and ependymal cells, and AQP1/4/9 in the spinal cord is
involved in edema caused by SCI (35). AQP1 levels were significantly
elevated in the SCI-induced injury site in rats, and AQP1 and
mechanical ectopic pain were significantly decreased following
melatonin treatment, suggesting that AQP1 may be associated with
chronic neuropathic pain following SCI (36). As a member of the aquaporin family,
AQP9 may also serve a role in neuropathic pain following SCI, but
the specific function and mechanism of action are unknown. The
present study identified that AQP9 expression was significantly
upregulated in CCI rats and that neuropathic pain was attenuated
following inhibition of AQP9, which was regulated through
MALAT1/miR-154-5p axis. This suggested that the aquaporin family
might be an important reference intervention target for the
treatment of neuropathic pain in humans, but this also needs
verification in human SCI patients.
Taken together, the results of the present study
suggested that MALAT1/miR-154-5p/AQP9 served an important role in
neuropathic pain and that the downregulation of MALAT1 attenuates
neuropathic pain and inhibits neuroinflammation. In addition,
MALAT1 inhibited neuropathic pain in rats by targeting the binding
of miR-154-5p to inhibit AQP9 expression, which provided a
potential prognostic marker and a potential target for neuropathic
pain.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD designed the present study. JW and CW performed
the experiments. JW analyzed the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was carried out in strict
accordance with the requirements in the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health. The
experiments were carried out strictly in line with the requirements
of the International Association for the Study of Pain (IASP). The
present study was approved by the ethics committee of Lishui
Municipal Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thomson S: Failed back surgery
syndrome-definition, epidemiology and demographics. Br J Pain.
7:56–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomson S and Jacques L: Demographic
characteristics of patients with severe neuropathic pain secondary
to failed back surgery syndrome. Pain Pract. 9:206–215. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu XG, Pang RP, Zhou LJ, Wei XH and Zang
Y: Neuropathic pain: Sensory nerve injury or motor nerve injury?
Adv Exp Med Biol. 904:59–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yalbuzdag SA, Erol AM, Sengul I, Celik C,
Solum S, Adilay HU and Gungor B: Temperament and character profile
in failed back surgery syndrome: A cross-sectional clinical study.
Turk Neurosurg. 26:912–917. 2016.PubMed/NCBI
|
|
5
|
Ellis A and Bennett DL: Neuroinflammation
and the generation of neuropathic pain. Br J Anaesth. 111:26–37.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadav R and Weng HR: EZH2 regulates spinal
neuroinflammation in rats with neuropathic pain. Neuroscience.
349:106–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hauptman N and Glavac D: MicroRNAs and
long non-coding RNAs: Prospects in diagnostics and therapy of
cancer. Radiol Oncol. 47:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morris KV and Mattick JS: The rise of
regulatory RNA. Nat Rev Genet. 15:423–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu S, Xie R, Liu X, Shou J, Gu W and Che
X: Long coding RNA XIST contributes to neuronal apoptosis through
the downregulation of AKT phosphorylation and is negatively
regulated by miR-494 in rat spinal cord injury. Int J Mol Sci.
18:E7322017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li ZX, Zhu QN, Zhang HB, Hu Y, Wang G and
Zhu YS: MALAT1: A potential biomarker in cancer. Cancer Manag Res.
10:6757–6768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y and Ma L: New insights into long
non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel).
11:E2162019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mei H and Liu Y, Zhou Q, Hu K and Liu Y:
Long noncoding RNA MALAT1 acts as a potential biomarker in cancer
diagnosis and detection: A meta-analysis. Biomark Med. 13:45–54.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia C, Liang S, He Z, Zhu X, Chen R and
Chen J: Metformin, a first-line drug for type 2 diabetes mellitus,
disrupts the MALAT1/miR-142-3p sponge to decrease invasion and
migration in cervical cancer cells. Eur J Pharmacol. 830:59–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Wu H, Wang F, Ye M, Zhu H and Bu
S: Long non-coding RNA MALAT1 expression in patients with
gestational diabetes mellitus. Int J Gynaecol Obstet. 140:164–169.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao Y, Peng C, Li J, Wu D and Wang X:
LncRNA MALAT1 is neuroprotective in a rat model of spinal cord
ischemia-reperfusion injury through miR-204 regulation. Curr
Neurovasc Res. 15:211–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie X, Ma L, Xi K, Zhang W and Fan D:
MicroRNA-183 suppresses neuropathic pain and expression of AMPA
receptors by targeting mTOR/VEGF signaling pathway. Cell Physiol
Biochem. 41:181–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Xu J, Zhu R and Liu L:
Down-regulation of miRNA-128 contributes to neuropathic pain
following spinal cord injury via activation of P38. Med Sci Monit.
23:405–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng Y, Zhu C, Ma L, Shao P, Qin C, Li P,
Cao Q, Ju X, Cheng G, Zhu Q, et al: miRNA-154-5p inhibits
proliferation, migration and invasion by targeting E2F5 in prostate
cancer cell lines. Urol Int. 98:102–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Sun S, Tong X, Ma Q, Di H, Fu T,
Sun Z, Cai Y, Fan W, Wu Q, et al: MiRNA-154-5p inhibits cell
proliferation and metastasis by targeting PIWIL1 in glioblastoma.
Brain Res. 1676:69–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damiano A, Zotta E, Goldstein J, Reisin I
and Ibarra C: Water channel proteins AQP3 and AQP9 are present in
syncytiotrophoblast of human term placenta. Placenta. 22:776–781.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jelen S, Parm Ulhøi B, Larsen A, Frøkiær
J, Nielsen S and Rützler M: AQP9 expression in glioblastoma
multiforme tumors is limited to a small population of astrocytic
cells and CD15(+)/CalB(+) leukocytes. PLoS One. 8:e757642013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv Y, Huang Q, Dai W, Jie Y, Yu G, Fan X,
Wu A and Miao Q: AQP9 promotes astrocytoma cell invasion and
motility via the AKT pathway. Oncol Lett. 16:6059–6064.
2018.PubMed/NCBI
|
|
23
|
Huang D, Feng X, Liu Y, Deng Y, Chen H,
Chen D, Fang L, Cai Y, Liu H, Wang L, et al: AQP9-induced cell
cycle arrest is associated with RAS activation and improves
chemotherapy treatment efficacy in colorectal cancer. Cell Death
Dis. 8:e28942017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung JM, Kim HK and Chung K: Segmental
spinal nerve ligation model of neuropathic pain. Methods Mol Med.
99:35–45. 2004.PubMed/NCBI
|
|
25
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press (US); Washington, DC: 2011
|
|
26
|
Breivik H: International Association for
the study of pain: Update on WHO-IASP activities. J Pain Symptom
Manage. 24:97–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beltramo M, Bernardini N, Bertorelli R,
Campanella M, Nicolussi E, Fredduzzi S and Reggiani A: CB2
receptor-mediated antihyperalgesia: Possible direct involvement of
neural mechanisms. Eur J Neurosci. 23:1530–1538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torrance N, Smith BH, Watson MC and
Bennett MI: Medication and treatment use in primary care patients
with chronic pain of predominantly neuropathic origin. Fam Pract.
24:481–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Torrance N, Smith BH, Bennett MI and Lee
A: The epidemiology of chronic pain of predominantly neuropathic
origin. Results from a general population survey. J Pain.
7:281–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiao W, Cao N and Yang L: MicroRNA-154
inhibits the growth and metastasis of gastric cancer cells by
directly targeting MTDH. Oncol Lett. 14:3268–3274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xin C, Zhang H and Liu Z: miR-154
suppresses colorectal cancer cell growth and motility by targeting
TLR2J. Mol Cell Biochem. 387:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pang X, Huang K, Zhang Q, Zhang Y and Niu
J: miR-154 targeting ZEB2 in hepatocellular carcinoma functions as
a potential tumor suppressor. Oncol Rep. 34:3272–3279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halsey AM, Conner AC, Bill RM, Logan A and
Ahmed Z: Aquaporins and their regulation after spinal cord injury.
Cells. 7:E1742018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nesic O, Lee J, Unabia GC, Johnson K, Ye
Z, Vergara L, Hulsebosch CE and Perez-Polo JR: Aquaporin 1-a novel
player in spinal cord injury. J Neurochem. 105:628–640. 2008.
View Article : Google Scholar : PubMed/NCBI
|