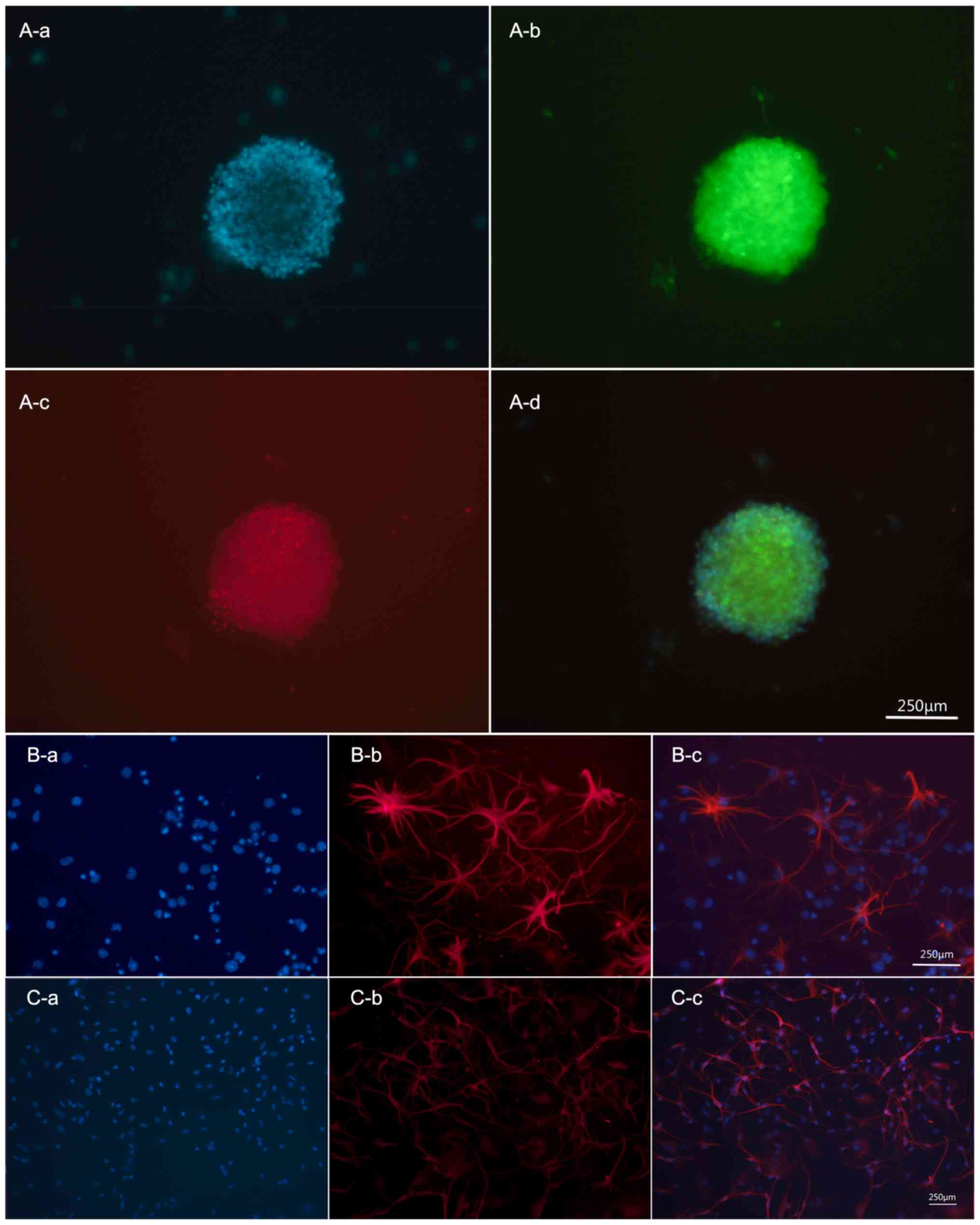
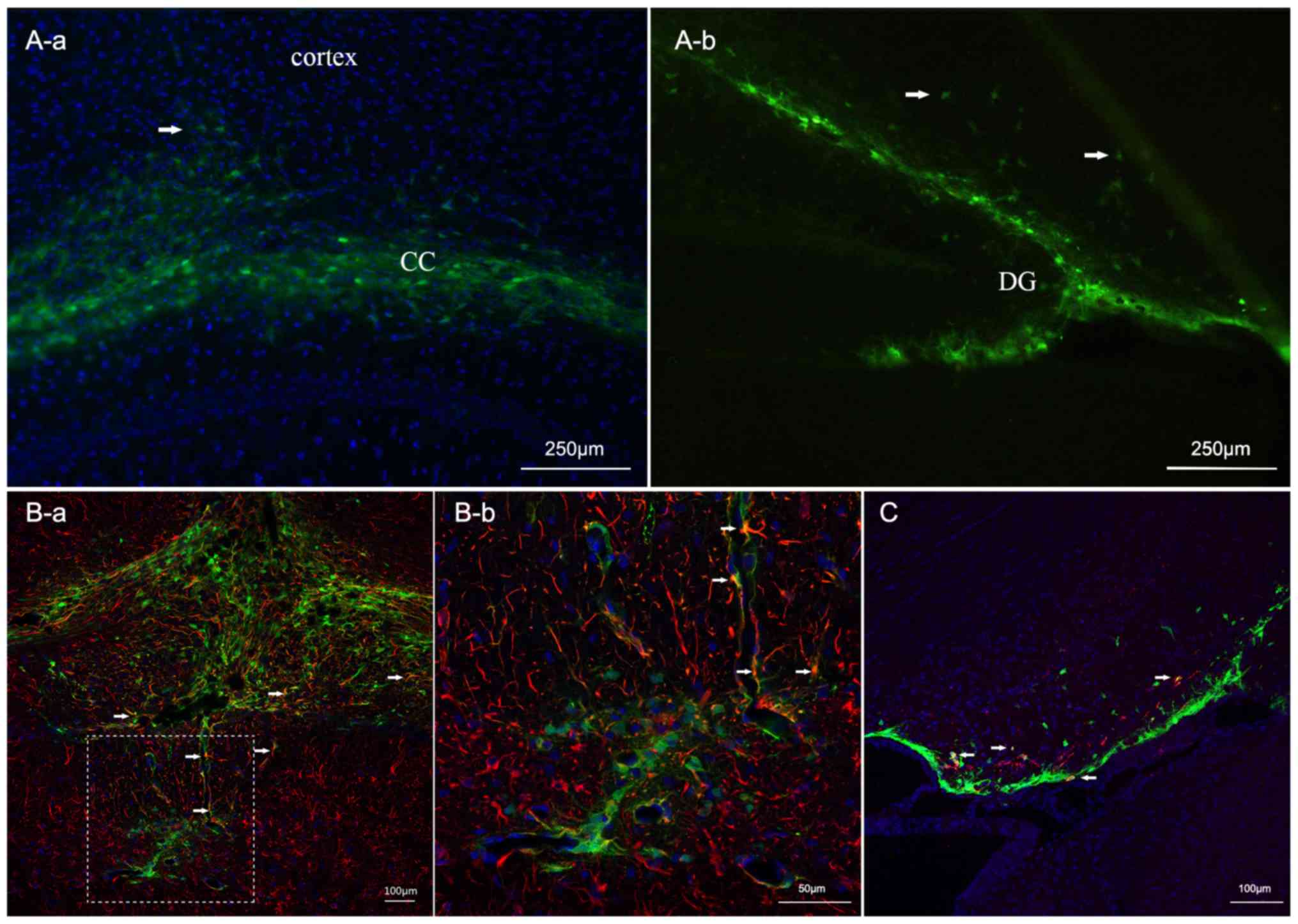
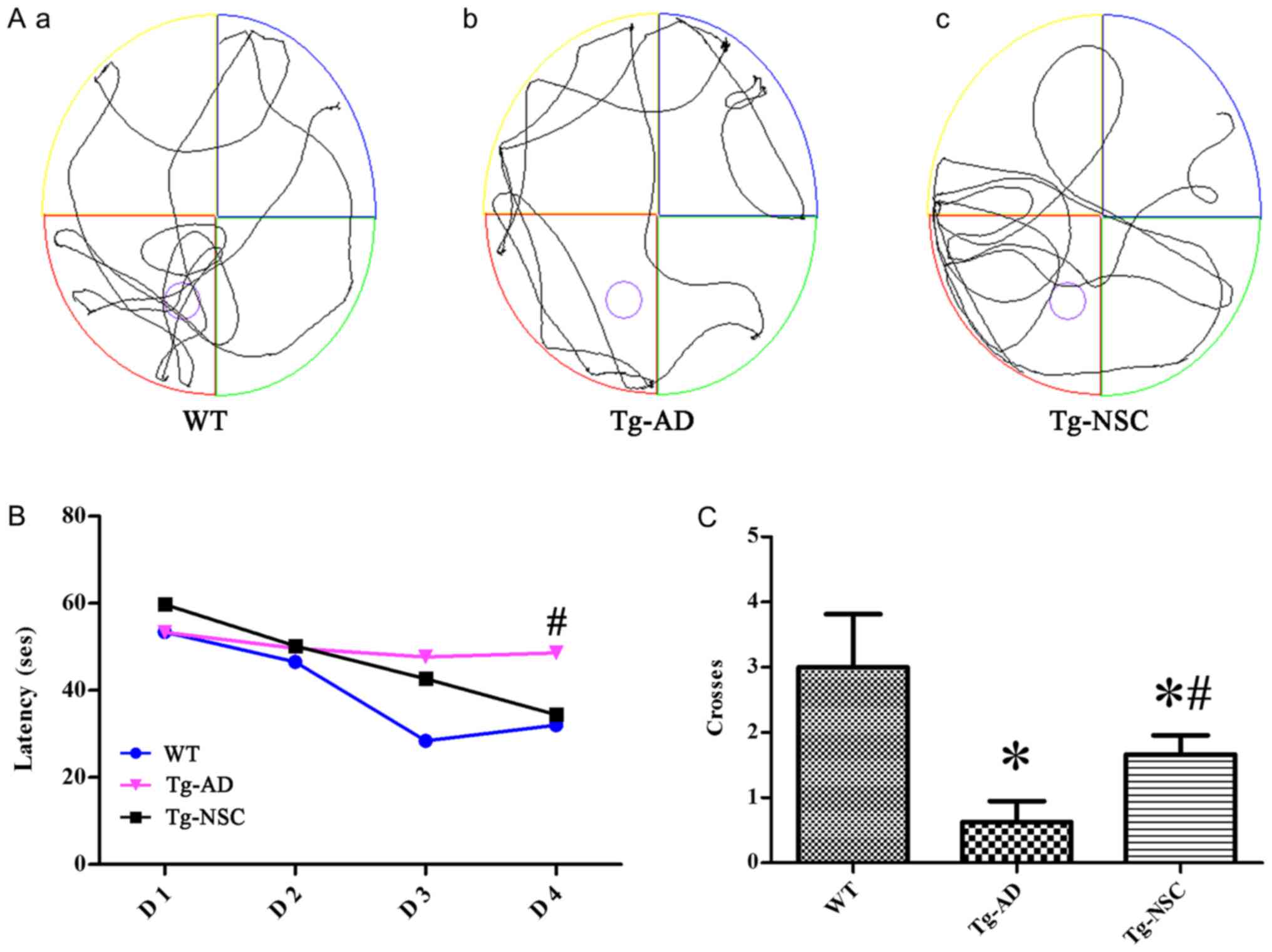
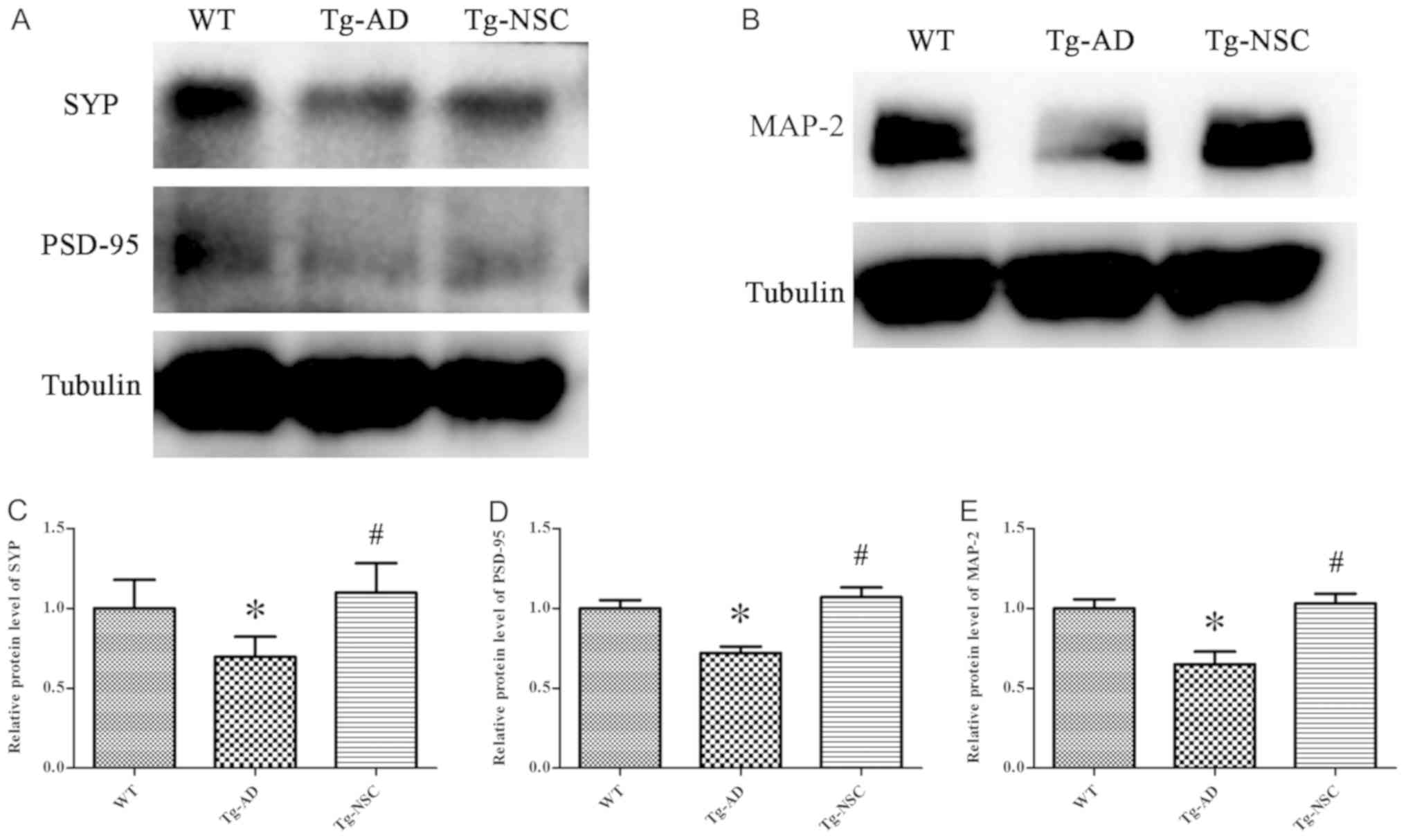
|
1
|
Anand R, Gill KD and Mahdi AA:
Therapeutics of Alzheimer's disease: Past, present and future.
Neuropharmacology. 76:27–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cattaneo A and Calissano P: Nerve growth
factor and Alzheimer's disease: New facts for an old hypothesis.
Mol Neurobiol. 46:588–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Small G and Bullock R: Defining optimal
treatment with cholinesterase inhibitors in Alzheimer's disease.
Alzheimers Dement. 7:177–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teng YD: Functional multipotency of stem
cells: Biological traits gleaned from neural progeny studies. Semin
Cell Dev Biol. pii:S1084–S9521. 2019.(Epub ahead of print).
|
|
5
|
Li XY, Bao XJ and Wang RZ: Potential of
neural stem cell-based therapies for Alzheimer's disease. J
Neurosci Res. 93:1313–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alipour M, Nabavi SM, Arab L, Vosough M,
Pakdaman H, Ehsani E and Shahpasand K: Stem cell therapy in
Alzheimer's disease: Possible benefits and limiting drawbacks. Mol
Biol Rep. 46:1425–1446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blurton-Jones M, Spencer B, Michael S,
Castello NA, Agazaryan AA, Davis JL, Müller FJ, Loring JF, Masliah
E and LaFerla FM: Neural stem cells genetically-modified to express
neprilysin reduce pathology in Alzheimer transgenic models. Stem
Cell Res Ther. 5:462014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Gu GJ, Zhang Q, Liu JH, Zhang B,
Guo Y, Wang MY, Gong QY and Xu JR: NSCs promote hippocampal
neurogenesis, metabolic changes and synaptogenesis in APP/PS1
transgenic mice. Hippocampus. 27:1250–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marsh SE and Blurton-Jones M: Neural stem
cell therapy for neurodegenerative disorders: The role of
neurotrophic support. Neurochem Int. 106:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Gu GJ, Shen X, Zhang Q, Wang GM
and Wang PJ: Neural stem cell transplantation enhances
mitochondrial biogenesis in a transgenic mouse model of Alzheimer's
disease-like pathology. Neurobiol Aging. 36:1282–1292. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang J: How close is the stem cell cure to
the Alzheimer's disease: Future and beyond? Neural Regen Res.
7:66–71. 2012.PubMed/NCBI
|
|
12
|
Whitehouse PJ, Price DL, Struble RG, Clark
AW, Coyle JT and Delon MR: Alzheimer's disease and senile dementia:
Loss of neurons in the basal forebrain. Science. 215:1237–1239.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller C and Remy S: Septo-hippocampal
interaction. Cell Tissue Res. 373:565–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitt U, Tanimoto N, Seeliger M,
Schaeffel F and Leube RE: Detection of behavioral alterations and
learning deficits in mice lacking synaptophysin. Neuroscience.
162:234–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dehmelt L and Halpain S: The MAP2/Tau
family of microtubule-associated proteins. Genome Biol. 6:2042005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shankar GM, Li S, Mehta TH, Garcia-Munoz
A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere
CA, et al: Amyloid-beta protein dimers isolated directly from
Alzheimer's brains impair synaptic plasticity and memory. Nat Med.
14:837–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu S, Okamoto S, Lipton SA and Xu H:
Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.
Mol Neurodegener. 9:482014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hampel H, Mesulam MM, Cuello AC, Farlow
MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo
E, Snyder PJ and Khachaturian ZS: The cholinergic system in the
pathophysiology and treatment of Alzheimer's disease. Brain.
141:1917–1933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D,
Choi J, Callaway EM and Xu X: Cell-type-specific circuit
connectivity of hippocampal CA1 revealed through Cre-dependent
rabies tracing. Cell Rep. 7:269–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Pan C, Xuan A, Xu L, Bao G, Liu F,
Fang J and Long D: Treatment efficacy of NGF nanoparticles
combining neural stem cell transplantation on Alzheimer's disease
model rats. Med Sci Monit. 21:3608–3615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mufson EJ, Counts SE, Fahnestock M and
Ginsberg SD: Cholinotrophic molecular substrates of mild cognitive
impairment in the elderly. Curr Alzheimer Res. 4:340–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu P, Jones LL, Snyder EY and Tuszynski
MH: Neural stem cells constitutively secrete neurotrophic factors
and promote extensive host axonal growth after spinal cord injury.
Exp Neurol. 181:115–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Gao Y, Zhang W and Xu JR: Regulation
and effects of neurotrophic factors after neural stem cell
transplantation in a transgenic mouse model of Alzheimer disease. J
Neurosci Res. 96:828–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conner JM, Franks KM, Titterness AK,
Russell K, Merrill DA, Christie BR, Sejnowski TJ and Tuszynski MH:
NGF is essential for hippocampal plasticity and learning. J
Neurosci. 29:10883–10889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mufson EJ, Counts SE, Perez SE and
Ginsberg SD: Cholinergic system during the progression of
Alzheimer's disease: Therapeutic implications. Expert Rev
Neurother. 8:1703–1718. 2008. View Article : Google Scholar : PubMed/NCBI
|