Introduction
Liver dysfunction is a global health issue and
currently the only successful treatment for end-stage liver disease
is liver transplantation (1).
However, due to donor shortage and immunological rejection, its
clinical application is limited (2). To this end, bio-artificial liver
support systems and hepatocyte transplantation are two potential
complementary therapies for patients with end-stage liver disease.
Developments in stem cells, particularly mesenchymal stem cells
(MSCs), have highlighted a new source of liver cells as they have
the ability to differentiate into hepatocyte-like cells via the
addition of cytokines in vitro (3).
MSCs can be isolated from various body tissues,
including amniotic fluid, umbilical cord placenta, bone marrow and
adipose tissue (4,5). Human umbilical cord (Hu)MSCs are
recognized as an ideal supply for cell therapy because of their low
immunogenicity, abundant source and freedom from ethical issues
(6). Our recent study showed the
efficacy of HuMSCs in regenerative medicine and that HuMSCs hold
numerous advantages over bone marrow-derived MSCs (BMSCs),
including higher potential for proliferation and differentiation
abilities in vitro (7).
However, the efficacy of hepatic differentiation of MSCs is still
insufficient for clinical application (8). Therefore, it is necessary to find a
new differentiation method to achieve a higher efficient
transdifferentiation.
Long noncoding RNAs (lncRNAs) are a class of RNAs
>200 nucleotides in length that cannot encode proteins. It has
recently been reported that some lncRNAs can play important roles
in cellular activities, including cell proliferation, self-renewal,
differentiation and apoptosis (9,10).
For example, HOTAIR improves MSC differentiation and is associated
with senescence-associated DNA methylation (11).
Studies on lncRNA cancer upregulated drug resistant
(CUDR) have mainly focused on cancer cells and other related
molecular mechanisms. It is a novel noncoding RNA gene, which was
found to influence the proliferation, apoptosis and cell cycle
progression of colorectal cancer cells (12). Moreover, CUDR has the ability to
promote liver cancer growth and hepatocyte-like stem cell malignant
transformation epigenetically by cooperating with set
domain-containing 1A, histone lysine methyltransferase (13). Little is known regarding the
expression of CUDR in hepatocytes or in the differentiation of
hepatocytes. A previous study has highlighted the role of CUDR in
embryo stem cell growth and hepatic differentiation (14). However, the function of CUDR in the
hepatic differentiation of MSCs remains unclear. The present study
demonstrated that expression of CUDR significantly increased during
the hepatic differentiation of HuMSCs, and that it promoted hepatic
differentiation. Moreover, these results showed that CUDR not only
regulated liver-enriched factors, but also inhibited the
Wnt/β-catenin pathway.
Materials and methods
Culture and differentiation of
HuMSCs
HuMSCs were purchased from Beijing Beina Chuanglian
Biotechnology Institute. Cells were cultured in 25-cm2
culture flasks containing HyClone™ Dulbecco's modified Eagle's
medium (HyClone; GE Healthcare Life Sciences), supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were grown at 37°C under 5% CO2
atmosphere. The culture medium was replaced every 3 days and the
HuMSCs were digested with trypsin (Gibco; Thermo Fisher Scientific,
Inc.) once they reached 70–80% confluency. The cells in the fourth
passage were used for further differentiation. Before hepatic
differentiation, the multipotency of the cultured HuMSCs was
confirmed by differentiation experiments. The cells were treated
with osteogenic medium containing L-glutamine, decamethasone,
ascorbate and β-glycerophosphate (Sigma-Aldrich; Merck KGaA) and
chondrogenic medium containing h-Insulin, L-glutamin,
dexamethasone, indomethacin and 3-isobuty-I-methyl-xanthine
(Sigma-Aldrich; Merck KGaA) according to previous studies (15,16).
Alizarin red staining
Cells were washed by PBS twice and fixed in 10%
paraformaldehyde for at 4°C for 10 min. Alizarin red (0.1%) was
added at 37°C for 30 min. Following washing in distilled water,
they were observed under an inverted microscope (magnification,
×100).
Type II collagen staining
Cells were fixed with 4% paraformaldehyde at 4°C for
30 min, permeabilized with 2% Triton X-100 and labeled with
monoclonal antibody anti-type II collagen antibody (SC-52658, Santa
Cruz Biotechnology, Inc.). They were observed under an inverted
microscope (magnification, ×100).
Hepatic differentiation
To induce hepatic differentiation, the growth medium
was replaced with differentiation medium described below when cells
in passage four reached 80% confluency, as based on a previous
protocol (3). Differentiation was
induced by treating MSCs with liver-specific growth factors: Days
0–2, Iscove's modified Dulbecco's medium (IMDM, Gibco; Thermo
Fisher Scientific, Inc.) with 20 ng/ml epidermal growth factor
(PeproTech, Inc.) and 10 ng/ml basic fibroblast growth factor
(bFGF; PeproTech, Inc.); days 3–9, IMDM supplemented with 20 ng/ml
hepatocyte growth factor (PeproTech, Inc.), 10 ng/ml bFGF and 0.61
g/ml nicotinamide (Sigma-Aldrich; Merck KGaA); from day 9 onwards,
IMDM containing 20 ng/ml oncostatin M (PeproTech, Inc.), 1 µmol/l
dexamethasone (Sigma-Aldrich; Merck KGaA) and 50 mg/ml
insulin/transferring/selenium (Sigma-Aldrich; Merck KGaA). The
hepatic differentiation medium was replaced every 3 days. The
progression of differentiation from HuMSCs to hepatocytes (at days
14 and 28) was analyzed.
Immunocytochemistry analysis
After induction, cells were fixed with 4%
paraformaldehyde for 30 min (Beyotime Institute of Biotechnology)
and blocked in 10% BSA (Gibco; Thermo Fisher Scientific, Inc.) at
37°C for 30 min. Slides were then incubated with primary antibodies
(ALB sc-271605, AFP sc-8399, CYP3A4 sc-365415 and β-catenin
sc-47724, Santa Cruz Biotechnology, Inc.), including mouse
monoclonal anti-albumin (ALB; 1:200), mouse monoclonal anti-α
fetoprotein (AFP; 1:200), mouse monoclonal anti-cytochrome P450 3A4
(CYP3A4; 1:200) and mouse monoclonal anti-β-catenin (1:200)
overnight at 4°C. After washing, cells were incubated with
FITC-conjugated (Dylight 594 and Alexa 488) goat anti-mouse
immunoglobulin G secondary antibody (1:1,000, sc-516140; Santa Cruz
Biotechnology, Inc.) at 37°C for 45 min. After rinsing, the nuclei
were stained with DAPI (Sigma-Aldrich; Merck KGaA) at 37°C for 5
min and cells were then observed with a fluorescence microscope
(Olympus Corporation) at ×200 magnification.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The cDNA was synthesized using
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.) in a
20 µl reaction containing 100–200 ng of total RNA. All samples were
reacted at 37°C for 60 min, heated at 95°C for 5 min, and finally
held at 4°C. The products were then quantified via RT-qPCR using
SYBR Green Master Mix (Takara Biotechnology Co., Ltd.) with the
primer sequences listed in Table
I. The analysis of the melting curve was achieved to exclude
nonspecific amplification products (16). Human GAPDH was used as an internal
control for PCR. Thermocycling conditions were: Initial
denaturation: 95°C for 30 sec, 95°C for 5 sec and 60°C for 34 sec
for 30 cycles. Fold changes were calculated using the relative
quantification (2−ΔΔCq) method (17).
 | Table I.Sequences of reverse
transcription-quantitative PCR primers. |
Table I.
Sequences of reverse
transcription-quantitative PCR primers.
| Genes | Primer sequence
(5′-3′) | Fragment length
(bp) | Annealing
temperature |
|---|
| ALB | F:
TGCTTGAATGTGCTGATGACAGGG |
|
|
|
| R:
AAGGCAAGTCAGCAGGCATCTCATC | 162 | 60 |
| AFB | F:
GAAACCCACTGGAGATGAACAGTC |
|
|
|
| R:
AAGTGGGATCGATGCAGGA | 190 | 60 |
| TAT | F:
TGAGCAGTCTGTCCACTGCCT |
|
|
|
| R:
ATGTGAATGAGGAGGATCTGAG | 359 | 60 |
| G6P | F:
GCTGGAGTCCTGTCAGGCATTGC |
|
|
|
| R:
TAGAGCTGAGGCGGAATGGGAG | 349 | 60 |
| CYP3A4 | F:
TGTGCCTGAGAACACCAGAG |
|
|
|
| R:
GCAGAGGAGCCAAATCTACC | 202 | 60 |
| α1AT | F:
CTGGGACAGTGAATCGACAATGC |
|
|
|
| R:
TCTGTTTCTTGGCCTCTTGGTG | 560 | 54 |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG |
|
|
|
| R:
AGGGCCATCCACAGTCTTC | 258 | 52 |
| HNF4α | F:
GCACCAACCTCAACGC |
|
|
|
| R:
AGGCTGCTGTCCTCATAG | 313 | 56 |
| CUDR | F:
ACGCTAACTGGCACCTTGTT |
|
|
|
| R:
CTCCGGACTGCTTCAAGTGT | 124 | 56 |
| HNF3β | F:
GCACCTGCAGATTCTGATTTT |
|
|
|
| R:
GACTTCCCTGCAACAACAGC | 66 | 60 |
| HNF6 | F:
CCTGGAGCAAACTCAAATCC |
|
|
|
| R:
TTCTTTCCTTTTGCATGCTG | 116 | 60 |
| CEBPα | F:
CAACACTTGTATCTGGCCTCTG |
|
|
|
| R:
CGAGCAAAACCAAAACAAAAC | 112 | 60 |
| β-catenin | F:
ATTGTCCACGCTGGATTTTC |
|
|
|
| R:
AGGTCTGAGGAGCAGCTTCA | 142 | 58 |
CUDR transfection
CUDR lentiviral plasmid LV5-CUDR-homo and negative
control vectors LV3-NC were purchased from Shanghai GenePharma Co.,
Ltd. Cells were seeded in 6-well plates (5×104
cells/well) and then incubated with 1×108 TU/ml
lentivirus (4 µl), 5 µg/ml polybrene (Shanghai GenePharma Co.,
Ltd.) and complete medium. After transfection, lentivirus-infected
HuMSCs were subjected to puromycin for selection. After 7 days of
selection, the stable transfected cell line was identified, and
overexpression of CUDR was confirmed by RT-qPCR analysis.
ELISA
The cell culture supernatants were collected after
hepatic differentiation of HuMSCs on 6-well plates. The human ALB
and blood urea nitrogen (BuN) concentrations of culture
supernatants were measured using ELISA kits (ALB, ab108788, Abcam;
BuN, SKT-213-192, StressMarq Biosciences Inc.), referring to the
manufacturer's protocol.
Western blotting
Cells were lysed in RIPA buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) and a protease inhibitor cocktail before
being heated at 95°C for 10 min. Then, the lysates were centrifuged
at 15,000 × g for 25 min at 4°C to remove the debris. 10 µg protein
per lane was separated by 10% SDS-PAGE and transferred to 0.45-µm
PVDF membranes. Primary antibodies against hepatocyte nuclear
factor 4α (HNF4α, sc-374299, Santa Cruz Biotechnology, Inc.),
hepatocyte nuclear factor 3β (HNF3β, sc-374376, Santa Cruz
Biotechnology, Inc.), hepatocyte nuclear factor 6 (HNF6, sc-365318,
Santa Cruz Biotechnology, Inc.), enhanced binding protein α (CEBPα,
sc-133239, Santa Cruz Biotechnology, Inc.), β-catenin (sc-47724,
Santa Cruz Biotechnology, Inc.) and GAPDH (all at 1:2,000 dilution)
were incubated overnight at 4°C and washed with TBS containing 0.1%
Tween-20. Secondary antibody (1:5,000; Santa Cruz Biotechnology,
Inc. goat anti-mouse IgG-HRP, sc-2005) was incubated with membranes
for 2 h at room temperature. Visualization was performed with an
enhanced chemiluminescence substrate (Thermo Fisher Scientific,
Inc.) and the band intensities were analyzed using Image J software
(version 1.8.0, National Institutes of Health) for quantitative
calculation.
Dual-luciferase reporter gene
assay
Cells were first seeded into 24-well plates and
co-transfected with TOP/FOP Flash plasmid and Renilla TK-Luciferase
vector (Shanghai GenePharma Co., Ltd.) with
Lipofectamine® 2000 reagent (11668027, Invitrogen).
Then, 48 h after transfection, cells were harvested and lysed for
luciferase assay (Promega Corporation). The luciferase activity of
each samples was normalized with its respective Renilla luciferase
activity.
Statistical analysis
The significant differences between mean values
obtained from at least three independent experiments were received.
Each value was presented as the mean ± SD. ANOVA followed by a
post-hoc Tukey's test was used for comparisons between various
groups, with P<0.05 considered statistically significant.
Statistical analysis was carried out using SPSS 16.0 software
(SPSS, Inc.).
Results
Hepatocyte differentiation of HuMSCs
in vitro
HuMSCs were successfully induced to osteoblasts and
chondrocytes, as evidenced by positive staining for alizarin red
and type II collagen, respectively (Fig. 1A). After treatment with hepatocyte
induction media, the morphology of HuMSCs observed by phase
contrast microscopy did not notably change until day 14. However,
from day 15, the cells were found to modify their phenotype from a
spindle to a polygonal shape, and granules in the cytoplasm also
gradually accumulated (Fig. 1B).
The progression of differentiation from HuMSCs to hepatocytes (at
days 14 and 28) was analyzed via RT-qPCR at the same time, and the
levels of hepatocyte-specific mRNAs such as ALB, AFP, CYP3A4 and
HNF4α were examined. It was shown that ALB, AFP, CYP3A4 and HNF4α
mRNA expression increased significantly during the period (Fig. 1C). The level of GAPDH was used to
normalize the results, and results obtained from HuMSCs cultured in
growth medium and primary hepatocytes were compared. Cells of day 0
were cultured in growth medium and cells of day 14,28 were cultured
in differentiation medium. Hepatocytes were acquired from Beijing
Beina Chuanglian Biotechnology Institute and were cultured in
L-DMEM. Additionally, the protein levels of ALB, AFP and CYP3A4
were analyzed via immunofluorescence staining after 28 days of
induction. These three proteins stained positive in HuMSC-derived
hepatocyte-like cells. Additionally, undifferentiated cells, as the
control group, were negative for AFP and CYP3A4, while the ALB
staining was lower than hepatocyte-like cells (Fig. 1D). The level of CUDR was also
examined during differentiation, and was gradually upregulated over
time (Fig. 1E).
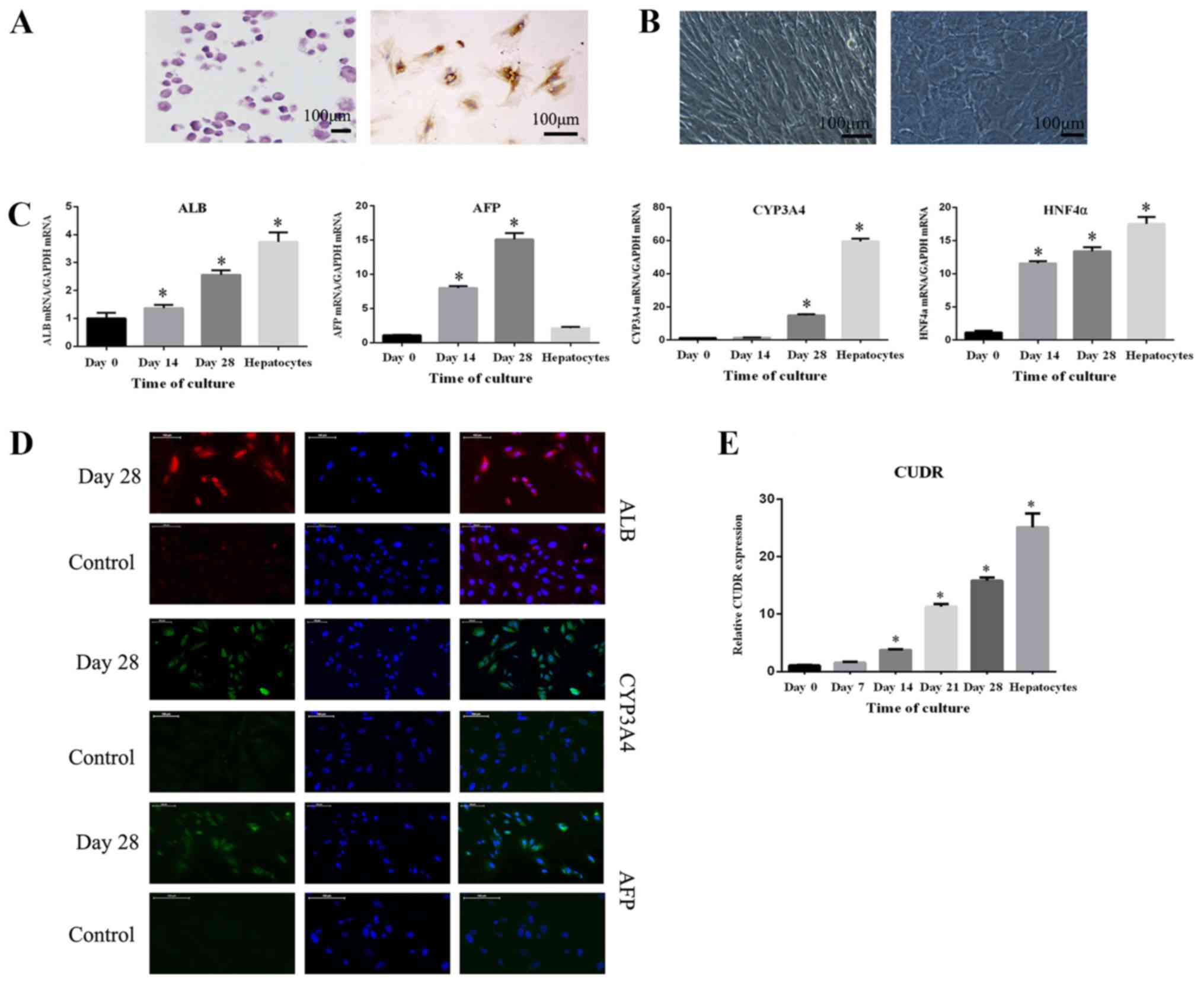 | Figure 1.Hepatic differentiation of HuMSCs. (A)
HuMSCs were identified by osteogenic and chondrogenic
differentiation. (B) Morphological changes observed as HuMSCs
differentiated into hepatocytes with the addition of cytokines. (C)
mRNA levels of ALB, AFP, CYP3A4 and HNF4α after differentiation
were analyzed via RT-qPCR. (D) ALB, AFP, CYP3A4 protein levels
after differentiation were analyzed by immunocytochemistry. (E)
Expression of CUDR was analyzed via RT-qPCR. All experiments were
performed three times independently. *P<0.05 vs. Day 0 group.
All experiments were performed three independent times. HuMSCs,
human umbilical cord mesenchymal stem cells; ALB, albumin; AFB, α
fetoprotein; CYP3A4, cytochrome P450 3A4; HNF4α, hepatocyte nuclear
factor 4α; RT-qPCR, reverse transcription-quantitative PCR; CUDR,
cancer upregulated drug resistant. |
Overexpression of CUDR in hepatic
differentiation
The overexpression of CUDR in hepatic
differentiation was further probed by using a lentiviral construct.
After confirming successful transfection by fluorescence imaging
(data not shown), HuMSCs were selected by puromycin for at least 7
days. The results of RT-qPCR demonstrated that CUDR remained highly
expressed during differentiation relative to the negative control
(Fig. 2A). To explore the role of
CUDR overexpression in hepatic differentiation, mRNAs of several
hepatic markers were compared between HuMSCs transfected with CUDR,
and HuMSCs transfected with the negative control, after 14 and 28
days of induction. As presented in Fig. 2C, the expression levels of ALB,
CYP3A4, tyrosine aminotransferase (TAT), glucose-6-phosphatase
(G6P) and α1-antitrypsin (α1AT) mRNA in the CUDR overexpression
group were higher than those in the control group (P<0.05) at
day 14 and day 28. The expression of AFP in the CUDR overexpression
group was lower than that in the control group at day 28,
indicating that these cells had a more mature status. Furthermore,
the ALB and BuN production at day 14 and day 28 showed a
significant increase in CUDR (Fig.
2B). The expression of ALB, CYP3A4 and AFP proteins were also
examined by immunostaining after 28 days of induction. Statistical
analysis demonstrated that the HuMSCs transfected with CUDR showed
higher expression of ALB and CYP3A4 compared with the control
group, while the expression of AFP protein level was lower compared
with the control group, which was consistent with the results of
RT-qPCR (Fig. 2D).
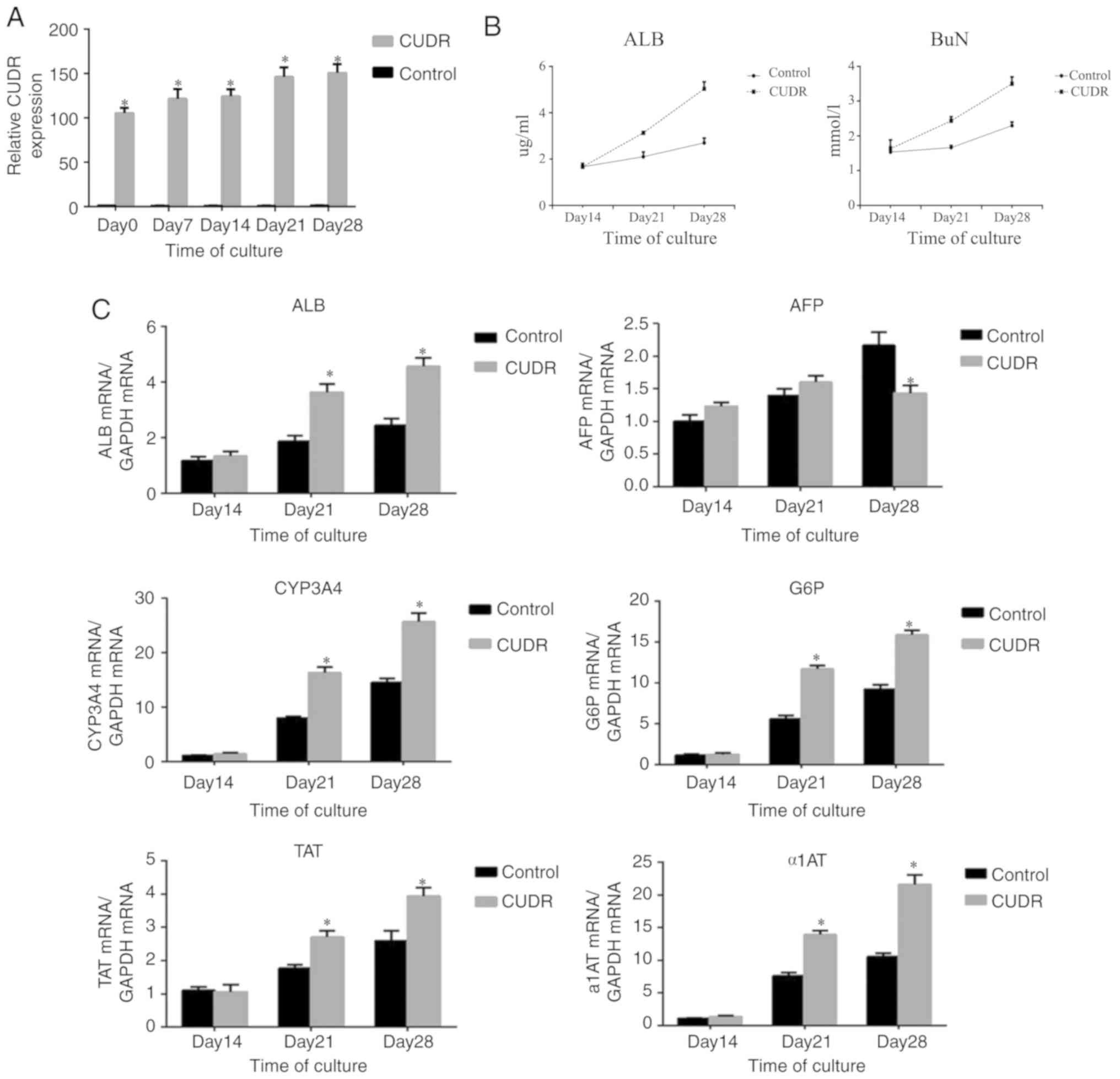 | Figure 2.Effect of CUDR overexpression on
hepatic differentiation. (A) RT-qPCR analysis of CUDR expression
levels in HuMSCs transduced with lentiviruses containing either
CUDR or negative control. (B) ALB and BuN concentration of the
supernatant were analyzed by ELISA. (C) Hepatic differentiation was
evaluated with reverse transcription-quantitative PCR analysis of
ALB, AFP, CYP3A4, G6P, TAT and α1AT. (D) Comparison of ALB, CYP3A4
and AFP protein between HuMSCs transfected with CUDR and cells
cultured in medium containing cytokines via immunocytochemistry.
*P<0.05 vs. control group. All experiments were performed three
independent times. HuMSCs, human umbilical cord mesenchymal stem
cells; ALB, albumin; AFB, α fetoprotein; CYP3A4, cytochrome P450
3A4; CUDR, cancer up-regulated drug resistant; BuN, blood urea
nitrogen; G6P, glucose-6-phosphatase; TAT, tyrosine
aminotransferase; α1AT, α1-antitrypsin. |
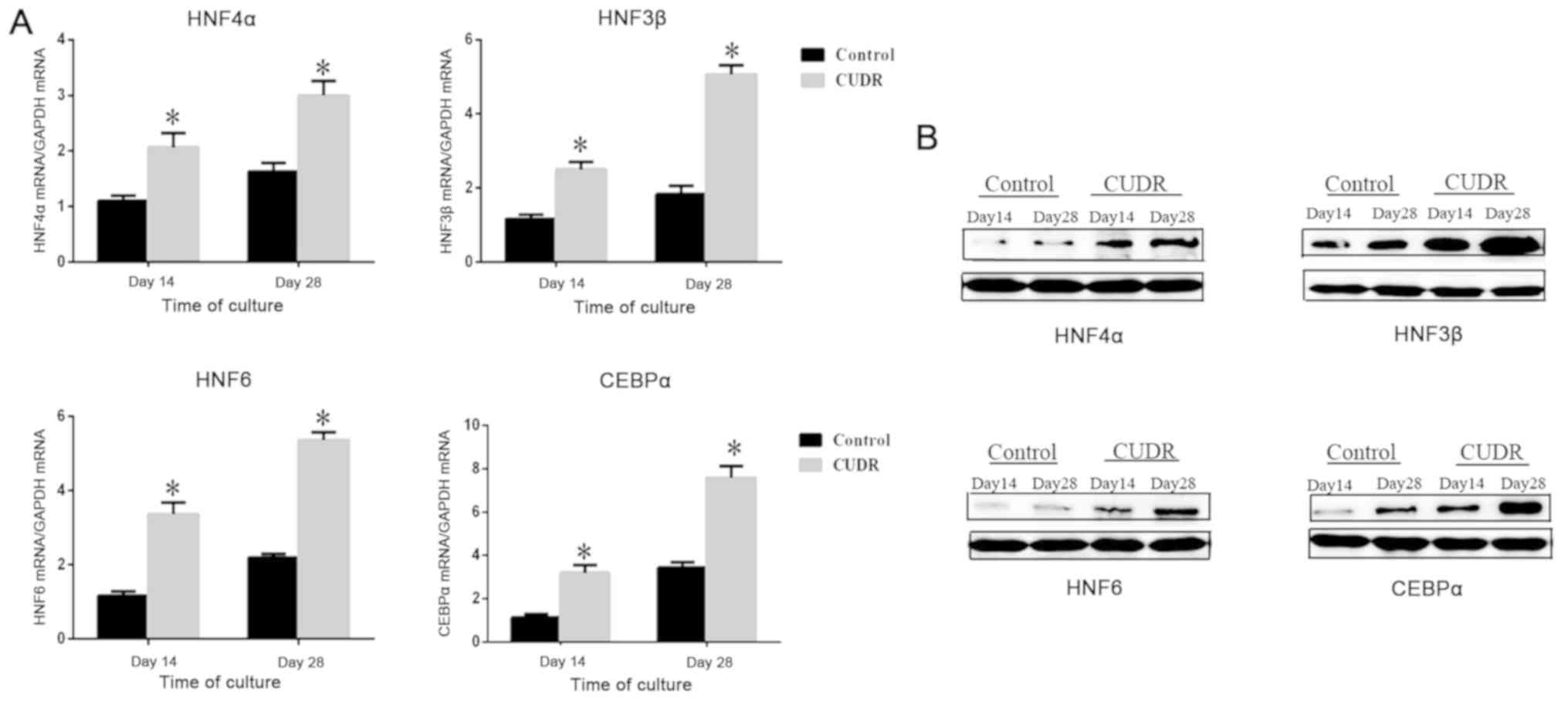
CUDR upregulates liver-enriched
transcriptional factors
It has been demonstrated by several studies that the
coordinated expression of liver-enriched transcriptional factors is
required for the hepatic differentiation and biological functions
of hepatocyte cells (18–20). It was shown in the control group
that the expression levels of HNF4α, HNF3β, HNF6 and CEBPα were low
at days 14 and 28. After the overexpression of CUDR, these genes
were expressed at a higher level on days 14 and 28 (Fig. 3A), which indicated their possible
relationship with CUDR. Protein levels of liver-enriched factors
were examined via western blotting and the results were in
accordance with RT-qPCR analysis (Fig.
3B).
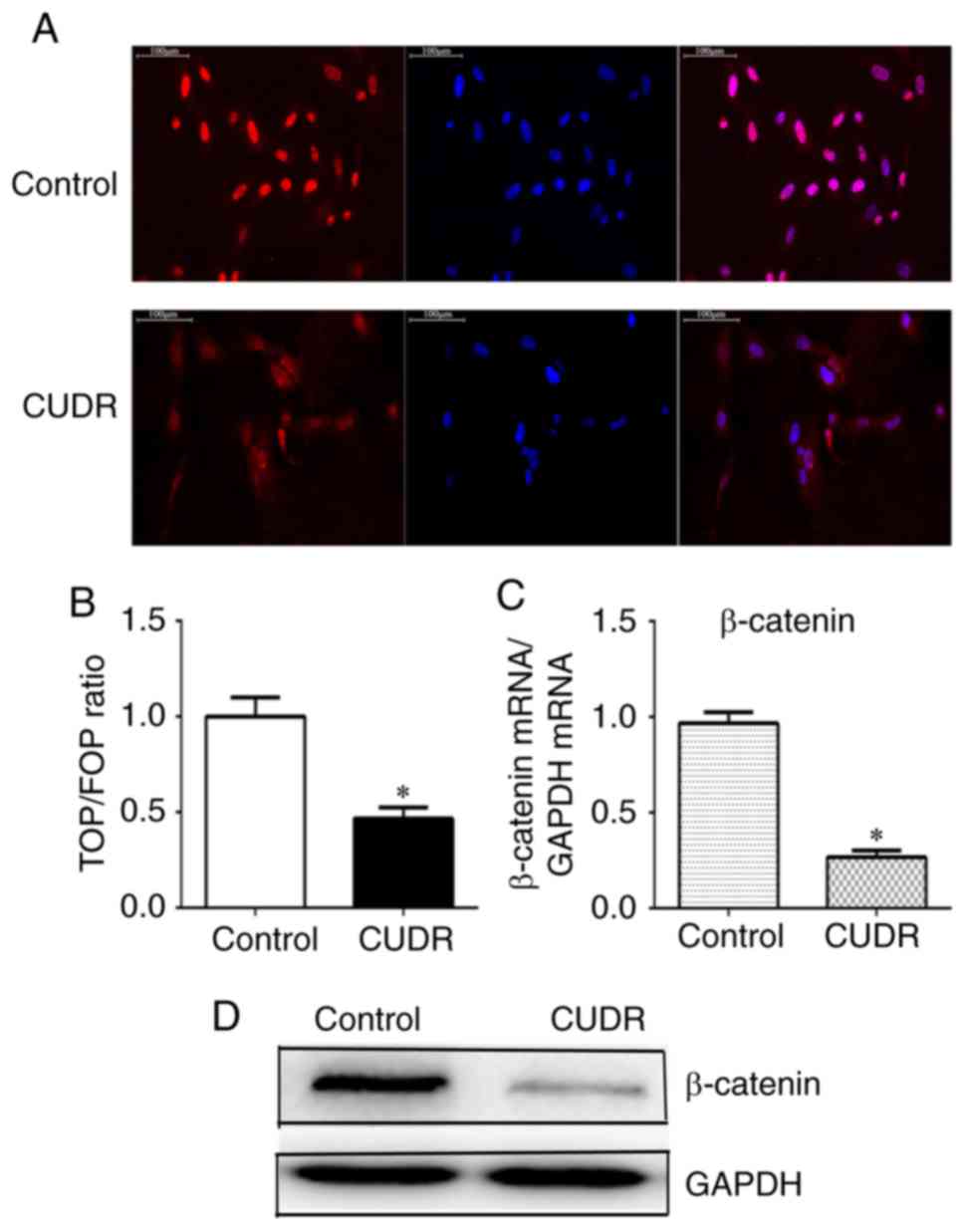
CUDR downregulates β-catenin
As is commonly known, nuclear translocation plays an
important role in Wnt/β-catenin signaling activation (21). To explore the mechanism underlying
the relationship between CUDR and increased hepatic
differentiation, the expression of β-catenin and the sub-cellular
localization in HuMSCs were examined in the presence or absence of
overexpression of CUDR. As shown in Fig. 4A, β-catenin was strongly expressed
and mostly localized in the nucleus in the control group. However,
following CUDR overexpression, the nuclear staining of β-catenin
was markedly reduced. The TOP/FOP reporter assay revealed that the
Wnt/β-catenin signaling was significantly downregulated by CUDR
overexpression (Fig. 4B). In line
with these data, overexpression of CUDR significantly suppressed
the expression of β-catenin mRNA and protein (Fig. 4C and D).
Discussion
It has been demonstrated that HuMSCs can
differentiate into hepatocyte-like cells under certain conditions
in vitro. HuMSCs have a major advantage over BMSCs and other
sources of stem cells as they can be obtained non-invasively from
umbilical cords. The fact that they are in vast abundance, free
from ethical problems, cause a less pronounced immune response and
offer the possibility to be cultured over a long time period in
vitro make HuMSCs the ideal choice for the present study
(6).
To generate hepatocyte-like cells, numerous
researchers have engaged in finding simpler and more efficient
procedures from various types of stem cells. In this study, HuMSCs
were induced under hepatic differentiation media that included
different cytokines and growth factors, which have been
demonstrated to be important during the differentiation into
hepatocyte-like cells. Then, hepatocyte-specific markers were
examined to confirm the hepatic features of hepatocyte-like cells.
Besides the changes of morphology that were examined by phase
contrast microscopy, expression of hepatocyte-related genes such as
AFP, ALB, CYP3A4 and HNF4α were also confirmed via RT-qPCR
analysis. Moreover, it was shown by immunofluorescence that ALB,
AFP and CYP3A4 were expressed at a translation level.
The importance of specific lncRNAs participating in
physiological and pathological processes, including the
differentiation of various cells has been shown. For instance,
Huang et al (22,23) demonstrated that H19 could improve
the osteogenic capacity of MSCs through the transforming growth
factor-β1/Smad family member 3/histone deacetylase signaling
pathway, while it inhibited adipocyte differentiation of BMSCs
through epigenetic modulation of histone deacetylases. The lncRNA
RAR related orphan receptor C contributes to SOX9 expression and
chondrogenic differentiation of HuMSCs (24). Although CUDR is known to be
involved in carcinogenesis at the transcriptional and
post-transcriptional levels, CUDR is seldom reported to regulate
hepatic differentiation (14). The
results of the present study showed that the expression of CUDR
gradually increased following the differentiation of HuMSCs. This
study attempted to focus on the overexpression of CUDR in the
hepatic differentiation of HuMSCs. In order to show this, the
lentiviral transduction procedure was used to overexpress CUDR in
HuMSCs, and the level of CUDR overexpression was examined using
RT-qPCR. This revealed a continuous high expression of CUDR in
transfected HuMSCs throughout the process of differentiation.
As expected, CUDR overexpression in HuMSCs resulted
in a significant upregulation of several genes related to hepatic
function. It was revealed by RT-qPCR that the expression levels of
ALB, CYP3A4, TAT, G6P and α1AT were higher than in the control
group, whereas AFP was expressed on a lower level, indicating a
more mature hepatocyte character (25). Furthermore, the secretion of ALB
and the production of urea in the transduced cells were detected
using ELISA. The results also demonstrated that CUDR overexpression
induced a more functional hepatocyte-like cell. Additionally, the
findings of immunofluorescence analysis of the ALB, AFP and CYP3A4
proteins were in accordance with the RT-qPCR results.
As commonly known, the coordinated expression of
liver-enriched transcriptional factors is required for the hepatic
differentiation and biological functions of hepatocytes (19,20).
In the present study, the liver-enriched factors mRNA HNF4α, HNF3β,
HNF6 and CEBPα began to increase after 14 days of induction as
determined via RT-qPCR analysis. Overexpression of CUDR upregulated
the liver-enriched factors compared with the control group, which
indicated that CUDR may improve the function of hepatocyte-like
cells via the regulation of liver-enriched factors.
Accumulating evidence indicates that the
downregulation of Wnt/β-catenin signaling contributes to the
hepatic differentiation of MSCs (26–28).
Several lncRNAs were found to participate in Wnt/β-catenin
signaling, including lnc liver regeneration 1, lnc transcription
factor 7, and lnc β-Catm (29–31).
The Wnt/β-catenin signaling pathway was focused on in the present
study as a possible mechanism for hepatic differentiation induced
by CUDR overexpression. It was shown that the overexpression of
CUDR prevented the translocation of β-catenin to the nucleus, and
suppressed the activity of this pathway. Therefore, the enhanced
hepatic differentiation of HuMSCs caused by CUDR may be linked to
the downregulation of the Wnt/β-catenin signaling pathway. However,
the mechanism of how CUDR affects β-catenin level and sub-cellular
localization needs further investigation.
To conclude, the findings of the present study
indicated that CUDR may be a prominent factor in the hepatic
differentiation of HuMSCs. This suggested that the overexpression
of CUDR could serve as a valuable procedure to produce efficient
and functional hepatocyte-like cells for transplantation.
Therefore, this could be very useful in improving hepatic
differentiation and providing a regenerative therapy for liver
disease. However, further studies are required to clarify the
particular mechanisms during hepatic differentiation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FQ conceived and designed the experiments. YY
performed the experiment and wrote the paper. ML, YS and JX
performed the experiments and analyzed the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable (HuMSCs were purchased from Beijing
Beina Chuanglian Biotechnology Institute).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meirelles Junior RF, Salvalaggio P,
Rezende MB, Evangelista AS, Guardia BD, Matielo CE, Neves DB,
Pandullo FL, Felga GE, Alves JA, et al: Liver transplantation:
History, outcomes and perspectives. Einstein (Sao Paulo).
13:149–152. 2015.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baertschiger RM, Serre-Beinier V, Morel P,
Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH and
Gonelle-Gispert C: Fibrogenic potential of human multipotent
mesenchymal stromal cells in injured liver. PLoS One. 4:e66572009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee KD, Kuo TK, Whang-Peng J, Chung YF,
Lin CT, Chou SH, Chen JR, Chen YP and Lee OK: In vitro hepatic
differentiation of human mesenchymal stem cells. Hepatology.
40:1275–1284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
In 't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetal or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Tantawy WH and Haleem EN: Therapeutic
effects of stem cell on hyperglycemia, hyperlipidemia, and
oxidative stress in alloxan-treated rats. Mol Cell Biochem.
391:193–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan CG, Zhang QJ and Zhou JR: Therapeutic
potentials of mesenchymal stem cells derived from human umbilical
cord. Stem Cell Rev. 7:195–207. 2011. View Article : Google Scholar
|
|
7
|
Yu YB, Song Y, Chen Y, Zhang F and Qi FZ:
Differentiation of umbilical cord mesenchymal stem cells into
hepatocytes in comparison with bone marrow mesenchymal stem cells.
Mol Med Rep. 18:2009–2016. 2018.PubMed/NCBI
|
|
8
|
Ek M, Soderdahl T, Kuppers-Munther B,
Edsbagge J, Andersson TB, Björquist P, Cotgreave I, Jernström B,
Ingelman-Sundberg M and Johansson I: Expression of drug
metabolizing enzymes in hepatocyte-like cells derived from human
embryonic stem cells. Biochem Pharmacol. 74:496–503. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu C, Zhang Y, Wang Q, Xu Z, Jiang J, Gao
Y, Gao M, Kang J, Wu M, Xiong J, et al: Long non-coding RNA GAS5
controls human embryonic stem cell self-renewal by maintaining
NODAL signalling. Nat Commun. 7:132872016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zhao Y, Bao X, Zhu X, Kwok YK, Sun
K, Chen X, Huang Y, Jauch R, Esteban MA, et al: LncRNA Dum
interacts with Dnmts to regulate Dppa2 expression during myogenic
differentiation and muscle regeneration. Cell Res. 25:335–350.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalwa M, Hanzelmann S, Otto S, Kuo CC,
Franzen J, Joussen S, Fernandez-Rebollo E, Rath B, Koch C, Hofmann
A, et al: The lncRNA HOTAIR impacts on mesenchymal stem cells via
triple helix formation. Nucleic Acids Res. 44:10631–10643. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Yang YN, Yuan HH, Zhang TT, Sui H,
Wei XL, Liu L, Huang P, Zhang WJ and Bai YX: UCA1, a long
non-coding RNA up-regulated in colorectal cancer influences cell
proliferation, apoptosis and cell cycle distribution. Pathology.
46:396–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Zheng Q, An J, Wu M, Li H, Gui X, Pu
H and Lu D: SET1A Cooperates With CUDR to promote liver cancer
growth and hepatocyte-like stem cell malignant transformation
epigenetically. Mol Ther. 24:261–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gui X, Li H, Li T, Pu H and Lu D: Long
Noncoding RNA CUDR Regulates HULC and β-catenin to govern human
liver stem cell malignant differentiation. Mol Ther. 23:1843–1853.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC,
Chen CP, Huang SW, Shyr YM, Tang KT and Chen TH: Transplantation of
insulin-producing cells derived from umbilical cord stromal
mesenchymal stem cells to treat NOD mice. Cell Transplant.
20:455–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciciarello M, Zini R, Rossi L, Salvestrini
V, Ferrari D, Manfredini R and Lemoli RM: Extracellular purines
promote the differentiation of human bone marrow-derived
mesenchymal stem cells to the osteogenic and adipogenic lineages.
Stem Cells Dev. 22:1097–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihailovic M, Petrovic M and Bogojevic D:
Correlation between acute phase-related expression of C/EBPbeta and
transcriptional regulation of the haptoglobin gene during rat liver
development. Gen Physiol Biophys. 19:317–321. 2000.PubMed/NCBI
|
|
19
|
Costa RH, Kalinichenko VV, Holterman AX
and Wang X: Transcription factors in liver development,
differentiation, and regeneration. Hepatology. 38:1331–1347. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kyrmizi I, Hatzis P, Katrakili N, Tronche
F, Gonzalez FJ and Talianidis I: Plasticity and expanding
complexity of the hepatic transcription factor network during liver
development. Genes Dev. 20:2293–2305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Zheng Y, Jia L and Li W: Long
Noncoding RNA H19 promotes osteoblast differentiation Via
TGF-β1/Smad3/HDAC signaling pathway by deriving miR-675. Stem
Cells. 33:3481–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Zheng Y, Jin C, Li X, Jia L and
Li W: Long Non-coding RNA H19 inhibits adipocyte differentiation of
bone marrow mesenchymal stem cells through epigenetic modulation of
histone deacetylases. Sci Rep. 6:288972016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barter MJ, Gomez R, Hyatt S, Cheung K,
Skelton AJ, Xu Y, Clark IM and Young DA: The long non-coding RNA
ROCR contributes to SOX9 expression and chondrogenic
differentiation of human mesenchymal stem cells. Development.
144:4510–4521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deutsch HF: Chemistry and biology of
alpha-fetoprotein. Adv Cancer Res. 56:253–312. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ke Z, Zhou F, Wang L, Chen S, Liu F, Fan
X, Tang F, Liu D and Zhao G: Down-regulation of Wnt signaling could
promote bone marrow-derived mesenchymal stem cells to differentiate
into hepatocytes. Biochem Biophys Res Commun. 367:342–348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimomura T, Yoshida Y, Sakabe T, Ishii K,
Gonda K, Murai R, Takubo K, Tsuchiya H, Hoshikawa Y, Kurimasa A, et
al: Hepatic differentiation of human bone marrow-derived UE7T-13
cells: Effects of cytokines and CCN family gene expression. Hepatol
Res. 37:1068–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida Y, Shimomura T, Sakabe T, Ishii K,
Gonda K, Matsuoka S, Watanabe Y, Takubo K, Tsuchiya H, Hoshikawa Y,
et al: A role of Wnt/beta-catenin signals in hepatic fate
specification of human umbilical cord blood-derived mesenchymal
stem cells. Am J Physiol Gastrointest Liver Physiol.
293:G1089–G1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu P, Wang Y, Huang G, Ye B, Liu B, Wu J,
Du Y, He L and Fan Z: lnc-β-Catm elicits EZH2-dependent β-catenin
stabilization and sustains liver CSC self-renewal. Nat Struct Mol
Biol. 23:631–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu D, Yang F, Yuan JH, Zhang L, Bi HS,
Zhou CC, Liu F, Wang F and Sun SH: Long noncoding RNAs associated
with liver regeneration 1 accelerates hepatocyte proliferation
during liver regeneration by activating Wnt/β-catenin signaling.
Hepatology. 58:739–751. 2013. View Article : Google Scholar : PubMed/NCBI
|