Introduction
Spinal cord injury (SCI) causes severe damage to the
central nervous system, resulting in irreversible motor and sensory
dysfunction below the injury area (1). In modern society, the global
incidence of SCI varied from 8 to 246 per million depending on the
country or region (2), and an
increasing number of paralyzed patients survive longer,
experiencing a variety of complications (3,4).
Currently there are no effective therapeutic treatments for SCI.
Methylprednisolone, which was previously used as first-aid measure
for acute SCI, is no longer recommended in the guidelines of
AANS/CNS as of 2013 due to an increased occurrence of complications
and no strong evidence of clinical efficacy (5). Rehabilitation, the only proven
effective treatment for SCI, is limited for early application as it
is only suitable for less serious injuries and requires active
cooperation from patients (6).
Thus, novel noninvasive treatments are required as early
interventions for patients with SCI.
The pathological process of SCI can be divided into
two stages; the primary and secondary injury (7). The primary injury is the original
tissue breakdown caused by contusion or compression, which occurs
immediately after SCI. The secondary injury is a series of
pathologic changes following the primary injury, including an
increase in the permeability of the blood spinal cord barrier, the
infiltration of inflammatory cells, excitotoxicity, demyelination
and neuronal apoptosis, which lasts for days to months. Two of the
most important components of the secondary injury in its early
stages are inflammation and oxidative stress. The inflammation
includes increased expression of pro-inflammatory factors,
including tumor necrosis factor-α (TNF-α) and interleukin-1β
(IL-1β), and a reduction in inflammatory mediators (8). The oxidative stress involves an
increase in the levels of nitric oxide (NO) and reactive oxygen
species (ROS) (9).
The use of low-frequency pulsed electromagnetic
fields (LPEMFs) is a noninvasive therapeutic method for various
diseases. Recent evidence has demonstrated that LPEMFs can prevent
inflammation and oxidative stress. LPEMF stimulation can suppress
the production of IL-1β and TNF-α in cultured nucleus pulposus
cells (10). Furthermore, LPEMFs
can reduce ROS levels and enhance antioxidative stress responses in
osteoblasts (11). LPEMFs exhibit
strong neuroprotective effects in the nervous system. In ischemic
stroke, LPEMFs can promote functional recovery by activation of the
brain derived neurotrophic factor/tropomyosin receptor kinase
B/protein kinase B signaling pathway (12). Additionally, LPEMFs can modulate
the expression of microRNAs and stimulate tissue regeneration in
in vitro models of Alzheimer's disease (13). Recent studies have revealed that
extremely low-frequency magnetic fields reduce iron-induced tissue
damage following SCI (14).
However, whether the administration of LPEMFs inhibits the
early-stage reaction of SCI secondary injury, inflammation and
oxidative stress, requires further exploration. In the present
study, neuroprotective effects of LPEMF stimulation on SCI model
rats were investigated. The changes in inflammation and oxidative
stress molecular markers were then compared, and whether this
protective effect was modulated by targeting heat shock protein 70
(HSP70) was examined. The results of the current study may provide
a noninvasive alternative therapeutic method for the early
treatment of SCI.
Materials and methods
Experimental animals
Adult female Wistar rats (230±20 g; n=60) used in
the current study were all provided by Tianjin Medical University
Animal Research Center (Tianjin, China; permission no.
SCXK-2012-0004). All animal experiments were approved by the Animal
Welfare Committee of Tianjin Medical University (Tianjin, China),
which is based on the NIH Guide for the Care and Use of Laboratory
Animals (15). The rats were
randomly distributed into three groups: Sham group, SCI group and
LPEMF group. In the sham group, rats underwent laminectomy, and the
spinal cord was not injured. In the SCI group, the spinal cord was
injured using the same procedure used in the LPEMF group on an
identical electromagnetic device, but without the application of
LPEMFs. In the LPEMF group, Wistar rats received the LPEMFs
treatment for 1 h per day from 24 h after SCI. Animals were
sacrificed on days 3, 7 and 14 after SCI, and the spinal cords were
harvested for further analysis.
Contusion SCI model
The standard New York University impactor machine
was used to induce a spinal cord contusion injury model as
described previously (16). Rats
were anesthetized with chloral hydrate (300 mg/kg), and a
laminectomy was performed to expose the T10 spinal cord. A metal
rod (10 g, 25 mm) was dropped onto the back side of the spinal
cord. A surveillance system was used to control the compression
force and velocity to maintain uniformity between animals.
Following the operation, the bladders of these rats were manually
emptied.
LPEMFs treatment
The BG100A-2 pulsed magnet field therapeutic
apparatus (Concord Beijing Medical Equipment Co., Ltd., Beijing,
China; patent no. ZL00101667.9) was used in the current study. The
apparatus contains seven coils arranged end-to-end beneath the
treatment table and a magnetic line of force was positioned across
the rats longitudinally. The frequency, power and duty cycle are
all adjustable and the apparatus can be monitored and controlled by
computer system. In the LPEMFs group, rats were exposed to LPEMFs
(frequency, 50 Hz; power, 2.5 mT; duty cycle, 40%) at 24 h after
SCI. Rats were placed into a transparent plastic chamber with
ventilation and were given time to explore for 1 h per day
(8:00-9:00 a.m.) for 14 days. In the SCI group, animals were placed
in the same chambers on the treatment table for 1 h per day without
exposure to LPEMFs.
Assessment of locomotor activity
Functional recovery of the animals was evaluated
using the Basso Beattie Bresnahan (BBB) locomotor rating scale
(17). The BBB was observed by two
groups, and the observers were blinded to the design of current
experiment (18). The BBB scores
of rats were recorded prior to contusion operation and at 1, 3, 5,
7 and 14 days post-injury (dpi).
Enzyme-linked immunosorbent assay
(ELISA)
The spinal cord tissue (10 mm block of spinal cord
surrounding the lesion center) was collected and homogenized at 14
dpi. Then, the tissue homogenate was centrifuged at a speed of
10,000 × g at 4°C for 20 min and the supernatant was collected for
determining protein concentration using a Pierce™ BCA
Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA,
USA). The commercially available ELISA kits were obtained from the
following companies: TNF-α ELISA kit (cat. no. ab46070; Abcam,
Cambridge, UK), IL-1β ELISA kit (cat. no. RA20422; Bio-Swamp,
Wuhan, China), superoxide dismutase (SOD) ELISA kit (cat. no.
706002; Cayman Chemical Company, Ann Arbor, MI, USA), catalase
(CAT) ELISA kit (cat. no. 11363727001; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Western blot analysis
After 14 days of treatment or SCI, the spinal cord
tissue around the lesion center was collected, harvested and
homogenized in radioimmunoprecipitation assay lysis buffer (cat.
no. P0013B; Beyotime Institute of Biotechnology, Shanghai, China).
The concentration of protein was detected using bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). Equal amounts
of protein samples (50 µg) from three individual animals in each
group were resolved using SDS-PAGE (12% gel) for separation and
then transferred to a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
non-fat milk and incubated with anti-inducible nitric oxide
synthase (iNOS; 1:250; cat. no. ab15323; Abcam) and anti-β-actin
(1:2,000; cat. no. ab8227; Abcam) overnight at 4°C. Then, the
membrane was incubated with secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibody (1:10,000; cat. no.
ab205718; Abcam) at 37°C for 1 h. BeyoECL Star (cat. no. P0018AM;
Beyotime Institute of Biotechnology) was used to develop the HRP
signal. Signals were captured using a ChemiDoc MP System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and quantified using ImageJ
software version 1.32 (National Institutes of Health, Bethesda, MD,
USA). The expression levels of iNOS were determined following
normalization to β-actin levels.
Immunohistochemistry analysis
Spinal cords were collected 14 days after injury and
samples were quickly frozen at −40°C immersed in 4%
paraformaldehyde. Transverse 10 µm thick sections of spinal cord
were used for immunohistochemistry analysis. Following
permeabilization in 0.25% Triton X-100/PBS for 10 min at room
temperature, sections were blocked with 10% goat serum (cat. no.
SL038; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 60 min at room temperature. Sections were
stained using anti-nuclear factor-κB (NF-κB) p65 (1:2,000; cat. no.
ab16502; Abcam) and anti-HSP70 (1:100; ab79852; Abcam) at 4°C
overnight. Samples were incubated with secondary HRP-conjugated
goat anti-rabbit antibody (1:10,000; cat. no. ab205718; Abcam) at
37°C for 1 h. The DAB Horseradish Peroxidase Color Development kit
(cat. no. P0202; Beyotime Institute of Biotechnology) was used for
signal development (sections were incubated at room temperature for
25 min). Then, 3 fields were randomly selected for each sample, and
the positive area in each field was collected and quantified using
ImageJ software version 1.32 (National Institutes of Health).
ROS assay
The spinal cord tissue (5 mm block of spinal cord
surrounding the lesion center) was collected and homogenized at 14
dpi. Subsequently, the tissue homogenate was centrifuged (1,500 × g
at 4°C for 20 min), and the supernatant was collected. A ROS assay
kit (cat. no. D6883; Sigma-Aldrich; Merck KGaA) was used to
determine the production of ROS. The supernatant was incubated with
2,7-dichlorodihydrofluorescein diacetate for 1 h and then washed
twice with PBS in ice. Fluorescence levels were detected at 480/530
nm.
Statistical analysis
The experimental data are expressed as the mean ±
standard error. A one-way analysis of variance followed by Tukey's
post hoc tests for multiple comparisons were used to analyze data
(SPSS 19.0 software; IBM Corp., Armonk, NY, USA). P<0.05 were
considered to indicate a statistically significant difference.
Results
Protective effects of LPEMFs on
locomotor recovery following SCI in rats
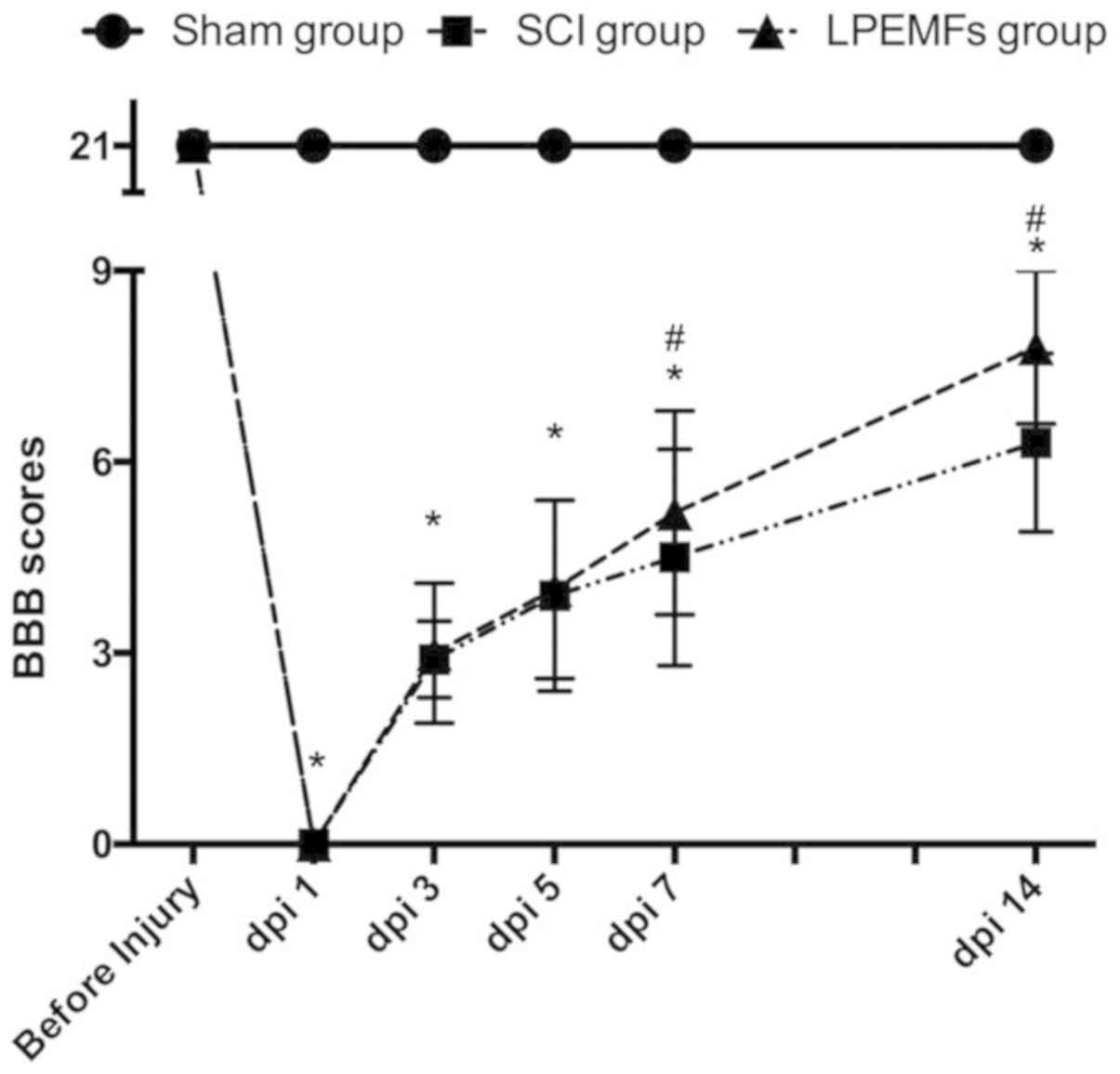
To evaluate whether the LPEMF treatment has
protective effects on motor function in SCI rats, the BBB locomotor
rating scale was used to measure behavior for 2 weeks. As presented
in Fig. 1, all the animals
exhibited full marks (21 points) in BBB scoring prior to the
injury. At dpi 1, the BBB scores dropped to 0. Following SCI, rats
exhibited spontaneous functional recovery over time and there were
no significant differences between the SCI group and the LPEMF
group before dpi 7. However, the rats exhibited better motor
function recovery after 7 dpi under LPEMF treatment compared with
SCI rats, and the BBB scores were significantly different ay dpi 7
and 14 (P<0.05). These results suggested that LPEMF exposure can
promote locomotor recovery in SCI rats (Fig. 1).
Protective effect of LPEMFs on
expression of pro-inflammatory cytokines in SCI rats
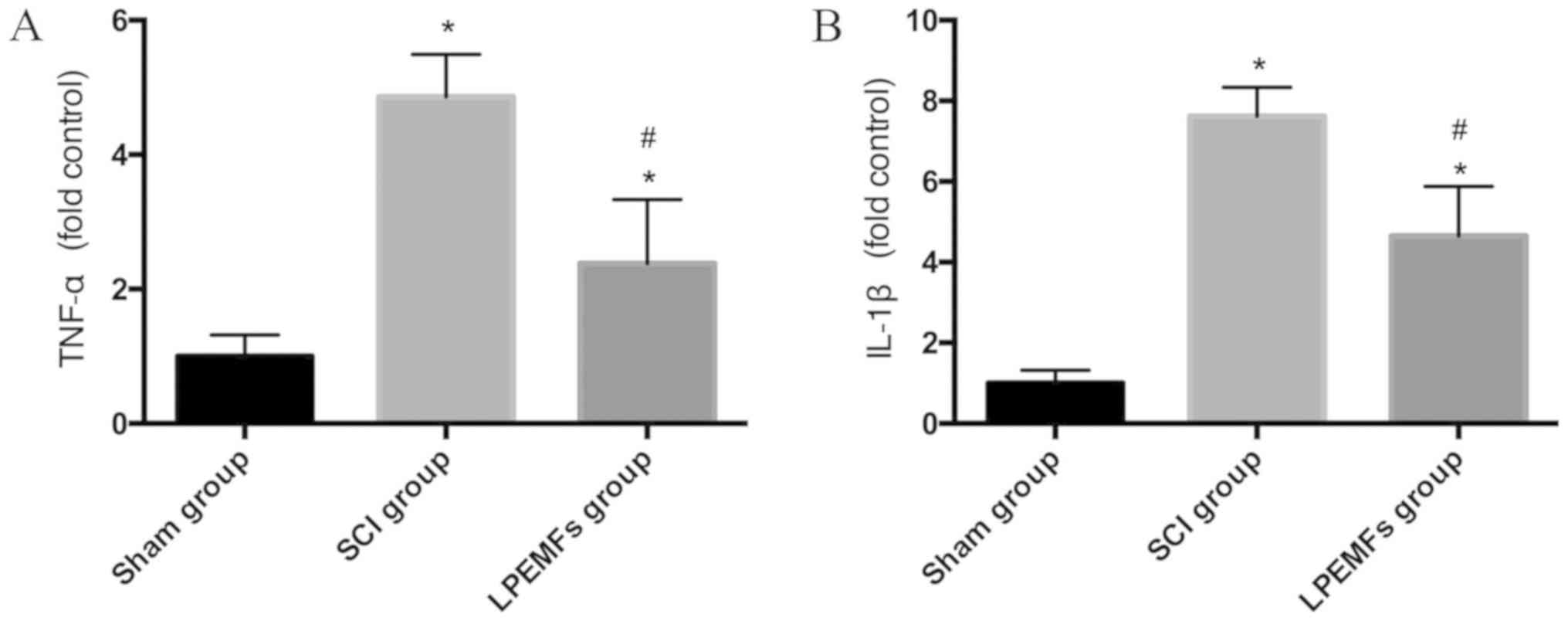
To determine whether LPEMFs suppressed inflammatory
reaction by decreasing the secretion of pro-inflammatory cytokines
in the injured spinal cord, the expression levels of TNF-α and
IL-1β were assessed. Following SCI, the inflammation markers TNF-α
and IL-1β were significantly increased compared with the Sham group
(Fig. 2A and B). However, after 2
weeks of LPEMF treatment, the expression of TNF-α and IL-1β were
decreased in comparison with the SCI group (Fig. 2A and B). These results indicated
that LPEMF treatment, to a certain extent, can alleviate the
inflammatory reaction following SCI.
Suppressive effect of LPEMFs on NF-κB
expression in injured spinal cord
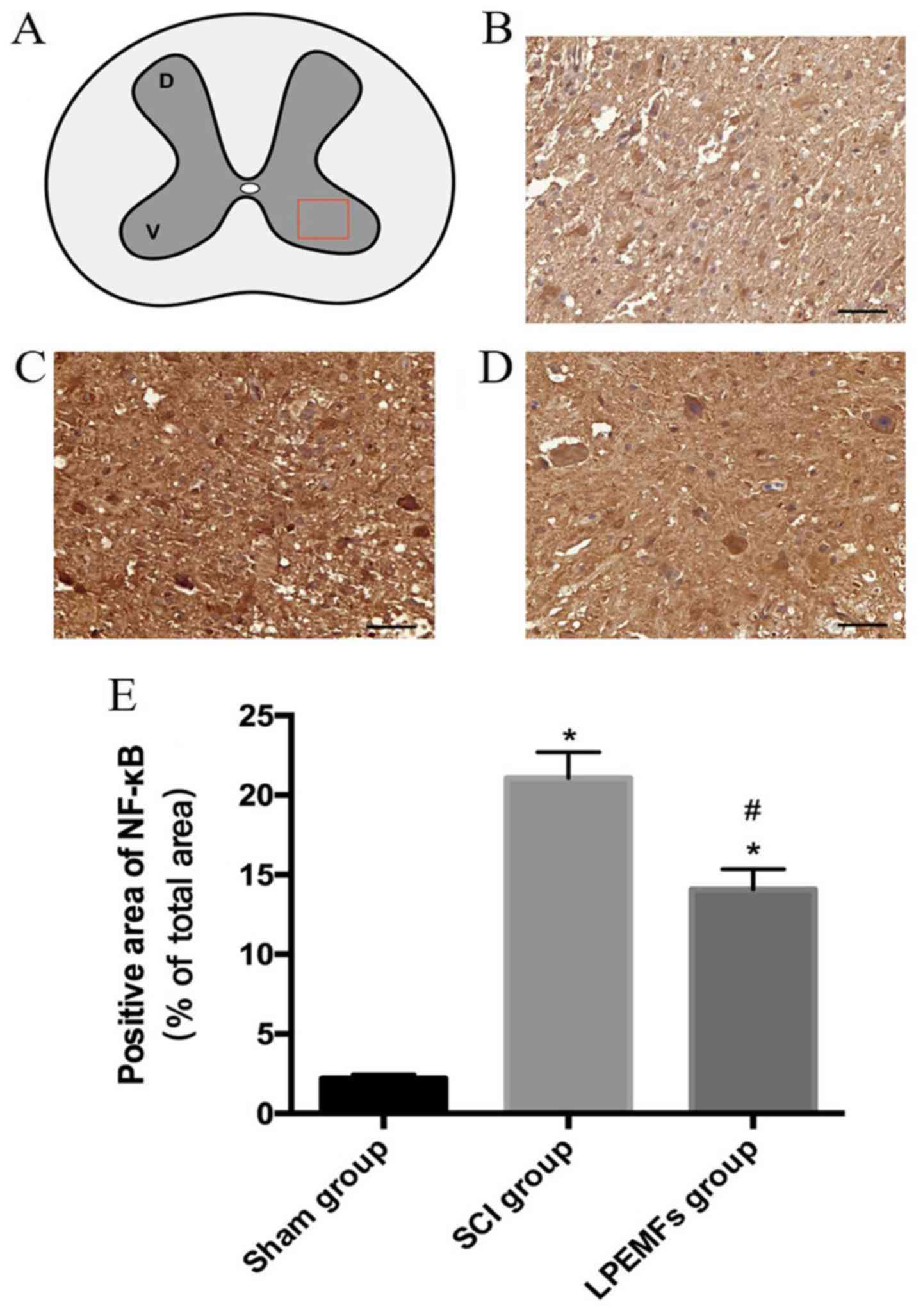
NF-κB is an important transcription factor that
stimulates inflammation (19). To
determine whether LPEMF treatment could suppress the expression of
NF-κB in the injured spinal cord, particularly in the ventral horn,
NF-κB protein levels were evaluated using immunohistochemistry
(Fig. 3A). In the sham group, the
NF-κB was difficult to detect (Fig.
3B). However, after 2 weeks of injury, there was a strong,
positive NF-κB signal in the ventral horn of the spinal cord, which
contains motor neurons (Fig. 3C).
By contrast, the administration of LPEMFs significantly reduced the
immunoreactivity of NF-κB in SCI rats (Fig. 3D). The quantified result was
consistent with observation of sections (Fig. 3E).
Suppressive effect of LPEMFs on iNOS
expression in injured spinal cord
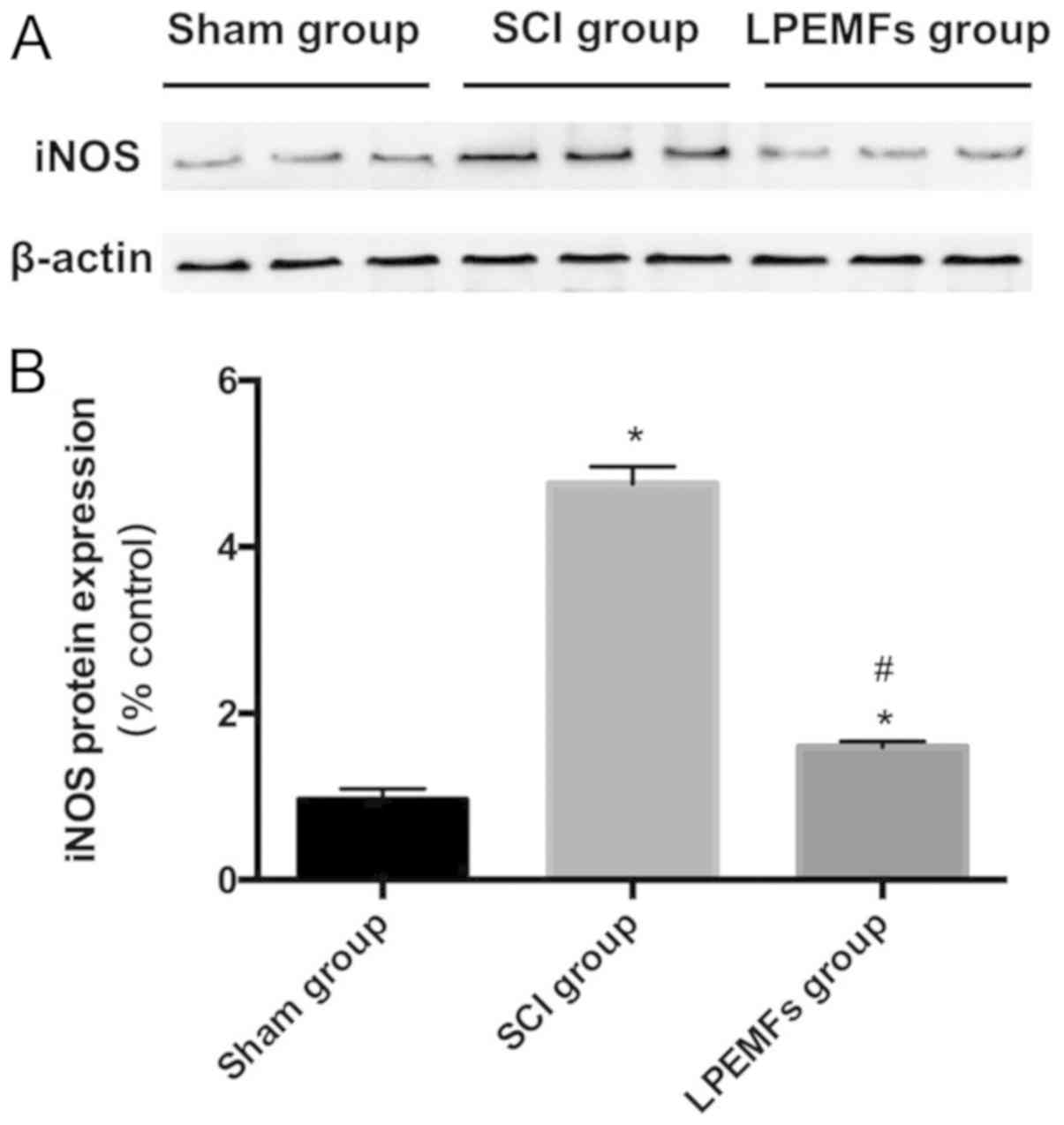
To determine the iNOS expression following SCI, the
iNOS was detected using western blot analysis. Significantly higher
levels of the iNOS protein were detected following SCI compared
with the Sham group. By contrast, the LPEMF treatment suppressed
the iNOS protein expression in the injured spinal cord (Fig. 4A and B). The decreased iNOS
expression indicated that the LPEMFs can alleviate oxidative stress
following SCI.
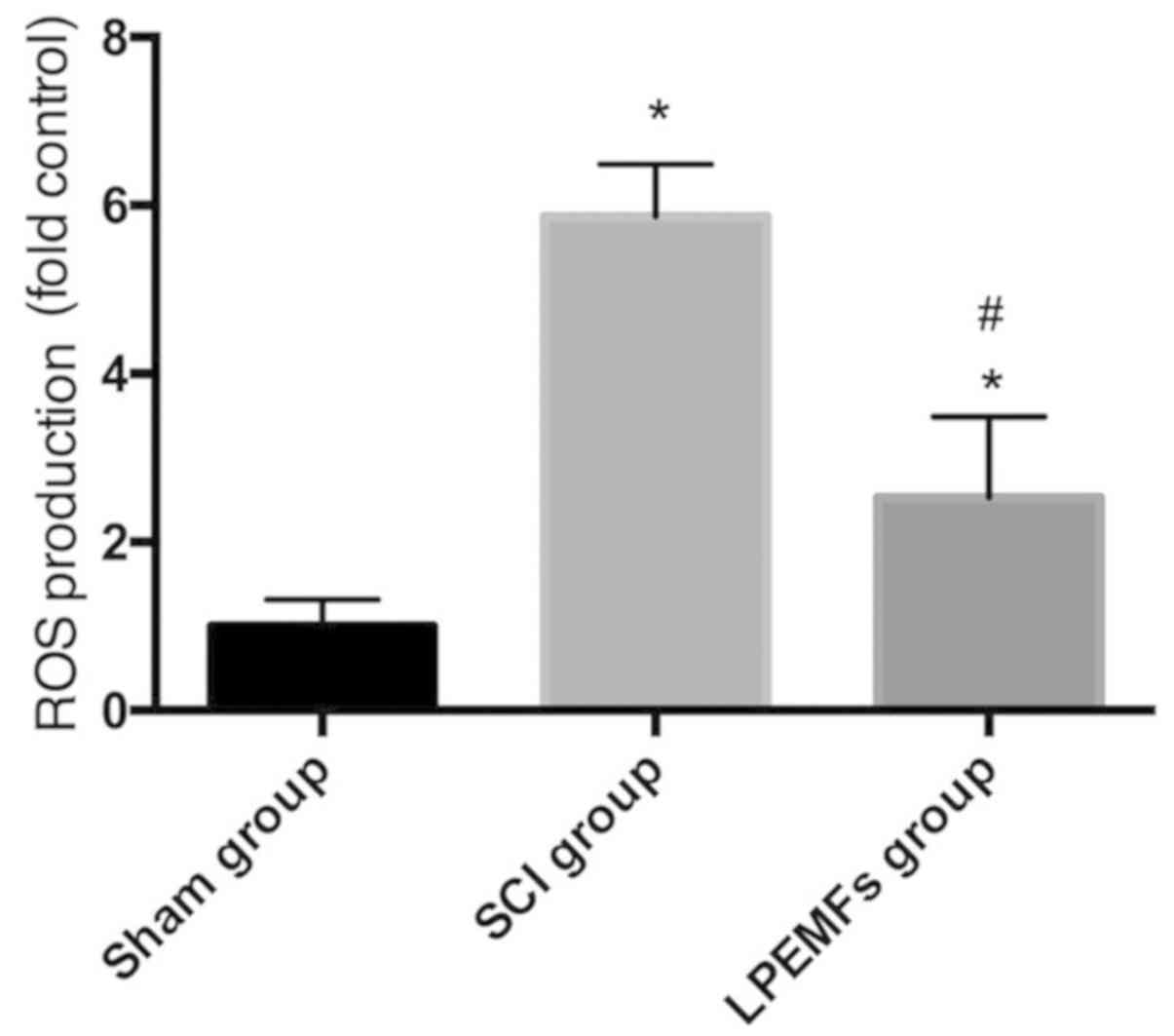
Protective effect of LPEMFs on ROS
production in SCI rats
To investigate the protective effect of LPEMFs on
ROS production in SCI rats, the ROS levels between groups were
measured. As demonstrated Fig. 5,
the injury induced strong ROS production in spinal cord tissue
compared with those in the uninjured Sham group. However, the LPEMF
administration reduced the ROS level significantly compared with
SCI rats. This result indicated that LPEMFs can alleviate the
oxidative stress by reducing ROS production following SCI.
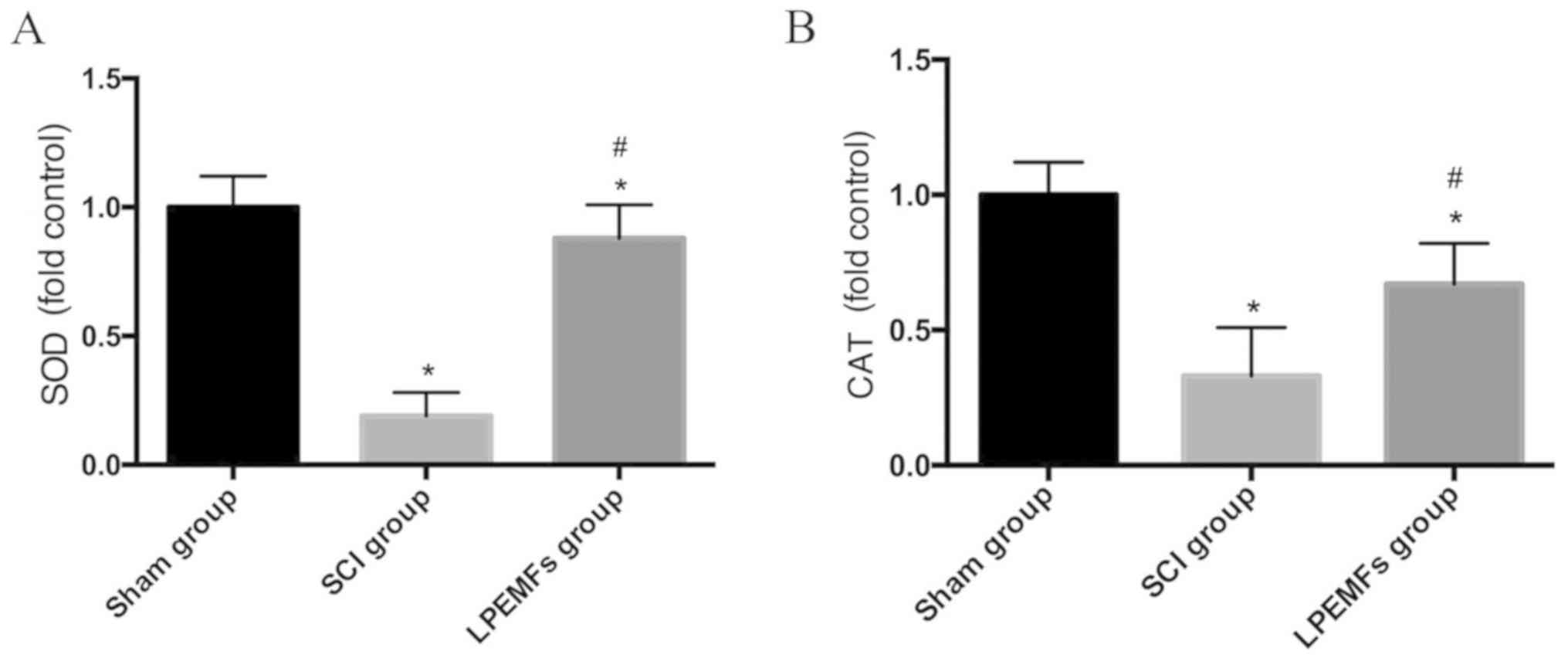
Protective effect of LPEMFs on
expression of antioxidant enzymes following SCI
To explore whether LPEMFs can alleviate oxidative
stress through upregulation of antioxidant enzymes, the expression
of SOD and CAT in spinal cord was measured using ELISA. Compared
with the intact spinal cord, the injured tissue exhibited decreased
expression of SOD and CAT. By contrast, the treatment of LPEMFs can
reversed this reduction to a certain extent (Fig. 6A and B). These results provided
evidence that the protective effect of LPEMFs on oxidative stress
may be attributed to the upregulation of antioxidant enzymes.
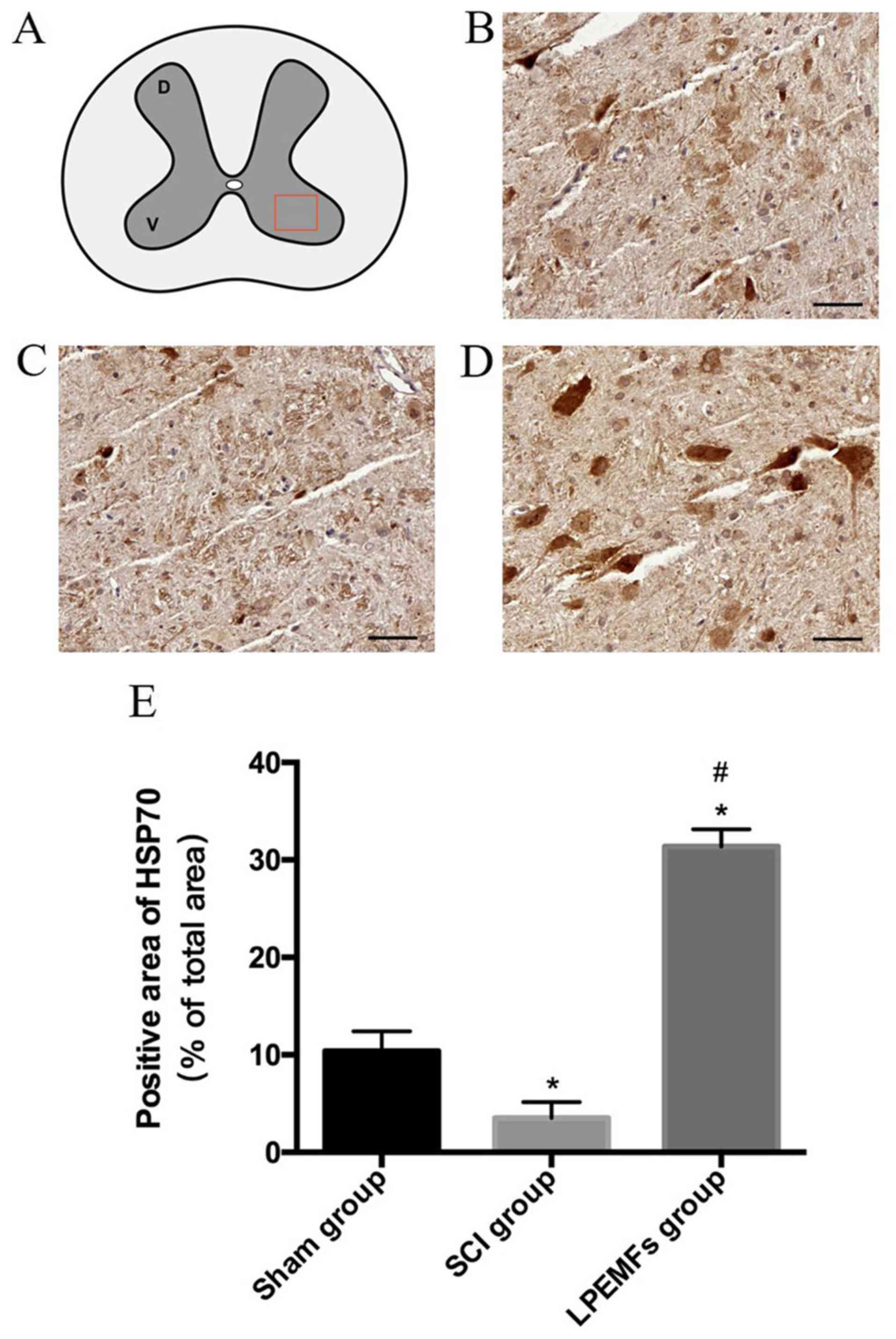
Protective effect of LPEMFs on
expression of HSP70 SCI rats
HSP70 is deemed as the protective agent for
inflammation and oxidative stress during tissue damage. In the
current study, the expression of HSP70 in the spinal cord following
injury with and without the administration of LPEMFs was examined.
Following SCI, the expression of HSP70 became scattered in the
ventral horn compared with the spinal cord in the Sham group.
However, the LPEMF treatment significantly increased the expression
of HSP70 in motor neurons (Fig.
7A-D). The quantified result was consistent with observations
(Fig. 7E). These results indicated
that the anti-inflammatory and antioxidative stress effects of
LPEMFs may associated with the high expression of HSP70.
Discussion
Electromagnetic fields (EMFs) have long been deemed
relevant for human health (20),
and recent studies have indicated that LPEMFs exhibit protective
effects in multiple pathologies, including Alzheimer's disease
(13), stroke (12), wound healing (21) and pain (22). The definition of low frequency is
<300 Hz and research has demonstrated that pulsed EMFs exhibited
greater therapeutic effects when applied with an amplitude <3 mT
and frequencies <100 Hz (23).
In the current study, LPEMFs with a 50 Hz frequency and 2.5 mT
amplitude were applied, which are suitable parameters for the
exploratory research. However, to the best of our knowledge, the
current study was first administration of LPEMFs in a contusion SCI
model, which is more clinically relevant than the transection model
(24,25). Unlike other disorders, the
pathological changes following SCI are complex due to the
microenvironment comprised of neurons, astrocytes,
oligodendrocytes, microglial cells and vascular endothelial cells
(26). It is well established that
the secondary injury is far more important than primary injury, due
the crucial role of the microenvironment in tissue preservation and
regeneration (7). In the current
study, LPEMF treatment improved the recovery of motor function in
the SCI rats and alleviated the inflammation and oxidative stress
in the damaged spinal cord, which indicated that LPEMFs promote
functional recovery, which may be associated with reduced secondary
injury following SCI.
Inflammatory cascades are activated during secondary
injury following SCI. High levels of pro-inflammatory factors are
released from spinal cord tissue (astrocytes and microglia) and
peripheral cells (neutrophils, monocytes and macrophages) to
increase vascular permeability. TNF-α and IL-1β pathophysiological
signaling pathways are two of the most important components in SCI
inflammatory cascades. The increased expression of TNF-α and IL-1β
can suppress cell survival and lead to cell death. The NF-κB
signaling pathway has been well established as the center of the
pathophysiology of inflammatory reactions induced by SCI (27). The NF-κB can be elevated by the
pro-inflammatory cytokines and chemokines following injury. The
activation of NF-κB, as a transcription factor, is required for the
upregulation of TNF-α and IL-1β. Therefore, this positive feedback
amplifies the inflammatory reaction and exacerbated the
microenvironment following SCI (28). Thus, the results of the current
study suggest that LPEMFs can reduce the expression of TNF-α, IL-1β
and NF-κB to alleviate the inflammatory reaction induced by SCI,
and this supports previous reports demonstrating LPEMF exposure
exhibits anti-inflammatory effects in synoviocytes, chondrocytes
and osteoblasts (29–31). However, in the current study,
inflammation was only detected at 14 dpi. It will be necessary to
use other time points to validate these results.
Following SCI, the production and elimination of
oxidative species are imbalanced, which results in tissue oxidative
stress (32). ROS and NO are two
main end-products of oxidative stress. Superoxides,
hydroxylradicals, hydrogen peroxides and peroxynitrites are the
principal components of ROS (33).
NO is synthesized by NOS, and the most abundant isoform is the
iNOS, which is located in a variety of cell types in the spinal
cord and can represent the production of NO (34). Antioxidant enzymes, including
superoxide dismutase (SOD) and catalase (CAT), act to reduce
oxidative stress. However, damage of the spinal cord impairs their
protective ability (35). In the
current study, the administration of LPEMFs significantly reduced
the levels of ROS and iNOS in the injured spinal cord. By contrast,
the expression of CAT and SOD were upregulated by LPEMFs, which can
protect damaged tissue. A previous study also demonstrated the
antioxidant protective effects of LPEMFs in neuronal cell lines by
elevating endogenous antioxidant properties (36), which was in accordance with the
current study.
Heat shock proteins have an important role in
transport and folding proteins, which is necessary for numerous
biological processes. HSP70 has been considered to protect against
cellular stress. HSP70 is also modulates inflammation and oxidative
stress (37,38). In an SCI model, HSP70 was
demonstrated to promote the survival of motor neurons (39). In the current study, LPEMFs
significantly promoted the expression of HSP70 in the ventral horn
of the injured spinal cord, where the motor neurons are located.
Therefore, the enhanced tolerance to inflammation and oxidative
stress may be mediated by upregulation of HSP70 following LPEMF
treatment. However, the causal association between HSP70 and
inflammation/oxidative stress requires further investigation.
In conclusion, the findings of the present study
revealed that the administration of LPEMFs reduces inflammation and
oxidative stress to promote functional recovery following SCI, and
the potential mechanism involves the activation of HSP70. The
findings provide new perspective for identifying novel noninvasive
therapeutic methods for early intervention following SCI.
Acknowledgements
The authors are grateful for the valuable
suggestions of Professor Xiaohong Kong from the 221 Laboratory,
School of Medicine, Nankai University (Tianjin, China).
Funding
The authors thank the following sources for funding
support: the NSFC program (grant nos. 81330042, 81472070, 81772342
and 81620108018), the Ministry of Science and Technology, China
(grant no. 2014DFR31210), and the Tianjin Science and Technology
Committee, China (grant nos. 13RCGFSY19000 and 14ZCZDSY00044).
Availability of data and materials
The data and materials used or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
SF, CXW, CYW and YL conceived and designed the
experiments. CYW and YL performed the experiments. YW, ZW and DS
provided critical reagents and scientific input. GN and QW
maintained the animals. CYW, YL, SF and CXW analyzed data and
prepared the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Welfare Committee of Tianjin Medical University (Tianjin, China),
which is based on the NIH Guide for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ropper AE and Ropper AH: Acute spinal cord
compression. N Eng J Med. 376:1358–1369. 2017. View Article : Google Scholar
|
|
2
|
Furlan JC, Sakakibara BM, Miller WC and
Krassioukov AV: Global incidence and prevalence of traumatic spinal
cord injury. Can J Neurol Sci. 40:456–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain NB, Ayers GD, Peterson EN, Harris MB,
Morse L, O'Connor KC and Garshick E: Traumatic spinal cord injury
in the United States, 1993–2012. JAMA. 313:2236–2243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller LE and Herbert WG: Health and
economic benefits of physical activity for patients with spinal
cord injury. Clinicoecon Outcomes Res. 8:551–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurlbert RJ, Hadley MN, Walters BC, Aarabi
B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC and Theodore N:
Pharmacological therapy for acute spinal cord injury. Neurosurgery.
72 Suppl 2:S93–S105. 2013. View Article : Google Scholar
|
|
6
|
Herzer KR, Chen Y, Heinemann AW and
González-Fernández M: Association between time to rehabilitation
and outcomes after traumatic spinal cord injury. Arch Phys Med
Rehabil. 97:1620–1627.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anwar MA, Al Shehabi TS and Eid AH:
Inflammogenesis of secondary spinal cord injury. Front Cell
Neurosci. 10:982016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye
FB, Cao Y and Lin FY: Systemic administration of exosomes released
from mesenchymal stromal cells attenuates apoptosis, inflammation,
and promotes angiogenesis after spinal cord injury in rats. J
Neurotrauma. 34:3388–3396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xun C, Mamat M, Guo H, Mamati P, Sheng J,
Zhang J, Xu T, Liang W, Cao R and Sheng W: Tocotrienol alleviates
inflammation and oxidative stress in a rat model of spinal cord
injury via suppression of transforming growth factor-β. Exp Ther
Med. 14:431–438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou J, Chen Y, Qian J and Yang H: Effect
of a low-frequency pulsed electromagnetic field on expression and
secretion of IL-1β and TNF-α in nucleus pulposus cells. J Int Med
Res. 45:462–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehnert S, Fentz AK, Schreiner A, Birk J,
Wilbrand B, Ziegler P, Reumann MK, Wang H, Falldorf K and Nussler
AK: Extremely low frequency pulsed electromagnetic fields cause
antioxidative defense mechanisms in human osteoblasts via induction
of •O2 and H2O2. Sci Rep.
7:145442017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urnukhsaikhan E, Mishig-Ochir T, Kim SC,
Park JK and Seo YK: Neuroprotective effect of low frequency-pulsed
electromagnetic fields in ischemic stroke. Appl Biochem Biotechnol.
181:1360–1371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Capelli E, Torrisi F, Venturini L, Granato
M, Fassina L, Lupo GF and Ricevuti G: Low-frequency pulsed
electromagnetic field is able to modulate miRNAs in an experimental
cell model of Alzheimer's disease. J Healthc Eng 2017.
25302702017.
|
|
14
|
Dey S, Bose S, Kumar S, Rathore R, Mathur
R and Jain S: Extremely low frequency magnetic field protects
injured spinal cord from the microglia- and iron-induced tissue
damage. Electromagn Biol Med. 36:330–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
NIH (National Institutes of Health U.S.A):
Guide for the Care and Use of Laboratory Animals. The National
Academies Press; Washington, DC: pp. 2462011
|
|
16
|
Zhou H, Li X, Wu Q, Li F, Fu Z, Liu C,
Liang Z, Chu T, Wang T, Lu L, et al: shRNA against PTEN promotes
neurite outgrowth of cortical neurons and functional recovery in
spinal cord contusion rats. Regen Med. 10:411–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei ZJ, Zhou XH, Fan BY, Lin W, Ren YM and
Feng SQ: Proteomic and bioinformatic analyses of spinal cord
injury-induced skeletal muscle atrophy in rats. Mol Med Rep.
14:165–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu L, Botchway BOA, Zhang S, Zhou J and
Liu X: Inhibition of NF-κB signaling pathway by resveratrol
improves spinal cord injury. Front Neurosci. 12:6902018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferroni L, Tocco I, De Pieri A, Menarin M,
Fermi E, Piattelli A, Gardin C and Zavan B: Pulsed magnetic therapy
increases osteogenic differentiation of mesenchymal stem cells only
if they are pre-committed. Life Sci. 152:44–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ottani V, De Pasquale V, Govoni P, Franchi
M, Zaniol P and Ruggeri A: Effects of pulsed
extremely-low-frequency magnetic fields on skin wounds in the rat.
Bioelectromagnetics. 9:53–62. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jorgensen WA, Frome BM and Wallach C:
Electrochemical therapy of pelvic pain: Effects of pulsed
electromagnetic fields (PEMF) on tissue trauma. Eur J Surg Suppl.
83–86. 1994.PubMed/NCBI
|
|
23
|
Kavand H, Haghighipour N, Zeynali B,
Seyedjafari E and Abdemami B: Extremely low frequency
electromagnetic field in mesenchymal stem cells gene regulation:
Chondrogenic markers evaluation. Artif Organs. 40:929–937. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng SQ, Zhou XF, Rush RA and Ferguson IA:
Graft of pre-injured sural nerve promotes regeneration of
corticospinal tract and functional recovery in rats with chronic
spinal cord injury. Brain Res 1209. 40–48. 2008. View Article : Google Scholar
|
|
25
|
Feng SQ, Kong XH, Guo SF, Wang P, Li L,
Zhong JH and Zhou XF: Treatment of spinal cord injury with
co-grafts of genetically modified Schwann cells and fetal spinal
cord cell suspension in the rat. Neurotox Res. 7:169–177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kjell J and Olson L: Rat models of spinal
cord injury: From pathology to potential therapies. Dis Model Mech.
9:1125–1137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cavalli G and Dinarello CA: Suppression of
inflammation and acquired immunity by IL-37. Immunol Rev.
281:179–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang
J, Liang W and Ma Z: Curcumin modulates TLR4/NF-κB inflammatory
signaling pathway following traumatic spinal cord injury in rats. J
Spinal Cord Med. 38:199–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varani K, De Mattei M, Vincenzi F, Gessi
S, Merighi S, Pellati A, Ongaro A, Caruso A, Cadossi R and Borea
PA: Characterization of adenosine receptors in bovine chondrocytes
and fibroblast-like synoviocytes exposed to low frequency low
energy pulsed electromagnetic fields. Osteoarthritis Cartilage.
16:292–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vincenzi F, Targa M, Corciulo C, Gessi S,
Merighi S, Setti S, Cadossi R, Goldring MB, Borea PA and Varani K:
Pulsed electromagnetic fields increased the anti-inflammatory
effect of A2A and A3 adenosine receptors in
human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One.
8:e655612013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ongaro A, Varani K, Masieri FF, Pellati A,
Massari L, Cadossi R, Vincenzi F, Borea PA, Fini M, Caruso A and De
Mattei M: Electromagnetic fields (EMFs) and adenosine receptors
modulate prostaglandin E(2) and cytokine release in human
osteoarthritic synovial fibroblasts. J Cell Physiol. 227:2461–2469.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Visavadiya NP, Patel SP, VanRooyen JL,
Sullivan PG and Rabchevsky AG: Cellular and subcellular oxidative
stress parameters following severe spinal cord injury. Redox Biol.
8:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Bazhin AV, Werner J and
Karakhanova S: Reactive oxygen species in the immune system. Int
Rev Immunol. 32:249–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sheng W, Zong Y, Mohammad A, Ajit D, Cui
J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, et al:
Pro-inflammatory cytokines and lipopolysaccharide induce changes in
cell morphology, and upregulation of ERK1/2, iNOS and
sPLA2-IIA expression in astrocytes and microglia. J
Neuroinflammation. 8:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Q, Chen Q, Ding Q, Yang Q, Peng Y, Lu
Y, Deng J and Xiong L: Sevoflurane postconditioning attenuates
spinal cord reperfusion injury through free radicals-mediated
up-regulation of antioxidant enzymes in rabbits. J Surg Res.
169:292–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vincenzi F, Ravani A, Pasquini S, Merighi
S, Gessi S, Setti S, Cadossi R, Borea PA and Varani K: Pulsed
electromagnetic field exposure reduces Hypoxia and inflammation
damage in neuron-like and microglial cells. J Cell Physiol.
232:1200–1208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacquier-Sarlin MR, Fuller K, Dinh-Xuan
AT, Richard MJ and Polla BS: Protective effects of hsp70 in
inflammation. Experientia. 50:1031–1038. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sevin M, Girodon F, Garrido C and de
Thonel A: HSP90 and HSP70: Implication in inflammation processes
and therapeutic approaches for myeloproliferative neoplasms.
Mediators Inflamm 2015. 9702422015.
|
|
39
|
Shabbir A, Bianchetti E, Cargonja R,
Petrovic A, Mladinic M, Pilipović K and Nistri A: Role of HSP70 in
motoneuron survival after excitotoxic stress in a rat spinal cord
injury model in vitro. Eur J Neurosci. 42:3054–3065. 2015.
View Article : Google Scholar : PubMed/NCBI
|