Introduction
Vitiligo is a common idiopathic disease that is
characterized by the destruction of melanocytes (1,2).
Although the prevalence of vitiligo is <1% globally, it may be
as high as 3% in some populations (2). At present, patients with vitiligo
have no other symptoms except for skin discoloration, but the
quality of life of some patients may be severely compromised
(3,4). Vitiligo is an autoimmune disease and
it has been reported that multiple immune response genes may be
involved in its development (5).
Previous studies have demonstrated that vitiligo may be caused by
T-cell-mediated oxidative stress and may involve certain mediators
such as tumor necrosis factor (TNF)-α, heat shock protein 70 and
interleukin-1β (6–8). The destruction of melanocytes is
caused by the imbalance of reactive oxygen species, which leads to
damage of skin melanocytes by free radicals, leading to structural
damage of proteins, apoptosis, activation of cytokines and
endoplasmic reticulum (ER) damage (6–8). The
ER is an important organelle, which is mainly responsible for
protein biosynthesis and folding. ER stress is characterized by
accumulation and aggregation of unfolded and/or misfolded proteins
in the ER lumen (9).
MicroRNAs (miRNAs/miRs) are small and highly
conserved regulatory RNA molecules ~22 nucleotides in length
(10). miRNAs can regulate gene
expression at the post-transcriptional level by targeting their
3′-untranslated region (UTR) to promote degradation or inhibit
translation of mRNA (11).
Accumulating evidence has demonstrated that miRNAs participate in
the development and progression of various human cancers (12–14).
The expression of miR-421 was found to be abnormal in several types
of cancer. A previous study demonstrated that miR-421 acted as a
tumor promoter in pancreatic cancer through targeting DPC4/mothers
against decapentaplegic homolog 4 (15). Wu et al (16) reported that miR-421 was
significantly upregulated in human gastric cancer tissues and
promoted proliferation of gastric cancer cells by downregulating
caspase-3 expression. However, the role of miR-421 in vitiligo
patients is unclear.
Receptor-interacting serine/threonine kinase 1
(RIPK1) is a crucial regulator of TNF receptor 1 (TNFR1) signaling
(17). RIPK1 plays a major role in
the pathogenesis and prognosis of liver diseases (18,19).
Previous research demonstrated that RIPK1-mediated necrotic
apoptosis may also occur in neurons, leading to the development of
neurodegenerative diseases (20).
However, the expression and role of RIPK1 in vitiligo patients
remain unclear.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT)/mammalian target of rapamycin (mTOR) pathway has been found
to be associated with cell survival in response to oxidative stress
(21). Growth factors may protect
against oxidative stress-induced apoptosis through activation of
the AKT and mTOR pathways (22–24).
In addition, indirect data indicated that α-melanocyte-stimulating
hormone (MSH) stimulates melanogenesis through the activation of
MEK/extracellular signal-regulated kinase (ERK) or PI3K/AKT
(25). Modulation of the
PI3K/AKT/mTOR signaling pathway may be a novel approach to the
clinical management of vitiligo (26). However, the association between
miR-421 and the PI3K/AKT/mTOR pathway in melanocytes under ER
stress remains unclear.
The aim of the present study was to determine the
role of miR-421 in vitiligo development and to explore the
underlying mechanism.
Materials and methods
Cell culture and transfection
Primary epidermal melanocytes were obtained from the
American Type Culture Collection (cat no. ATCC®
PCS-200-013) and cultured in Medium 254 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with human melanocyte growth
supplement (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. Primary epidermal melanocytes (1×106
cells per well) were transfected with the inhibitor control
(5′-CAGUACUUUUGUGUAGUACAA-3′; Guangzhou Ribobio Co., Ltd.), miR-421
inhibitor (5′-GCGCCCAAUUAAUGUCUGUUGAU-3′; Guangzhou Ribobio Co.,
Ltd.), 0.2 µM control-shRNA (cat no. sc-108060; Santa Cruz
Biotechnology, Inc.), 0.2 µM RIPK1-shRNA (cat no. sc-44326-SH;
Santa Cruz Biotechnology, Inc.), miR-421 inhibitor + control-shRNA,
or miR-421 inhibitor + RIPK1-shRNA for 24 h using Lipofectamine
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. The transfection efficiency was
determined by reverse transcription-quantitative PCR (RT-qPCR) 24 h
after cell transfection.
ER stress induction
To establish ER stress in human melanocytes, the
cells were treated with 3 µM tunicamycin (TM; Sigma-Aldrich; Merck
KGaA) for 48 h according to a previous study (9).
RT-qPCR analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific Inc.) and stored at
−80°C. Subsequently, total RNA was reverse transcribed into
complementary DNA using a reverse transcription kit (Vazyme)
according to the manufacturer's protocol. RT-qPCR was carried out
by SYBR Green PCR Master Mix (Vazyme) following the manufacturer's
protocols. U6 or GAPDH was used for normalization. The primer
sequences used were as follows: U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH, forward
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse 5′-CTCCACGACGTACTCAGCG-3′;
miR-421, forward 5′-CTCACTCACATCAACAGACATTAATT-3′ and reverse
5′-TATGGTTGTTCTGCTCTCTGTGTC-3′; and RIPK1, forward
5′-AGGCTTTGGGAAGGTGTCTC-3′ and reverse 5′-CGGAGTACTCATCTCGGCTTT-3′.
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C
for 15 sec and annealing/elongation at 60°C for 30 sec. The
relative expression of genes was calculated by the
2−ΔΔCq method (27).
Each experiment was performed in triplicate.
Western blotting assay
After treatment, cells were washed three times with
ice-cold phosphate-buffered saline and then treated with RIPA lysis
solution (Beijing Solarbio Science & Technology Co., Ltd.) for
30 min to extract cellular proteins. Equal amounts (40 µg/lane) of
protein were separated by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then transferred to polyvinylidene fluoride
membranes (EMD Millipore). The membranes were blocked with 5%
skimmed milk in TBST containing 0.1% Tween at room temperature for
2 h, followed by incubation with primary antibodies at 4°C
overnight. Subsequently, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody at room
temperature for 2 h. The protein band was visualized using the
enhanced chemiluminescence method using ECL reagent (EMD
Millipore). GAPDH served as the loading control for normalization.
The primary antibodies were as follows: Anti-protein kinase
RNA-like endoplasmic reticulum kinase (PERK; cat no. 5683;
1:1,000), anti-α subunit of eukaryotic translation initiation
factor 2 (eIF2α; cat no. 5324; 1:1,000), anti-C/EBP homologous
protein (CHOP; cat no. 2895; 1:1,000), anti-RIPK1 (cat no. 3493;
1:1,000), anti-phosphorylated (p)-AKT (cat no. 4060; 1:1,000) and
anti-p-mTOR (cat no. 5536; 1:1,000) and they were purchased from
Cell Signaling Technology, Inc. Anti-p-PI3K was obtained from
Biogot Technology, Co., Ltd. (cat no. BS4811; 1:1,000). The
secondary antibodies were as follows: Anti-mouse IgG,
HRP-conjugated antibody (cat no. 7076; 1:1,000) and anti-rabbit
IgG, HRP-conjugated antibody (cat no. 7074; 1:1,000) were purchased
from Cell Signaling Technology, Inc. Protein expression was
quantified by performing AlphaView 3.4.0 software
(ProteinSimple).
Flow cytometry analysis
Cell apoptosis detection was performed using the
Annexin-V/propidium iodide (PI) Apoptosis Detection kit (Beyotime
Institute of Biotechnology). Briefly, human melanocytes were plated
into 6-well plates at a density of 2×105 cells per well.
On the following day, the cells were transfected with inhibitor
control, miR-421 inhibitor, miR-421 inhibitor + control-shRNA, or
miR-421 inhibitor + RIPK1-shRNA. After transfection for 24 h, the
cells were treated with 3 µM TM for 48 h. Subsequently, the cells
were collected, centrifuged with low temperature at high speed
(1,000 × g; 5 min; 4°C) and re-suspended in 100 µl fluorescein
isothiocyanate (FITC)-binding buffer. Subsequently, ~5 µl
ready-to-use Annexin V-FITC (BD Bioscience) and 5 µl PI were added
to the buffer and the cells were incubated for 30 min at room
temperature in the dark. Annexin V-FITC and PI fluorescence were
assessed by BD FACSCalibur flow cytometer (BD Biosciences; Becton,
Dickinson and Company). FlowJo software (version 7.6.1; FlowJo LLC)
was used to analyze the data.
Dual-luciferase reporter assay
The bioinformatics software TargetScan 7.2
(http://www.targetscan.org/vert_72/)
was used to predict the association between miR-421 and RIPK1, and
the results demonstrated that miR-421 had binding sites for RIPK1.
Subsequently, to confirm the binding sites between miR-421 and
RIPK1, a dual-luciferase reporter assay was performed. In brief,
luciferase reporter plasmids (psi-CHECK2) containing the wild-type
as well as the mutant 3′UTRs of RIPK1, were manufactured by TsingKe
Biotech. Human melanocytes were co-transfected with the wild-type
or mutant 3′UTR luciferase reporter plasmids and the miR-421 mimic
or the mimic control, respectively, using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were harvested
after transfection for 24 h and the luciferase activity was
measured using the Dual-glo luciferase assay system (Promega
Corporation) following the manufacturer's protocol. Firefly
luciferase was used as a normalization control.
MTT assay
Cell viability was evaluated using the MTT assay
(Beijing Solarbio Science & Technology Co., Ltd.). Human
melanocytes seeded in 96-well plates were treated according to the
purpose of the experiment and 20 µl MTT reagent was added into each
well for another 4 h of incubation at 37°C. Subsequently, 150 µl
dimethyl sulfoxide was added into each well and shaken for 15 min.
The optical density values were read at 490 nm using a microplate
reader.
Statistical analysis
All data are presented as the mean ± standard
deviation. The SPSS 22.0 software (IBM Corp.) was used for
statistical analysis and differences between groups were determined
by Student's t-test or one way-analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-421 in human
melanocytes induced by ER stress
To evaluate the role of miR-421 in human melanocytes
induced by ER stress, the levels of miR-421 expression were
measured by RT-qPCR. Human melanocytes were treated with 3 µM TM
for 48 h. A western blotting assay demonstrated that the expression
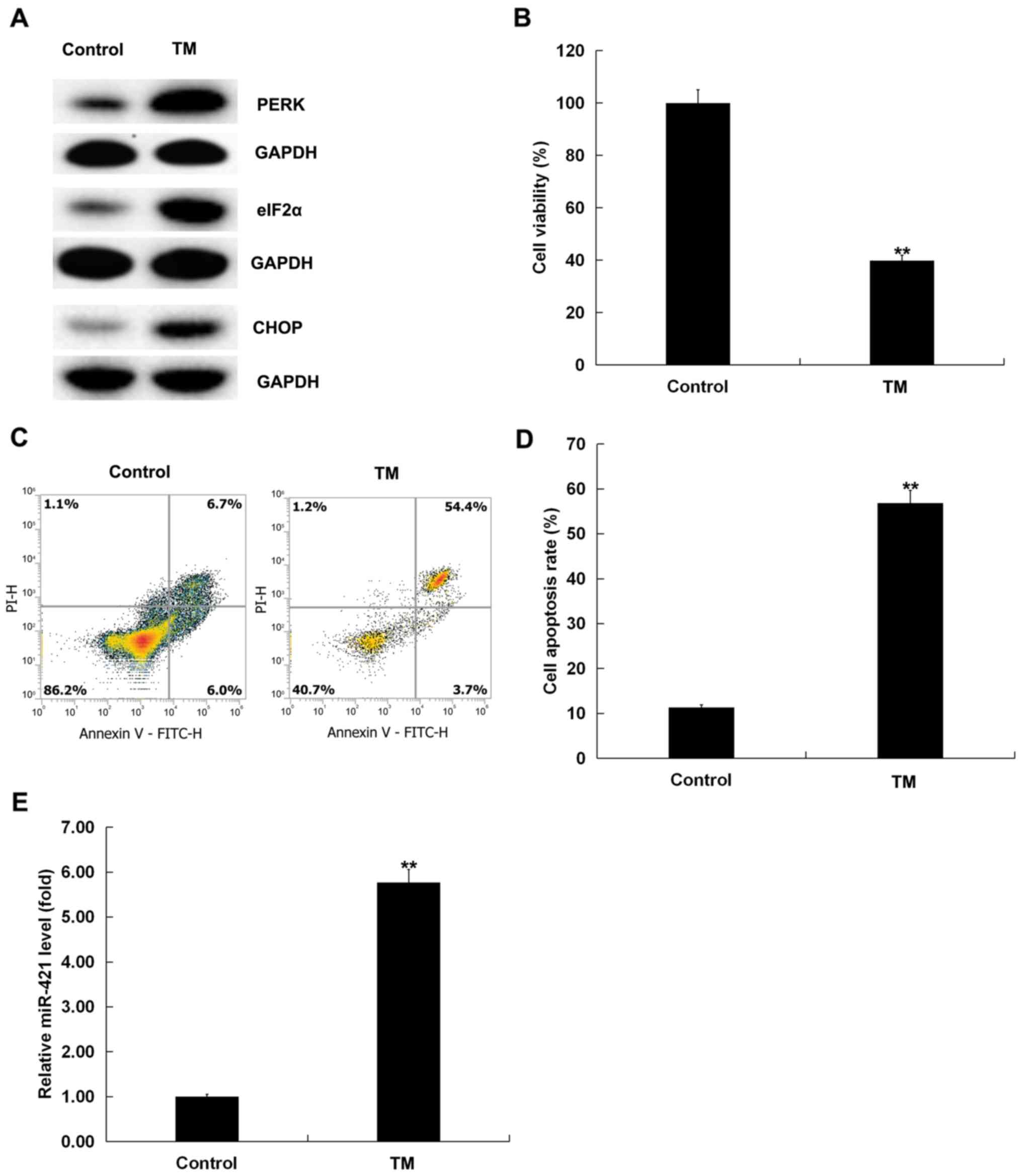
of ER stress-related proteins, such as PERK, eIF2α and CHOP, was
upregulated (Fig. 1A), indicating
that 3 µM TM activated ER stress in human melanocytes. MTT assay
and flow cytometry indicated that TM significantly inhibited the
viability (P<0.01; Fig. 1B) and
induced apoptosis (P<0.01; Fig. 1C
and D) of human melanocytes. Moreover, an RT-qPCR assay was
performed to detect the expression of miR-421 in human melanocytes.
RT-qPCR analysis demonstrated that miR-421 expression was
significantly upregulated in TM-induced human melanocytes compared
with the control group (P<0.01; Fig. 1E). Taken together, these findings
confirmed that miR-421 expression was increased in human
melanocytes induced by ER stress.
RIPK1 is a direct target gene of
miR-421
In order to study the underlying molecular
mechanism, the TargetScan bioinformatic predication algorithms were
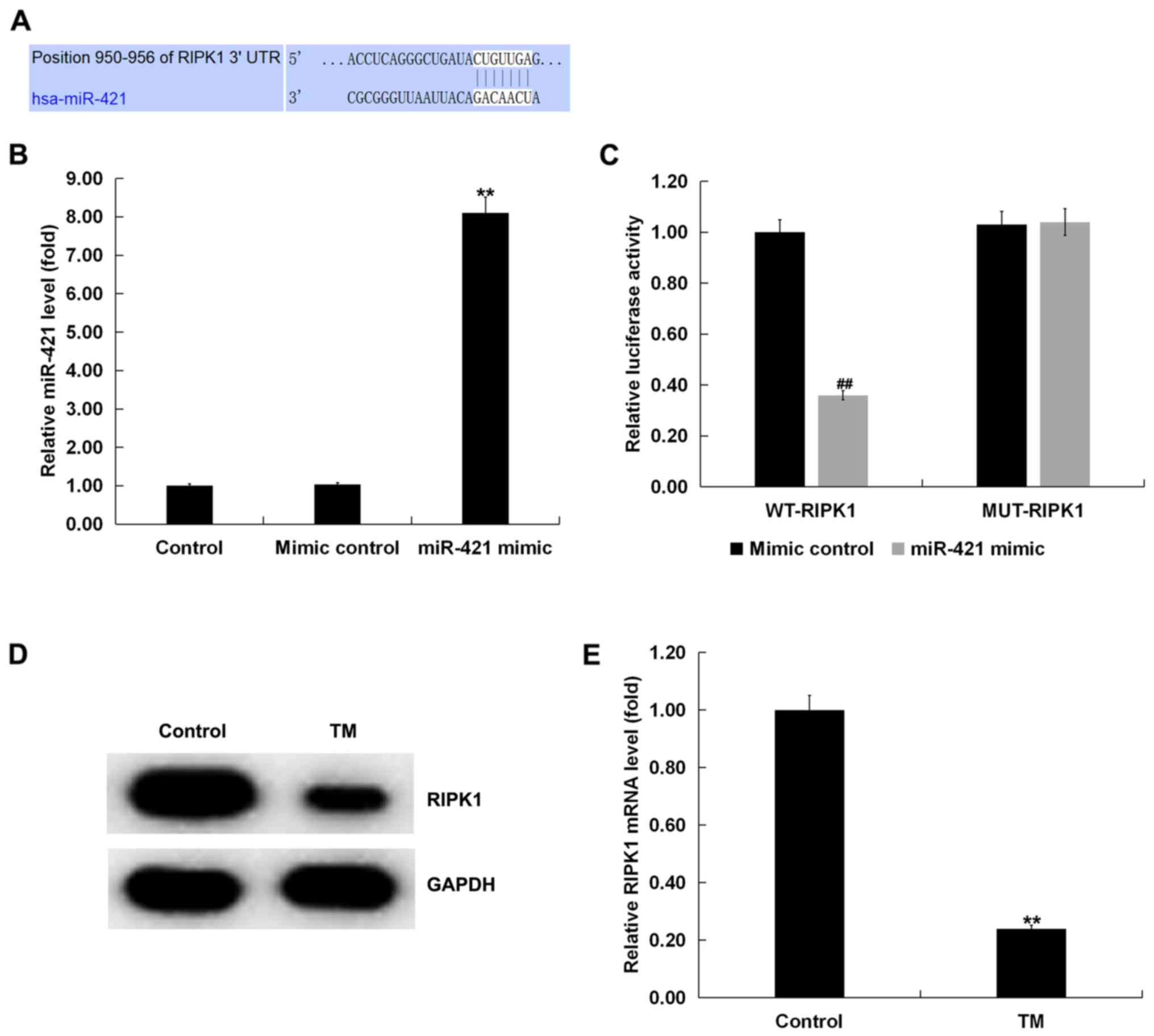
used to predict the potential targets of miR-421. The results
revealed the binding sites between RIPK1 and miR-421 (Fig. 2A). Subsequently, a dual-luciferase
reporter assay was performed to confirm the binding sites between
RIPK1 and miR-421. It was observed that the miR-421 mimic
significantly increased the level of miR-421 in human melanocytes
compared with the control group (Fig.
2B). miR-421 co-transfection with wild-type RIPK1 3′-UTR
reporter inhibited the luciferase activity, but miR-421 exerted no
effect on the reporter containing the mutant sequence (Fig. 2C). Therefore, RIPK1 was confirmed
as a direct target gene of miR-421.
The expression of RIPK1 in TM-induced human
melanocytes was next examined. Western blotting and the RT-qPCR
assay demonstrated that, compared with the control group, TM
significantly reduced the expression of RIPK1 in human melanocytes
(Fig. 2D and E).
Effect of miR-421 inhibitor on the
expression of ER stress-related proteins in TM-induced human
melanocytes
Human melanocytes were transfected with the
inhibitor control, miR-421 inhibitor, control-shRNA, RIPK1-shRNA or
miR-421 inhibitor + RIPK1-shRNA for 24 h and then treated with TM
(3 µM) for 48 h. The results indicated that, compared with the
control group, the miR-421 inhibitor significantly reduced the
expression of miR-421 in human melanocytes (Fig. 3A). Compared with the control group,
RIPK1-shRNA significantly decreased the expression of RIPK1 in
human melanocytes (Fig. 3B and C).
In addition, RIPK1 expression was increased by the miR-421
inhibitor, which was obviously abolished by RIPK1-shRNA (Fig. 3D and E).
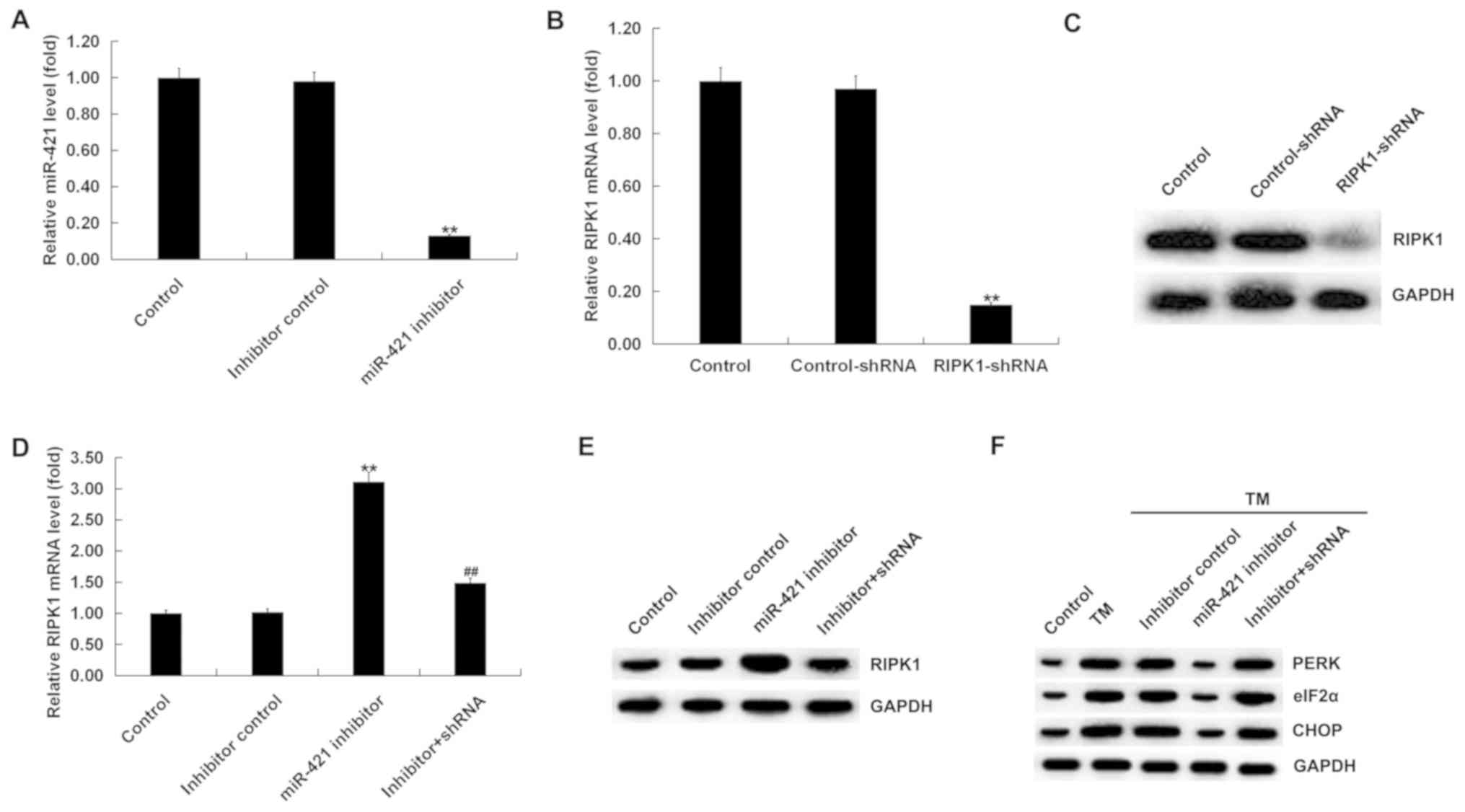 | Figure 3.Effect of miR-421 inhibitor on the
expression of ER stress-related proteins in TM-induced human
melanocytes. (A) RT-qPCR analysis detected the expression of
miR-421 in human melanocytes transfected with inhibitor control or
miR-421 inhibitor for 24 h. (B) RT-qPCR and (C) western blotting
assays detected the expression of RIPK1 in human melanocytes
transfected with control-shRNA or RIPK1-shRNA for 24 h. (D) RT-qPCR
assay and (E) western blotting assay were performed to detect the
expression of RIPK1 in human melanocytes. (F) Western blot analysis
of the protein expression of PERK, eIF2α and CHOP in human
melanocytes. Data were presented as the mean ± standard deviation.
**P<0.01 vs. the control; ##P<0.01 vs. miR-421 inhibitor. ER,
endoplasmic reticulum; TM, tunicamycin; RIPK1, receptor-interacting
serine/threonine kinase 1; PERK, protein kinase RNA-like
endoplasmic reticulum kinase; eIF2α, α subunit of eukaryotic
translation initiation factor 2; CHOP, C/EBP homologous protein;
RT-qPCR, reverse transcription-quantitative PCR; miR, microRNA; sh,
short hairpin. |
The expression of ER stress-related proteins was
next examined. A western blotting assay revealed that, compared
with the control group the protein expression of PERK, eIF2α and
CHOP was markedly increased in the TM treatment group; compared
with the TM treatment group, the protein expression of PERK, eIF2α
and CHOP was obviously decreased in the TM + miR-421 inhibitor
group, and this effect was eliminated by RIPK1-shRNA (Fig. 3F).
Effect of the miR-421 inhibitor on ER
stress-induced human melanocyte damage
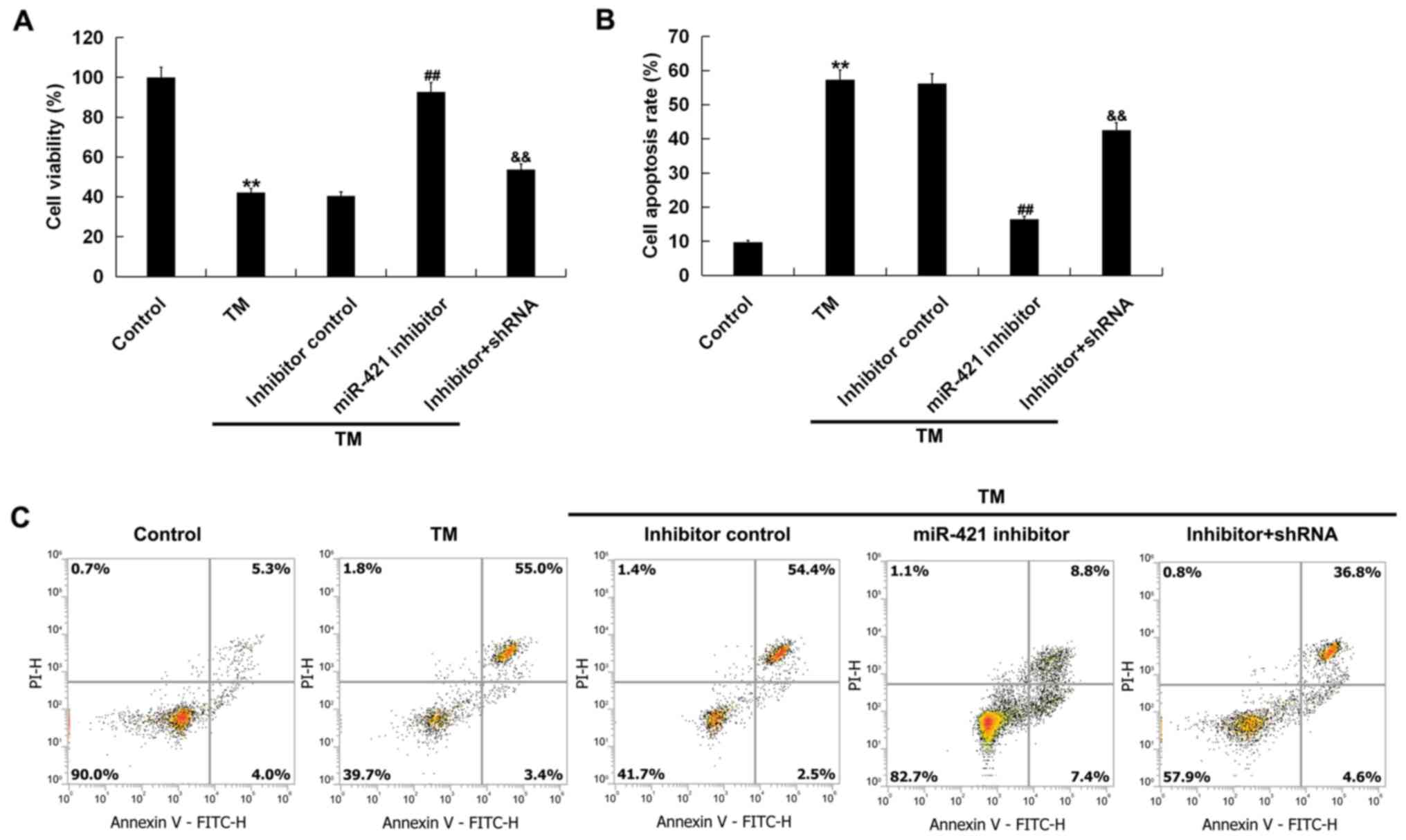
In order to investigate the effect of low expression
of miR-421 on ER stress-induced damage of human melanocytes, an MTT
assay and flow cytometry were performed. The results indicated
that, compared with the control group, cell viability (Fig. 4A) was decreased and cell apoptosis
(Fig. 4B and C) was increased in
the TM treatment group. Compared with the TM treatment group, the
miR-421 inhibitor significantly increased the viability of human
melanocytes (Fig. 4A) and
decreased cell apoptosis (Fig. 4B and
C). All these changes were notably reversed by RIPK1-shRNA.
Effect of the miR-421 inhibitor on the
PI3K/AKT/mTOR signaling pathway in TM-induced human
melanocytes
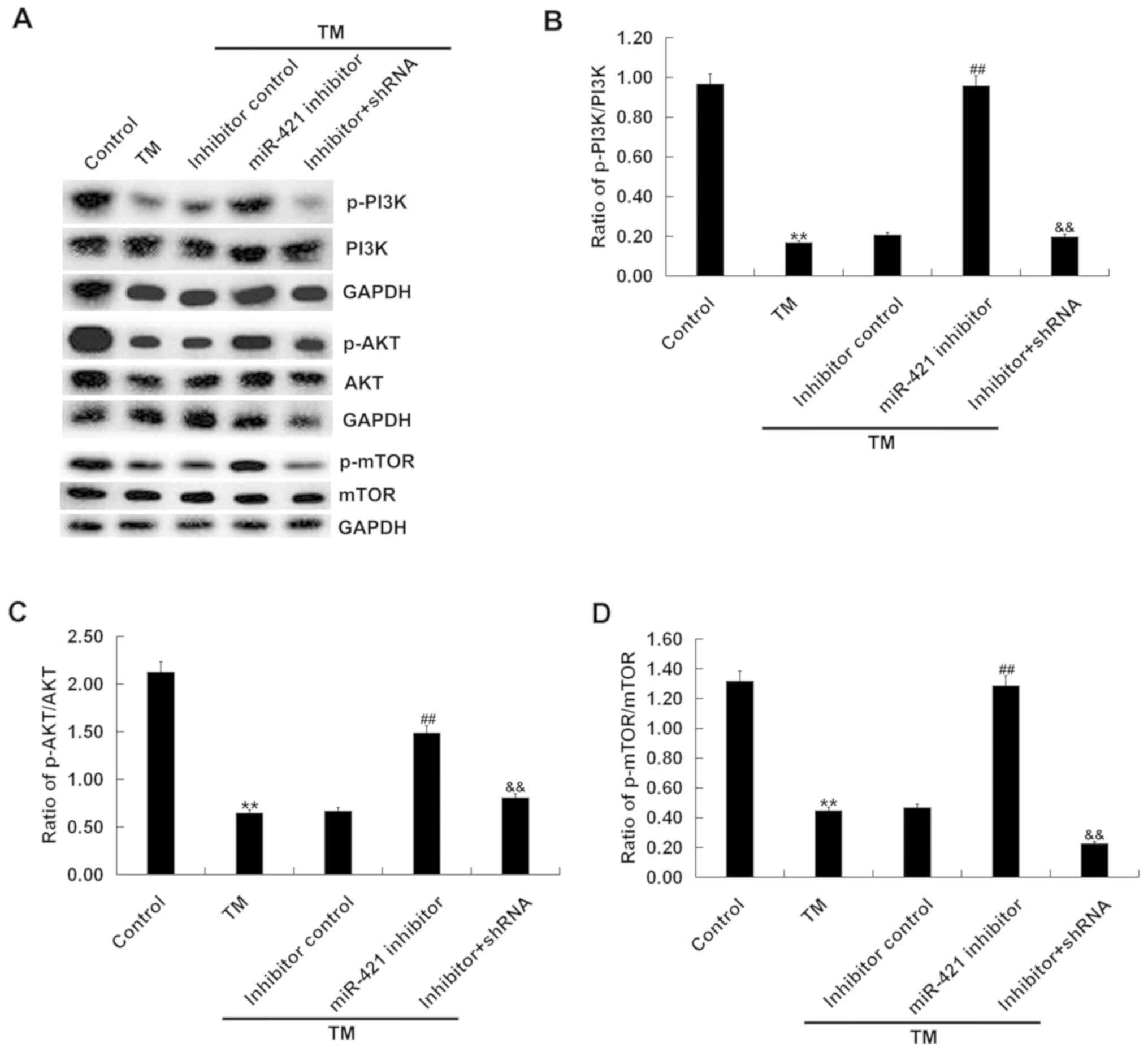
To investigate whether PI3K/AKT/mTOR signaling was
involved in the effect of the miR-421 inhibitor on TM-induced human
melanocytes, the expression of p-PI3K, PI3K, p-AKT, AKT, mTOR and
p-mTOR in human melanocytes was examined. The western blot assay
revealed that, compared with the control group, the protein
expression of p-PI3K, PI3K, p-AKT, AKT, mTOR and p-mTOR and the
ratio of p-PI3K/PI3K, p-AKT/AKT and p-mTOR/mTOR were significantly
decreased in the TM treatment group and, compared with the TM
treatment group, the miR-421 inhibitor significantly increased the
protein expression of p-PI3K, p-AKT and p-mTOR in the TM + miR-421
inhibitor group. The effect of the miR-421 inhibitor on TM-induced
human melanocytes was markedly eliminated by RIPK1-shRNA (Fig. 5). Taken together, these findings
indicate that miR-421 inhibitor activated the PI3K/AKT/mTOR
signaling pathway in TM-induced human melanocytes.
Discussion
The present study demonstrated that the expression
of the ER stress-related proteins PERK, eIF2α and CHOP was
upregulated in human melanocytes treated with 3 µM TM for 48 h.
Moreover, TM inhibited human melanocyte viability and induced
apoptosis. The results demonstrated that TM increased the
expression of miR-421 in human melanocytes. To explore the role of
miR-421, bioinformatics analysis was performed to predict its
potential target genes. TargetScan predicted that RIPK1 was a
potential target gene of miR-421. Dual-luciferase reporter gene
assay further verified the association between miR-421 and RIPK1.
The expression of RIPK1 was downregulated in human melanocytes
treated with 3 µM TM for 48 h. Next, the effect of the
downregulation of miR-421 on ER stress-induced damage of human
melanocytes was investigated. miR-421 inhibitor reduced the
expression of the ER stress-related proteins PERK, eIF2α and CHOP
in human melanocytes treated with TM. In addition, the miR-421
inhibitor promoted human melanocyte viability and decreased
apoptosis, and these effects were reversed by RIPK1-shRNA. Finally,
it was observed that the effect of the miR-421 inhibitor on human
melanocytes was associated with the PI3K/AKT/mTOR signaling
pathway.
Vitiligo is a non-continuous skin disease
characterized by loss of pigment-producing skin cells
(melanocytes), causing progressive skin depigmentation (28). At present, the treatment of
vitiligo is mainly focused on preventing the development of the
disease and achieving re-pigmentation of the non-pigmented area.
Phototherapy is currently the preferred method of vitiligo
treatment, but corticosteroids, surgery or local immunomodulators
are also considered as treatment options for vitiligo (29–31).
As a type of oxidative stress, ER stress is affected by adverse
factors such as ischemia, hypoxia, hypoglycemia, drugs or poisons,
which cause unfolded proteins to accumulate in the lumen of the ER.
When the ability of the cell to repair is exceeded, the unfolded
protein-mediated apoptosis may be induced.
It was previously demonstrated that miRNAs are
involved in a number of key developmental pathways and different
miRNAs may be associated with different diseases, such as
inflammatory diseases, infections, developmental disorders and
cancer (32). It has been reported
that miR-211 plays a key role in the pathophysiology of human
vitiligo and may be located at the apex of the normal melanocyte
gene network (33). Shi et
al (34) reported that
overexpression of miR-25 promoted
H2O2-induced melanocyte destruction and led
to melanocyte dysfunction. miR-421, a specific miRNA, has been
shown to promote the development of neuroblastoma through targeting
the tumor suppressor Menin (35).
Wu et al (16) indicated
that miR-421 may regulate cell apoptosis via mediating caspase-3 in
gastric cancer. However, to the best of our knowledge, there is
currently no report on the role of miR-421 in vitiligo development.
The results of the present study demonstrated that miR-421
expression was upregulated in human melanocytes induced by ER
stress.
RIPK1 is a crucial regulator of TNFR1 signaling
(17). It has been reported that
RIPK1 is a target gene of miR-24-3p (36). In the present study, it was
demonstrated by the dual-luciferase reporter assay that RIPK1 is a
target of miR-421. Moreover, RIPK1 expression was downregulated in
TM-induced human melanocytes.
ER stress activates the unfolded protein response
pathways by inducing PERK, which increases the phosphorylation
level of eIF2α and the transcription activation of CHOP (37). Apoptosis mediators, such as PERK,
CHOP and eIF2α, are involved in ER stress-associated cell death
(38). These events indicate that
ER stress activation of PERK, eIF2α and CHOP protein expression
plays a critical role in TM-induced human melanocyte apoptosis.
Furthermore, PERK, eIF2α and CHOP protein expression inhibition may
inhibit ER stress-induced human melanocyte apoptosis, thus playing
a protective role in vitiligo. As expected, the present study
revealed that TM significantly increased the protein level of PERK,
CHOP and eIF2α in human melanocytes. The miR-421 inhibitor reduced
the expression of the ER stress-related proteins PERK, eIF2α and
CHOP in human melanocytes treated with TM, and these effects were
reversed by RIPK1-shRNA. Next, it was observed that low expression
of miR-421 relieved ER stress-induced human melanocyte damage, but
this effect was reversed by RIPK1-shRNA.
The PI3K/AKT/mTOR signaling pathway has been found
to be associated with cell survival (21). Growth factors may protect against
oxidative stress-induced apoptosis through activation of the AKT
and mTOR pathways (22–24). AKT/mTOR complex activation
suppresses ultraviolet-induced skin cell damage (39). PSORI-CM02, an empirically developed
Chinese medicinal formula optimized from Yin Xie Ling, successfully
treated psoriasis by inducing autophagy via inhibition of the
PI3K/Akt/mTOR pathway (40). The
PI3K/AKT/mTOR pathway has also been reported to play a critical
role in atopic dermatitis (41).
In addition, indirect data indicated that α-MSH stimulated
melanogenesis through the activation of the MEK/ERK or PI3K/AKT
pathways (25). Modulation of the
PI3K/AKT and mTOR pathway may be a novel approach to the clinical
management of vitiligo (26). A
previous study indicated that Nrf2 negatively regulates
melanogenesis by modulating the PI3K/Akt signaling pathway
(42). The α-MSH-induced
activation of the mTORC1 pathway, inhibited by rapamycin, helps to
maintain dendrites of melanocytes under oxidative stress (26). Sustained PI3K activity can protect
melanocytes from apoptosis, thereby indicating that the PI3K/AKT
pathway plays a pivotal role in melanocyte survival (43). In the present study, it was
investigated whether the miR-421 inhibitor affected the
PI3K/AKT/mTOR signaling pathway in human melanocytes under ER
stress and the results were the first to prove that ER stress
inhibited the PI3K/AKT/mTOR signaling pathway in human melanocytes
which was significantly activated by miR-421 inhibitor, while this
effect was eliminated by RIPK1-shRNA.
In conclusion, the miR-421 inhibitor may protect
human melanocytes from ER stress-induced cell damage through
regulating PI3K/AKT/mTOR signaling and ER stress signaling by
targeting RIPK1. The findings of the present study may provide a
new therapeutic target and a theoretical basis for the treatment of
vitiligo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81773335, 81803131
and 81602755), Zhejiang Provincial Natural Science Foundation
(grant no. LY18H110001) and Zhejiang Basic Public Welfare Research
Project (grant no. LGF18H110002).
Availability of data and materials
All data sets used and/or generated during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XS and TW contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. BH, GR and AX contributed to data
collection and statistical analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ezzedine K, Eleftheriadou V, Whitton M and
van Geel N: Vitiligo. Lancet. 386:74–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain A, Mal J, Mehndiratta V, Chander R
and Patra SK: Study of oxidative stress in vitiligo. Indian J Clin
Biochem. 26:78–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverberg JI and Silverberg NB:
Association between vitiligo extent and distribution and
quality-of-life impairment. JAMA Dermatol. 149:159–164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karelson M, Silm H and Kingo K: Quality of
life and emotional state in vitiligo in an Estonian sample:
Comparison with psoriasis and healthy controls. Acta Derm Venereol.
93:446–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin Y, Birlea SA, Fain PR, Gowan K,
Riccardi SL, Holland PJ, Mailloux CM, Sufit AJ, Hutton SM,
Amadi-Myers A, et al: Variant of TYR and autoimmunity
susceptibility loci in generalized vitiligo. New Engl J Med.
362:1686–1697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alghamdi KM, Khurrum H, Taieb A and
Ezzedine K: Treatment of generalized vitiligo with anti-TNF-α
agents. J Drugs Dermatol. 11:534–539. 2012.PubMed/NCBI
|
|
7
|
Manga P, Elbuluk N and Orlow SJ: Recent
advances in understanding vitiligo. F1000Res. 5(pii): F1000 Faculty
Rev. 22342016. View Article : Google Scholar
|
|
8
|
Eleftheriadou V, Whitton ME, Gawkrodger
DJ, Batchelor J, Corne J, Lamb B, Ersser S, Ravenscroft J and
Thomas KS: Future research into the treatment of vitiligo: Where
should our priorities lie? Results of the vitiligo priority setting
partnership. Br J Dermatol. 164:530–536. 2011.PubMed/NCBI
|
|
9
|
Luan Q, Jin L, Jiang CC, Tay KH, Lai F,
Liu XY, Liu YL, Guo ST, Li CY, Yan XG, et al: RIPK1 regulates
survival of human melanoma cells upon endoplasmic reticulum stress
through autophagy. Autophagy. 11:975–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Šahmatova L, Tankov S, Prans E, Aab A,
Hermann H, Reemann P, Pihlap M, Karelson M, Abram K, Kisand K, et
al: MicroRNA-155 is dysregulated in the skin of patients with
vitiligo and inhibits melanogenesis-associated genes in melanocytes
and keratinocytes. Acta Derm Venereol. 96:742–747. 2016.PubMed/NCBI
|
|
11
|
Bijkerk R, de Bruin RG, van Solingen C,
van Gils JM, Duijs JM, van der Veer EP, Rabelink TJ, Humphreys BD
and van Zonneveld AJ: Silencing of microRNA-132 reduces renal
fibrosis by selectively inhibiting myofibroblast proliferation.
Kidney Int. 89:1268–1280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zu Y, Yang Y, Zhu J, Bo X, Hou S, Zhang B,
Qiu J and Zheng J: MiR-146a suppresses hepatocellular carcinoma by
downregulating TRAF6. Am J Cancer Res. 6:2502–2513. 2016.PubMed/NCBI
|
|
13
|
Li Q, Zhang X, Li N, Liu Q and Chen D:
miR-30b inhibits cancer cell growth, migration, and invasion by
targeting homeobox A1 in esophageal cancer. Biochem Biophys Res
Commun. 485:506–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian W, Wang G, Liu Y, Huang Z, Zhang C,
Ning K, Yu C, Shen Y, Wang M, Li Y, et al: The miR-599 promotes
non-small cell lung cancer cell invasion via SATB2. Biochem Biophys
Res Commun. 485:35–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao J, Zhang S, Zhou Y, Liu C, Hu X and
Shao C: MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer.
Biochem Biophys Res Commun. 406:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu JH, Yao YL, Gu T, Wang ZY, Pu XY, Sun
WW, Zhang X, Jiang YB and Wang JJ: MiR-421 regulates apoptosis of
BGC-823 gastric cancer cells by targeting caspase-3. Asian Pac J
Cancer Prev. 15:5463–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schneider AT, Gautheron J, Feoktistova M,
Roderburg C, Loosen SH, Roy S, Benz F, Schemmer P, Büchler MW,
Nachbur U, et al: RIPK1 Suppresses a TRAF2-dependent pathway to
liver cancer. Cancer Cell. 31:94–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saeed WK and Jun DW: Necroptosis: An
emerging type of cell death in liver diseases. World J
Gastroenterol. 20:12526–12532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao C and Wan Y: Parameters of protection
against ultraviolet radiation-induced skin cell damage. J Cell
Physiol. 220:277–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao C, Huang X, Han Y, Wan Y, Birnbaumer
L, Feng GS, Marshall J, Jiang M and Chu WM: Galpha(i1) and
Galpha(i3) are required for epidermal growth factor-mediated
activation of the Akt-mTORC1 pathway. Sci Signal. 2:ra172009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao C, Lu S, Jiang Q, Wang WJ, Song X,
Kivlin R, Wallin B, Bagdasarian A, Tamakloe T, Chu WM, et al: EGFR
activation confers protections against UV-induced apoptosis in
cultured mouse skin dendritic cells. Cell Signal. 20:1830–1838.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng LB, Cheng L, Bi HE, Zhang ZQ, Yao J,
Zhou XZ and Jiang Q: Alpha-melanocyte stimulating hormone protects
retinal pigment epithelium cells from oxidative stress through
activation of melanocortin 1 receptor-Akt-mTOR signaling. Biochem
Biophys Res Commun. 443:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kadekaro AL, Kavanagh R, Kanto H, Terzieva
S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G,
Shertzer HG, et al: Alpha-Melanocortin and endothelin-1 activate
antiapoptotic pathways and reduce DNA damage in human melanocytes.
Cancer Res. 65:4292–4299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan J, Lin F, Zhang W, Xu A, DeGiorgis J,
Lu H and Wan Y: Novel approaches to vitiligo treatment via
modulation of mTOR and NF-κB pathways in human skin melanocytes.
Int J Biol Sci. 13:391–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Le Poole IC, Das PK, van den Wijngaard RM,
Bos JD and Westerhof W: Review of the etiopathomechanism of
vitiligo: A convergence theory. Exp Dermatol. 2:145–153. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gawkrodger DJ, Ormerod AD, Shaw L,
Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J and Young
K: Vitiligo: Concise evidence based guidelines on diagnosis and
management. Postgrad Med J. 86:466–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gianfaldoni S, Wollina U, Tirant M,
Tchernev G, Lotti J, Satolli F, Rovesti M, França K and Lotti T:
Herbal compounds for the treatment of vitiligo: A review. Open
Access Maced J Med Sci. 6:203–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lotti T, Wollina U, Tchernev G, Valle Y,
Lotti J, França K, Satolli F, Rovesti M, Tirant M, Lozev I, et al:
An innovative therapeutic protocol for Vitiligo: Experience with
the use of fraxel herbium laser, topical latanoprost and successive
irradiation with UVA-1 Laser. Open Access Maced J Med Sci. 6:49–51.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehrgou A and Akouchekian M: Therapeutic
impacts of microRNAs in breast cancer by their roles in regulating
processes involved in this disease. J Res Med Sci. 22:1302017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spiegelman VS and Elcheva IA: Metabo-miR:
miR-211 regulates mitochondrial energy metabolism in vitiligo. J
Invest Dermatol. 137:1828–1830, 137. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K,
Ge R, Dai W, Wang G, Gao T and Li C: Oxidative stress-induced
overexpression of miR-25: The mechanism underlying the degeneration
of melanocytes in vitiligo. Cell Death Differ. 23:496–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Li W, Zhang JG, Li HY and Li YM:
Downregulation of tumor suppressor menin by miR-421 promotes
proliferation and migration of neuroblastoma. Tumour Biol.
35:10011–10017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan H, Qi J, Fan BY, Zhang J, Su FF and
Wang HT: MicroRNA-24-3p attenuates myocardial ischemia/reperfusion
injury by suppressing RIPK1 expression in mice. Cell Physiol
Biochem. 51:46–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostaticregulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Zhang Y, Fu H, Huang H, Lu Q, Qin H,
Wu Y, Huang H, Mao G, Wei Z and Liao P: Hes1 knockdown exacerbates
ischemic stroke following tMCAO by increasing ER stress-dependent
apoptosis via the PERK/eIF2α/ATF4/CHOP signaling pathway. Neurosci
Bull. Jul 15–2019.(Epub ahead of print). View Article : Google Scholar
|
|
39
|
Umeda J, Sano S, Kogawa K, Motoyama N,
Yoshikawa K, Itami S, Kondoh G, Watanabe T and Takeda J: In vivo
cooperation between Bcl-xL and the phosphoinositide 3-kinase-Akt
signalingpathway for the protection of epidermal keratinocytes from
apoptosis. FASEB J. 17:610–620. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yue L, Ailin W, Jinwei Z, Leng L, Jianan
W, Li L, Haiming C, Ling H and Chuanjian L: PSORI-CM02 ameliorates
psoriasis in vivo and in vitro by inducing autophagy via inhibition
of the PI3K/Akt/mTOR pathway. Phytomedicine. 64:1530542019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arshad Z, Rezapour-Firouzi S, Mohammadian
M and Ebrahimifar: The sources of essential fatty acids for
allergic and cancer patients; a connection with insight into
mammalian target of rapamycin: A narrative review. Asian Pac J
Cancer Prev. 19:2391–2401. 2018.PubMed/NCBI
|
|
42
|
Shin JM, Kim MY, Sohn KC, Jung SY, Lee HE,
Lim JW, Kim S, Lee YH, Im M, Seo YJ, et al: Nrf2 negatively
regulates melanogenesis by modulating PI3K/Akt signaling. PLoS One.
9:e960352014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Larribere L, Khaled M, Tartare-Deckert S,
Busca R, Luciano F, Bille K, Valony G, Eychene A, Auberger P,
Ortonne JP, et al: PI3K mediates protection against TRAIL-induced
apoptosis in primary human melanocytes. Cell Death Differ.
11:1084–1091. 2004. View Article : Google Scholar : PubMed/NCBI
|