Introduction
With the rapid development of social economies,
standards are increasing and drinking alcohol has become a common
social activity (1). In this a
social environment, drinking behavior is continuously increasing,
and if an individual drinker suffers from a psychopathological
condition, drinking can easily develop into alcohol abuse and
dependence. Alcohol consumption has resulted in a series of
far-reaching social and medical issues (2). Diseases caused by alcohol abuse are
attracting increasing attention from the medical community
(3,4). Alcohol-associated diseases are often
reported, and alcoholism is associated serious systemic system
damage and causes social dysfunction and mental health disorders,
resulting in a series of social and medical problems (5,6).
Chronic prostatitis (CP) is a common urological
disease in men. Patients typically present with pain in the lower
abdomen, perineum, scrotum, penis, lumbosacral area and other areas
of discomfort (7). Numerous
patients also experience different degrees of sexual dysfunction,
neuropsychiatric symptoms, fatigue and insomnia. Although CP does
not cause a significant threat to life, it can seriously affect the
quality of life of patients, particularly for patients with mental
health issues (8,9). However, the pathogenesis and
pathophysiological changes that cause CP remain unclear (10,11).
Currently, there are no effective drugs or methods to treat the
disease, and it is imperative to identify safe and cost-effective
treatments. A previous study reported that CP may be caused by
changes in hormone secretion levels and immunological dysfunction
(12).
Drinking is a habit closely associated with male
patients. Moderate drinking is able to promote blood circulation to
improve the function of the human body but a high degree of
drinking can affect human health. Alcohol can also increase the
severity of prostate congestion. In addition to the direct effects
of alcohol on the nervous system, the prostate is also very
sensitive to alcohol, and it is prone to prostate disease (13). Currently, there is limited research
on the effect of alcohol on prostatitis.
In the present study, it was attempted to
investigate the association of ethanol consumption and CP, where
rats with CP were treated with or without ethanol, to explore the
mechanism and role of alcohol CP (14). A chronic non-bacterial prostatitis
model was successfully established in rats. In conclusion, ethanol
increased the inflammatory responses in the prostate of rats with
non-bacterial prostatitis.
Materials and methods
Animals
Healthy male Sprague Dawley (SD) rats (n=24; age,
6–7 weeks; weight, 180–200 g), were purchased from the Animal
Experimental Center of Shanghai Jiaotong University (Shanghai,
China). Animal room temperature control was at 25°C, humidity
control was at 60%, and 12-h light cycle was used to simulate the
normal circadian physiology. Drinking water and standard food were
available to animals following sterilization. The establishment of
non-bacterial prostatitis model was performed as reported in a
previous study (15). The rats
were injected intraperitoneally with 3 mg/ml complete Freund's
adjuvant, 1mg purified prostaglandin and 0.1 ml DPT vaccine to
induce non-bacterial prostatitis in rats (16,17).
The Bioethics Committee of Shanghai Jiaotong University approved
all animal experiments, which were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf),
the Ethical Certificate number was SYXK (Shanghai) 2011–0128. Rats
were divided into four groups (6 rats/group): A (normal SD rats), B
(ethanol-drinking rats), C (chronic non-bacterial prostatitis rats)
and D (ethanol-drinking chronic non-bacterial prostatitis
rats).
Blood tests
Blood was collected from heart by cardiocentesis.
The white blood cells (WBC), and concentrations of IgG, serum
testosterone (serum T) and dihydrotestosterone (DHT) were analyzed
by Animal blood routine detector (Acon, Shanghai, China).
Preparation of rat prostate protein
purification solution
A total of 10 SD rats were sacrificed, the body
weight of each rat having been recorded. Following disinfection and
dissection, the prostate was exposed, removed and put into iced
saline. The weight of the prostate was recorded. The prostate index
was the weight of prostate/body weight. The tissue was cleansed and
diced, followed by homogenization. Samples were centrifuged for 30
min (20,000 × g) at 4°C. A suction tube was used to remove the
upper adipose tissue, and then 3 ml supernatant was placed in
cryopreservation storage. Bicinchoninic protein assay was used for
protein concentration quantification (Beyotime Institute of
Biotechnology, Haimen, China) and the protein was diluted to a
concentration of 50 mg/ml (18).
Hematoxylin and eosin (H&E)
staining
After the rats were sacrificed, a sample of prostate
was fixed in 4% formalin for 24 h then placed in 70–100% graded
ethanol series the subsequent day. The prostate was embedded in
paraffin followed by dehydration and was sectioned at 4 µm of
thickness. The prostate sections were deparaffinized and rehydrated
(100–70%), then stained with hematoxylin (15 min) and eosin (2 min)
at room temperature, and observed under an inverted microscope
(Nikon TS-100-F; Nikon Corporation, Tokyo, Japan) (19).
Measurement of inflammatory
factors
The prostate tissue of each group was immersed in a
physiological saline solution containing 0.5% Triton X-100 and
homogenized in a glass homogenizer on an ice-water bath. The
prostate tissue was equilibrated into 10% prostate tissue
homogenate and centrifuged at 300 × g for 15 min at 4°C. The
supernatant was collected according to the ELISA kit instructions
to measure tumor necrosis factor (TNF)-α (catalog number ab46070;
Abcam, Cambridge, UK), inducible nitric oxide synthase (iNOS)
(catalog number: MBS263618; MyBioSource, Inc., San Diego, CA, USA)
and total antioxidant capacity (T-AOC) kit (catalog number STA-360;
Cell Biolabs, Inc., San Diego, CA, USA).
Evaluation of prostatic
inflammation
Inflammatory scores (20) were given to the prostatic tissue of
rats in each group. The standard of evaluation was the ratio of the
area of the lesion to the total area (5 visual fields were randomly
selected from each slice). No lesion present was denoted as 0, 25%
was denoted as 1 point, 50% was denoted as 2 points, 75% was
denoted as 3 points and 100% was denoted as 4 points (21).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously described
(9), followed by the use of SYBR
Premix Ex Taq II kit (catalog number RR82LR; Takara Bio, Inc.,
Otsu, Japan). mRNA was extracted from the prostate tissue of rats
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). PCR was performed with the following thermocycling
conditions: 5 min at 95°C, followed by 40 cycles of 95°C for 30
sec, 55°C for 30 sec and 72°C for 30 sec, with a final holding step
at 4C. The thermocycler used in the present study was the
StepOnePlus™ Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primers were obtained from Funengbio
Co. (Shanghai, China). Primers sequences were as follows:
α1a-adrenoreceptor (AR), forward, TAGCCTFTCACCGACACCTG and reverse,
GGAGGTCGGCCACCG; α1b-AR, forward, CTCAACCCCATCATCTACCCA and
reverse, CTCAACCCCATCATCTACCCA; α1d-AR, forward, AGCGCTTCTGCGGTATCA
and reverse, CAGGTAGAAGGAGCACACGG; GAPDH, forward,
TCTAGACGGCAGGTCAGGTCCAC and reverse, CCACCCATGGCAAATTCCATGGCA.
RT-qPCR was performed using an Applied Biosystems 7500 real-time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The results were analyzed using Light Cycler Software version 3.5
(Idaho Technology Inc., Salt Lake City, UT, USA). The
2−ΔΔCq method was performed to calculate the relative
expression (22).
Statistical analysis
The data are presented as the mean ± standard
deviation and analyzed using GraphPad prim 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA). The statistical significance was
determined by one-way analysis of variance followed by the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Body weight in each group of SD
rats
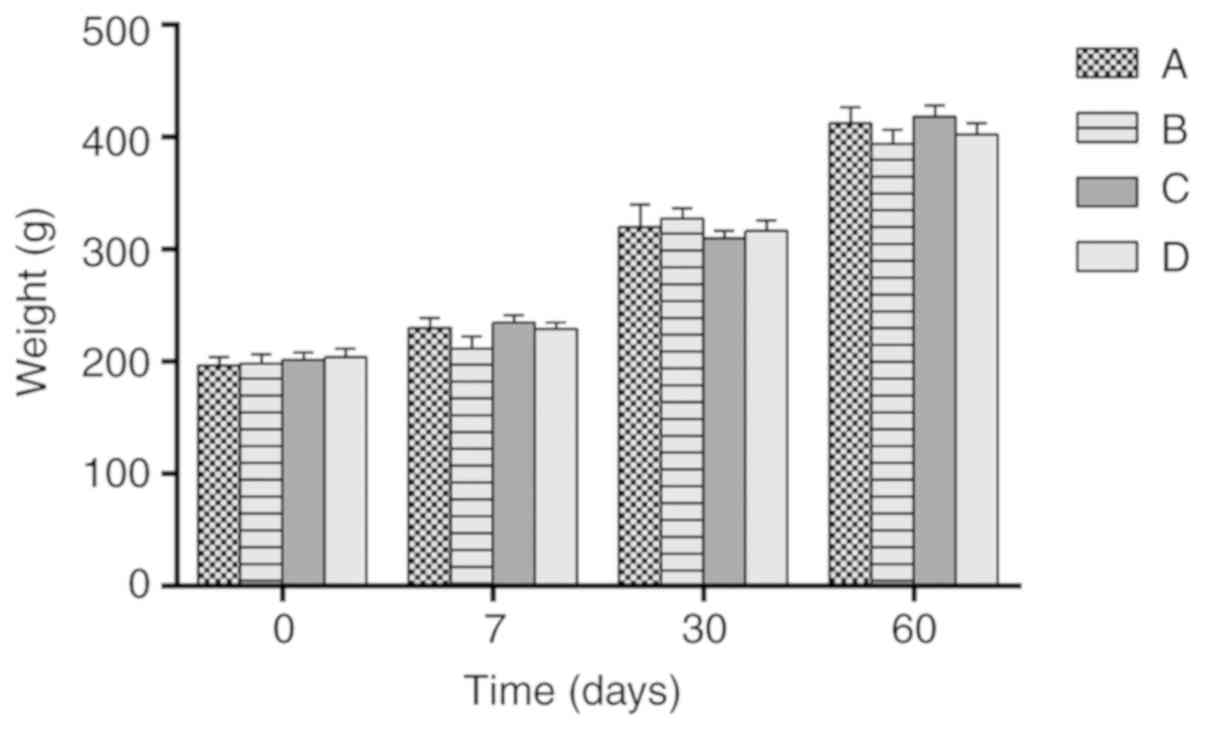
As shown in Fig. 1,
there were no significant differences in body weight among the
groups.
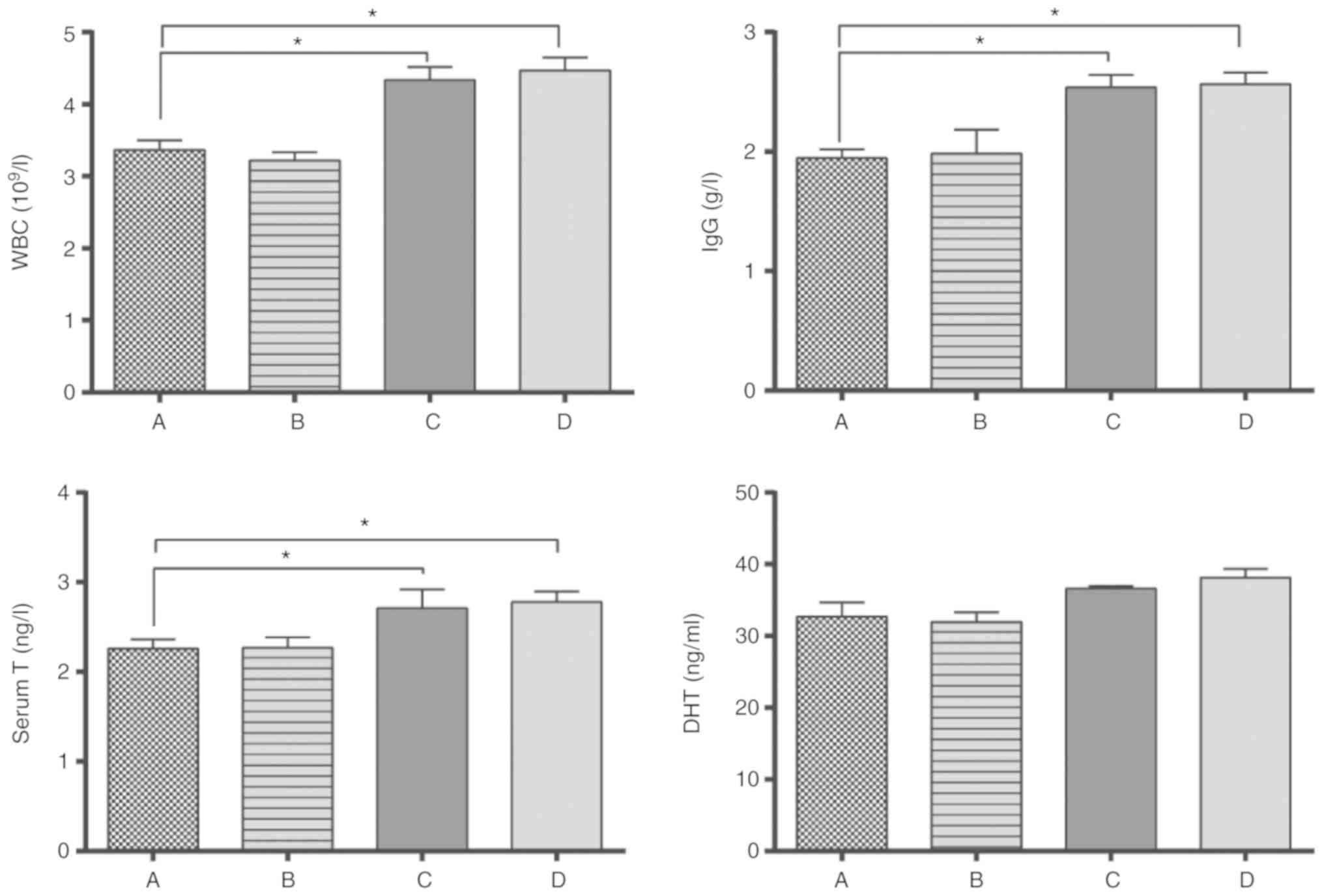
Blood tests were performed on all
rats
Compared with the normal control group A, there was
no significant difference in the blood parameters of group B
(P>0.05; Fig. 2).
There were significant differences in blood
parameters between groups C, D and A, B. The total count of white
blood cells (WBC), and concentrations of IgG, serum testosterone
(serum T) in group C and Group D were higher compared with Group A
and Group B (P<0.05). No difference in the expression of DHT,
important for development and maintenance of the prostate gland and
seminal vesicles, was observed among the 4 groups.
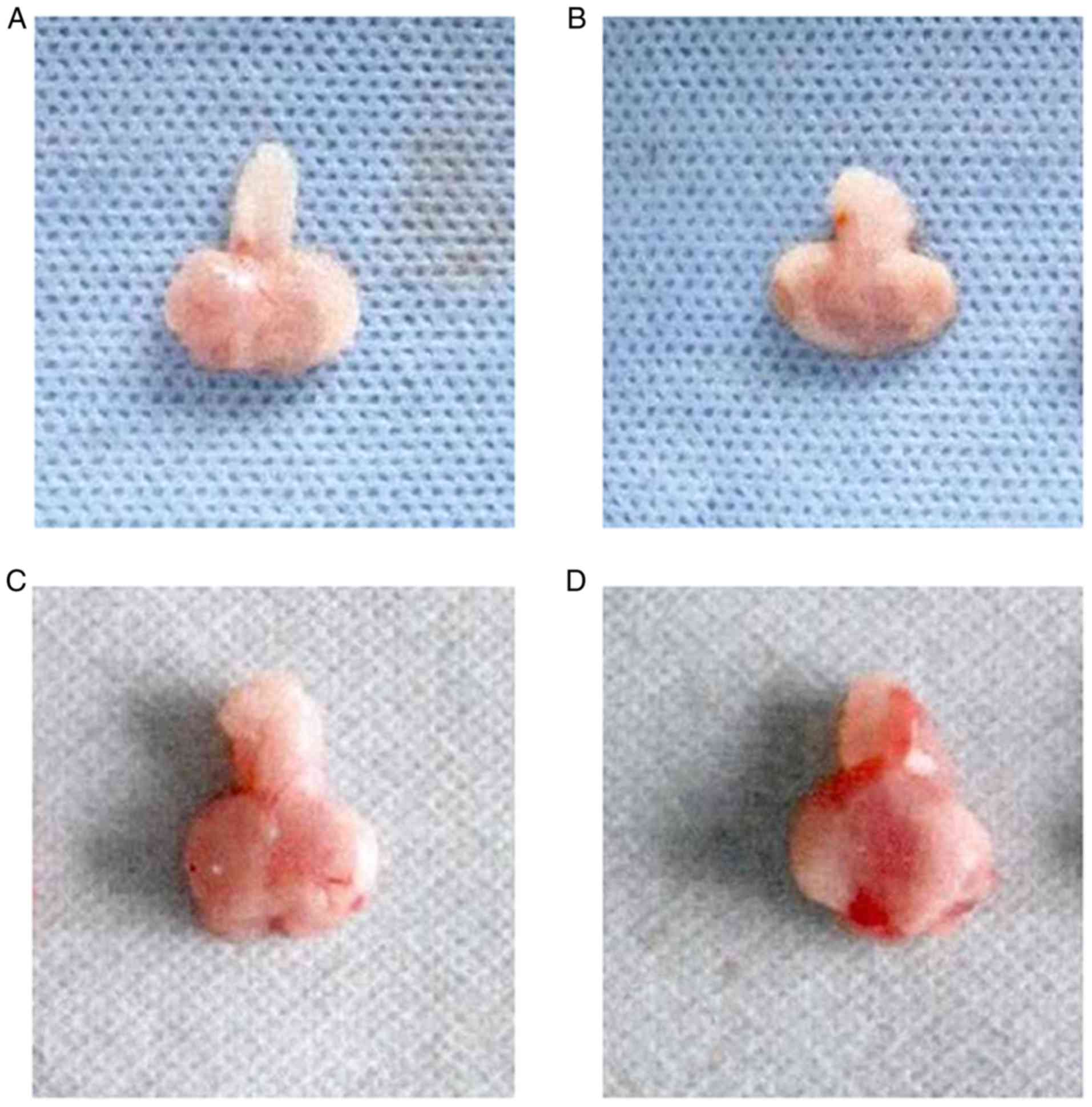
Prostate morphology in each group of
rats
There was no difference in the morphology of the
prostate between groups A and B. The surface of the organ was
smooth, the capsules were intact without edema, there were no
glandular congestions and prostatic fluids were clear (Fig. 3A and B). In groups C and D,
prostates exhibited edema and adhesions, had enlarged volume and
local congestion of glands. The congestion and volume of the
prostate in group D was higher than in group C (Fig. 3C and D).
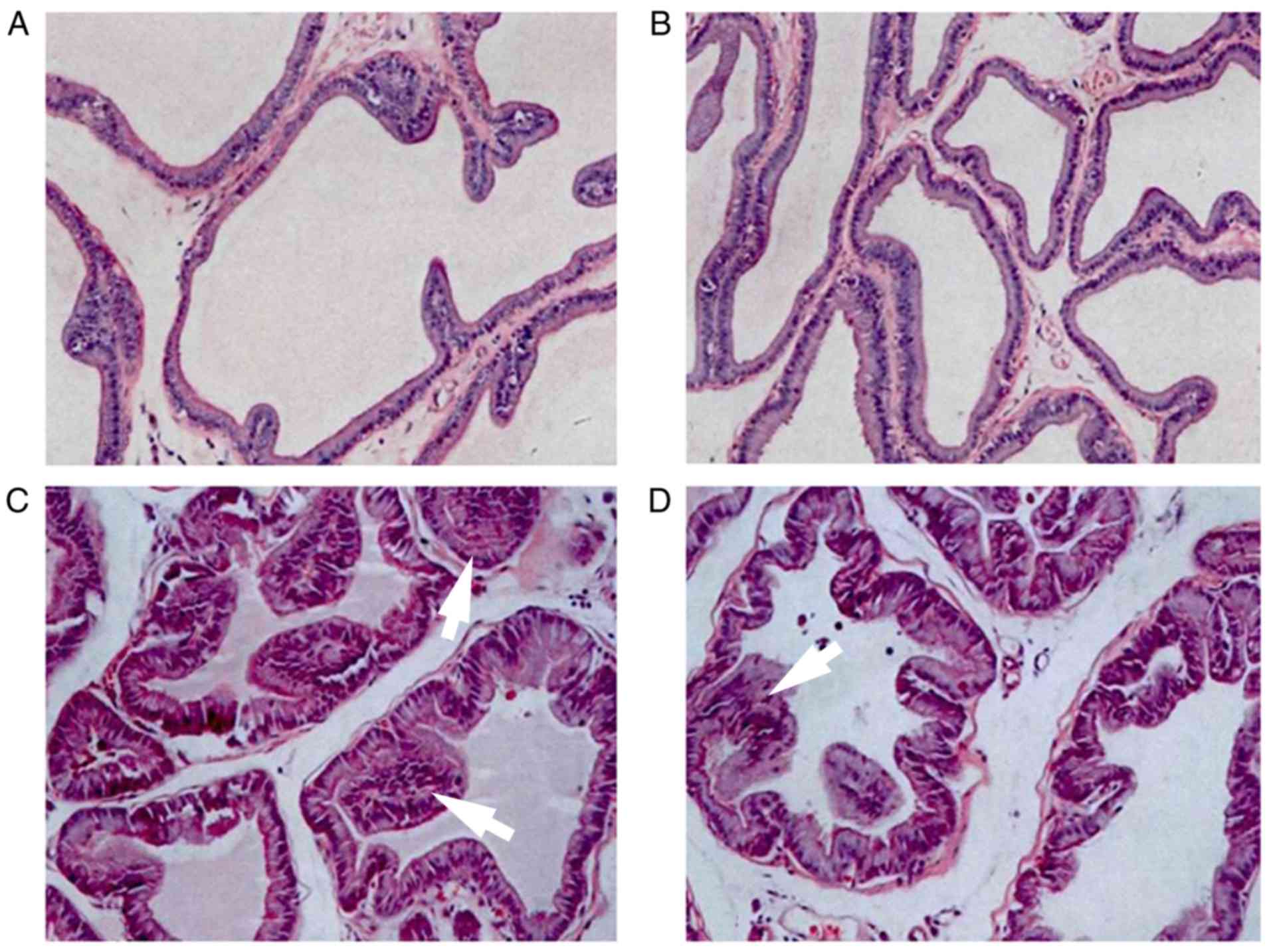
Pathological examination of the
prostate
H&E staining indicated that the cells in groups
A and B sections were neatly arranged, with no obvious infiltration
of inflammatory cells. The epithelial cells were arranged in rows
as either cubic or columnar epithelium (Fig. 4A and B). In group C, there was
hyperplasia of the prostate epithelium, infiltration of
interstitial inflammatory cells, and a certain degree of
interstitial edema and enlargement. Group D exhibited infiltration
of inflammatory cells, while the expansion of the prostate capsule
and interstitial blood vessels was marked and accompanied by
glandular injury (Fig. 4C and
D).
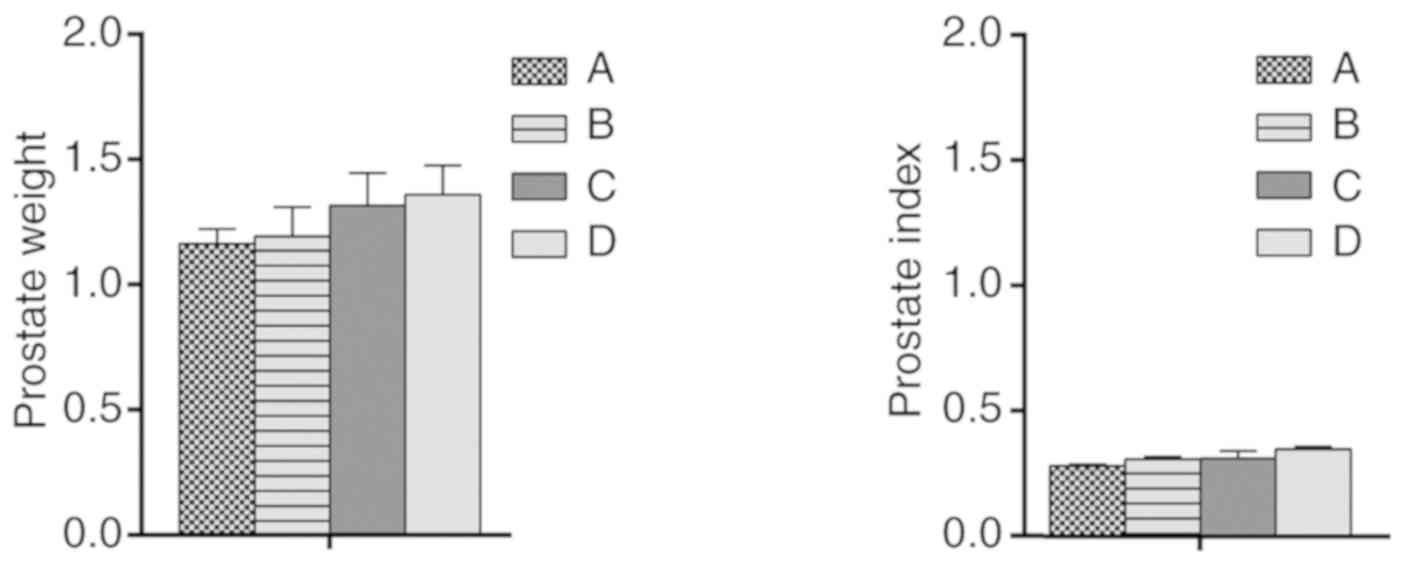
Wet weight and index of the
prostate
There were no significant differences in prostate
wet weight and prostate index among the groups (Fig. 5).
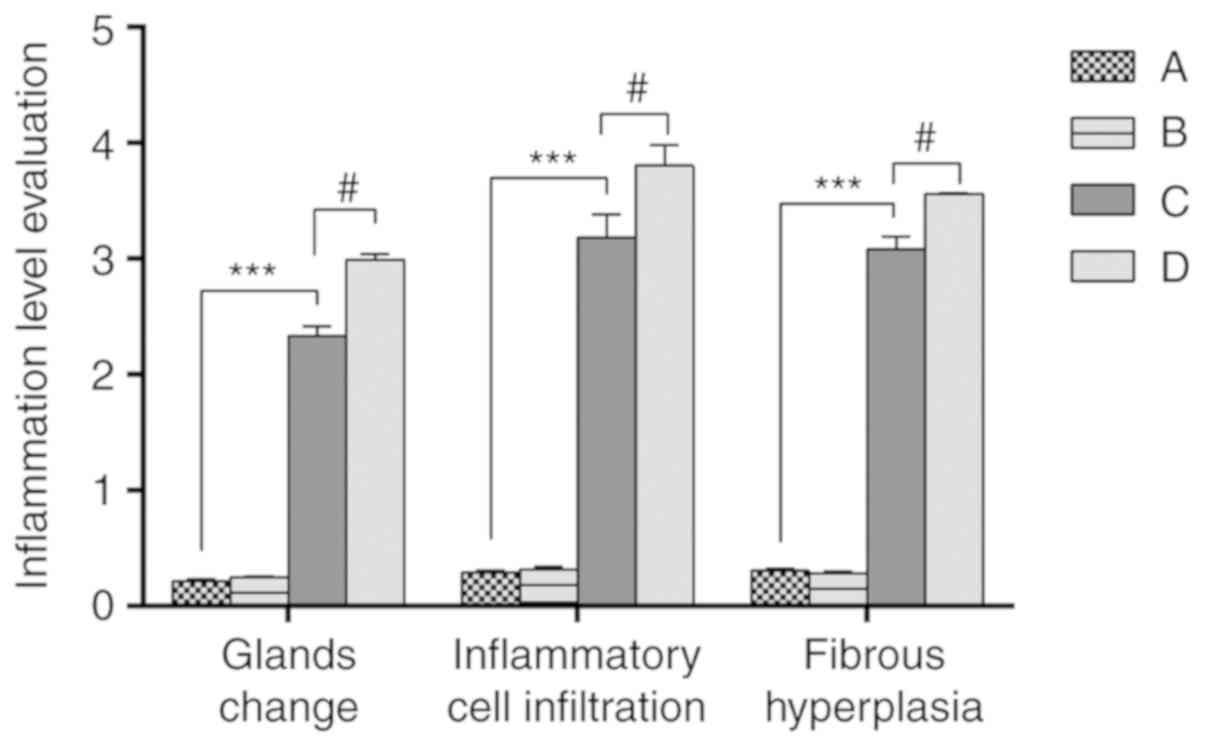
Evaluation of prostatic
inflammation
Inflammatory scores (20) were given to the prostatic tissue of
rats in each group (Fig. 6). The
results demonstrated that there was no significant difference in
inflammation between groups A and B. The inflammation scores of
groups C and D were significantly higher than those of group A, and
the inflammation scores of group D were significantly higher than
that of group C.
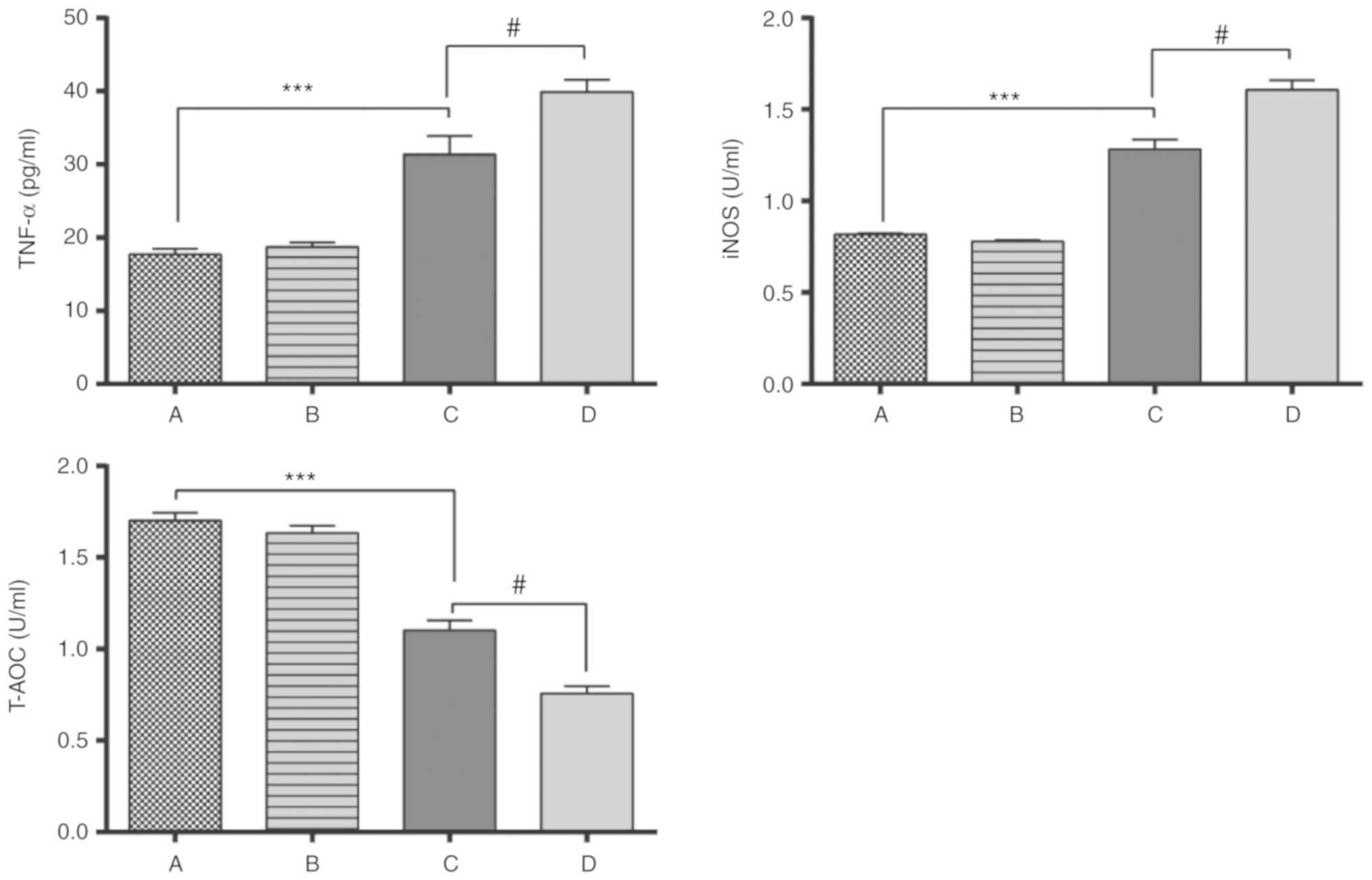
Expression of inflammatory factors in
prostate
The expression of TNF-α and iNOS in groups C and D
were significantly higher than those in groups A and B. The T-AOC
levels in groups C and D was significantly lower than that in group
A, while the expression of T-AOC in group D was significantly lower
than that in group C (P<0.05; Fig.
7), which indicated that the total antioxidant capacity of
group C was higher compared with group D.
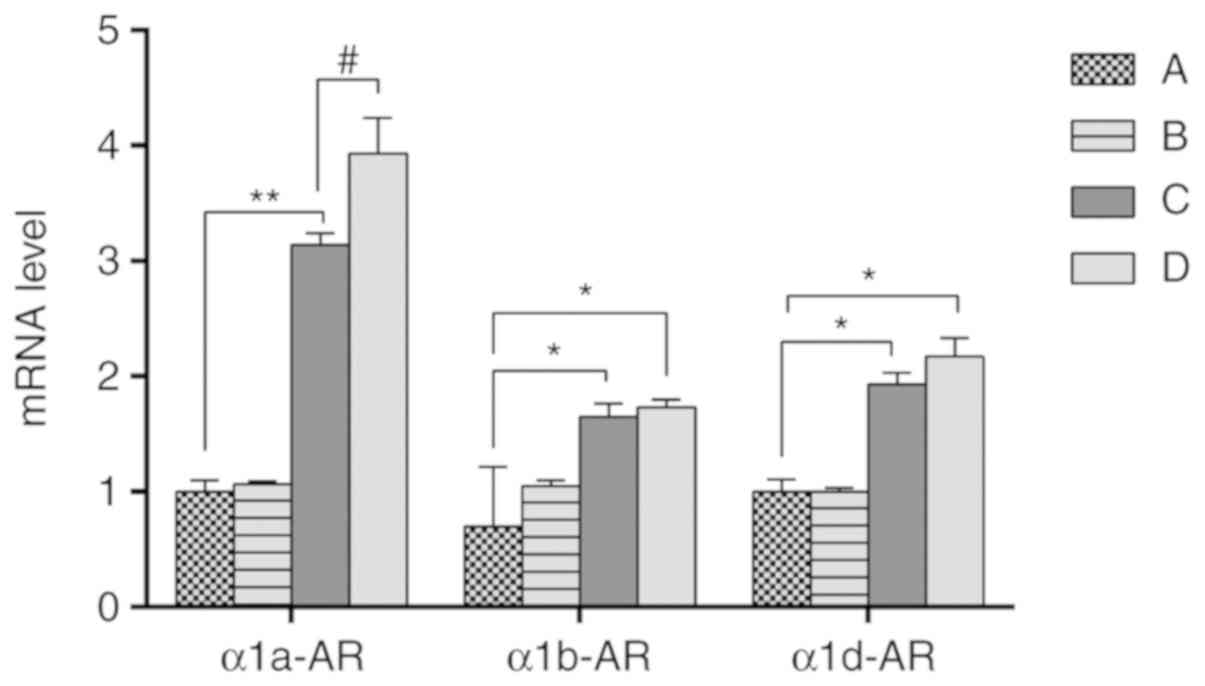
mRNA expression levels of α1a-AR,
α1b-AR and α1d-AR in the prostate
The mRNA expression levels of α1a-AR, α1b-AR, and
α1d-AR in the prostate of each group were detected by RT-qPCR
(Fig. 8). The results demonstrated
that there was no significant difference in mRNA expression of
α1a-AR, α1b-AR, and α1d-AR between groups A and B. While the
expression of α1a-AR, α1b-AR, and α1d-AR in group C and group D
were higher compared with group A and group B. Among the expression
of α1a-AR, α1b-AR, and α1d-AR, the fold change of α1a-AR was most
significant, which indicated that the expression of α1a-AR may be
the most important subtype among the 3 genes for inflammation.
Discussion
In the present study, SD rats were injected
intraperitoneally with purified prostaglandin with double
immunoadjuvant to induce non-bacterial prostatitis in rats
(16,17). This rat model is a good model for
analyzing the effects of ethanol (23,24).
The weight of the rats did not significantly differ among the
groups, indicating that the body weight of the rats was stable
during the experiment. However, internal changes were observed. The
number of WBC, the concentration of serum T and the concentration
of IgG in groups C and D were increased compared with the control
(group A).
Additionally, when the prostates of ethanol-exposed
rats with prostatitis were removed, the prostates were damaged, and
enlarged with local congestion of glands and edema. H&E
staining revealed that blood vessels were outstretched and glands
were damaged in group D. These results indicated that there was
inflammation in rats treated with ethanol. Significant glandular
changes, severe inflammatory infiltration and hyperplasia of
fibrous tissue was observed in the prostates of group D. The
expression of TNF-α, iNOS in group D rats were the highest, while
the expression of T-AOC was much lower in group D group compared
with group A. To further analyze inflammation levels, the mRNA
expression levels of α1a-AR, α1b-AR, and α1d-AR were investigated
via RT-qPCR (25,26). α1a-AR expression was highest in
groups D, which indicated that α1a-AR may be the most important
mRNA subtype in these rats.
In the present study, the association between
ethanol and non-bacterial prostatitis in rats was explored. The
findings indicated that ethanol may accelerate the inflammatory
response in arts with prostatitis, which may provide some useful
information for clinical research.
However, this study has limitations. For example,
the detailed signaling pathways involved remain unclear, and
potential target molecules that could be exploited to prevent
non-bacterial prostatitis also need to be identified. Future
studies should investigate the underlying mechanism and target
molecules involved in non-bacterial prostatitis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Youth Fund of
Shanghai Municipal Commission of Health and Family Planning (grant
no. 2011-Y132). Clinical research and cultivation fund of Renji
Hospital (grant no. PYXJS16-009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY designed this study. FL and XX performed all of
the experiments. LL helped to collect the data. ZW produced the
figures. LC helped with the analysis and interpretation of data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Bioethics Committee of Shanghai Jiaotong
University approved all animal experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rehm J, Samokhvalov AV and Shield KD:
Global burden of alcoholic liver diseases. J Hepatol. 59:160–168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JH, Friso S and Choi SW: Epigenetic
mechanisms underlying the link between non-alcoholic fatty liver
diseases and nutrition. Nutrients. 6:3303–3325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YJ, Song GH and Liu GT: Investigation
of the effect of traditional Chinese medicine on pain and
inflammation in chronic nonbacterial prostatitis in rats.
Andrologia. 48:714–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao HF, Li X and Jiang XZ: Heat shock
protein 9-mediated inflammation reaction in patients with chronic
prostatitis with erectile dysfunction. Eur Rev Med Pharmacol Sci.
20:4185–4189. 2016.PubMed/NCBI
|
|
5
|
Testino G: Alcoholic diseases in
hepato-gastroenterology: A point of view. Hepatogastroenterology.
55:371–377. 2008.PubMed/NCBI
|
|
6
|
Zhang DF, Zhang F, Zhang J, Zhang RM and
Li R: Protection effect of trigonelline on liver of rats with
non-alcoholic fatty liver diseases. Asian Pac J Trop Med.
8:651–654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson RU, Wise D, Sawyer T, Glowe P and
Orenberg EK: 6-day intensive treatment protocol for refractory
chronic prostatitis/chronic pelvic pain syndrome using myofascial
release and paradoxical relaxation training. J Urol. 185:1294–1299.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahin S, Bicer M, Eren GA, Tas S, Tugcu V,
Tasci AI and Cek M: Acupuncture relieves symptoms in chronic
prostatitis/chronic pelvic pain syndrome: A randomized,
sham-controlled trial. Prostate Cancer Prostatic Dis. 18:249–254.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zibara K, Awada Z, Dib L, El-Saghir J,
Al-Ghadban S, Ibrik A, El-Zein N and El-Sabban M: Anti-angiogenesis
therapy and gap junction inhibition reduce MDA-MB-231 breast cancer
cell invasion and metastasis in vitro and in vivo. Sci Rep.
5:125982015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamura H, Koie T, Soma O, Matsumoto T,
Imai A, Hatakeyama S, Yoneyama T, Hashimoto Y and Ohyama C:
Eviprostat has an identical effect compared to pollen extract
(Cernilton) in patients with chronic prostatitis/chronic pelvic
pain syndrome: A randomized, prospective study. BMC Urol.
15:1202015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin JX, Wang HZ, Zhai ZX, Ma BL, Li QF,
Xiao N, Wang ZP and Rodriguez R: Transrectal microwave
thermotherapy causing a short-time influence on sperm quality in
Chinese chronic nonbacterial prostatitis patients. Asian J Androl.
19:548–553. 2016.
|
|
12
|
Krsmanovic A, Tripp DA, Nickel JC, Shoskes
DA, Pontari M, Litwin MS and McNaughton-Collins MF: Psychosocial
mechanisms of the pain and quality of life relationship for chronic
prostatitis/chronic pelvic pain syndrome (CP/CPPS). Can Urol Assoc
J. 8:403–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zijoo R, Dirweesh A, Ordonez FM and Kaji
A: Spontaneous Rupture of Urinary Bladder in a Young Alcoholic
Male. J Med Cases. 7:245–247. 2016. View Article : Google Scholar
|
|
14
|
Yatkin E, Bernoulli J, Lammintausta R and
Santti R: Fispemifene
(Z-2-{2-(4-(4-chloro-1,2-diphenylbut-1-enyl)-phenoxy)ethoxy}-ethanol),
a novel selective estrogen receptor modulator, attenuates glandular
inflammation in an animal model of chronic nonbacterial
prostatitis. J Pharmacol Exp Ther. 327:58–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keetch DW, Humphrey P and Ratliff TL:
Development of a mouse model for nonbacterial prostatitis. J Urol.
152:247–250. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui D, Han G, Shang Y, Mu L, Long Q and Du
Y: The effect of chronic prostatitis on zinc concentration of
prostatic fluid and seminal plasma: A systematic review and
meta-analysis. Curr Med Res Opin. 31:1763–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee G: Chronic Prostatitis: A possible
cause of hematospermia. World J Mens Health. 33:103–108. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magistro G, Wagenlehner FM, Grabe M,
Weidner W, Stief CG and Nickel JC: Contemporary management of
chronic prostatitis/chronic pelvic pain syndrome. Eur Urol.
69:286–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai C, Yu X, Zhuo H, Zhou N, Xie Y, He J,
Peng Y, Xie X, Luo G, Zhou S, et al: Anti-tumor immune response of
folate-conjugated chitosan nanoparticles containing the IP-10 gene
in mice with hepatocellular carcinoma. J Biomed Nanotechnol.
10:3576–3589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zdrodowska-Stefanow B,
Ostaszewska-Puchalska I, Badyda J and Galewska Z: The evaluation of
markers of prostatic inflammation and function of the prostate
gland in patients with chronic prostatitis. Arch Immunol Ther Exp
(Warsz). 56:277–282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delongchamps NB, de la Roza G, Chandan V,
Jones R, Sunheimer R, Threatte G, Jumbelic M and Haas GP:
Evaluation of prostatitis in autopsied prostates-is chronic
inflammation more associated with benign prostatic hyperplasia or
cancer? J Urol. 179:1736–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kutch JJ, Yani MS, Asavasopon S, Kirages
DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C,
Farmer MA, et al: Altered resting state neuromotor connectivity in
men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP:
Research Network Neuroimaging Study. Neuroimage Clin. 8:493–502.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thakur V, Talwar M and Singh PP: Low free
to total PSA ratio is not a good discriminator of chronic
prostatitis and prostate cancer: An Indian experience. Indian J
Cancer. 51:335–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giannantoni A and Proietti S: Chronic
prostatitis: How to give our best without apposite vagueness. BJU
Int. 116:499–500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zheng T, Tu X, Chen X, Wang Z,
Chen S, Yang Q, Wan Z, Han D, Xiao H, et al: Erectile dysfunction
in chronic prostatitis/chronic pelvic pain syndrome: Outcomes from
a multi-center study and risk factor analysis in a single center.
PLoS One. 11:e01530542016. View Article : Google Scholar : PubMed/NCBI
|