Introduction
Lung cancer is one of the most prevalent and
aggressive types of cancer worldwide, with an extremely high
mortality rate (1). Regardless of
the advances in surgical techniques and chemotherapy, the outcomes
of patients with lung cancer remain poor, and the 5-year survival
rate is only ~15% (2). Metastasis
is the most important risk for cancer-associated mortality in lung
cancer (3). Among all cases,
non-small cell lung cancer (NSCLC) accounts for almost 85%
(4). Therefore, to improve the
therapy available to patients, exploration of the molecular
mechanisms underlying NSCLC development and progression is urgently
required.
MicroRNAs (miRNAs) are a type of short noncoding
RNAs measuring 19–22 nucleotides long, which function by
recognizing specific complementary sites in the 3′-untranslated
region (3′-UTR) of target mRNAs (5). Through directing the degradation of
target mRNAs, miRNAs are involved in the regulation of gene
expression. Accumulating evidence has indicated that miRNAs serve
crucial functions in multiple biological processes, including
proliferation, migration, development and survival (6). In previous decades, miRNAs have been
demonstrated to participate in tumorigenesis and an increasing
number of previous studies have suggested that dysregulation of
miRNAs is associated with the pathogenesis of cancer (7). Furthermore, a previous study has
indicated that miRNAs exert vital roles in NSCLC occurrence and
progression (8). For example,
miR-520a-3p inhibits cell growth and metastasis of non-small cell
lung cancer through the phosphoinositide 3′-kinase/protein kinase B
(AKT)/mechanistic target of rapamycin signaling pathway (9). miR-224 enhances invasion and
metastasis by targeting homeobox D10 in non-small cell lung cancer
cells (10). Therefore, the
identification of important miRNAs in NSCLC will contribute to
understanding of the pathogenesis and novel drug
identification.
A recent study indicated that miR-671-3p inhibits
the development of breast cancer (11). The role of miR-671-3p in NSCLC
remains largely unknown. The present study aimed to investigate the
biological function of miR-671-3p in NSCLC progression. It was
identified that miR-671-3p was significantly upregulated in NSCLC
tissues compared with adjacent normal tissues. Furthermore, it was
demonstrated that miR-671-3p inhibited NSCLC cell proliferation and
invasion via directly targeting cyclin D2 (CCND2). In conclusion,
the present study demonstrated that miR-671-3p exerts
tumor-suppressive roles in NSCLC, suggesting miR-671-3p may be a
promising therapeutic target.
Materials and methods
Patient samples
A total of 43 NSCLC cancer and corresponding
adjacent tissue specimens were obtained from patients with NSCLC
who underwent curative resection at Ningbo No. 2 Hospital (Ningbo,
China). No patients had undergone chemotherapy or radiotherapy
prior to surgery. Fresh samples were collected at the time of
surgery, and were rapidly frozen in liquid nitrogen and stored at
−80°C until use. The clinicopathological characteristics of the
patients with NSCLC are summarized in Table I. Informed consent was signed by
each patient. The present study was approved by the Ethics
Committee of Ningbo No. 2 Hospital.
 | Table I.Associations between miR-671-3p
expression and clinicopathological characteristics of patients with
non-small cell lung cancer. |
Table I.
Associations between miR-671-3p
expression and clinicopathological characteristics of patients with
non-small cell lung cancer.
|
| miR-671-3p
expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | Lower (n=21) | Higher (n=22) | P-value |
|---|
| Sex |
|
| 0.719 |
| Male | 15 | 17 |
|
|
Female | 6 | 4 |
|
| Age, years |
|
| 0.373 |
| ≤60 | 14 | 12 |
|
|
>60 | 7 | 11 |
|
| Lymph node
metastasis |
|
| 0.015 |
|
Negative | 6 | 15 |
|
|
Positive | 15 | 7 |
|
| Tumor size, cm |
|
| 0.033 |
| ≤3 | 8 | 16 |
|
|
>3 | 13 | 6 |
|
| Tumor node metastasis
stage |
|
| 0.034 |
| I/II | 7 | 15 |
|
| III | 14 | 7 |
|
Cell culture
Human NSCLC A549, H1299, H1650 and H1975 cell lines
and the non-tumorigenic human bronchial epithelial NL20 cell line
were purchased from the American Type Culture Collection (Manassas,
VA, USA). All the cells were cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
penicillin (100 U/ml), streptomycin (100 U/ml) (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), and 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in 5% CO2.
miRNA transfection
miR-671-3p mimics, miR-671-3p negative control
(miR-NC), miR-671-3p inhibitors and miR-671-3p inhibitor negative
control (anti-miR-NC) were purchased from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). These were individually and transiently
transfected with A549 cells at a final concentration of 100 nM
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The miR-671-3p inhibitors were modified
antisense oligonucleotides designed specifically to bind to and
inhibit endogenous miR-671-3p with a rare off-target effect:
miR-NC: 5′-ACAUCUGCGUAAGAUUCGAGUCUA-3′; miR-671-3p mimics:
5′-UCCGGUUCUCAGGGCUCCACC-3′; anti-miR-NC:
5′-GCGTAACTAATACATCGGATTCGT-3′; miR-671-3p inhibitors:
5′-GGUGGAGCCCUGAGAACCGGA-3′. The efficiency was measured 48 h after
transfection, by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) as described below.
RT-qPCR
Total RNA was isolated from tissues or cultured
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.). RNA quality and concentration was measured using a Nanodrop
2000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington,
DE, USA). A total of 1 µg RNA was used for cDNA synthesis using a
PrimeScript 1st Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu,
Japan). qPCR was performed using the miRNA Q-PCR Detection kit
(GeneCopoeia, Inc., Rockville, MD, USA). The PCR thermocycling
conditions were: 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 34 sec. The RT-qPCR data were analyzed using the
2−∆∆Cq method (12) and
relative to the small nuclear RNA U6 or GAPDH levels. RT primers
were as follows: GAPDH forward (F), 5′-ACAACTTTGGTATCGTGGAAGG-3′;
GAPDH reverse (R), 5′-GCCATCACGCCACAGTTTC-3′; U6 F,
5′-CTCGCTTCGGCAGCACA-3′; U6 R, 5′-AACGCTTCACGAATTTGCGT-3′;
miR-671-3p F, 5′-CTGGCTGGACAGAGTTGTCAT-3′; miR-671-3p R,
5′-TCCGGTTCTCAGGGCTCCACC-3′; CCND2 F, 5′-TACCTGGACCGTTTCTTGGC-3′;
CCND2 R, 5′-AGGCTTGATGGAGTTGTCGG-3′.
Western blot analysis
Western blot was performed as previously described
(13). In brief, tumor cells were
lysed with lysis buffer (0.5 mol/l Tris-HCl, pH 7.4, 1.5 mol/l
NaCl, 2.5% deoxycholic acid, 10% NP-40 and 10 mmol/l EDTA) in the
presence of cocktail protease inhibitors (Thermo Fisher Scientific,
Inc.). The lysates were collected by centrifugation at 16,000 × g
for 20 min at 4°C. Protein concentration was determined by Bio-Rad
Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
A total of 50 mg protein/lane was separated by 12% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane (Thermo Fisher
Scientific, Inc.) The membrane was blocked with 5% non-fat dry milk
(Yili Group, Beijing, China) for 1 h at room temperature, followed
by incubation with primary antibodies for 2 h at room temperature:
Anti-Cyclin D2 (1:1,000; cat. no. ab207604; Abcam, Cambridge, MA,
USA) and anti-GAPDH (1:1,000; cat. no. ab9485; Abcam). Following
washing, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. ab7090; Abcam) at room temperature for 1 h. An enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.) was used to
perform chemiluminescence detection.
Cell Counting Kit-8 (CCK-8)
proliferation assays
Each group of A549 cells was collected at 24, 48, 72
and 96 h following transfection. Then, cells were incubated with 10
µl CCK-8 reagent (Beyotime Institute of Biotechnology, Haimen,
China) for 2 h at 37°C. Next, absorbance at 450 nm was measured at
each time point using an enzyme immunoassay analyzer. The
experiment was conducted in three separate wells for each sample,
and performed in triplicate.
Transwell invasion assays
To measure cell invasion, a Transwell invasion
chamber coated with Matrigel® (Corning Incorporated,
Corning, NY, USA) at 37°C for 30 min was used to determine the cell
invasion ability. Following fixation with 4% paraformaldehyde for 1
h at room temperature, the cells that had invaded the membrane were
stained with 0.1% crystal violet for 30 min at room temperature and
counted. The number of cells that had invaded through the Matrigel
was counted in 5 fields of triplicate membranes at magnification,
×100, using an inverted light microscope (Olympus Corporation,
Tokyo, Japan).
Luciferase assay
The potential targets and binding sites of
miR-671-3p were analyzed using TargetScan7 tool (http://www.targetscan.org/vert_71/). The 3′-UTR
of the CCND2 was obtained by gene synthesis, and inserted
downstream of the luciferase reporter gene in a pmirGLO vector
(Promega Corporation, Madison, WI, USA). For the luciferase
reporter assay, A549 cells (2×104/well) were seeded in a
24-well plate and incubated for 24 h prior to transfection. Next,
firefly luciferase constructs containing the 3′-UTR and miR-671-3p
mimics or the corresponding negative controls were co-transfected
into A549 cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were collected at 48 h after
transfection, and measured using the Dual-Luciferase Reporter
System (Promega Corporation), according to manufacturer's
protocols. The pRL-TK Renilla luciferase activity was used
for normalization.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism v. 6 (GraphPad
Software, Inc., La Jolla, CA, USA). A Student's t-test and one-way
analysis of variance followed by Tukey's post-hoc test were used to
analyze two or multiple groups, respectively. Kaplan-Meier curves
were used to analyze survival rate and log-rank tests was used to
calculate the corresponding P-values. Associations between
miR-671-3p expression and clinicopathological characteristics of
patients with NSCLC were analyzed using a χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-671-3p is downregulated in NSCLC
tissues
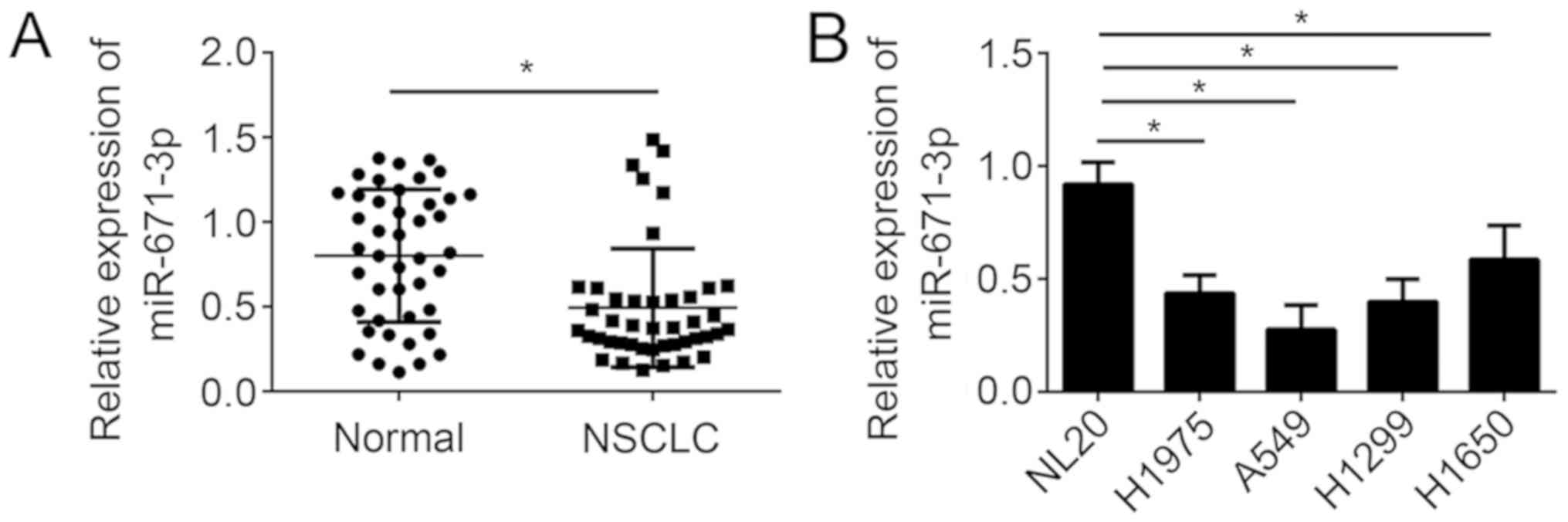
In order to explore the function of miR-671-3p in
NSCLC progression, the expression of miR-671-3p in 43 pairs of
NSCLC tissues and adjacent normal tissues was first analyzed by
RT-qPCR. The results demonstrated that miR-671-3p expression was
significantly downregulated in NSCLC tissues compared with the
adjacent normal tissues (Fig. 1A).
Consistently, it was identified that the expression of miR-671-3p
was also markedly downregulated in the NSCLC A549, H1975, H1299 and
H1650 cell lines compared with the non-tumorigenic human bronchial
epithelial NL20 cell line (Fig.
1B). Furthermore, the NSCLC tissues were divided into
miR-671-3p high and miR-671-3p low groups using the median value of
miR-671-3p level as the cut-off value. pTNM staging designations
were made according to the postsurgical pathological staging system
according to the 7th edition of the TNM classification of malignant
tumors (14). It was observed that
the level of miR-671-3p was negatively associated with tumor size,
Tumor Node Metastasis stage and metastasis (Table I). Taken together, these results
suggested that miR-671-3p may be involved in NSCLC progression.
miR-671-3p overexpression inhibits
NSCLC cell proliferation and invasion
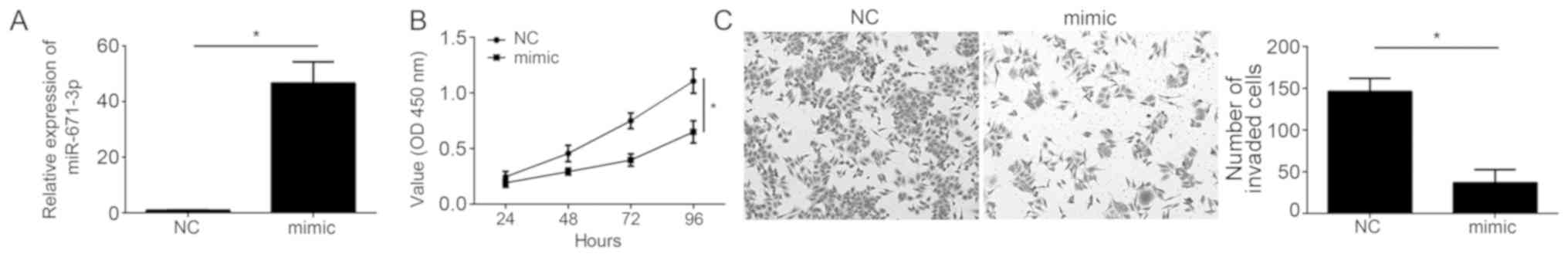
To additionally investigate the biological functions
of miR-671-3p, miR-671-3p was overexpressed in A549 cells, which
exhibited the lowest level of miR-671-3p among all measured cell
lines. RT-qPCR analysis indicated that miR-671-3p levels were
significantly upregulated following transfection with miR-671-3p
mimics in A549 cells (Fig. 2A).
Then, CCK-8 and Transwell invasion assays were performed. The
results indicated that ectopic expression of miR-671-3p
significantly inhibited the proliferation and decreased the
invading cell number (Fig. 2B and
C), suggesting that miR-671-3p exerts a tumor-suppressive role
in NSCLC.
miR-671-3p inhibition promotes NSCLC
cell proliferation and invasion
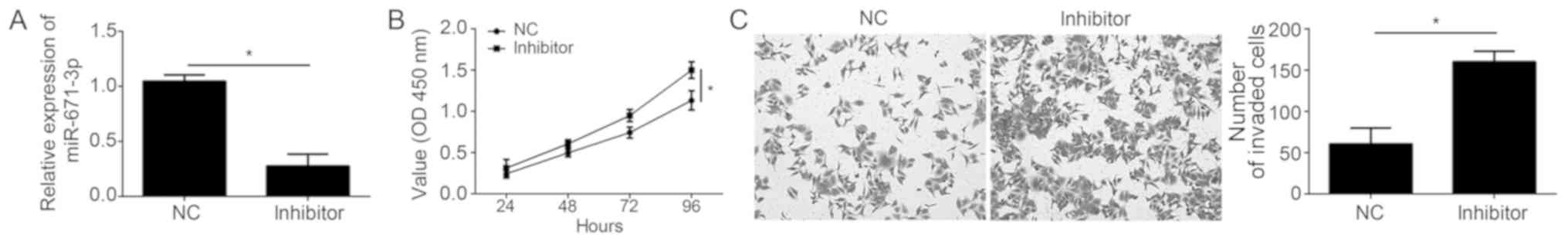
To additionally confirm the role of miR-671-3p in
NSCLC, experiments using miR-671-3p inhibitors were performed. As
demonstrated by the RT-qPCR assay results, miR-671-3p expression
was significantly downregulated in A549 cells following
transfection with miR-671-3p inhibitors compared with the negative
control (Fig. 3A). The CCK-8 assay
revealed that miR-671-3p inhibition led to a decreased
proliferation ability in A549 cells (Fig. 3B). Furthermore, knockdown of
miR-671-3p significantly inhibited the invasion of A549 cells
(Fig. 3C).
CCND2 is a target of miR-671-3p
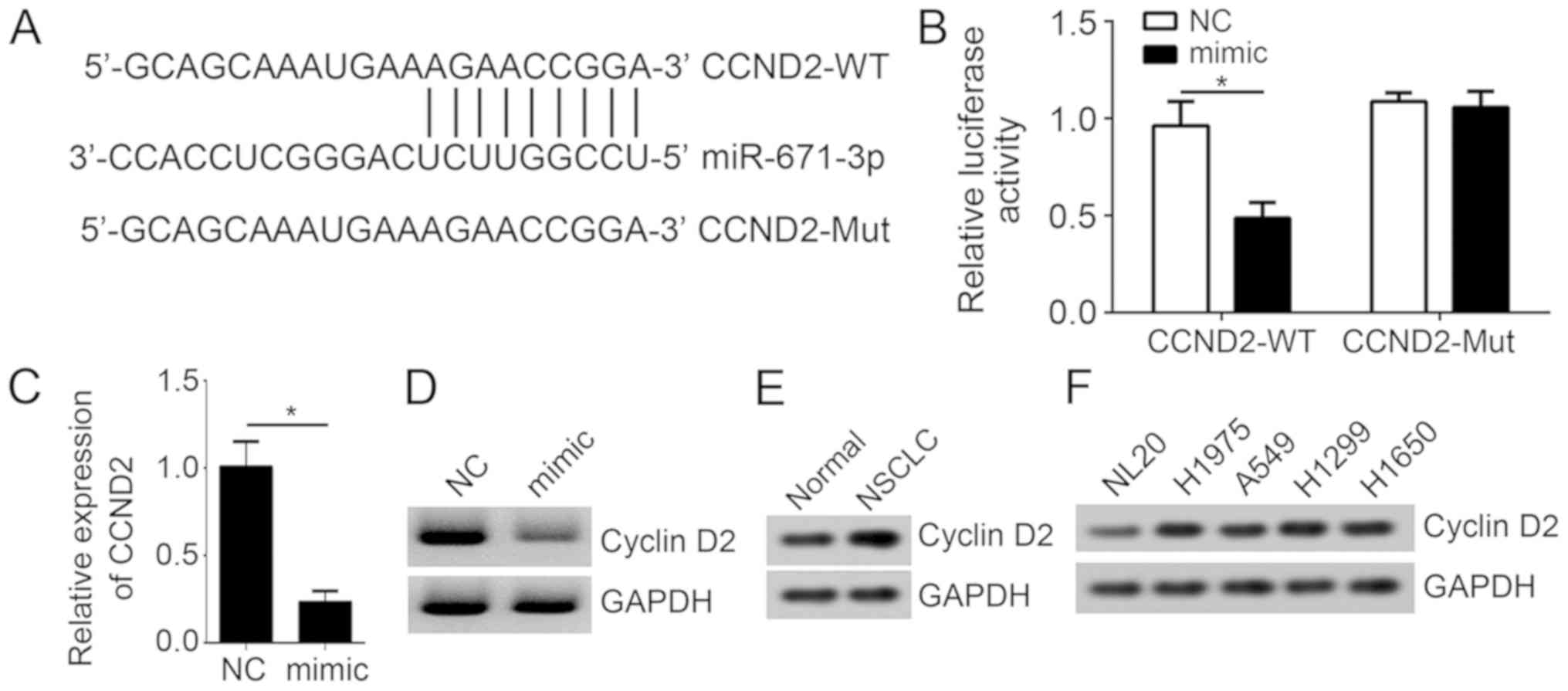
The present study then aimed to determine the
mechanism of miR-671-3p in NSCLC. The potential target of
miR-671-3p was identified using TargetScan7. The results implied
that CCND2 may be a target of miR-671-3p, as a potential binding
site of miR-671-3p in the CCND2 3′-UTR was identified (Fig. 4A). To validate this prediction, a
luciferase reporter assay was then performed. It was identified
that the overexpression of miR-671-3p significantly repressed the
luciferase intensity of CCND2-3′-UTR-wild type in A549 cells
(Fig. 4B). Notably, mutation of
the predicted site in CCND2 3′-UTR abrogated the effect of
miR-671-3p (Fig. 4B), indicating
that miR-671-3p interacts with CCND2 mRNA directly. In addition,
RT-qPCR and western blot analyses suggested that the overexpression
of miR-671-3p markedly decreased the mRNA and protein levels of
CCND2 in A549 cells (Fig. 4C and
D). The expression levels of CCND2 were also upregulated in
NSCLC tissues and cell lines (Fig. 4E
and F). Taken together, these results suggested that CCND2 was
directly targeted by miR-671-3p in NSCLC cells.
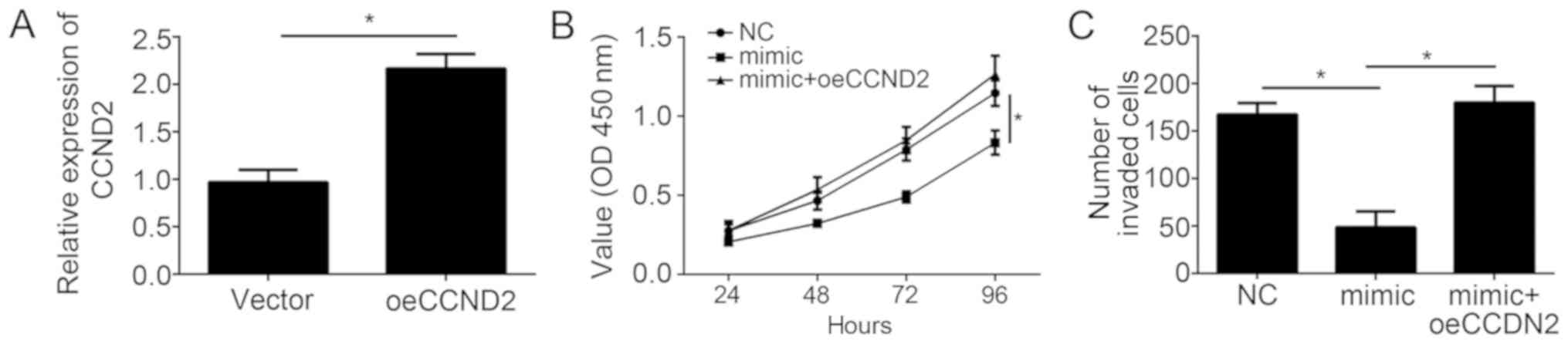
CCND2 restoration rescued the effects
of miR-671-3p overexpression
To confirm the role of CCND2 in the process of
miR-671-3p-mediated NSCLC progression, rescue experiments were
performed. The efficiency of CCND2 overexpression was first
validated by RT-qPCR. The results revealed that the CCND2 mRNA
level was significantly upregulated in A549 cells following
transfection with the pCDNA3-CCND2 vector (Fig. 5A). Then, CCK-8 and Transwell
invasion assays were conducted. The results demonstrated that
miR-671-3p overexpression significantly inhibited the proliferation
and invasion of NSCLC cells (Fig. 5B
and C). However, restoration of CCND2 markedly rescued the
inhibitory effects of miR-671-3p overexpression in A549 cells
(Fig. 5B and C). In conclusion,
these results demonstrated that miR-671-3p exerts its roles via
directly targeting CCND2 in NSCLC cells.
Discussion
Lung cancer has become a major public health
challenge due to its high incidence and mortality (15). Among all lung cancer cases, NSCLC
accounts for ~85% (1).
Nevertheless, the molecular mechanism underlying lung cancer
progression remains poorly understood. In previous decades, with
advances in surgical techniques and the development of chemical
drug therapies, the outcomes of NSCLC patients have been improved
(16). However, the 5-year
survival rate of patients with NSCLC is only 15%, even following
treatment (17). Therefore,
determination of the molecular mechanism and development of novel
therapeutic strategies are urgently required. In the present study,
the association between miR-671-3p expression and NSCLC progression
was demonstrated. It was identified that miR-671-3p expression was
significantly downregulated in NSCLC tissues compared with adjacent
normal tissues. Overexpression of miR-671-3p significantly
suppressed the proliferation and invasion of NSCLC cells, and vice
versa. These data suggested that miR-671-3p serves as a tumor
suppressor, and implies that miR-671-3p may be a potential target
for NSCLC treatment.
An increasing number of miRNAs have been recognized
as oncogenes or tumor suppressors, which suggests that miRNAs may
be promising targets for cancer intervention (18). For example, miR-30a was
significantly downregulated in osteosarcoma, and suppressed
osteosarcoma proliferation and metastasis by targeting myocyte
enhancer factor 2D (19).
miR-12528 was demonstrated to regulate tumorigenesis and metastasis
in lung cancer by targeting insulin-like growth factor 1 receptor
(20). miR-502 mediates esophageal
cancer TE1 cell proliferation by promoting AKT phosphorylation
(21). Therefore, it is crucial to
investigate the association between miRNA and cancer. A previous
study indicated that miR-671 promotes prostate cancer cell
proliferation by targeting tumor suppressor sex-determining region
Y-box 6 (22). A recent study
indicated that miR-671-3p suppressed breast cancer progression
(11). However, the function of
miR-671-3p in NSCLC remains unclear. In the present study, the data
demonstrated that miR-671-3p serves as a tumor suppressor. Using
CCK-8 and Transwell assays, it was revealed that miR-671-3p
inhibited NSCLC cell proliferation and invasion.
miRNAs have been demonstrated to target mRNAs for
the regulation of gene expression in cancer (23). Through bioinformatics analysis, the
present study identified that miR-671-3p may target CCND2. Using a
luciferase reporter assay, the direct interaction between
miR-671-3p and CCND2 mRNA was validated. Furthermore, it was
demonstrated that miR-671-3p overexpression significantly inhibited
the expression of CCND2 in NSCLC tissues. Previous studies imply
that CCND2 is a classical oncogene in various types of cancer
(24). CCND2 upregulation may lead
to proliferation and metastasis of cancer cells: For example, Wang
et al (25) indicated that
miR-1297 represses the growth and metastasis of colorectal cancer
by suppressing CCND2 expression. CCND2 was also demonstrated to
participate in NSCLC progression (24). Consistent with these previous
studies, the present study also indicated that CCND2 exerts
oncogenic roles in NSCLC. It was identified that the restoration of
CCND2 expression may significantly attenuate the inhibitory effects
of miR-671-3p mimics on NSCLC cell proliferation and invasion.
Notably, according to the results of the target prediction,
miR-671-3p may also target other genes. Whether other potential
genes are involved in this process requires additional
investigation.
In conclusion, the present study provided novel
evidence to suggest the tumor suppressor role of miR-671-3p in
NSCLC. Furthermore, CCND2 was identified as a novel target of
miR-671-3p in NSCLC. Future studies will focus on whether
miR-671-3p may serve as a biomarker and therapeutic target for
NSCLC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YY and XF initiated and designed the study, and
analyzed and interpreted the results. YZ performed RT-qPCR
analysis. XF wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
Ningbo No. 2 Hospital and all enrolled patients provided written
informed consent.
Patient consent for publication
All patients within the present study provided
consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS: Ten years of progress in
non-small cell lung cancer. J Natl Compr Canc Netw. 10:292–295.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao F, Liu D, Guo Y, Shi B, Song Z, Tian
Y, Zhang Z and Liang C: Survival rate and prognostic factors of
surgically resected clinically synchronous multiple primary
non-small cell lung cancer and further differentiation from
intrapulmonary metastasis. J Thorac Dis. 9:990–1001. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Du J, Jiang R and Li L:
MicroRNA-214 inhibits the proliferation and invasion of lung
carcinoma cells by targeting JAK1. Am J Transl Res. 10:1164–1171.
2018.PubMed/NCBI
|
|
5
|
Saumet A and Lecellier CH: microRNAs and
personalized medicine: Evaluating their potential as cancer
biomarkers. Adv Exp Med Biol. 888:5–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren P, Gong F, Zhang Y, Jiang J and Zhang
H: MicroRNA-92a promotes growth, metastasis and chemoresistance in
non-small cell lung cancer cells by targeting PTEN. Tumour Biol.
37:3215–3225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv X, Li CY, Han P and Xu XY:
MicroRNA-520a-3p inhibits cell growth and metastasis of non-small
cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur Rev
Med Pharmacol Sci. 22:2321–2327. 2018.PubMed/NCBI
|
|
10
|
Li S, Zhang J, Zhao Y, Wang F, Chen Y and
Fei X: miR-224 enhances invasion and metastasis by targeting HOXD10
in non-small cell lung cancer cells. Oncol Lett. 15:7069–7075.
2018.PubMed/NCBI
|
|
11
|
Xiong DD, Chen H, He RQ, Lan AH, Zhong JC,
Chen G, Feng ZB and Wei KL: MicroRNA-671-3p inhibits the
development of breast cancer: A study based on in vitro
experiments, in-house quantitative polymerase chain reaction and
bioinformatics analysis. Int J Oncol. 52:1801–1814. 2018.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang B, Li M, Ji F and Nie Y:
MicroRNA-219 exerts a tumor suppressive role in glioma via
targeting Sal-like protein 4. Exp Ther Med. 14:6213–6221.
2017.PubMed/NCBI
|
|
14
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions, : The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onat S, Ates G, Avci A, Yildiz T, Birak A,
Akgul Ozmen C and Ulku R: The role of mediastinoscopy in the
diagnosis of non-lung cancer diseases. Ther Clin Risk Manag.
13:939–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu YX, Wang Y and Liu H: Overexpression of
PTEN suppresses non-small-cell lung carcinoma metastasis through
inhibition of integrin αVβ6 signaling. Am J Transl Res.
9:3304–3314. 2017.PubMed/NCBI
|
|
17
|
Wang S, Zhang B, Li C, Cui C, Yue D, Shi
B, Zhang Q, Zhang Z, Zhang X and Wang C: Prognostic value of number
of negative lymph node in patients with stage II and IIIa non-small
cell lung cancer. Oncotarget. 8:79387–79396. 2017.PubMed/NCBI
|
|
18
|
Esmatabadi MJD, Farhangi B, Montazeri M,
Monfared H, Sistani RN and Sadeghizadeh M: Up-regulation of miR-21
decreases chemotherapeutic effect of dendrosomal curcumin in breast
cancer cells. Iran J Basic Med Sci. 20:350–359. 2017.PubMed/NCBI
|
|
19
|
Du L, Chen T, Zhao K and Yang D: miR-30a
suppresses osteosarcoma proliferation and metastasis by
downregulating MEF2D expression. Onco Targets Ther. 11:2195–2202.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeon SH, Yoo JK, Kim CM, Lim ES, Lee SJ,
Lee JM, Oh SH and Kim JK: The novel hsa-miR-12528 regulates
tumourigenesis and metastasis through hypo-phosphorylation of AKT
cascade by targeting IGF-1R in human lung cancer. Cell Death Dis.
9:4932018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Pan X and Hu Z: MiR-502 mediates
esophageal cancer cell TE1 proliferation by promoting AKT
phosphorylation. Biochem Biophys Res Commun. 501:119–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Wang Z, Sun D, Zhou X, Wei X, Hou W,
Ding Y, Ma Y and Hou Y: miR-671 promotes prostate cancer cell
proliferation by targeting tumor suppressor SOX6. Eur J Pharmacol.
823:65–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang F, Wei K, Qin Z, Liu W, Shao C, Wang
C, Ma L, Xie M, Shu Y and Shen H: MiR-598 suppresses invasion and
migration by negative regulation of derlin-1 and
epithelial-mesenchymal transition in non-small cell lung cancer.
Cell Physiol Biochem. 47:245–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YL, Wang J, Zhang CY, Shen YQ, Wang HM,
Ding L, Gu YC, Lou JT, Zhao XT, Ma ZL and Jin YX: MiR-146a-5p
inhibits cell proliferation and cell cycle progression in NSCLC
cell lines by targeting CCND1 and CCND2. Oncotarget. 7:59287–59298.
2016.PubMed/NCBI
|
|
25
|
Wang Y, Xue J, Kuang H, Zhou X, Liao L and
Yin F: microRNA-1297 inhibits the growth and metastasis of
colorectal cancer by suppressing cyclin D2 expression. DNA Cell
Biol. 36:991–999. 2017. View Article : Google Scholar : PubMed/NCBI
|