Introduction
Liver fibrosis continues to be a major health
problem worldwide and is associated with significant morbidity and
mortality. If left untreated, fibrosis will develop into liver
cirrhosis, leading to organ failure and ultimately death (1,2). The
cytokine connective tissue growth factor (CTGF) is key in promoting
liver fibrosis and plays an important role in the pathogenesis of
liver fibrosis (3). Despite the
advances in our understanding of the pathogenesis of fibrotic
processes, the therapeutic effect of drugs used in the treatment of
liver fibrosis is insufficient. Therefore, there is an urgent need
for specific and effective antifibrotic therapies.
Gene therapy is a novel therapeutic tool that may
provide a solution to managing chronic diseases and genetic
disorders. RNA interference is a novel therapeutic strategy that
may aid in the management of liver fibrosis through silencing the
expression of specific genes in diseased cells (4,5). Its
capacity to effectively knock down the target gene with high
sequence specificity makes the use of small interfering RNA (siRNA)
a promising therapeutic strategy. In previous years, there has been
considerable interest in siRNA-based gene therapy for the treatment
of liver fibrosis (6,7). However, gene delivery is a major
challenge in gene therapy. Currently, viral vectors remain the
primary gene delivery system utilized in gene therapy. However,
they present important limitations, including non-specificity,
immunogenicity to target cells, toxicity and enzymatic degradation
(8). Therefore, the development of
non-viral carriers is of considerable interest at present.
Currently, a wide variety of non-viral carriers have
been developed, and mainly lipids, polymers and nanoparticles (NPs)
have been utilized as siRNA delivery systems (9). NPs, which are nanostructured entities
with adaptable size, shape, surface and biological properties, have
been widely used in biomedical applications and diagnosis (10,11).
Numerous NP core material formulations, including gold, silica,
carbon, semiconductors and metal oxides, are being evaluated as
potential siRNA carriers (12–16).
Among them, magnetic iron oxide (Fe3O4) NPs
are emerging as one of the most potent non-viral gene carriers, and
have extensive applications in various biomedical fields due to
their high efficiency, low toxicity and relatively simple
experimental use (17–20).
In the present study, human hepatic stellate cells
LX-2 were selected as target cells of fibrosis to analyze the
antifibrotic efficiency of polyethyleneimine (PEI)-functionalized
Fe3O4 NPs combined with CTGF siRNA, which may
serve as a basis for the development of future therapeutics for
hepatic fibrosis.
Materials and methods
Characterization of
PEI-Fe3O4 NPs
PEI-Fe3O4 NPs (Nanjing
Nanoeast Biotech Co., Ltd.) were used as carriers for siRNA
delivery. The NPs were dispersed in water at pH 7.4, obtaining a
final concentration of 1 mg/ml, a hydrodynamic diameter of 48±5 nm
and a ζ-value of +30.5±4.87 mV. The morphology of
PEI-Fe3O4 NPs and
PEI-Fe3O4/siRNA was characterized using a
transmission electron microscope (TEM). Samples for TEM imaging
were prepared by placing a drop of the NP suspension onto a copper
grid and drying it at ambient temperature overnight. The samples
were then visualized with a H-7000FA microscope, operating at 75 kV
and 200,000× magnification (Hitachi, Ltd.).
Preparation of
PEI-Fe3O4/siRNA complexes
siRNA targeting human CTGF (Genbank accession number
NM-001901) was synthesized and purified by Shanghai GenePharma Co.,
Ltd. It is a 21-bp double-stranded RNA oligo and has the following
sequence: Sense 5′-CCGACUGGAAGACACGUUUTT-3′ and antisense
5′-AAACGUGUCUUCCAGUCGGTT-3′. In addition, a cyanine (Cy)3-labeled
negative control (NC) siRNA was used for evaluating the efficiency
of transfection (sense 5′-Cy3-UUCUCCGAACGUGUCACGUTT-3′ and
antisense 5′-ACGUGACACGUUCGGAGAATT-3′). A NC siRNA duplex was also
prepared with the same sequence but without Cy3 labeling. The siRNA
was complexed with PEI-Fe3O4 at different
weight ratios of Fe to siRNA ranging from 0:1 to 64:1. The
resulting mixture was incubated at room temperature for 30 min to
allow composite formation. All complexes in the solution were
freshly prepared prior to subsequent experimentation.
Gel retardation assay
NC siRNA was mixed with
PEI-Fe3O4 at various Fe:siRNA weight ratios.
While maintaining a uniform concentration of NC siRNA, samples of
PEI-Fe3O4:siRNA complexes were prepared at
weight ratios of 0:1, 2:1, 4:1, 8:1, 16:1, 32:1 and 64:1 (Fe mass
to NC siRNA mass). The resulting mixtures were incubated at room
temperature for 30 min. Then, 20 µl of samples were loaded into 3%
w/v agarose gel. The siRNA bands were visualized by 3% agarose gel
electrophoresis stained with ethidium bromide (EtBr) and images
were captured under UV illumination.
Heparin decomplexation assay
NC siRNA was complexed with
PEI-Fe3O4 at a Fe:siRNA weight ratio of 8:1
for 30 min at room temperature. Various amounts of heparin (LEO
Pharma A/S) were added (heparin:siRNA weight ratio of 0:1, 25:1,
50:1, 100:1, 200:1 and 400:1) and the mixtures were further
incubated at room temperature for 30 min. After centrifugation for
15 min at 12,000 × g at 4°C, the supernatants were analyzed by 3%
agarose gel electrophoresis and the released siRNA was visualized
by EtBr staining and photographed under ultraviolet
illumination.
Serum protection assay
NC siRNA-loaded PEI-Fe3O4
complexes prepared at a Fe:siRNA weight ratio of 8:1 were selected
for a serum protection assay. Complexes containing 0.5 µg siRNA
were incubated with Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences) supplemented with 50% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) for 2, 4,
8, 16 and 24 h at 37°C. The siRNA remaining in the complex was
displaced with heparin at a heparin/siRNA weight ratio of 200:1 to
ensure complete release of siRNA. Naked siRNA acted as a control
and was treated in the same manner. The released siRNA was
visualized by 3% agarose gel electrophoresis stained with EtBr.
Culture of LX-2 cells
The human hepatic stellate cell line LX-2 was
obtained from Procell Life Science & Technology Co., Ltd. LX-2
cells were routinely maintained in DMEM supplemented with 10% FBS
and 1% (v/v) penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere in 5%
CO2.
Cytotoxicity assay
The cytotoxicity of PEI-Fe3O4
and NC siRNA complexes was evaluated by MTT assay. LX-2 cells were
treated with PEI-Fe3O4/siRNA complexes and
PEI-Fe3O4 NPs at concentrations 10–80 µg
Fe/ml. After incubation for 6 h, the culture medium was replaced
with fresh medium, and the cells were cultured for another 24 or 48
h. Then, LX-2 cells were incubated with MTT (Sigma-Aldrich; Merck
KGaA) for an additional 4 h. Next, the medium in each well was
removed and 100 µl/well dimethyl sulfoxide was added to dissolve
the internalized purple formazan crystals. The optical density at
490 nm was measured using a microplate reader (BioTek Instruments,
Inc.). All experiments were performed in triplicate and repeated ≥3
times.
Transfection efficiency
A Cy3-labeled siRNA (red fluorescence) was used to
verify transfection efficiency. The transfection efficiency of
PEI-Fe3O4 NPs was measured and compared with
that of the naked siRNA and the standard transfection reagent
Lipofectamine® 2000 (Lipo 2000; Invitrogen; Thermo
Fisher Scientific, Inc.). LX-2 cells were seeded into a 6-well
plate (1.5×105 cells/well) and treated with
PEI-Fe3O4/Cy3-siRNA at a concentration of 15
µg Fe/ml and Cy3-siRNA at a 100 nM siRNA concentration at 37°C for
2 h. Magnetofection was performed by placing a magnetic sheet
(Nanjing Nanoeast Biotech Co., Ltd.) under the plates for 4 h. The
medium was replaced after 6 h, and the transfection efficiency was
evaluated after 24 h. As a control experiment, the cells were also
treated with Lipo 2000/Cy3-siRNA complexes. The transfection
procedures were performed in accordance with the manufacturer's
protocols. The transfection efficacy was evaluated by observation
of the red fluorescent cells vs. total cells using fluorescence
microscopy (magnification, ×200) and flow cytometry (BD
Biosciences). Briefly, after transfection, the cells were rinsed
with phosphate-buffered saline (PBS, pH 7.4), harvested, fixed in
4% formaldehyde solution at room temperature for 10 min, and
subsequently analyzed by flow cytometer (Accuri C6 flow cytometer,
BD Biosciences). Data analyses were performed using the FlowJo 10.2
software package (FlowJo LLC). The fixed cells were stained with 1
µg/ml DAPI (Sigma-Aldrich; Merck KGaA) at room temperature for 5
min. The fluorescence images were obtained with a Leica DMI 3000B
fluorescence microscope (Leica Microsystems Inc.) magnification,
×200. The experiments were repeated ≥3 times.
Intracellular localization of siRNA in
LX-2 cells
To determine successful siRNA internalization, LX-2
cells (1.5×105) were seeded in a 6-well tissue culture
plate and cultured for 24 h (70–80% confluence). Lipo 2000 was used
as a positive control for siRNA transfection, and the transfection
procedure was performed in accordance with the manufacturer's
protocols. The cells in each well were incubated at 37°C for 6 h in
medium with 10% FBS and free Cy3-siRNA (100 nM), or with
PEI-Fe3O4/Cy3-siRNA (15 µg Fe/ml). Upon
incubation, the cells were washed twice with PBS (pH 7.4) and fixed
for 10 min in PBS containing 4% (w/v) formaldehyde (pH 7.4).
Following two further PBS washing steps, the cell nuclei were
stained with 500 µl DAPI (1 µg/ml; Sigma-Aldrich; Merck KGaA) in
PBS for 5 min. Then, the cells were examined under a confocal
microscope magnification, ×1200 (TCS SP5; Leica Microsystems
GmbH).
Transforming growth factor (TGF)-β1
treatment
LX-2 cells were seeded in a 24-well plate at a
density of 1×106 cells/well and grown for 24 h until
they reached 70–80% confluence. In total, three separate
experimental groups were studied: Blank (no reagent) + TGF-β1
(PeproTech, Inc.); NC siRNA transfection + TGF-β1; and CTGF siRNA
transfection + TGF-β1 groups. Freshly prepared
PEI-Fe3O4/siRNA complexes (Fe:siRNA weight
ratio of 8:1) at a concentration of 15 µg Fe/ml were incubated with
the cells under standard culture conditions. After 6 h of
transfection, the culture medium was removed from each well and
replaced by medium with TGF-β1 (final concentration of 5 ng/ml) for
24 or 48 h.
Immunocytochemical staining for
α-smooth muscle actin (SMA)
Immunocytochemical staining for α-SMA in LX-2 cells
was carried out using a horseradish peroxidase-conjugated
streptavidin system (Dako; Agilent Technologies, Inc.). In brief,
the cells were fixed in 4% formaldehyde for 30 min at 37°C and then
washed three times with PBS. Subsequently, cells were blocked with
3% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 30 min at
room temperature. The cells were treated with 1:100 dilution of the
primary antibody, α-SMA monoclonal antibody (cat. no. 412021;
Nichirei Corporation) at 4°C overnight. The cells were washed in
PBS and then incubated with a biotinylated secondary antibody
(diluted 1:100, cat. no. 5220-0336, Seracare Life Sciences) and
streptavidin peroxidase for 60 min at room temperature.
Subsequently, the cells were developed with DAB and
H2O2, followed by counterstaining with
hematoxylin for 3 min at room temperature (Sigma-Aldrich; Merck
KGaA). Then, images were captured of the cells via an Olympus IX71
inverted microscopy (Olympus Corporation, magnification ×400).
Reverse transcription-quantitative PCR
(RT-qPCR)
After treatment for 48 h, total RNA was extracted
from LX-2 cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. RNA samples were converted into first-strand cDNA with
PrimeScript™ RT Reagent kit with gDNA Eraser (Takara Bio, Inc.).
RT-qPCR analysis was performed with the StepOne™ Real-Time PCR
Detection System (Thermo Fisher Scientific, Inc.) using
SYBR® Premix Ex Taq™ (Takara Bio, Inc.). The primer
sequences used to amplify the desired cDNA were as follows: CTGF
forward 5′-GGAAAAGATTCCCACCCAAT-3′, and reverse
5′-TGCTCCTAAAGCCACACCTT-3′; type collagen I forward,
5′-CCCGGGTTTCAGAGACAACTTC-3′, and reverse
5′-TCCACATGCTTTATTCCAGCAAT-3′; tissue inhibitor of matrix
metalloproteinase-1 (TIMP-1) forward, 5′-GCTTCTGGCATCCTGTTGTTG-3′,
and reverse, 5′-CTTCTGGTGTCCCCACGAACT-3; and β-actin forward,
5′-AGAGCTACGAGCTGCCTGAC-3′, and reverse 5′-AGCACTGTGTTGGCGTACAG-3′.
The thermal cycling conditions were as follows: cDNA synthesis, 15
min at 50°C and 5 sec at 85°C; reverse transcriptase inactivation,
10 min at 95°C; thermal cycling and detection (up to 40 cycles), 15
sec at 95°C and 60 sec at 60°C (data collection). The mRNA
expression of the target gene was evaluated against β-actin mRNA.
The quantification cycle (Cq) value was calculated using the
2−ΔΔCq method (21)
(IQ5 software version 2.0; Bio-Rad Laboratories, Inc.). The
experiments were repeated three times.
Western blot analysis
Western blotting was performed on the total protein
extracts of the LX-2 cells 48 h after treatment. For the total
protein fraction, the harvested cells were washed three times with
ice-cold PBS and lysed in RIPA buffer (Thermo Fisher Scientific,
Waltham, MA, USA), and then centrifuged at 12,000 × g at 4°C for 30
min. The total protein concentration of the supernatant was
determined using the Bradford method with a Bio-Rad protein assay
kit II (Bio-Rad Laboratories, Inc.). Briefly, equal amounts (30 µg)
of protein samples were separated by 10% SDS-PAGE and subsequently
electrotransferred onto polyvinylidene fluoride membranes (EMD
Millipore). The membranes were blocked with 5% fat-free milk in PBS
plus 0.05% (v/v) Tween-20 at 37°C for 2 h. After blocking, the
membranes were incubated with 1:1,000 dilution of anti-CTGF (cat.
no. ab6992; Abcam), anti-TIMP-1 (cat. no. ab61224; Abcam),
anti-collagen I (cat. no. ab34710; Abcam) and anti-β-actin (cat.
no. ab8226; Abcam) antibodies overnight at 4°C with gentle rocking.
After 3 further washing steps with TBS, the membranes were
incubated with 1:5,000-diluted horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (cat. no. SA00001-2;
ProteinTech Group, Inc.). The antibody-antigen complexes were
visualized using the enhanced chemiluminescence detection system
(Pierce™ ECL Plus Western Blotting Substrate; Thermo Fisher
Scientific, Inc.). All western blotting experiments were performed
≥3 times. Western blot data were quantitatively analyzed using
Image Lab 2.0 software (Bio-Rad Laboratories, Inc.). The relative
protein abundance in each sample was normalized to that of
β-actin.
Statistical analysis
The results were expressed as the mean ± SD. One-way
ANOVA followed by Tukey's post hoc test was carried out for the
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS 16.0 software (SPSS, Inc.).
Results
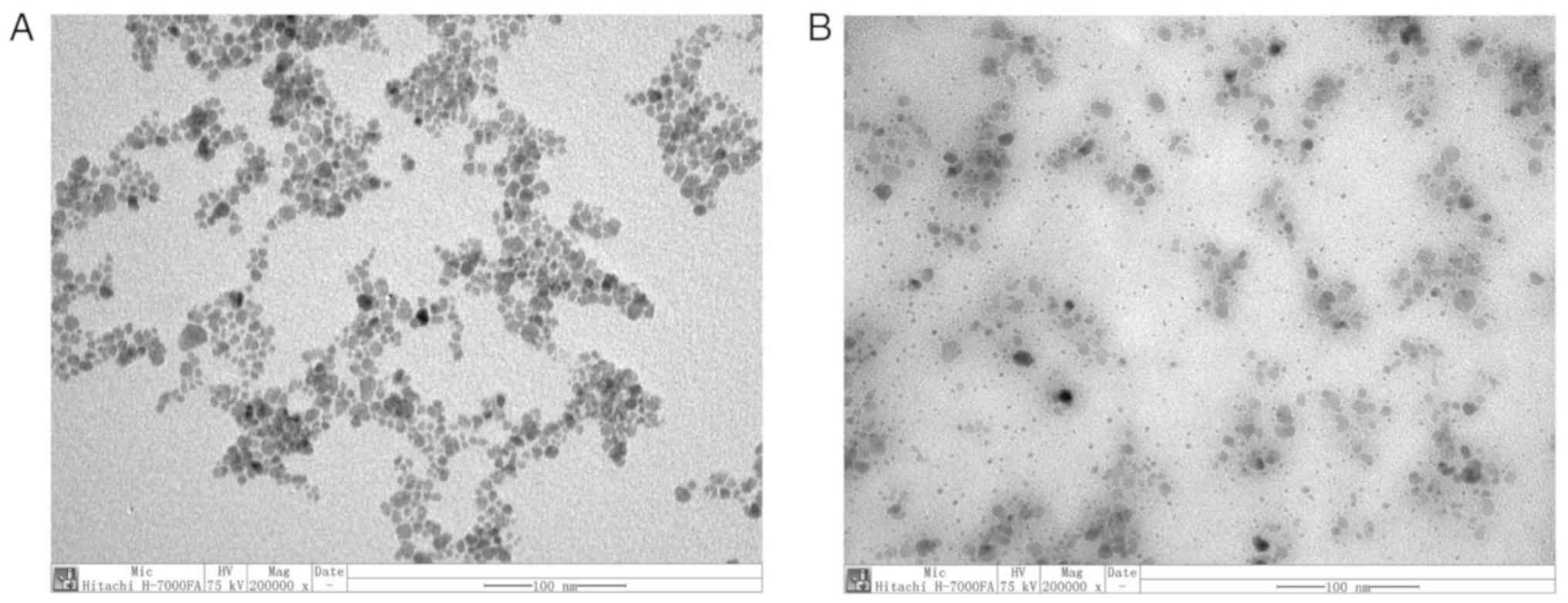
TEM images of
PEI-Fe3O4 NPs and
PEI-Fe3O4/siRNA complexes
TEM observations were performed to assess the
morphology of PEI-Fe3O4 NPs and their
association with siRNA. The images indicated that
PEI-Fe3O4 NPs and
PEI-Fe3O4/NC siRNA complexes (Fe:siRNA weight
ratio of 8:1) were of spherical morphology and well-dispersed
(Fig. 1).
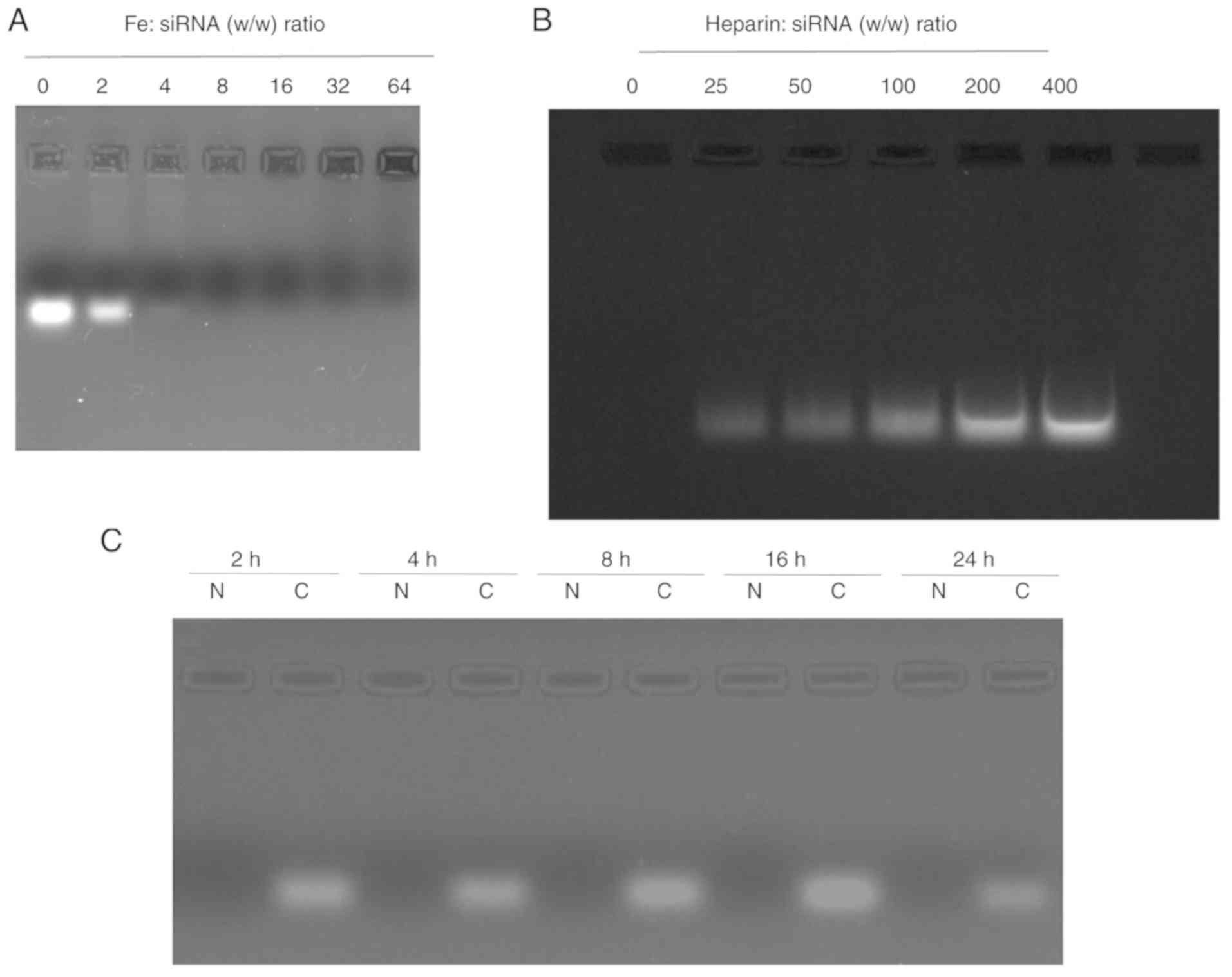
siRNA-loading capacity of
PEI-Fe3O4 NPs
The migration of naked NC siRNA and
PEI-Fe3O4/siRNA complexes at weight ratios
ranging from 0:1 to 64:1 are shown in Fig. 2A. The siRNA band tended to
disappear in the gel as the weight ratio of
PEI-Fe3O4 to siRNA increased, indicating that
binding occurred. When the ratio of Fe mass to siRNA mass was
>4, the migration of siRNA was completely blocked, indicating
that the siRNA was largely complexed with the
PEI-Fe3O4 NPs. At a weight ratio of 8:1, no
free siRNA was detected in the gel retardation assay. Therefore,
the PEI-Fe3O4 NPs exhibited stable binding
capability. In the following experiments, 8:1 was selected as the
optimized ratio of PEI-Fe3O4/siRNA
complexes.
Heparin induces complex
dissociation
To investigate the effect of
PEI-Fe3O4 NPs on complex dissociation,
PEI-Fe3O4/siRNA complexes were challenged by
exposure to different quantities of heparin. As expected, the
complex was dissociated in the presence of negatively charged
heparin molecules, and the amount of siRNA released increased with
increasing levels of heparin (Fig.
2B).
Serum protection assay
As shown in Fig.
2C, naked NC siRNA degradation was completely degraded within 2
h of incubation. By contrast, the
PEI-Fe3O4/siRNA complexes displayed
significant stability under the same conditions, with no notable
degradation detected within 24 h. Therefore, these data suggested
that PEI-Fe3O4 had strong siRNA affinity and
effectively protected siRNA from the enzymatic activity of serum
components.
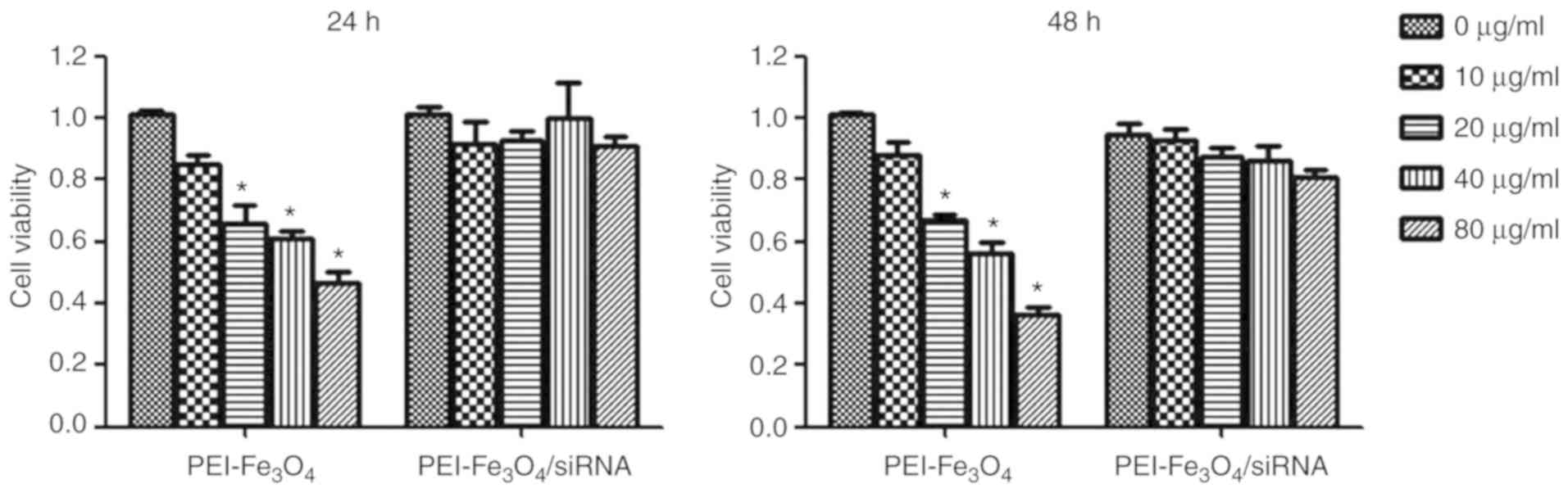
Cytotoxicity assay
The MTT results indicated that the
PEI-Fe3O4/siRNA complexes had no obvious
cytotoxic effect on LX-2 cells when added in the range of 0–80 µg
Fe/ml for 24 or 48 h, while empty PEI-Fe3O4
exhibited a significant cytotoxicity on LX-2 cells in a
concentration-dependent manner (Fig.
3).
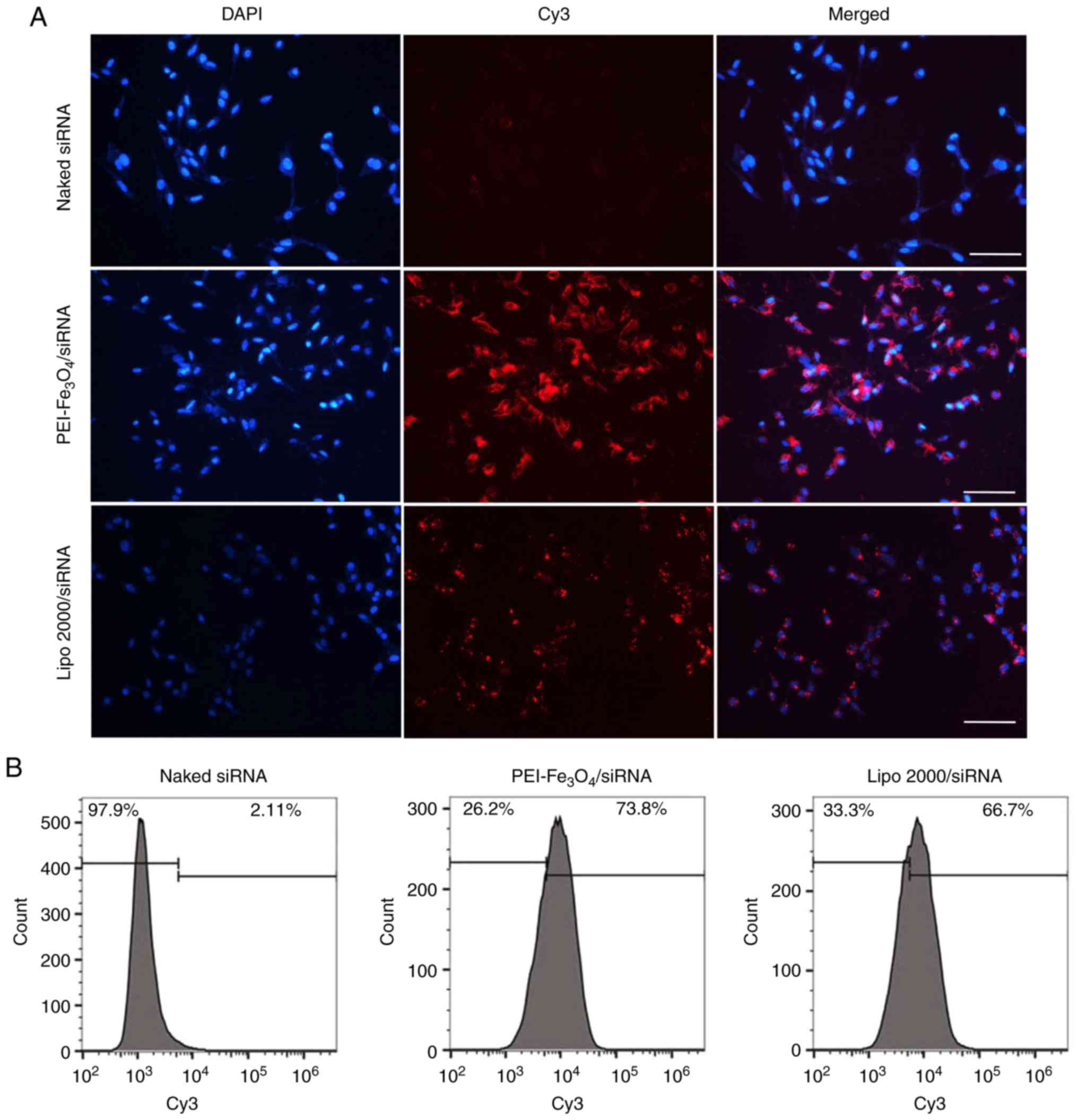
Transfection efficiency
Fluorescence microscopy was performed to examine
cellular uptake of siRNA when complexed with
PEI-Fe3O4 NPs. When the cells were
transfected with PEI-Fe3O4/Cy3-siRNA
complexes for 6 h, red fluorescence was observed inside the cells
exposed to the complexes (Fig.
4A), demonstrating that Cy3-siRNA has been delivered into the
cells. To exclude nonspecific uptake of siRNA, the cells were
incubated with Cy3-siRNA alone. However, no siRNA-derived
fluorescence was observed inside the cells.
Transfection efficiency was assessed by quantifying
the number of red fluorescence-expressing cells with flow
cytometry. As shown in Fig. 4B,
siRNA alone translated into almost no uptake in LX-2 cells, which
also confirmed the limitation of siRNA alone in cellular uptake.
PEI-Fe3O4 NPs achieved 73.8% uptake of siRNA,
which is comparable with that of Lipo 2000 (liposome transfection,
66.7%), which is one of the most efficient commercially available
transfection reagents.
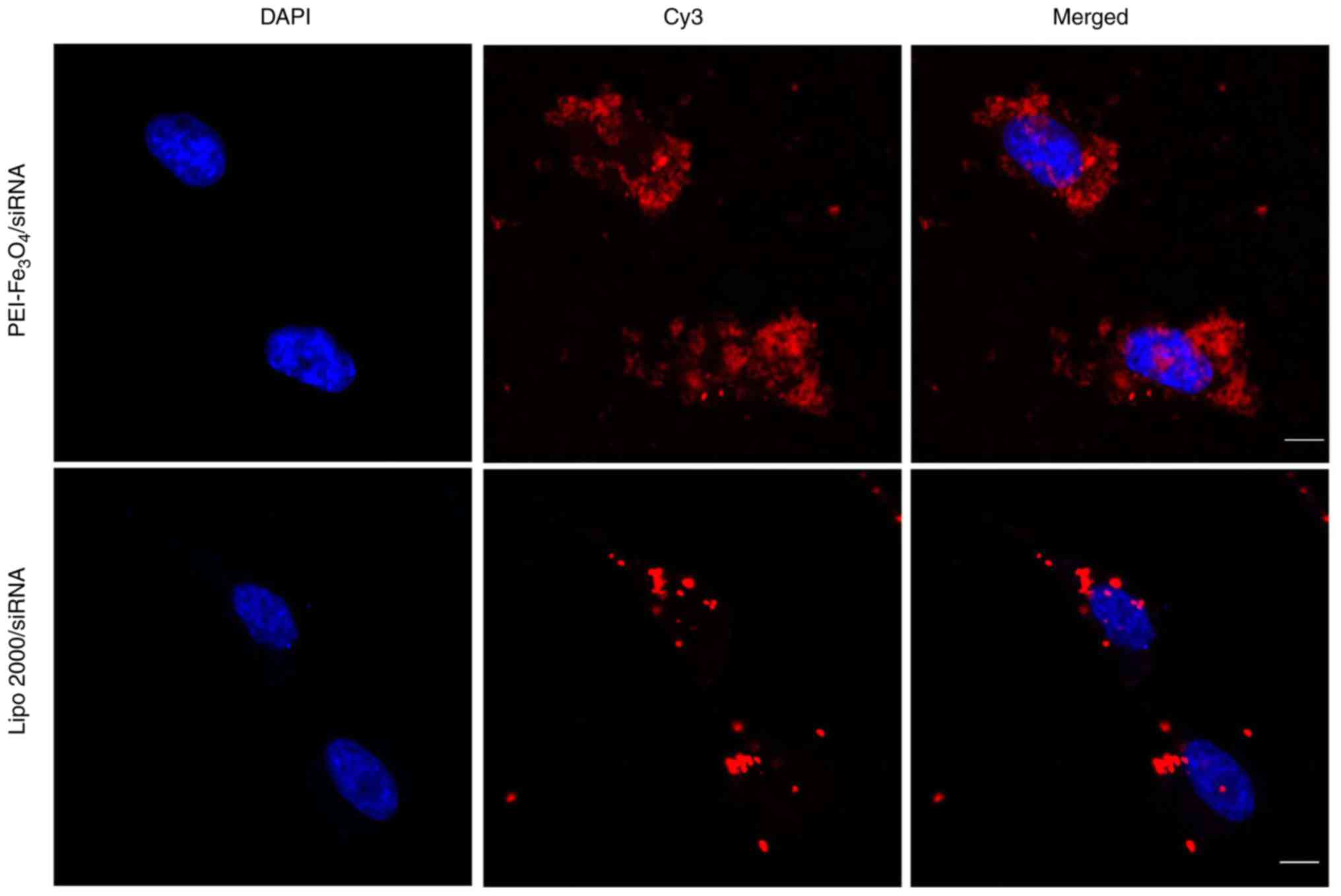
Intracellular localization of
Cy3-siRNA in LX-2 cells
The cellular localization of siRNA is shown in
Fig. 5. The overlay images
revealed that the red fluorescence was predominantly localized in
the perinuclear region of LX-2 cells treated with
PEI-Fe3O4/Cy3-siRNA complexes, which
indicates disruption of endosomes and release of Cy3-siRNA into the
cytoplasm of the cells. The siRNAs that were delivered by Lipo 2000
were also localized to regions near the nuclear membrane and were
distributed in a non-homogeneous pattern at the periphery of the
nucleus.
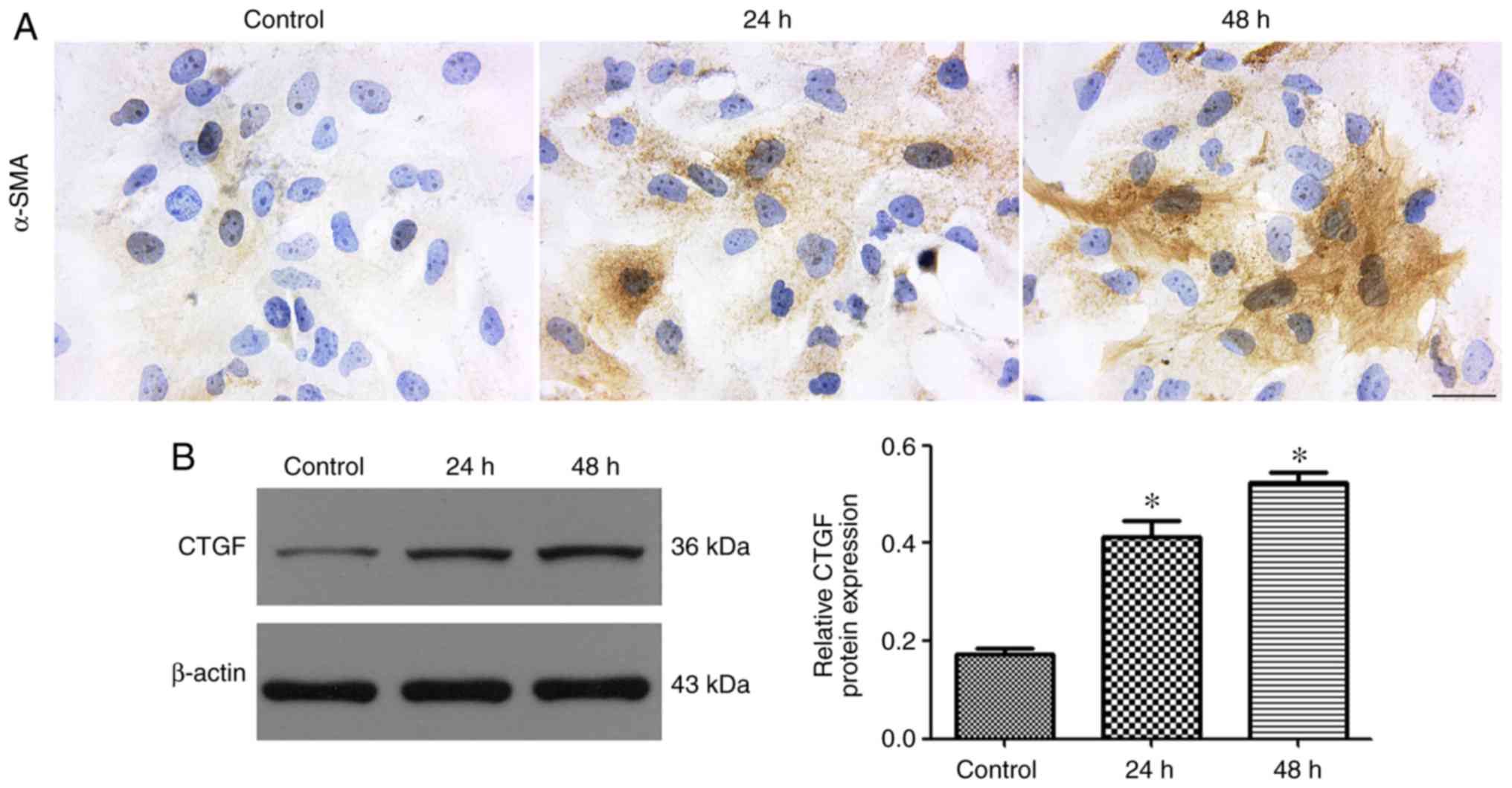
Activation of LX-2 cells mediated by
TGF-β1
The expression of α-SMA is a characteristic feature
of activated liver stellate cells and considered as a marker for
the triggering of hepatic fibrosis (22). Immunocytochemical staining for
α-SMA demonstrated strong positive staining in LX-2 cells
stimulated with TGF-β1 (Fig. 6A).
CTGF acts both as a profibrotic marker and a downstream effector of
TGF-β. The western blot results showed that LX-2 cells treated with
TGF-β1 exhibited a markedly upregulated expression of CTGF
(Fig. 6B).
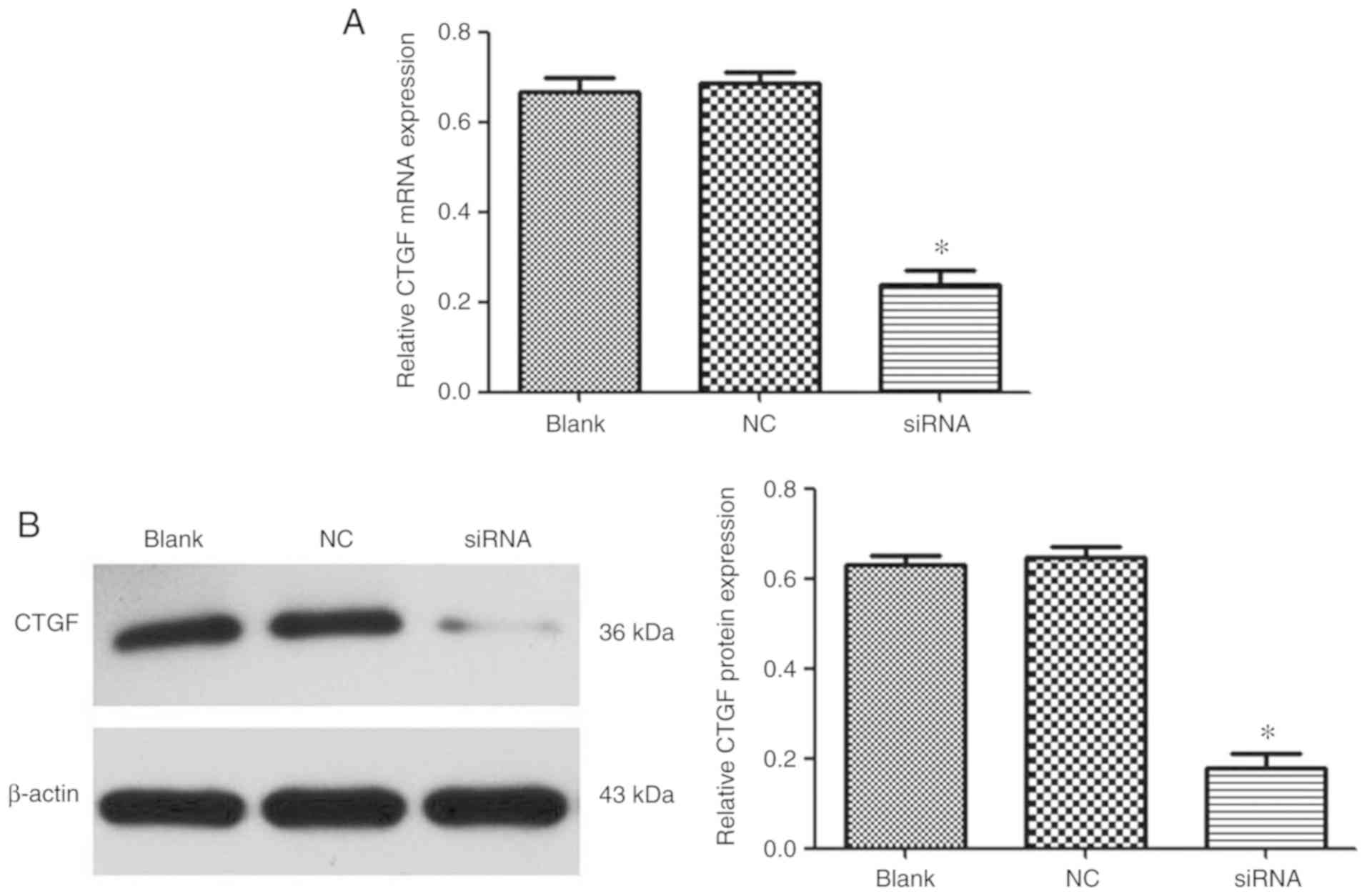
Evaluation of gene silencing
efficiency mediated by PEI-Fe3O4/CTGF-siRNA
complexes in activated LX-2 cells
The mRNA and protein expression levels of CTGF were
measured by RT-qPCR and western blotting, respectively. As shown in
Fig. 7,
PEI-Fe3O4/CTGF-siRNA complexes achieved 62
and 71% gene silencing efficiency at the mRNA and protein level,
respectively. This result indicated that
PEI-Fe3O4 could efficiently deliver siRNA to
cells and induce specific gene silencing.
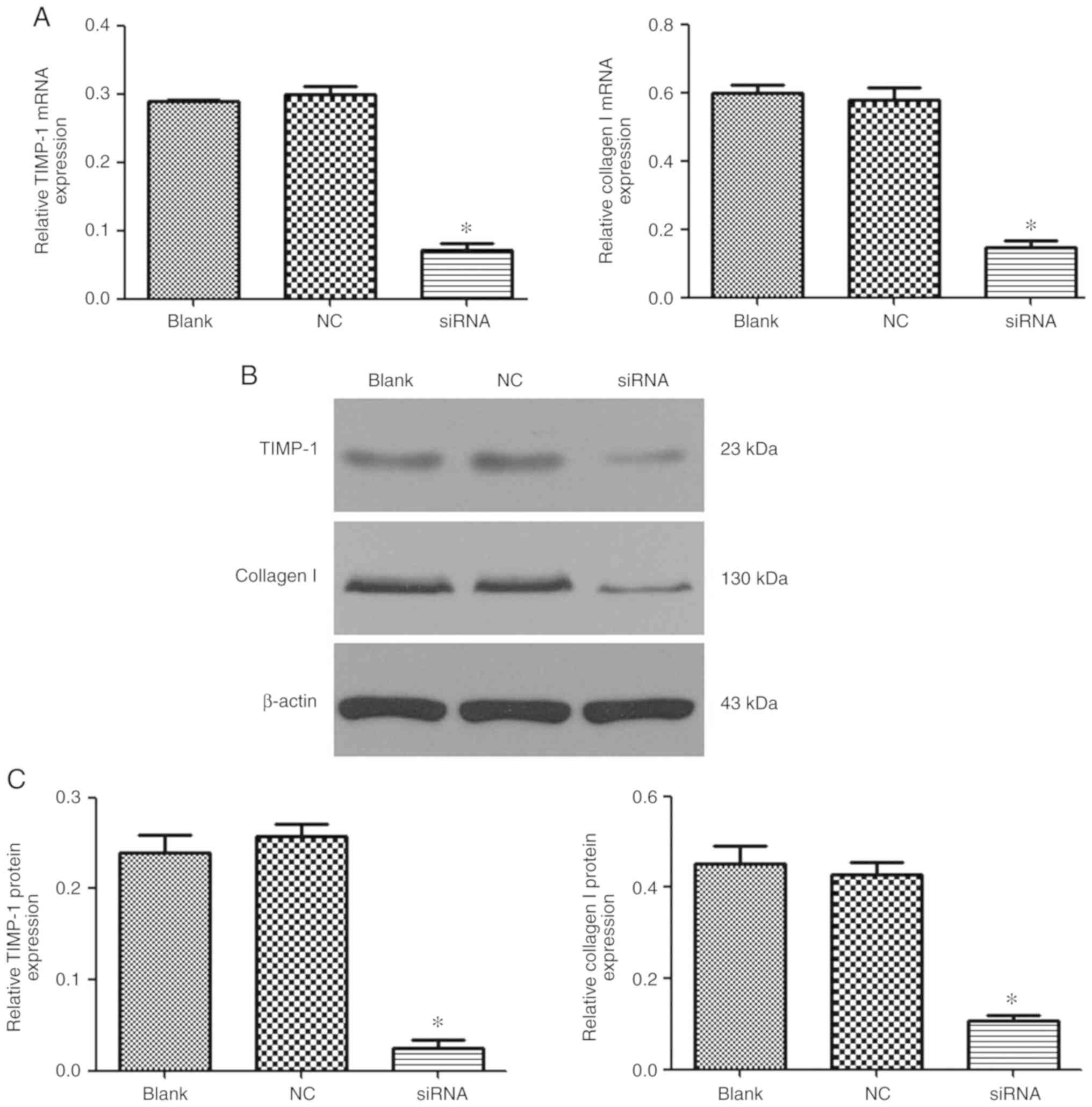
Effect of
PEI-Fe3O4/CTGF-siRNA complexes on type I
collagen and TIMP-1 expression in activated LX-2 cells
The ability of
PEI-Fe3O4/CTGF-siRNA complexes to reduce mRNA
and protein expression levels of collagen I and TIMP-1 was analyzed
via RT-qPCR and western blot analysis. As shown in Fig. 8, the collagen I mRNA and protein
expression levels in CTGF-knockdown LX-2 cells were markedly
reduced, while no interference effects were observed in cells
treated with siRNA-NC only. Similarly, TIMP-1 gene transcription
and protein expression were also significantly knocked down by
PEI-Fe3O4/CTGF-siRNA.
Discussion
Liver fibrosis is the final stage of all chronic
hepatic diseases, and may develop into cirrhosis and hepatic
carcinoma, eventually inducing liver failure (23,24).
Previous studies have shown that hepatic fibrosis is a reversible
wound-healing process, and effective drugs against fibrosis should
be used as early as possible (25,26).
However, there is an imperative requirement to develop a radical
therapy for hepatic fibrosis, and new effective drugs for fibrosis
should be applied in the clinic.
siRNAs are expected to serve as a powerful molecular
therapy for the treatment of hepatic fibrosis (4,7,27).
Previously, several lines of evidence have indicated that CTGF is a
central mediator and promising therapeutic target in hepatic
fibrosis (28–30). However, an effective and safe
delivery vector is considered as the key to successful delivery of
siRNA into target cells. Gene delivery system-based NPs could be an
efficient treatment strategy for the management of liver fibrosis
(31). In the present study,
PEI-Fe3O4 NPs were used as the CTGF siRNA
carrier, and their anti-fibrosis effect in vitro was
investigated.
Agarose gel electrophoresis is one of the simplest
methods available to visualize a change in electromobility of DNA
and RNA strands before and after complexation with cationic
polymers (32). In the present
study, the migration of siRNA was completely retarded in the
PEI-Fe3O4/siRNA complexes, at a mass ratio of
8:1. This indicated that positively charged
PEI-Fe3O4 NPs effectively interacted with
negatively charged siRNA molecules via electrostatic interaction.
In addition, PEI-Fe3O4/siRNA complexes were
notably more stable than naked siRNA in the presence of serum. The
ability of a nanocarrier to protect its cargo from nuclease
degradation is an important property for efficient gene delivery.
siRNA must be protected from nuclease digestion for maximum
activity in cells.
The cytotoxicity of gene carriers is one of their
main disadvantages, and needs to be considered. Therefore, it is
important to evaluate the cytotoxicity of the
PEI-Fe3O4/siRNA complexes. An MTT assay
revealed that the PEI-Fe3O4/siRNA complexes
had no obvious toxicity in LX-2 cells, while empty
PEI-Fe3O4 NPs showed dose-dependent
cytotoxicity. It is well known that PEI is a type of typical
cationic polymer. The toxicity of PEI-Fe3O4
NPs could be associated with the positively charged nature of the
PEI. The reduced toxicity of PEI-Fe3O4/siRNA
may be partially attributed to mild cationic charge upon the
loading of siRNA. These data illustrated that
PEI-Fe3O4 NPs could be a genetic drug carrier
for further studies.
In order to investigate whether
PEI-Fe3O4 NPs can successfully deliver
Cy3-labeled siRNA into cells, siRNA transfection efficiency was
evaluated via flow cytometry and confocal microscopy. Flow
cytometric analyses showed that the transfection efficiency of
PEI-Fe3O4/Cy3-siRNA was strongly improved
compared with that of naked Cy3-siRNA. Fluorescence from Cy3 was
obviously detected in the cytoplasm, suggesting that
PEI-Fe3O4 NPs could effectively carry
Cy3-siRNA and lead to a subsequent disruption of the endosome.
These results suggested that PEI-Fe3O4 NPs
could be effective carriers for further studies.
Activated hepatic stellate cells (HSCs) are
considered to be the major effector cells in the development of
hepatic fibrosis (33). TGF-β1
participates in the initiation and maintenance of fibrogenesis in
the liver (34). Stimulation of
activated HSCs by TGF-β1 is considered to be the key fibrogenic
response in liver fibrosis (35).
In the present study, the immunocytochemical staining result
suggested that TGF-β1 treatment increased α-SMA protein expression,
which is an important surrogate marker for activated myofibroblasts
during liver fibrogenesis compared with the non-treated groups
(36). CTGF expression was also
remarkably upregulated in LX-2 cells stimulated with TGF-β1.
Moreover, the data demonstrated that the vector efficiently
conveyed PEI-Fe3O4/siRNA into HSCs and
inhibited the gene expression of CTGF in activated LX-2 cells.
Activated HSCs not only secrete excess type I
collagen, but also exhibit markedly increased TIMP expression,
leading to a shift towards excessive extracellular matrix (ECM)
synthesis and fibrogenesis (37).
Previous studies have suggested that the downregulation of CTGF
expression may inhibit CTGF- and TGF-β1-mediated ECM production
both in vitro and in vivo (31,38).
The present study observed that the siRNA knockdown of CTGF
delivered by PEI-Fe3O4 NPs could
significantly attenuate TIMP-1 and type I collagen expression in
activated LX-2 cells. The data demonstrated that disruption of CTGF
expression mediated by PEI-Fe3O4 NPs inhibits
the production of ECM. Therefore,
PEI-Fe3O4/CTGF-siRNA showed a remarkable
antifibrotic effect in vitro.
Overall, the data of the present study indicated
that PEI-Fe3O4/CTGF-siRNA complexes can be
used as a safe and effective method to deliver siRNA to target
cells in vitro, providing a basis for gene delivery in
vivo. However, additional studies using animal models are
required to gain further insight into the biological effects of
hepatic fibrosis.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81402640 and 81502816), the
Natural Science Foundation of Hubei province (grant no. 2014CFB406)
and the Health and Family Planning commission of Wuhan city (grant
no. WX15B23).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY and QW participated in the study design, wrote
the paper and submitted the manuscript, and QW revised the
manuscript, QY, XX, LZ, TX and QW performed the experiments. QY
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atta HM: Reversibility and heritability of
liver fibrosis: Implications for research and therapy. World J
Gastroenterol. 21:5138–5148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kisseleva T and Brenner DA: Hepatic
stellate cells and the reversal of fibrosis. J Gastroenterol
Hepatol. 21 (Suppl 3):S84–S87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gressner OA and Gressner AM: Connective
tissue growth factor: A fibrogenic master switch in fibrotic liver
diseases. Liver Int. 28:1065–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salazar-Montes AM, Hernández-Ortega LD,
Lucano-Landeros MS and Armendariz-Borunda J: New gene therapy
strategies for hepatic fibrosis. World J Gastroenterol.
21:3813–3825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Rodriguez A and Valverde AM: RNA
interference as a therapeutic strategy for the treatment of liver
diseases. Curr Pharm Des. 21:4574–4586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng K, Yang N and Mahato RI: TGF-beta1
gene silencing for treating liver fibrosis. Mol Pharm. 6:772–779.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Omar R, Yang J, Liu H, Davies NM and Gong
Y: Hepatic stellate cells in liver fibrosis and siRNA-based
therapy. Rev Physiol Biochem Pharmacol. 172:1–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motoyama H, Ogawa S, Kubo A, Miwa S,
Nakayama J, Tagawa Y and Miyagawa S: In vitro reprogramming of
adult hepatocytes into insulin-producing cells without viral
vectors. Biochem Biophys Res Commun. 385:123–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Shum KT, Burnett JC and Rossi JJ:
Nanoparticle-based delivery of RNAi therapeutics: Progress and
challenges. Pharmaceuticals (Basel). 6:85–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poilil Surendran SP, Thomas RG, Moon MJ
and Jeong YY: Nanoparticles for the treatment of liver fibrosis.
Int J Nanomedicine. 12:6997–7006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li TT, Shen X, Chen Y, Zhang CC, Yan J,
Yang H, Wu CH, Zeng HJ and Liu YY: Polyetherimide-grafted
Fe3O4@SiO2 nanoparticles as
theranostic agents for simultaneous VEGF siRNA delivery and
magnetic resonance cell imaging. Int J Nanomedicine. 10:4279–4291.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirazi AN, Paquin KL, Howlett NG, Mandal
D and Parang K: Cyclic peptide-capped gold nanoparticles for
enhanced siRNA delivery. Molecules. 19:13319–13331. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J,
Che E, Hu L, Zhang Q, Jiang T and Wang S: Mesoporous silica
nanoparticles in drug delivery and biomedical applications.
Nanomedicine. 11:313–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reina G, Gismondi A, Carcione R, Nanni V,
Peruzzi C, Angjellari M, Chau NDQ, Canini A, Terranova ML and
Tamburri E: Oxidized and amino-functionalized nanodiamonds as
shuttle for delivery of plant secondary metabolites: Interplay
between chemical affinity and bioactivity. Appl Surface Sci.
470:744–754. 2019. View Article : Google Scholar
|
|
15
|
Getz T, Qin J, Medintz IL, Delehanty JB,
Susumu K, Dawson PE and Dawson G: Quantum dot-mediated delivery of
siRNA to inhibit sphingomyelinase activities in brain-derived
cells. J Neurochem. 139:872–885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta AK and Gupta M: Synthesis and
surface engineering of iron oxide nanoparticles for biomedical
applications. Biomaterials. 26:3995–4021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu W, He QG and Jiang CZ: Magnetic iron
oxide nanoparticles: Synthesis and surface functionalization
strategies. Nanoscale Res Lett. 3:397–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao S, Castro R, Rodrigues J, Shi X and
Tomás H: PAMAM dendrimer/pDNA functionalized-magnetic iron oxide
nanoparticles for gene delivery. J Biomed Nanotechnol.
11:1370–1384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saeed M, Ren W and Wu A: Therapeutic
applications of iron oxide based nanoparticles in cancer: Basic
concepts and recent advances. Biomater Sci. 6:708–725. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Zou S, Gao J, Liang J, Zhou H, Liang
L and Wu W: Block copolymer conjugated Au-coated
Fe3O4 nanoparticles as vectors for enhancing
colloidal stability and cellular uptake. J Nanobiotechnology.
15:562017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedman SL: Cytokines and fibrogenesis.
Semin Liver Dis. 19:129–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trautwein C, Friedman SL, Schuppan D and
Pinzani M: Hepatic fibrosis: Concept to treatment. J Hepatol. 62 (1
Suppl):S15–S24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schuppan D, Surabattula R and Wang XY:
Determinants of fibrosis progression and regression in NASH. J
Hepatol. 68:238–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lipson KE, Wong C, Teng Y and Spong S:
CTGF is a central mediator of tissue remodeling and fibrosis and
its inhibition can reverse the process of fibrosis. Fibrogenesis
Tissue Repair. 5 (Suppl 1):S242012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hao C, Xie Y, Peng M, Ma L, Zhou Y, Zhang
Y, Kang W, Wang J, Bai X, Wang P and Jia Z: Inhibition of
connective tissue growth factor suppresses hepatic stellate cell
activation in vitro and prevents liver fibrosis in vivo. Clin Exp
Med. 14:141–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang
S, Zhou H, Lu H and Jin Y: Inhibition of connective tissue growth
factor by siRNA prevents liver fibrosis in rats. J Gene Med.
8:889–900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taymouri S and Taheri A: Use of
nanotechnology in diagnosis and treatment of hepatic fibrosis: A
review. Curr Drug Deliv. 13:662–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Southern EM: Detection of specific
sequences among DNA fragments separated by gel electrophoresis. J
Mol Biol. 98:503–517. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J and Zern MA: Hepatic stellate cells:
A target for the treatment of liver fibrosis. J Gastroenterol.
35:665–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang N, Dang S, Shi J, Wu F, Li M, Zhang
X, Li Y, Jia X and Zhai S: Caffeic acid phenethyl ester attenuates
liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction
of autophagy pathway. Biochem Biophys Res Commun. 486:22–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang G and Brigstock DR: Regulation of
hepatic stellate cells by connective tissue growth factor. Front
Biosci (Landmark Ed). 17:2495–2507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yuhua Z, Wanhua R, Chenggang S, Jun S,
Yanjun W and Chunqing Z: Disruption of connective tissue growth
factor by short hairpin RNA inhibits collagen synthesis and
extracellular matrix secretion in hepatic stellate cells. Liver
Int. 28:632–639. 2008. View Article : Google Scholar : PubMed/NCBI
|