Introduction
Osteosarcoma is the most common type of malignant
bone cancer, which originates from long bones, including the
humerus, femur and tibia, and contributes to cancer-related
mortality in adolescents (1,2).
Although survival rates have improved with the current standard
treatment strategies, such as radiotherapy, chemotherapy and
surgery, 80% of cases of osteosarcoma still progress to metastasis
(3). Therefore, there is an urgent
requirement to develop effective and safe compounds, which exert
minimal cytotoxicity on healthy tissue that could be potential
future therapies for patients with osteosarcoma.
The extracellular matrix (ECM) acts as a support
system for normal cells and tissues. In the context of
osteosarcoma, which progresses rapidly and can lead to metastasis,
aggressive cancer cells can damage ECM components, thus easily
invading normal tissues and causing irreversible damage; ECM
breakdown is mainly modulated by proteases, specifically matrix
metalloproteinases (MMPs), such as MMP-2 and MMP-9 (4–6);
activation of MMPs are understood to promote the invasion of
osteosarcoma (7,8). Previous studies have reported that
the PI3K/AKT signaling pathway is associated with the metastasis of
osteosarcoma (9–11); the deregulation of this pathway
serves an important role in the tumorigenesis, proliferation,
invasion, apoptosis and metastasis of cancer cells (12–16).
Thus, the PI3K/AKT signaling pathway may represent a possible
target for cancer therapy in osteosarcoma.
Alantolactone (ALT) is a biologically natural
compound derived from Inula helenium (17). Previous studies have reported that
ALT promotes numerous biological effects, including
anti-inflammatory and antioxidant functions (18,19).
It has also been observed to suppress several types of human
cancer, including gastric, pancreatic and breast cancer cells
(19–21). It is reported that these anticancer
effects of ALT are mediated through the PI3K/AKT signaling pathway
(22). Thus, it was hypothesized
that ALT may represent a potential agent for the treatment of
osteosarcoma. In the present study, the effect of ALT on the
apoptosis, proliferation, and invasion of osteosarcoma cells,
alongside the underlying mechanism associated with these effects
was evaluated.
Materials and methods
Reagents
ALT and Cell Counting Kit (CCK)-8 were obtained from
MedChem Express. Primary antibodies against PI3K (4249),
phosphorylated (p)-AKT (4060), AKT (9272), cyclin D1 (2978), p27
(3686), Bax (5023), Bcl-2 (15071) and β-actin (3700) were purchased
from Cell Signaling Technology, Inc., primary antibodies against
cleaved caspase-3 and cleaved caspase-8 were purchased from Abcam,
and primary antibodies against MMP-2 and MMP-9 were obtained from
ProteinTech Group, Inc. The Annexin V-FITC/propidium iodide (PI)
apoptosis detection kit and was purchased from Nanjing KeyGen
Biotech Co., Ltd. RPMI-1640 medium and FBS were purchased from
Hyclone; GE Healthcare Life Sciences.
Cell culture and reagents
The human osteosarcoma U2OS and HOS cell lines were
obtained from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences. U2OS and HOS cells were cultured in RPMI-1640
medium and DMEM (Gibco; Thermo Fisher Scientific, Inc.),
respectively, supplemented with 10% FBS and 1%
penicillin/streptomycin, and maintained in a humidified atmosphere
of 37°C and 5% CO2.
CCK-8 assay
The U2OS and HOS cell lines were seeded at a density
of 8×103 cells/well in 96-well plates and cells were
subsequently exposed to a range of concentrations of ALT (2.5, 5,
10, 20, 40, 80 or 160 µM) for 24 and 48 h at 37°C with 5%
CO2. The CCK-8 assay was used to assess the viability of
osteosarcoma cells following drug treatment. A total of 10 µl CCK-8
kit solution was added to each well and the cells were subsequently
incubated for a further 4 h at 37°C with 5% CO2, after
which the optical density of the cell lysates was measured at 450
nm. GraphPad Prism version 7.0 software (GraphPad Software, Inc.)
was used to calculate the median lethal concentration of ALT
(IC50) for osteosarcoma cells.
Colony formation assay
U2OS and HOS cells were collected and seeded in
6-well plates at a density of 1×103 cells/well.
Following cell adherence, the culture medium was replaced with each
cell line's respective media containing a range of ALT
concentrations (U2OS, 0, 5, 10 or 20 µM; HOS, 0, 15, 30 or 60 µM)
and the cells were further incubated for a further 8 days in a
humidified cell incubator at 37°C with 5% CO2. Following
incubation, colonies were first fixed with 4% paraformaldehyde for
30 mins at room temperature and then stained with 0.1% crystal
violet for 5 min at room temperature. Colonies (>50 cells) were
visualized using an optical microscope (magnification, ×10).
Hoechst 33258 staining assay
To evaluate the apoptotic rates of ALT-treated U2OS
cells, the Hoechst 33258 kit (Beyotime Institute of Biotechnology)
was used for nuclear staining. Bright blue nuclear staining
indicated nuclear pyknosis, which is a characteristic of apoptotic
cells (23). Following treatment
with ALT (0, 5, 10 or 20 µM) for 48 h, the U2OS cells were fixed
with 4% paraformaldehyde for 30 min at room temperature. The U2OS
cells were rinsed with PBS three times both before and after
staining with Hoechst 33258 (10 µg/ml; 5 min at room temperature))
in the dark. An Eclipse TS100 fluorescence microscope (Nikon
Corporation; magnification, ×20 and ×40) was used to visualize the
changes in the nuclear morphology of ALT-treated U2OS cells.
Flow cytometric analysis
A total of 5×105 U2OS cells/well were
seeded in 6-well plates and incubated with a range of ALT
concentrations (0, 5, 10 or 20 µM) for 48 h at 37°C with 5%
CO2. Following cellular adherence to the plates, the
U2OS cells were harvested and rinsed with pre-chilled PBS (4°C). To
further evaluate ALT-induced apoptosis of the U2OS cells, an
Annexin V-FITC/ PI kit was used, according to the manufacturer's
protocol. Flow cytometry cell sorting equipment (Navios EX flow
cytometer; Beckman Coulter, Inc. and FlowJo v. 10.4; FlowJo LLC)
was used to analyze the apoptosis of ALT-treated U2OS cells. The
cells stained with Annexin V (+) were considered early apoptotic
and the cells stained with PI (+) were late apoptotic cells.
Wound healing assay
The effect of ALT on the migratory ability of U2OS
cells was assessed using a scratch wound healing assay. A total of
5×105 U2OS cells/well were seeded into 6-well plates and
cultured to 90% confluence prior to incubation at 37°C with 5%
CO2 with 5% serum and a range of ALT concentrations (0,
5, 10 or 20 µM) for 48 h. The wound scratch was performed by 10 µl
pipette tip. The wound area was visualized at the 0, 12, 24 and 48
h time points using an optical microscope (magnification, ×10) and
the width of the wound was analyzed using ImageJ software (v. d
1.47; National Institutes of Health). The Recovered wound area (%)
of each time point (12, 24 and 48 h) was calculated as The wound
area of 0 h-the wound area of each time point)/The wound area of 0
h.
Matrigel invasion assay
Matrigel-coated (BD Biosciences) Transwell chambers
were used to detect the invasive ability of U2OS cells exposed to
0, 5, 10 or 20 µM ALT. A total of 2×104 U2OS cells/well
were plated in the upper chambers of Transwell plates in 100 µl
FBS-free RPMI-1640 medium containing the previously indicated
concentrations of AL, 200 µl RPMI-1640 medium containing 20% FBS
was placed in the lower chambers. Following incubation for 48 h at
37°C with 5% CO2, the non-invasive cells remaining in
the upper chambers were removed. The invasive cells in the lower
chambers were fixed with 4% paraformaldehyde for 30 min and
subsequently stained with 0.1% crystal violet for 20 min at room
temperature. Stained cells were counted using an optical microscope
(magnification, ×20).
Western blotting
A total of 5×105 U2OS cells/well were
seeded into 6-well plates and incubated with a range of ALT
concentrations (0, 5, 10 or 20 µM) for 48 h at 37°C with 5%
CO2. Total protein was extracted from U2OS cells using
RIPA lysis buffer containing 1% protease and phosphorylase
inhibitor (all Beyotime Institute of Biotechnology). Protein
samples were maintained on ice for 30 min and subsequently
centrifuged (15,000 × g for 10 min at 4°C). Total protein was
quantified using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology) and the extracted proteins were mixed with loading
buffer and boiled at 95°C for 5 min for denaturation. Proteins (40
µg) were separated via SDS-PAGE on a 12% gel. The separated
proteins were subsequently transferred onto a 0.22-µm PVDF membrane
and blocked for 2 h with 5% non-fat dry milk at room temperature.
The membranes were incubated with the following primary antibodies
overnight at 4°C: Anti-PI3K, anti-AKT, anti-p-AKT, anti-cyclin D1,
anti-p27, anti-Bax, anti-Bcl-2, anti-cleaved caspase-3,
anti-cleaved caspase-8, anti-MMP-2, anti-MMP-9 and anti-β-actin,
all the primary antibodies were diluted in the primary antibody
diluent (Beyotime Institute of Biotechnology) at a concentration of
1%. Membranes were washed three times with TBS-0.1% Tween 20.
Following the primary antibody incubation, membranes were incubated
with anti-rabbit antibodies (14708, Cell Signaling Technology,
Inc.) which were diluted in the secondary antibody diluent
(Beyotime Institute of Biotechnology) at a concentration of 0.02%,
for 1 h at room temperature. Protein bands were visualized using
the BioSpectrum Imaging system and ImageJ software (v. d 1.47;
National Institutes of Health) was used for densitometry.
Statistical analysis
All data are expressed as the mean ± SD from ≥3
independent experimental repeats. Statistical analyses were
performed using GraphPad Prism software (version 7.0; GraphPad
Software, Inc.). Significant differences between groups were
determined by using one-way ANOVA, followed by a Tukey's post hoc
test, P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity of ALT in osteosarcoma
cell lines
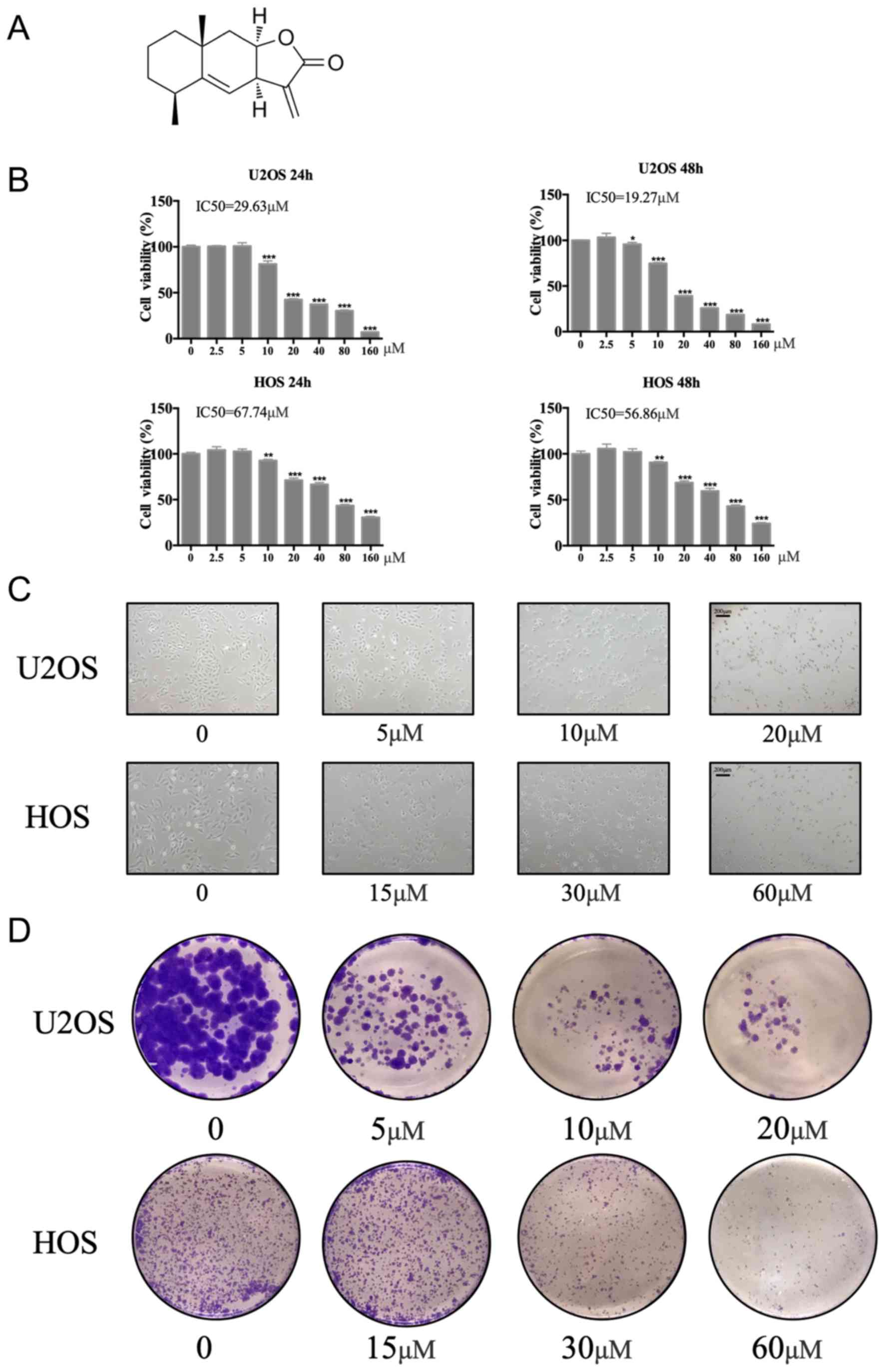
The chemical structure of ALT is presented in
Fig. 1A. Cellular viability of
U2OS and HOS cells was evaluated in the presence of a range of ALT
concentrations for 24 and 48 h using the CCK-8 assay.
Concentrations of 20 or 60 µM ALT significantly reduced the
viability of U2OS cells and HOS cells respectively, compared with
the untreated cells (0 µM; Fig.
1B). The IC50 of ALT in U2OS and HOS cell lines was
determined as 29.63 and 67.74 µM at 24 h, and 19.57 and 56.86 µM at
48 h, respectively, for each cell line. Concentrations of 20 or 60
µM ALT presented the half maximal inhibitory concentration, so
these concentrations were chosen as the high concentrations in the
subsequent experiment.
ALT suppresses the proliferation of
osteosarcoma cells
Low concentrations (5 µM) of ALT slightly decreased
the number of cells and induced the shrinkage of HOS and U2OS cells
compared with untreated cells, whereas higher concentrations (10
and 20 µM) not only decreased the number of osteosarcoma cells, but
also made the shrinkage more serious (Fig. 1C). In addition, the colony
formation assay demonstrated that ALT markedly reduced the
colony-forming ability of U2OS and HOS cells compared with
untreated cells (Fig. 1D).
ALT promotes apoptosis of osteosarcoma
cells
Therapeutic candidates with antitumor activity often
exert cellular cytotoxicity through promoting apoptosis of cancer
cells (24,25). As U2OS and HOS cell lines were both
collected from young Caucasian female patients with osteosarcoma,
this peculiarity also lead to some similarities on tumorigenicity
and drug resistance, therefore, subsequent experiments were only
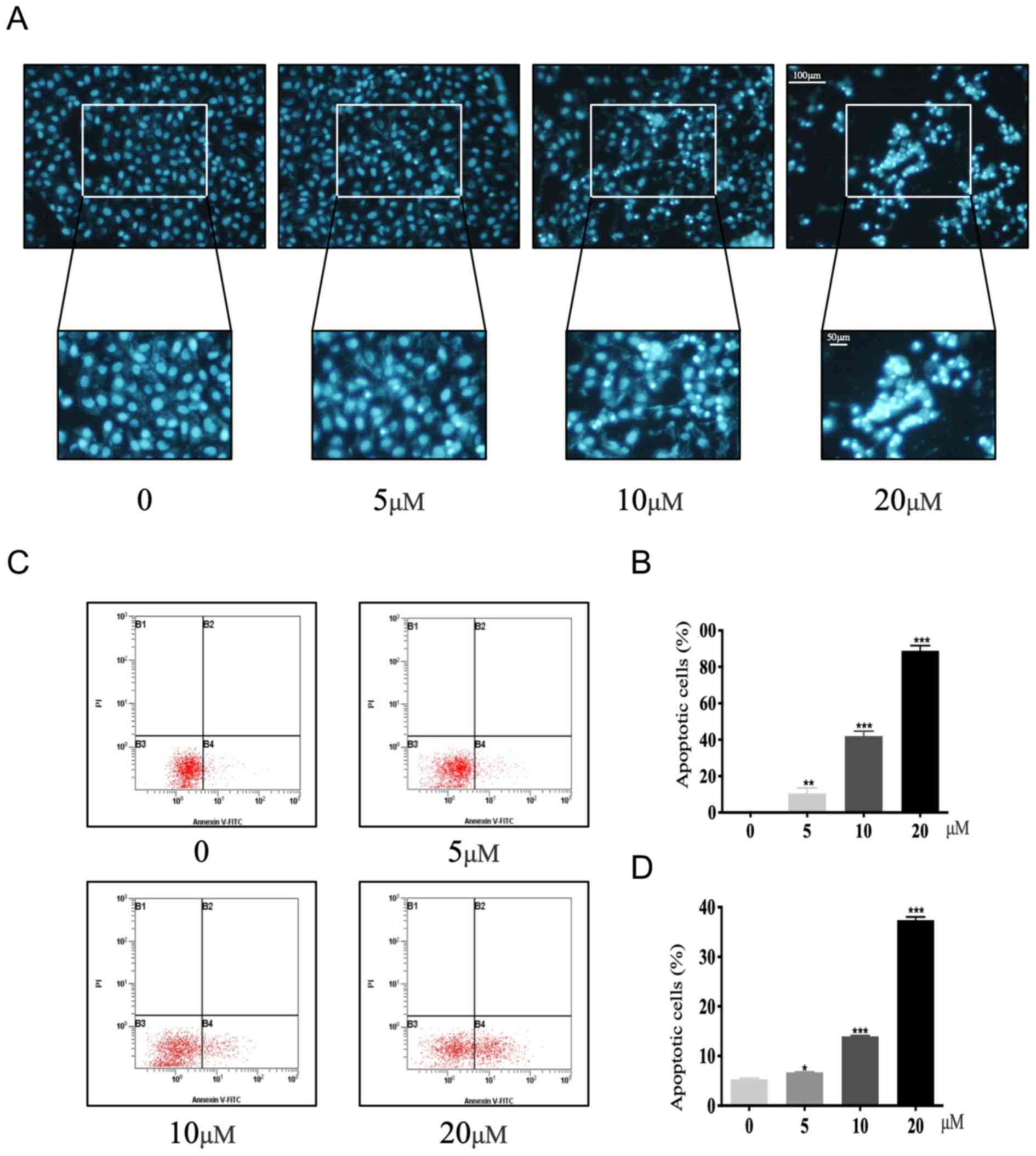
performed on U2OS cells. Hoechst 33258 staining, which detects
condensed chromatin, demonstrated that the number of apoptotic U2OS
cells increased with increasing concentrations of ALT compared with
untreated cells (Fig. 2A and B).
The rate of apoptosis of U2OS cells following ALT administration
was also evaluated using Annexin V-FITC/PI double staining and flow
cytometry (Fig. 2C and D). The
percentage of apoptotic cells was 6.7±0.12 (5 µM), 13.9±0.36 (10
µM) and 37.4±0.45 (20 µM) compared to 0 µM (5.33±0.20)-for ALT
concentrations of 0, 5, 10 and 20 µM respectively (Fig. 2C and D). These data demonstrated
that ALT markedly enhanced the apoptosis of osteosarcoma cells in a
dose-dependent manner.
ALT inhibits the migratory and
invasive ability of osteosarcoma cells
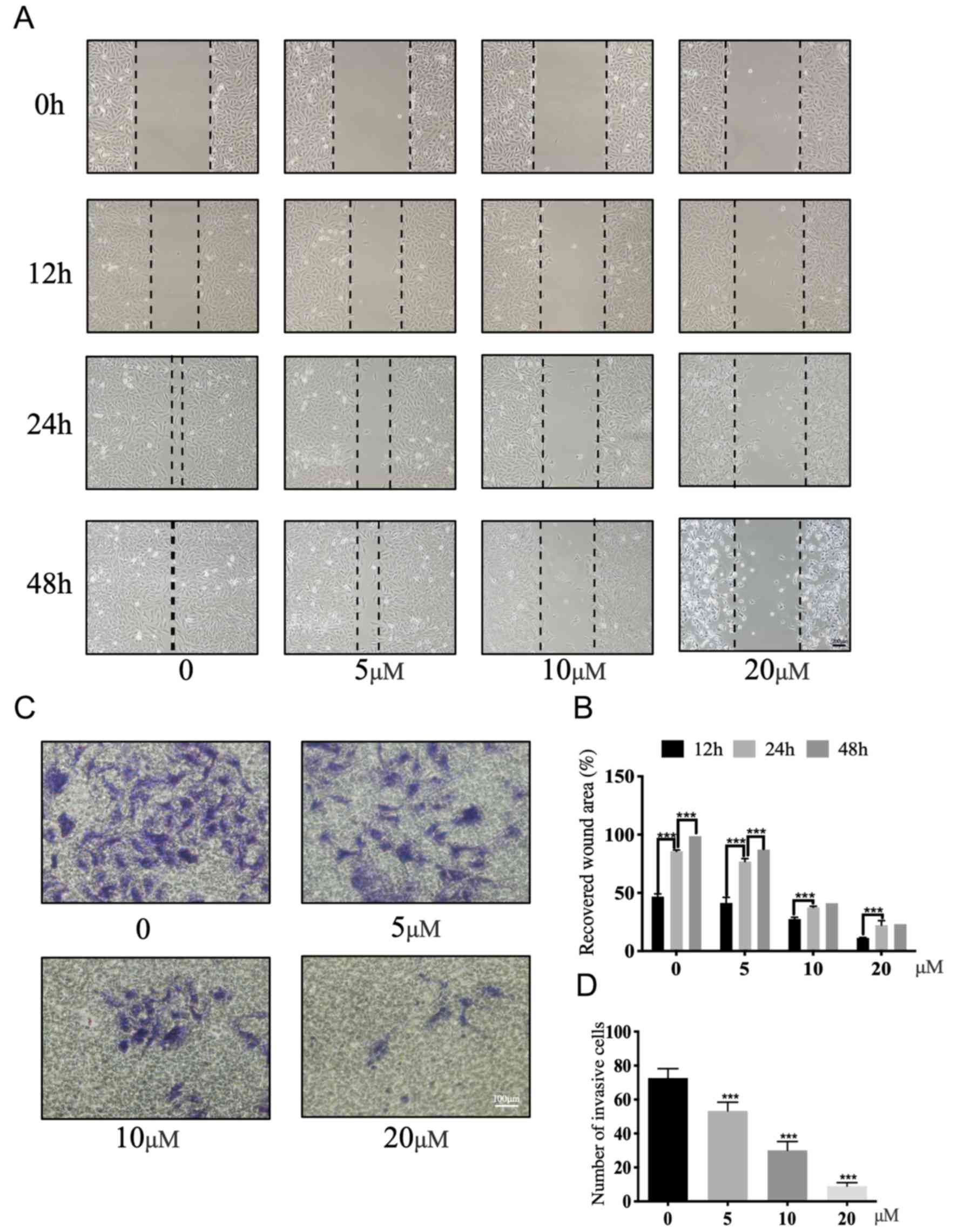
A wound-healing assay was performed to evaluate the
effect of ALT on the migratory ability of U2OS cells. ALT
significantly suppressed the migration of the U2OS cells in a
dose-dependent manner compared with untreated cells (Fig. 3A and B). Subsequently, a Matrigel
assay was performed to assess the effect of ALT on the invasive
potential of U2OS cells. The number of invading cells significantly
decreased following exposure to ALT for 48 h compared with the
untreated cells (Fig. 3C and D).
Overall, these results demonstrated that ALT significantly
suppressed the invasion and migration of U2OS cells.
Inhibitory mechanisms of ALT in
osteosarcoma cells
To evaluate the mechanisms underlying ALT-mediated
inhibition of osteosarcoma, western blotting was performed. High
concentration of ALT-treated cells demonstrated significantly
reduced expression levels of cyclin D1 and increased expression of
p27 compared with the untreated cells (Fig. 4A and B). Cyclin D1 and p27 modulate
the progression through the G1 and S phases of the cell cycle
(26,27), and thus these changes may suggest
the reason for inhibition of cell proliferation.
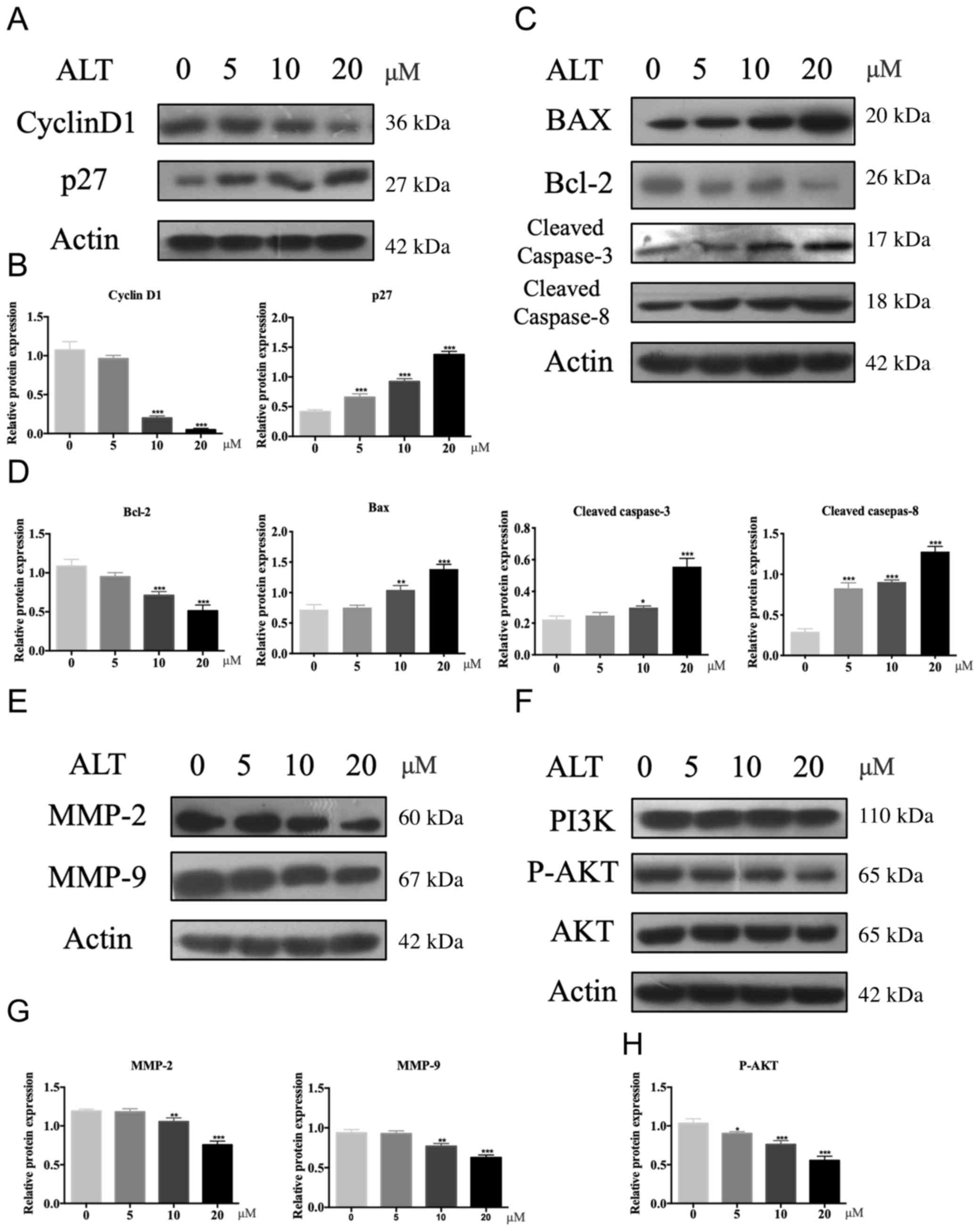 | Figure 4.Potential inhibitory mechanism of ALT
on osteosarcoma cells. (A-H) Western blotting and semi-quantitative
analysis of relative protein expression of (A and B) cyclin D1 and
p27. (C and D) Bax, Bcl-2, cleaved caspase-3 and cleaved caspase-8,
(E and G) MMP-2 and MMP-9, and (F and H) PI3K, p-AKT and AKT in
U2OS cells treated with a range of ALT concentrations. The
expression of cyclin D1, p27, BAX, Bcl-2, cleaved caspase-3,
cleaved caspase-8, MMP-2 and MMP-9 were relative to β-actin, the
expression of p-AKT was relative to AKT. Data are presented as the
mean ± SD of three independent experimental repeats. *P<0.05,
**P<0.01 and ***P<0.001. ALT, alantolactone; MMP, matrix
metalloproteinase; p, phosphorylated. |
It has previously been reported that ALT promotes
apoptosis in osteosarcoma (28–30);
therefore, the effect of ALT on the expression levels on Bcl-2,
Bax, cleaved caspase-3 and cleaved caspase-8, which all serve
important roles in the process of apoptosis was explored. The
expression levels of cleaved caspase-3 and cleaved caspase-8 were
significantly upregulated in the ALT-treated U2OS cells compared
with the untreated cells (Fig. 4C and
D). In addition, ALT treatment reduced the expression levels of
Bcl-2 and increased the expression levels of Bax compared with
untreated cells (Fig. 4C and
D).
MMPs are strongly associated with cellular invasion
in osteosarcoma (31–33); therefore, the effect of ALT on
MMP-2 and MMP-9 was evaluated. The data revealed that ALT decreased
the expression of MMP-2 and MMP-9 in a dose-dependent manner
compared with untreated cells (Fig. 4E
and G).
Previous studies have reported that the PI3K/AKT
signaling pathway serves an important role in osteosarcoma
(11,34,35);
PI3K/AKT modulates the proliferation, invasion, migration and
apoptosis of osteosarcoma cell lines (36–39).
Thus, the effect of ALT on the PI3K/AKT signaling pathway was
investigated to confirm whether it is the mechanism by which ALT
affects osteosarcoma cells. ALT demonstrated a significant ability
to reduce the activation of p-AKT, which was observed through
significantly decreased protein expression levels in U2OS cells
compared with untreated cells (Fig. 4F
and H) although no effect on the expression of total PI3K was
observed. These findings suggested that the cytotoxic effect of ALT
is closely associated with regulation of the PI3K/AKT signaling
pathway.
Discussion
Osteosarcoma is the most common type of primary bone
cancer and affects a large number of adolescents; the incidence of
osteosarcoma in the Netherlands was ~0.55/100,000 (using the
European Standardized Rate, ESR) (40). Current standard therapy regimens,
such as surgical resection and chemotherapy only delay osteosarcoma
progression and are unable to prevent tumor metastasis (41). Therefore, patient mortality rates
for osteosarcoma remain high, and effective and safe therapeutic
agents are urgently required to improve patient outcomes.
ALT is the main biologically active compound derived
from Inula helenium (42).
ALT has been widely researched as a potential candidate for the
treatment of several types of cancer, and previous in vivo
studies have reported its antiproliferative, anti-metastatic and
anti-invasive activity in a variety of cell lines (19,20,42).
The present study aimed to evaluate the effect of ALT on
osteosarcoma and to determine whether it could be used as a
potential future treatment strategy for this disease. It was
demonstrated that ALT inhibited the proliferation of osteosarcoma
cell lines in a dose-dependent manner. Previous studies have
reported the role of cyclin D1 and p27 in cell proliferation
(27,43,44);
thus, the effect of ALT on these two proteins was further
investigated. The data revealed that ALT decreased the expression
of cyclin D1 and increased the expression of p27. In addition, ALT
significantly reduced the invasive and migratory ability of
osteosarcoma cells, which was attributed to the observed inhibitory
effect of ALT over MMP-2 and MMP-9 expression levels. Regarding
apoptosis, it was revealed that ALT could promote the apoptosis of
osteosarcoma cells in a dose-dependent manner. The Bcl-2 protein
family has previously been shown to regulate apoptosis through the
release of pro-apoptotic factors (45,46)
and Bax activation increases osteosarcoma cell sensitivity
(47). A previous study
demonstrated that the downregulation of Bcl-2 and upregulation of
BAX significantly promotes cell apoptosis (48). The data from the present study
demonstrated that ALT significantly inhibited the expression of
Bcl-2 and increased the activation of BAX, thus promoting the
apoptosis of osteosarcoma cells. The role of caspase-3 and
caspase-8 in apoptosis has also been previously explored (49,50).
Western blot analysis was used to evaluate the effect of ALT on
these apoptosis-related proteins and revealed that ALT promoted the
apoptosis of osteosarcoma cells through modulation of these
apoptotic-related factors.
Cyclin D1 and p27 are vital mediators of cell death
(43,51,52).
Previous studies have reported a crucial role for cyclin D1 and p27
in modulating the G1 and S phase of cells; cell cycle arrest at the
G1/S phase leads to cell apoptosis (53,54).
Cyclin D1 is repressed by the p53 protein (55), thus p53 is eventually responsible
for growth arrest-induced cell apoptosis. p53 is also able to
directly activate Bax to facilitate permeabilization of the
mitochondria and it serves a role in the downregulation of Bcl-2
expression, which contributes to DNA damage (56,57).
In addition, p53 promotes apoptosis through caspase-3 and −8
(58,59). Thus, the reduced protein expression
of cyclin D1 and increased expression of p27 caused by ALT provides
an explanation for the pro-apoptotic effect of ALT. Future work
should aim to explore the effect of ALT on the p53 pathway, which
was not undertaken in the current study. The findings from the
present study suggested that ALT may suppress the proliferation,
invasion, and migration of osteosarcoma cell lines, while promoting
their apoptosis. However, the underlying mechanism of action of ALT
was unclear.
Accumulating evidence has indicated an important
role of the PI3K/AKT signaling pathway in osteosarcoma (11,34,35);
PI3K/AKT signaling modulates the proliferation, invasion, migration
and apoptosis of osteosarcoma cell lines (36–39,60).
Previous studies revealed that alantolactone is a specific
inhibitor in several diseases via the PI3K/AKT signaling pathway
(7,8). The downregulation of AKT decreases
the downstream signaling factors, caspase-8 and caspase-3, which
are responsible for cell apoptosis (61). Protein expression levels of cyclin
D1, which is a prominent regulator of cell cycle progression from
G1 to S phase (62), were also
decreased following AKT downregulation, which has been demonstrated
to inhibit cell proliferation (63). Furthermore, invasion-related
proteins MMP-2 and MMP-9 are modulated by AKT (64). Other inhibitors of AKT have also
demonstrated an ability to suppress osteosarcoma proliferation,
invasion and migration (65,66).
Consequently, this study explored whether the antitumor activities
of ALT were mediated through the PI3K/AKT signaling pathway. The
findings indicated that ALT significantly suppressed the expression
of AKT, thus, it may act through this mechanism to inhibit
osteosarcoma progression.
Nonetheless, the study has several limitations.
Firstly, osteosarcoma is composed of several types of cells;
therefore, the effect of ALT on other osteosarcoma cell lines
should be investigated. Secondly, the antitumor activity of ALT was
only investigated in vitro, and its in vivo
protective effect on osteosarcoma remains unclear. In conclusion,
the present study demonstrated that the protective effect of ALT on
osteosarcoma was mediated through the inhibition of cellular
proliferation, migration and invasion, in addition to promoting
apoptosis, and that these effects may be explained through the
inhibitory effect of ALT on the PI3K/AKT signaling pathway. The
present study represents a potential, future therapeutic strategy
for osteosarcoma treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Medical and
Health Technology Project of Zhejiang Province (grant no.
2019PY073), The Science and Technology Research on Public Welfare
Project of Ningbo, Zhejiang Province (grant no. 2019C50050) and The
Scientific Technology Project of Agriculture and Social Development
of Yingzhou, Ningbo, Zhejiang Province (grant no. 20180137).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and JC conceived the study; YZ and QW conducted
the experiments; JH wrote the manuscript and performed statistical
analysis; YZ and JC analyzed the results and created the figures.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finkel MP, Reilly CA Jr and Biskis BO:
Pathogenesis of radiation and virus-induced bone tumors. Recent
Results Cancer Res. 92–103. 1976.PubMed/NCBI
|
|
2
|
Li X, Liu X, Fang J, Li H and Chen J:
microRNA-363 plays a tumor suppressive role in osteosarcoma by
directly targeting MAP2K4. Int J Clin Exp Med. 8:20157–20167.
2015.PubMed/NCBI
|
|
3
|
Rosen G: The current management of
malignant bone tumours: Where do we go from here? Med J Aust.
148:373–377. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chien MH, Lin CW, Cheng CW, Wen YC and
Yang SF: Matrix metalloproteinase-2 as a target for head and neck
cancer therapy. Expert Opin Ther Targets. 17:203–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin CW, Chou YE, Chiou HL, Chen MK, Yang
WE, Hsieh MJ and Yang SF: Pterostilbene suppresses oral cancer cell
invasion by inhibiting MMP-2 expression. Expert Opin Ther Targets.
18:1109–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nawaz M, Shah N, Zanetti BR, Maugeri M,
Silvestre RN, Fatima F, Neder L and Valadi H: Extracellular
vesicles and matrix remodeling enzymes: The emerging roles in
extracellular matrix remodeling, progression of diseases and tissue
repair. Cells. 7:E1672018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad Ø and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang HG, Kim HS, Kim KJ, Oh JH, Lee MR,
Seol SM and Han I: RECK expression in osteosarcoma: Correlation
with matrix metalloproteinases activation and tumor invasiveness. J
Orthop Res. 25:696–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Li M, Zhu X, Bai Y and Yang C:
Knockdown of Akt sensitizes osteosarcoma cells to apoptosis induced
by cisplatin treatment. Int J Mol Sci. 12:2994–3005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Montero CM, Wygant JN and McIntyre
BW: PI3-K/Akt-mediated anoikis resistance of human osteosarcoma
cells requires Src activation. Eur J Cancer. 42:1491–1500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai M, Cong L, Han Y and Tu G: CIP2A is
overexpressed in osteosarcoma and regulates cell proliferation and
invasion. Tumour Biol. 35:1123–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding L, He S and Sun X: HSP70 desensitizes
osteosarcoma cells to baicalein and protects cells from undergoing
apoptosis. Apoptosis. 19:1269–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY,
Luo QF, Shu Y, Liu ZL and Huang SH: Knockdown of aurora-B inhibits
osteosarcoma cell invasion and migration via modulating
PI3K/Akt/NF-κB signaling pathway. Int J Clin Exp Pathol.
7:3984–3991. 2014.PubMed/NCBI
|
|
15
|
Zhou R, Zhang Z, Zhao L, Jia C, Xu S, Mai
Q, Lu M, Huang M, Wang L, Wang X, et al: Inhibition of mTOR
signaling by oleanolic acid contributes to its anti-tumor activity
in osteosarcoma cells. J Orthop Res. 29:846–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang HS, Chang JB, Lin JH, Lin JP, Hsu
SC, Liu CM, Liu JY, Wu PP, Lu HF, Au MK and Chung JG: Deguelin
inhibits the migration and invasion of U-2 OS human osteosarcoma
cells via the inhibition of matrix metalloproteinase-2/-9 in vitro.
Molecules. 19:16588–16608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X and Zhang HM: Alantolactone
induces gastric cancer BGC-823 cell apoptosis by regulating
reactive oxygen species generation and the AKT signaling pathway.
Oncol Lett. 17:4795–4802. 2019.PubMed/NCBI
|
|
18
|
Chun J, Choi RJ, Khan S, Lee DS, Kim YC,
Nam YJ, Lee DU and Kim YS: Alantolactone suppresses inducible
nitric oxide synthase and cyclooxygenase-2 expression by
down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling
pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol.
14:375–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng H, Yang L, Kang Y, Chen M, Lin S,
Xiang Y, Li C, Dai X, Huang X, Liang G and Zhao C: Alantolactone
sensitizes human pancreatic cancer cells to EGFR inhibitors through
the inhibition of STAT3 signaling. Mol Carcinog. 58:565–576. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Cao X, Kong Y, Wang S, Xia Y, Bi R
and Liu J: Apoptosis-promoting and migration-suppressing effect of
alantolactone on gastric cancer cell lines BGC-823 and SGC-7901 via
regulating p38MAPK and NF-κB pathways. Hum Exp Toxicol.
38:1132–1144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Liu M, Wang S, He Y, Huo Y, Yang Z
and Cao X: Alantolactone induces apoptosis and suppresses migration
in MCF-7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2
signaling pathways. Int J Mol Med. 42:1847–1856. 2018.PubMed/NCBI
|
|
22
|
Kang X, Wang H, Li Y, Xiao Y, Zhao L,
Zhang T, Zhou S, Zhou X, Li Y, Shou Z, et al: Alantolactone induces
apoptosis through ROS-mediated AKT pathway and inhibition of
PINK1-mediated mitophagy in human HepG2 cells. Artif Cells Nanomed
Biotechnol. 47:1961–1970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ioannou YA and Chen FW: Quantitation of
DNA fragmentation in apoptosis. Nucleic Acids Res. 24:992–993.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Zhang Q, Bao J, Huang J and Zhang
H: Apiosporamide, a 4-hydroxy-2-pyridone alkaloid, induces
apoptosis via PI3K/Akt signaling pathway in osteosarcoma cells.
Onco Targets Ther. 12:8611–8620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Sun S, Chen J, Ren P, Hu Y, Cao
Z, Sun H and Ding Y: Oxymatrine induces mitochondria dependent
apoptosis in human osteosarcoma MNNG/HOS cells through inhibition
of PI3K/Akt pathway. Tumor Biol. 35:1619–1625. 2014. View Article : Google Scholar
|
|
26
|
Ni Y, Schmidt KR, Werner BA, Koenig JK,
Guldner IH, Schnepp PM, Tan X, Jiang L, Host M, Sun L, et al: Death
effector domain-containing protein induces vulnerability to cell
cycle inhibition in triple-negative breast cancer. Nat Commun.
10:28602019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YW, Morita I, Ikeda M, Ma KW and
Murota S: Connexin43 suppresses proliferation of osteosarcoma U2OS
cells through post-transcriptional regulation of p27. Oncogene.
20:4138–4149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muhammad T, Ikram M, Ullah R, Rehman SU
and Kim MO: Hesperetin, a citrus flavonoid, attenuates lps-induced
neuroinflammation, apoptosis and memory impairments by modulating
TLR4/NF-κB signaling. Nutrients. 11:E6482019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun WJ, Huang H, He B, Hu DH, Li PH, Yu
YJ, Zhou XH, Lv Z, Zhou L, Hu TY, et al: Romidepsin induces G2/M
phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis
induction through JNK/c-Jun/caspase3 pathway in hepatocellular
carcinoma cells. Biochem Pharmacol. 127:90–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu
Y, Tian N, Huang Y, Xue E, Wang X and Xu H: Apoptosis, senescence,
and autophagy in rat nucleus pulposus cells: Implications for
diabetic intervertebral disc degeneration. J Orthop Res.
31:692–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho HJ, Lee TS, Park JB, Park KK, Choe JY,
Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, et al: Disulfiram
suppresses invasive ability of osteosarcoma cells via the
inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol.
40:1069–1076. 2007.PubMed/NCBI
|
|
32
|
Bjørnland K, Winberg JO, Odegaard OT,
Hovig E, Loennechen T, Aasen AO, Fodstad O and Maelandsmo GM:
S100A4 involvement in metastasis: Deregulation of matrix
metalloproteinases and tissue inhibitors of matrix
metalloproteinases in osteosarcoma cells transfected with an
anti-S100A4 ribozyme. Cancer Res. 59:4702–4708. 1999.PubMed/NCBI
|
|
33
|
Jin J, Cai L, Liu ZM and Zhou XS:
MiRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hou CH, Lin FL, Tong KB, Hou SM and Liu
JF: Transforming growth factor alpha promotes osteosarcoma
metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem
Pharmacol. 89:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang A, He S, Sun X, Ding L, Bao X and
Wang N: Wnt5a promotes migration of human osteosarcoma cells by
triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell
Int. 14:152014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takagi S, Takemoto A, Takami M, Oh-Hara T
and Fujita N: Platelets promote osteosarcoma cell growth through
activation of the platelet-derived growth factor receptor-Akt
signaling axis. Cancer Sci. 105:983–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shukla A, Alsarraj J and Hunter K:
Understanding susceptibility to breast cancer metastasis: The
genetic approach. Breast Cancer Manag. 3:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long XH, Zhang GM, Peng AF, Luo QF, Zhang
L, Wen HC, Zhou RP, Gao S, Zhou Y and Liu ZL: Lapatinib alters the
malignant phenotype of osteosarcoma cells via downregulation of the
activity of the HER2-PI3K/AKT-FASN axis in vitro. Oncol Rep.
31:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goedhart LM, Ho VKY, Dijkstra PDS,
Schreuder HWB, Schaap GR, Ploegmakers JJW, van der Geest ICM, van
de Sande MAJ, Bramer JA, Suurmeijer AJH and Jutte PC: Bone sarcoma
incidence in the Netherlands. Cancer Epidemiol. 60:31–38. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reed DR, Hayashi M, Wagner L, Binitie O,
Steppan DA, Brohl AS, Shinohara ET, Bridge JA, Loeb DM, Borinstein
SC and Isakoff MS: Treatment pathway of bone sarcoma in children,
adolescents, and young adults. Cancer. 123:2206–2218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu YR, Cai QY, Gao YG, Luan X, Guan YY,
Lu Q, Sun P, Zhao M and Fang C: Alantolactone, a sesquiterpene
lactone, inhibits breast cancer growth by antiangiogenic activity
via blocking VEGFR2 signaling. Phytother Res. 32:643–650. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kranenburg O, van der Eb AJ and Zantema A:
Cyclin D1 is an essential mediator of apoptotic neuronal cell
death. EMBO J. 15:46–54. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Hu H, Song L, Cai L, Wei R and
Jin W: Epirubicin-mediated expression of miR-302b is involved in
osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett.
222:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eliseev RA, Dong YF, Sampson E, Zuscik MJ,
Schwarz EM, O'Keefe RJ, Rosier RN and Drissi MH: Runx2-mediated
activation of the Bax gene increases osteosarcoma cell sensitivity
to apoptosis. Oncogene. 27:3605–3614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Diff. 6:99–104. 1999.
View Article : Google Scholar
|
|
50
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Katayose Y, Kim M, Rakkar AN, Li Z, Cowan
KH and Seth P: Promoting apoptosis: A novel activity associated
with the cyclin-dependent kinase inhibitor p27. Cancer Res.
57:5441–5445. 1997.PubMed/NCBI
|
|
52
|
Sofer-Levi Y and Resnitzky D: Apoptosis
induced by ectopic expression of cyclin D1 but not cyclin E.
Oncogene. 13:2431–2437. 1996.PubMed/NCBI
|
|
53
|
Shishodia S, Amin HM, Lai R and Aggarwal
BB: Curcumin (diferuloylmethane) inhibits constitutive NF-κB
activation, induces G1/S arrest, suppresses proliferation, and
induces apoptosis in mantle cell lymphoma. Biochem Pharmacol.
70:700–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181-182:475–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rocha S, Martin AM, Meek DW and Perkins
ND: p53 represses cyclin D1 transcription through down regulation
of Bcl-3 and inducing increased association of the p52 NF-κB
subunit with histone deacetylase 1. Mol Cell Biol. 23:4713–4727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu Yl, Mehew JW, Heckman CA, Arcinas M and
Boxer LM: Negative regulation of bcl-2 expression by p53 in
hematopoietic cells. Oncogene. 20:240–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cregan SP, MacLaurin JG, Craig CG,
Robertson GS, Nicholson DW, Park DS and Slack RS: Bax-dependent
caspase-3 activation is a key determinant in p53-induced apoptosis
in neurons. J Neurosci. 19:7860–7869. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ding HF, Lin YL, McGill G, Juo P, Zhu H,
Blenis J, Yuan J and Fisher DE: Essential role for caspase-8 in
transcription-independent apoptosis triggered by p53. J Biol Chem.
275:38905–38911. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sheng L, Tang T, Liu Y, Ma Y, Wang Z, Tao
H, Zhang Y and Qi Z: Inducible HSP70 antagonizes cisplatin-induced
cell apoptosis through inhibition of the MAPK signaling pathway in
HGC-27 cells. Int J Mol Med. 42:2089–2097. 2018.PubMed/NCBI
|
|
61
|
Liu YW, Yang T, Zhao L, Ni Z, Yang N, He F
and Dai SS: Activation of adenosine 2A receptor inhibits neutrophil
apoptosis in an autophagy-dependent manner in mice with systemic
inflammatory response syndrome. Sci Rep. 6:336142016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S transition. Mol
Cell Biol. 15:3463–3469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kline CL, Van den Heuvel AP, Allen JE,
Prabhu VV, Dicker DT and El-Deiry WS: ONC201 kills solid tumor
cells by triggering an integrated stress response dependent on ATF4
activation by specific eIF2α kinases. Sci Signal. 9:ra182016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang L, Zhang ZG, Zhang RL, Gregg SR,
Hozeska-Solgot A, LeTourneau Y, Wang Y and Chopp M: Matrix
metalloproteinase 2 (MMP2) and MMP9 secreted by
erythropoietin-activated endothelial cells promote neural
progenitor cell migration. J Neurosci. 26:5996–6003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kuijjer ML, van den Akker BE, Hilhorst R,
Mommersteeg M, Buddingh EP, Serra M, Bürger H, Hogendoorn PC and
Cleton-Jansen AM: Kinome and mRNA expression profiling of
high-grade osteosarcoma cell lines implies Akt signaling as
possible target for therapy. BMC Med Genomics. 7:42014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hideshima T, Catley L, Yasui H, Ishitsuka
K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson
PG and Anderson KC: Perifosine, an oral bioactive novel
alkylphospholipid, inhibits Akt and induces in vitro and in vivo
cytotoxicity in human multiple myeloma cells. Blood. 107:4053–4062.
2006. View Article : Google Scholar : PubMed/NCBI
|