Introduction
Alzheimer's disease (AD) is the most common
neurodegenerative disorder, affecting over 5 million people in the
U.S. alone (Alzheimer's Association, 2016). It has become
increasingly clear that AD is associated with multiple causes. In
addition to the deposition of β-amyloid (Aβ) proteins and
neurofibrillary tangles, inflammation and oxidative stresses,
metabolic disorders, impaired calcium ion channels, mitochondrial
dysfunction and the lack of neurotrophic factors are also linked
with its pathomechanism (1).
Collectively, these pathologies ultimately lead to the loss of
cholinergic neurons and synapses (2). Despite decades of research, there is
no effective treatment to cure AD (3).
In recent decades, significant progress has been
made in the treatment of neurodegenerative diseases by neural stem
cell-based therapy. Neural stem cells (NSCs) have a strong
potential of self-renewal and multi-differentiation. Furthermore,
they can differentiate into neurons, astrocytes and
oligodendrocytes (4). NSC
transplantation may be an effective method to cure
neurodegenerative diseases by repairing and replenishing functional
neurons (5,6). Several studies have revealed that
transplanting NSC can alleviate the learning and memory impairment
in an AD mouse model (7,8). It has been revealed that transplanted
NSC survived, migrated, and differentiated into neurons (only a
small amount). In addition, NSC can secrete neurotrophic factors
(9), inhibit inflammation, enhance
mitochondrial functions (10) and
even promote the activation of endogenous NSCs (11). Unfortunately, little is known about
the influence of NSC transplantation on cholinergic neurons in the
basal forebrain of APP/PS1 transgenic mice.
Cholinergic neurons are closely related to cognition
and memory functions. In AD, the loss of cholinergic neurons in the
basal forebrain leads to a decrease in the number of cholinergic
fibers in the hippocampus and the neocortex (12). Nevertheless, synaptic loss is the
principal correlate of disease progression and loss of cholinergic
neurons thus causing cognitive deficits (13). However, limited literature has
reported the association between neural stem cell transplantation
and basal forebrain cholinergic neurons. In the present study,
enhanced green fluorescent protein (EGFP)-labeled NSCs were
bilaterally transplanted into the hippocampus of 12-month-old
APP/PS1 transgenic mice and age-matched wild-type (WT) mice, the
effects of neural stem cell transplantation on basal forebrain
cholinergic neurons and the recovery of synaptic impairment and its
relationship with cognitive functions were investigated.
Materials and methods
Materials
The following reagents were used in the present
study: Dulbecco's modified Eagle's medium/F-12 (1:1) and fetal
bovine serum (FBS) were purchased from HyClone; GE Healthcare Life
Sciences; B27 supplements and Accutase were obtained from Gibco;
Thermo Fisher Scientific; epidermal growth factor (EGF) and basic
fibroblast growth factor (b-FGF) were obtained from PeproTech,
Inc.; nestin (cat. no. ab6142), β-tubulin III (cat. no. ab78078),
GFAP (cat. no. ab10062), and choline acetyl transferase (ChAT)
antibodies (cat. no. ab6168) were purchased from Abcam;
postsynaptic density protein 95 (PSD95) (cat. no. 3450),
synaptophysin (SYP) (cat. no. 5461), MAP2 (cat. no. 4542), and
doublecortin (DCX) (cat. no. 4604) antibodies were obtained from
Cell Signaling Technology, Inc.; Alexa Fluor™ 568 goat anti-mouse
IgG (cat. no. A-11004) and Alexa Fluor 594 donkey anti-rabbit IgG
(cat. no. A32754) were purchased from Thermo Fisher Scientific,
Inc.; ChAT antibody (cat. no. DF6964) and HRP-conjugated goat
anti-rabbit IgG (cat. no. S0001) were obtained from Affinity
Biologicals, Inc.
Animals
In total, 24 APP/PS1 (APPswe, PSEN1dE9) double
transgenic mice (age, 5 months; weight, 25–35 g) were obtained from
the Experimental Animal Center (Guangdong, China) (certificate no.
44007200038817), and were then maintained in our laboratory (Animal
experiment center, Guangzhou Medical University) for another 7
months. In total, three EGFP-labeled mice (age, 50 days; weight,
15–20 g) were provided by the Nanjing Biomedical Research Institute
[certificate no. SCXK (SU) 2015-0001]. When the mice suffered
trauma, infection and weight loss (>20%), they were not suitable
for the experiment and were euthanized by cervical dislocation.
Embryos (n=8–10) of 12.5–14.5 days were used in this study. All
animals were kept in separate cages at room temperature (~22–24°C)
with a 12-h light/dark cycle and free access to water and food. All
procedures were approved by the Animal Ethics Committee of the
Second Affiliated Hospital of Guangzhou Medical University
(certification no. A2018-016).
Preparation of NSCs
NSCs were derived from the embryonic brain
(E12.5-14.5 days) of pregnant EGFP-labeled mice. The pregnant mice
were fixed in supine position, the abdomen was sterilized twice
with iodine, then cut with sterile scissors, and the entire uterus
was removed and placed in alcohol. The mouse embryo was washed
twice in ethyl alcohol prior to placing it in D-Hank's balanced
salt solution. The fetal mouse brain was separated from septal
areas, the cerebellum and olfactory bulb were excised, the meninges
and blood vessels were stripped, and then washed in cold D-Hank's
balanced salt solution. After dissection and digestion, samples
were re-suspended in Dulbecco's modified Eagle's medium/F-12 (1:1)
(HyClone; GE Healthcare Life Sciences) medium containing 20 ng/ml
of epidermal growth factor (EGF), 20 ng/ml of basic fibroblast
growth factor (b-FGF; both from PeproTech, Inc.) and 2% B27 (Gibco;
Thermo Fisher Scientific, Inc.). Cells (1×105/ml) were
inoculated into 25-ml culture flasks and maintained at 37°C in an
incubator with 5% CO2 and 95% humidity.
To assess the differentiation ability of NSCs, P2
neurospheres were dissociated with Accutase (Gibco; Thermo Fisher
Scientific, Inc.) and inoculated into 24-well plates pre-coated
with polylysine (Sangon Biotech, Inc.). When cells adhered closely
to the plate, one plate was used to assess stem cell properties.
The other was used to investigate the differentiation ability after
5 days with fetal bovine serum (FBS) co-cultured by cell
immunofluorescence staining. In short, cells were rinsed three
times (5 min each time) in phosphate-buffered saline (PBS) solution
prior to incubation in 0.3% Triton X-100 to destabilize cell
membranes. Then, the cells were incubated in 5% bovine serum
albumin (BSA) at room temperature for 2 h. Mouse anti-nestin
antibody, mouse anti-β-tubulin III antibody, mouse anti-GFAP
antibody (all 1:300, Abcam) were applied and incubated overnight at
4°C. After washing the primary antibodies, Alexa Fluor 568 goat
anti-mouse IgG (1:500) was added and samples were incubated at RT
for 2 h. DAPI (Wuhan Boster Biological Technology, Ltd.) staining
was then performed for 15 min. Images were captured with the use of
a fluorescence microscope (magnification, ×200-400).
Surgical transplantation of NSCs into
the hippocampus
P2 generation NSCs were used in the experiment.
Before transplantation, neurospheres were dissociated with Accutase
and washed twice in PBS. Viable cells were counted using trypan
blue and the density of living cells was adjusted to
1×105 cells/µl in PBS. Bilateral hippocampal sites were
selected for transplantation according to the Mouse Brain
Stereotaxic Atlas. Twenty-four 12-month-old male APP/PS1 transgenic
mice were randomly divided into two groups: The AD model group
(Tg-AD group), the NSC-transplanted group (Tg-NSC group). Twelve
wild-type mice of the same age were used as the normal control
group (WT group). The WT and Tg-AD group were injected with 5 µl
PBS in the bilateral hippocampus and the NSC group was injected
with 5 µl NSC suspension (re-suspended by PBS). In brief, mice were
anaesthetized by intraperitoneal injection of 1% pentobarbital
sodium (50 mg/kg), which was prepared with 0.9% normal saline and
fixed with the use of a stereotaxic apparatus. Each mouse
hippocampus was injected bilaterally with 5 µl of either vehicle
(Tg-AD group and WT group) or NSCs (Tg-NSC group) at a rate of 1
µl/min. The injection site was at anteroposterior (AP): −2.06 mm,
mediolateral (ML): ±1.85 mm, and dorsoventral (DV): −2.50 mm
relative to bregma. The needle was maintained in place for an
additional 5 min to allow the liquid to diffuse into the tissue.
After injection, the incision was sealed and sterilized prior to
placing the mice in single cages.
Morris water-maze (MWM)
Ten days after NSC transplantation, Morris water
maze (MWM) was used to assess hippocampal-dependent learning and
memory. MWM training was comprised of 2 procedures: The place
navigation test and the spatial probe test. All the trials were run
in a 1-m diameter circular pool, divided into 4 equal quadrants by
different symbols. A hidden platform was placed 1.5 cm underneath
the surface of the opaque water. Swimming paths were recorded by a
computerized video imaging analysis system. The place navigation
test lasted 4 days. Mice were placed into the water quadrants
facing the wall of the pool to record the escape latency while
searching for the platform for 60 sec. When the mouse failed to
locate the platform within 60 sec, it was guided to stay on the
platform for 10 sec. On the fifth day, the platform was removed,
and the time spent in the target quadrant and the frequency of
crossing through the platform place were recorded.
Western blotting
Brain tissue samples were harvested 2 weeks after
NSC transplantation. Tissues were lysed in cold lysis buffer (1X
PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% SDS; RIPA)
containing proteinase inhibitors. The protein concentration was
measured using a bicinchoninic protein assay kit. An equal amount
of protein samples (50 µg) was loaded in each lane. The protein
samples (concentration, 5 µg/µl; volume, 10 µl) were submitted to
10% SDS-PAGE prior to electrotransfer on a PVDF membrane. The
membrane was incubated in 5% non-fat milk at room temperature for 1
h and washed three times with TBST (0.01 M TBS and 0.1% Tween-20).
The specific primary antibodies: Rabbit anti-ChAT (1:1,000), rabbit
anti-Map2 (1:1,000), rabbit anti-PSD95 (1:1,000), rabbit anti-SYP
(1:1,000), mouse anti-β-tubulin III (1:1,000) were incubated with
the membrane overnight at 4°C. After washing in TBST to remove
residual antibodies, horseradish peroxidase (HRP) conjugated
secondary antibodies (goat anti-rabbit IgG; 1:2,000 and rabbit
anti-mouse 1:1,000) were applied for 2 h at room temperature. The
membrane was then washed with TBST prior to film exposition and
development using an ECL kit (Bioworld Technology, Inc.). The
protein expression levels were analyzed using ImageJ (version
1.4.3.67; National Institutes of Health).
Immunohistochemistry
Mice were anesthetized and perfused transcardially
with saline buffer and a pre-cooled 4% paraformaldehyde (PFA)
solution. The whole brain was removed and fixed overnight in 4%
PFA. Then, dehydration was performed with the use of a sucrose
gradient and brains were cut into 30-µm thick slices. Slices of
basal forebrain and hippocampus were rinsed three times (5 min each
time) in PBS and incubated in 0.3% Triton X-100 to weaken cell
membranes. Slices were then incubated in 5% bovine serum albumin
(BSA) at room temperature for 1 h, and ChAT, GFAP and DCX
antibodies (1:300) were added prior to incubation overnight at 4°C.
Alexa Fluor™ 594 donkey anti-rabbit IgG (1:500) was then added
after washing the primary antibodies and the samples were incubated
at RT for 2 h. DAPI (Wuhan Boster Biological Technology, Ltd.)
staining was carried out for 15 min prior to imaging with the use
of a fluorescence microscope (magnification, ×200 and ×400).
Statistical analysis
All statistical analyses were computed using the
SPSS 16.0 software (SPSS, Inc.). The data were expressed as the
mean ± standard deviation (SD). Multiple group comparisons were
achieved by single-effect analysis of variance (one-way ANOVA)
followed by post hoc Fisher's LSD multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mouse EGFP-NSCs have self-renewal and
multi-differentiation abilities in vitro
The stem cells used in the present study were
produced from 12.5 to 14.5-day-old mice embryos expressing EGFP.
Immunohistochemical analysis revealed co-expression of EGFP and
Nestin, a marker of NSCs, in undifferentiated NSCs (Fig. 1A). When incubated with FBS for 5
days, NSCs could differentiate into glial cells and neurons
expressing the positive marker GFAP and β-tubulin III, respectively
(Fig. 1B and C).
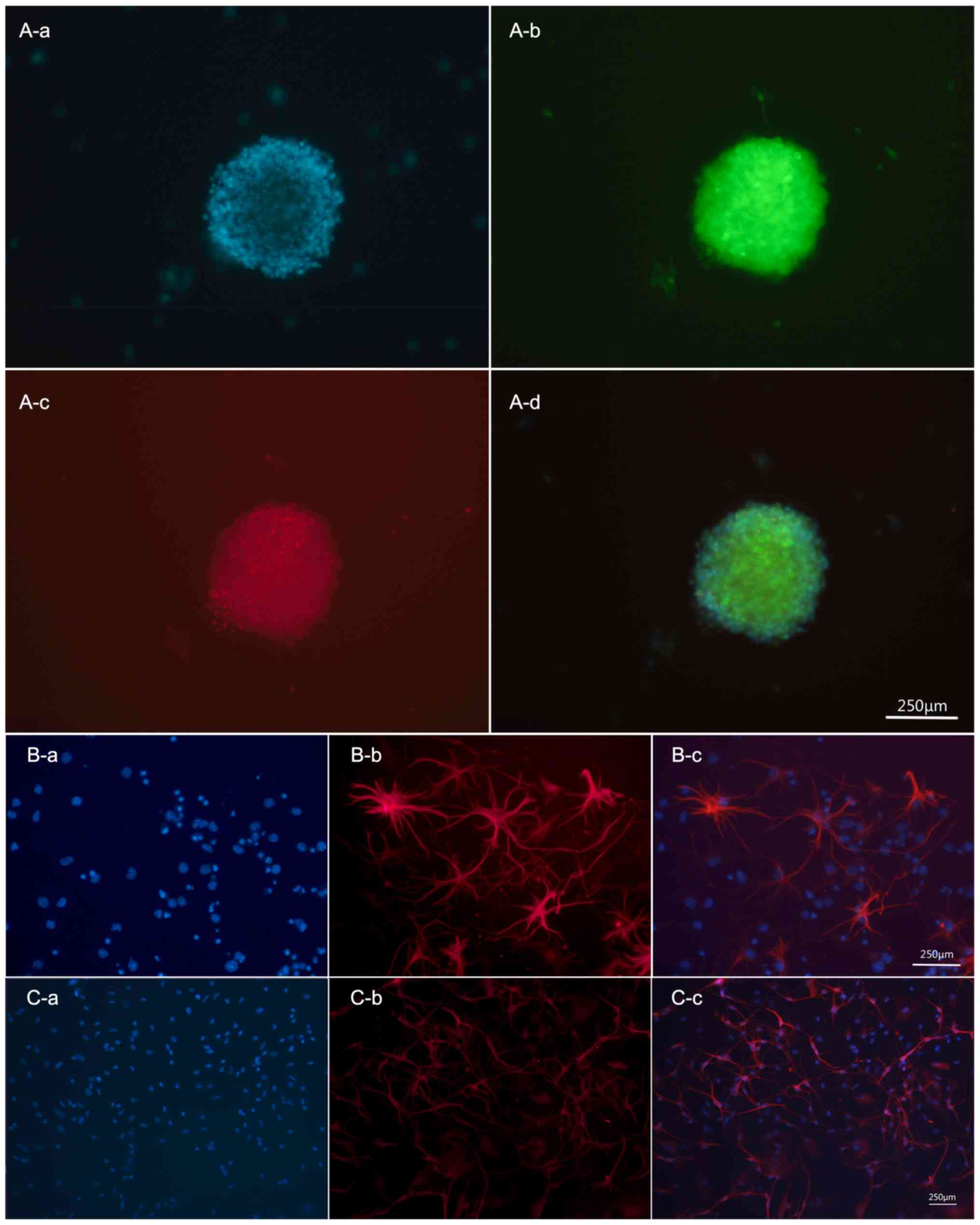 | Figure 1.Mouse EGFP-NSCs have the ability of
self-renewal and multi-differentiation in vitro. (A)
Cultured NSCs were amassed as neurospheres and co-expressed EGFP
(green) and Nestin (red). Subpart A-d is a merge image, shows DAPI
(blue fluorescence, A-a) + EGFP (green fluorescence, A-b) + Nestin
(red fluorescence, A-c). (B) NSCs differentiated into astrocyte,
expression of GFAP-positive markers (red). Subpart B-c is a merge
image, shows DAPI (blue fluorescence, B-a) + GFAP (red
fluorescence, B-b). (C) NSCs differentiated into neurons,
expression of Tuj1-positive markers (red). Subpart C-c is a merge
image, shows DAPI (blue fluorescence, C-a) + Tuj1 (red
fluorescence, C-b). Scale bar, 100 µm. EGFP, enhanced green
fluorescent protein; NSCs, neural stem cells. |
Survival, differentiation and
migration of NSCs in vivo
EGFP-NSCs were prepared to better track the fate of
engrafted cells in vivo. Migration and differentiation were
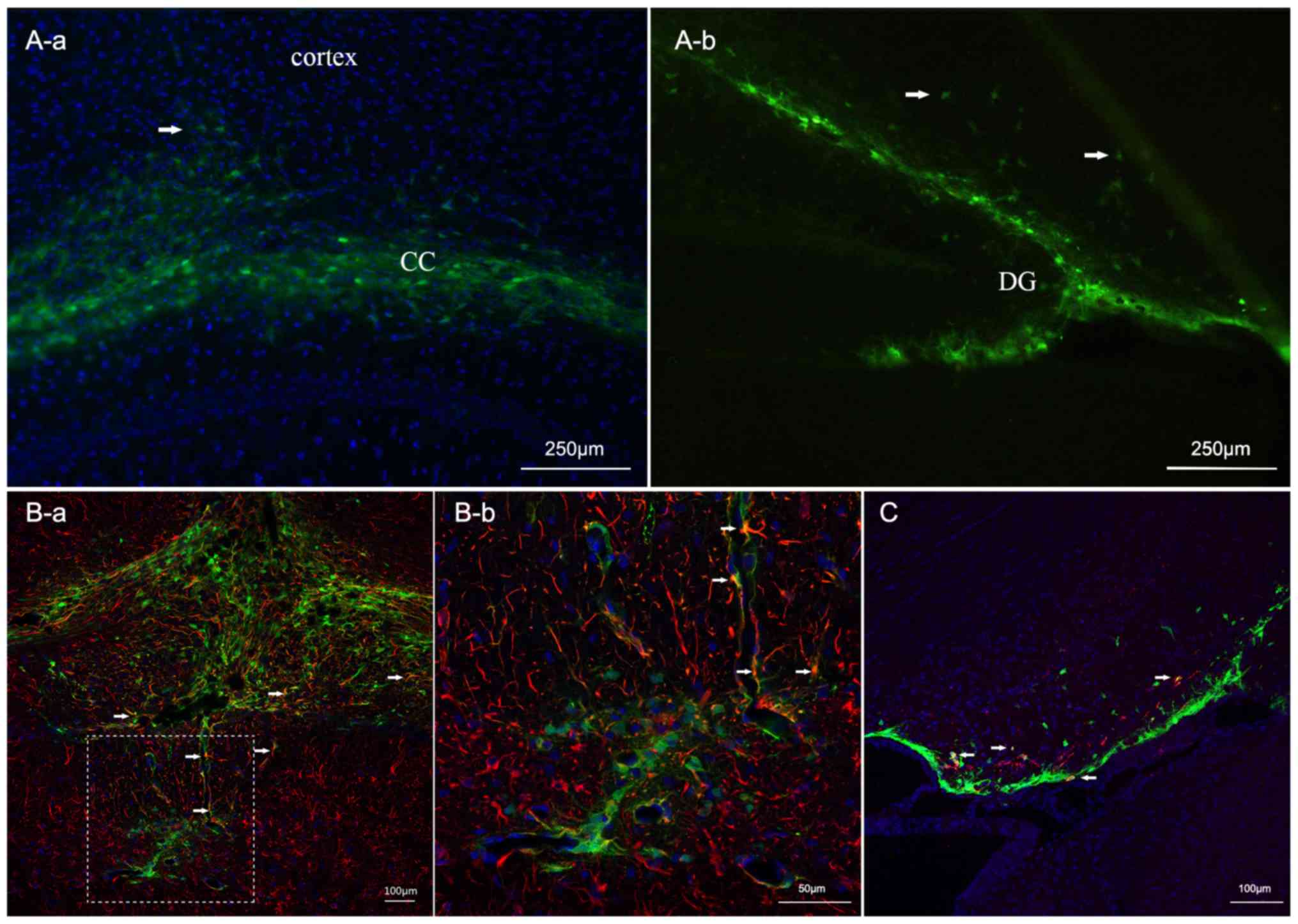
observed two weeks after transplantation. As presented in Fig. 2, engrafted cells survived and
partly remained at the injection site. Conversely, some cells
migrated to surrounding regions including the corpus callosum and
the adjacent cortex (Fig. 2A).
Some cells experienced morphologic changes. To assess their
phenotypes, astrocytes and immature neurons were labeled with GFAP
or DCX. Confocal microscopy analysis revealed that a small portion
of engrafted cells differentiated into GFAP + astrocytes. Others
differentiated into DCX + neurons (Fig. 2B and C).
NSC transplantation alleviates
cognitive impairment
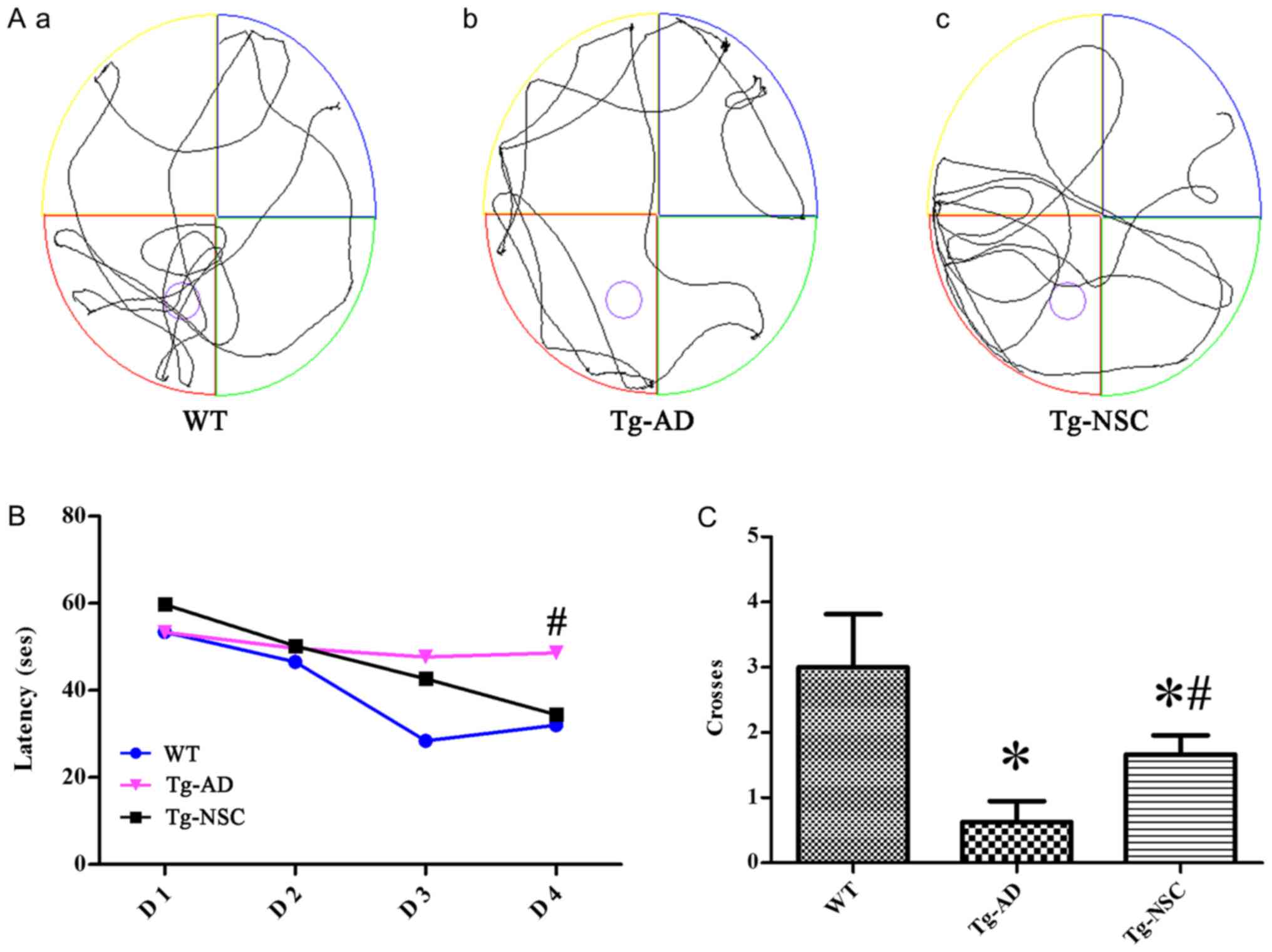
To assess whether NSC transplantation can alleviate
cognitive impairment, the MWM was implemented to assay spatial
learning and memory ability (Fig.
3A). As anticipated, during the four-day navigation task,
shorter latencies could be observed in the three groups. However,
this reduction was less pronounced in the Tg-AD group and the
latency was significantly shorter in the Tg-NSC group compared to
the Tg-AD group on the last trial day (Fig. 3B, P<0.05). The crossing times of
the original platform were significantly ameliorated in the Tg-NSC
group compared to Tg-AD group. However, it was still lower than
those observed in the WT group (Fig.
3C, P<0.05).
NSC transplantation increases the
levels of cognitive-related proteins
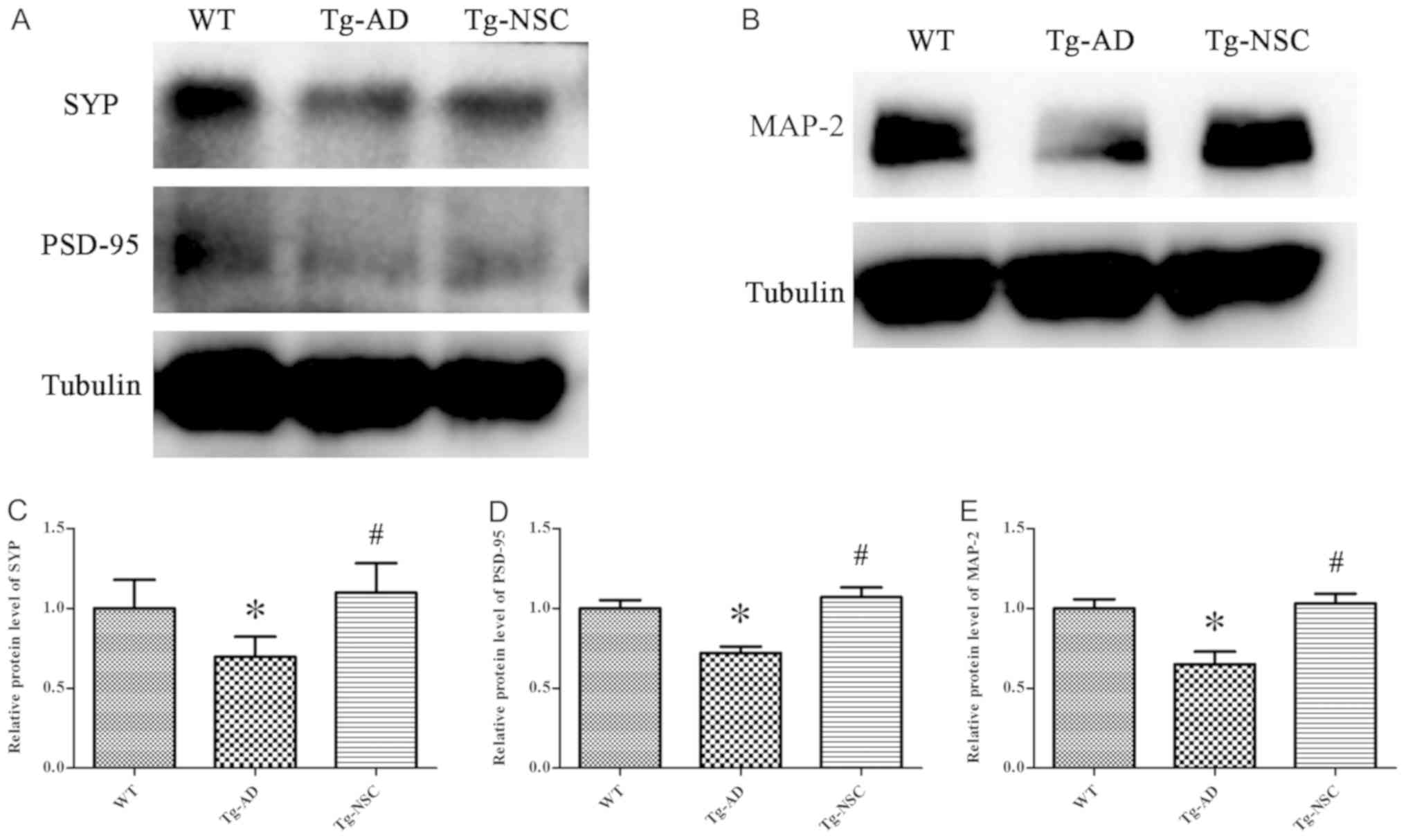
Synaptic damage is one of the most important
pathological features of AD, which is highly correlated with
cognitive deficit (14). MAP-2 is
overexpressed in dendrites and involved in microtubule assembly.
This protein can regulate microtubule networks in axons and neuron
dendrites that may be critical for neurogenesis (15). In the present study, the levels of
SYP, PSD-95 and MAP-2 proteins were significantly increased in the
Tg-NSC group compared with the Tg-AD group and no difference could
be observed in comparison with the WT group (Fig. 4, P<0.05).
NSC transplantation protects
cholinergic neurons in the basal forebrain
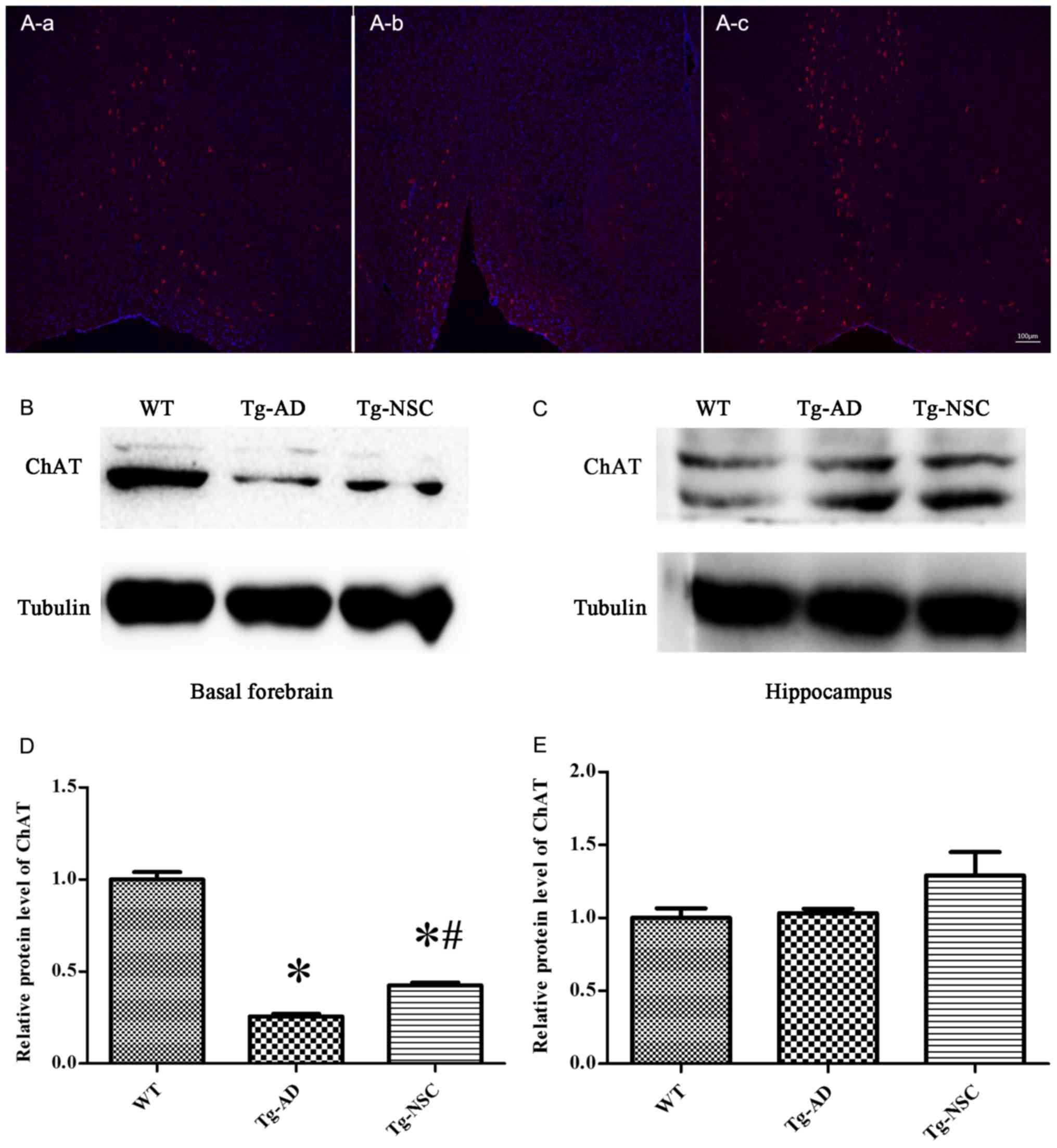
In order to determine whether NSC transplantation
restored atrophic cholinergic neurons in the AD mouse model,
ChAT-positive neurons and ChAT protein expression were analyzed.
The results are displayed in Fig.
5. NSC transplantation markedly increased the levels of ChAT
proteins and ChAT-positive neurons in the basal forebrain. There
were significant differences compared with the control group
(Tg-AD). However, ChAT protein levels were still lower compared to
the WT group (Fig. 5A and B). NSC
transplantation increased ChAT protein levels in the basal
forebrain, but not in the hippocampus (Fig. 5C).
Discussion
In the present study, MWM was used to detect
hippocampal-dependent learning and memory. Furthermore, confocal
microscopy was applied to investigate the migration and
differentiation of transplanted cells and ChAT-positive neurons.
Western blotting was performed to assess the levels of SYP, PSD-95,
MAP-2 and ChAT proteins in the hippocampus and the basal forebrain.
These analyses revealed that engrafted cells survived, migrated and
differentiated in the brains of AD mice. Moreover, learning and
memory ability were rescued by NSC transplantation. The mechanism
of NSC transplantation may be related to the restoration of the
synaptic impairment and the cholinergic neuron recovery in the
basal forebrain.
APP/PS1 transgenic mice are useful in studying
neurological brain disorders and have been widely used to mimic the
pathology of AD. Mice develop significant impairment after the age
of nine months. In the present study, 12-month-old APP/PS1 mice
were used. It was revealed that Tg-AD mice exhibited cognitive
defects and that the learning ability was significantly increased
after NSC transplantation. However, it was still lower than that of
the corresponding WT control mice.
Previous studies revealed that the accumulation of
pathological Aβ proteins causes synaptic loss related with
AD-associated cognitive deficit (16). Moreover, synaptic loss is the
principal correlate of the loss of cholinergic neurons (2). Thus, in the present study, several
important protein markers associated with the synaptic plasticity
were selected. SYP is a marker of presynaptic proteins. SYP k.o.
mice exhibited retarded learning with slightly impaired memory
performance (14). PSD-95 is
another important synaptic protein. This protein is a constituent
of the postsynaptic complex and plays an important role in synaptic
plasticity and the stabilization of synaptic changes during
long-term potentiation (17). In
the present study, it was revealed that the protein levels of SYP
and PSD-95 significantly increased in the hippocampus of the Tg-NSC
group compared to the Tg-AD group. Accordingly, the cognitive
function was also improved. In addition, MAP-2 protein levels were
also increased in the hippocampus after NSC transplantation. The
present results indicated that NSC transplantation can restore
synaptic impairment in accordance with previous research.
Another important typical feature of AD is the
damage of cholinergic neurons in the basal forebrain (18). Evidence has revealed that
cholinergic neurons from the medial septum and the diagonal band of
Broca (MSDB), within the basal forebrain projections (~65%) to the
hippocampus, provide the main source of acetylcholine in the
hippocampus (19). Cholinergic
hypofunction induces the loss of cortical and hippocampus
cholinergic innervation and may cause cognitive disorders. In the
present study, NSCs were transplanted into the basal forebrain and
hippocampus of an AD rat model with basal forebrain lesion. Damaged
neurons were restored and replenished (P75 was used as a marker of
cholinergic neurons), and learning and memory abilities were
improved (20). It was revealed
that NSC transplantation increased the levels of ChAT protein in
the basal forebrain, but not in the hippocampus. Moreover,
immunofluorescence analysis demonstrated that ChAT-positive neurons
were restored in the Tg-NSC group with a significant improvement
compared to the Tg-AD group. Moreover, no difference could be
observed in comparison with the WT group. These results were
inconsistent with previous research revealing that dysfunction of
cholinergic neurons, rather than cholinergic cell loss, is
impacted. In this study, the number of ChAT-positive neurons was
unchanged, however, trkA and p75NTR-containing neurons, which
co-localize with ChAT, were significantly reduced in the nucleus
basalis (21).
Numerous studies have revealed that NSC
transplantation alleviates learning and memory impairment in AD
mouse models. The possible mechanism may be related to the
engrafted cell itself or to the secretion or play a bystander role
by transplanted cells (11; 22). In the present study, the recovery
of injured synapses and atrophic cholinergic neurons in the basal
forebrain were observed. However, interactions between these
improved pathologies remain poorly defined. Transplanted NSCs
survived, migrated, and differentiated into GFAP and DCX-positive
neural cells. This differentiation was limited. It was assumed that
neuronal replacement by grafted cells is not possible. Some studies
have suggested that it is challenging that transplanted cells could
differentiate into target neurons and then be projected to the
appropriate region to form appropriate synaptic connections
(9). The beneficial effects that
were observed in this AD model may be explained by cell complement
or only protection rather than replacement. The recovery of
cholinergic functions is most likely due to NSCs themselves, which
can release neurotrophic factors (22,23),
and be uptaken by axonal terminals and retrogradely be transported
into the cell body. These factors protect basal forebrain
cholinergic neurons (24).
Notably, although cholinergic fiber projections to the hippocampus
decreased in AD, ChAT protein levels increased in the hippocampus.
Similar to previous studies, it can be suggested that the
upregulation of hippocampal ChAT in MCI cases may be due to the
replacement of denervated glutamatergic synapses by cholinergic
input arising from the septum (25). Collectively, the present data
indicated that AD transgenic mice display cognitive impairment
associated with damage or loss of cholinergic neurons in the basal
forebrain, and that cell transplantation may enhance ChAT protein
levels and restore cholinergic neurons.
In conclusion, the present study revealed that NSC
transplantation can protect basal forebrain cholinergic neurons and
restore synaptic impairment as well as eventually improve learning
and memory functions in an APP/PS1 transgenic (Tg) mouse model.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of Guangdong Province (2016A030310275).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QZ performed experiments, and collected and
interpreted the data. NZ interpreted the data and drafted the
manuscript. NH and RJ collected the data and performed the
statistical analysis. HL fed the animals and performed the
experiments. AX searched the literature, analyzed the data and
revised the manuscript. DL designed the study and searched the
literature. YC designed the study and revised the manuscript.
Ethics approval and consent to
participate
All procedures were approved by The Animal Ethics
Committee of the Second Affiliated Hospital of Guangzhou Medical
University (approval no. A2018-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Anand R, Gill KD and Mahdi AA:
Therapeutics of Alzheimer's disease: Past, present and future.
Neuropharmacology. 76:27–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cattaneo A and Calissano P: Nerve growth
factor and Alzheimer's disease: New facts for an old hypothesis.
Mol Neurobiol. 46:588–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Small G and Bullock R: Defining optimal
treatment with cholinesterase inhibitors in Alzheimer's disease.
Alzheimers Dement. 7:177–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teng YD: Functional multipotency of stem
cells: Biological traits gleaned from neural progeny studies. Semin
Cell Dev Biol. pii:S1084–S9521. 2019.(Epub ahead of print).
|
|
5
|
Li XY, Bao XJ and Wang RZ: Potential of
neural stem cell-based therapies for Alzheimer's disease. J
Neurosci Res. 93:1313–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alipour M, Nabavi SM, Arab L, Vosough M,
Pakdaman H, Ehsani E and Shahpasand K: Stem cell therapy in
Alzheimer's disease: Possible benefits and limiting drawbacks. Mol
Biol Rep. 46:1425–1446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blurton-Jones M, Spencer B, Michael S,
Castello NA, Agazaryan AA, Davis JL, Müller FJ, Loring JF, Masliah
E and LaFerla FM: Neural stem cells genetically-modified to express
neprilysin reduce pathology in Alzheimer transgenic models. Stem
Cell Res Ther. 5:462014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Gu GJ, Zhang Q, Liu JH, Zhang B,
Guo Y, Wang MY, Gong QY and Xu JR: NSCs promote hippocampal
neurogenesis, metabolic changes and synaptogenesis in APP/PS1
transgenic mice. Hippocampus. 27:1250–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marsh SE and Blurton-Jones M: Neural stem
cell therapy for neurodegenerative disorders: The role of
neurotrophic support. Neurochem Int. 106:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Gu GJ, Shen X, Zhang Q, Wang GM
and Wang PJ: Neural stem cell transplantation enhances
mitochondrial biogenesis in a transgenic mouse model of Alzheimer's
disease-like pathology. Neurobiol Aging. 36:1282–1292. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang J: How close is the stem cell cure to
the Alzheimer's disease: Future and beyond? Neural Regen Res.
7:66–71. 2012.PubMed/NCBI
|
|
12
|
Whitehouse PJ, Price DL, Struble RG, Clark
AW, Coyle JT and Delon MR: Alzheimer's disease and senile dementia:
Loss of neurons in the basal forebrain. Science. 215:1237–1239.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller C and Remy S: Septo-hippocampal
interaction. Cell Tissue Res. 373:565–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitt U, Tanimoto N, Seeliger M,
Schaeffel F and Leube RE: Detection of behavioral alterations and
learning deficits in mice lacking synaptophysin. Neuroscience.
162:234–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dehmelt L and Halpain S: The MAP2/Tau
family of microtubule-associated proteins. Genome Biol. 6:2042005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shankar GM, Li S, Mehta TH, Garcia-Munoz
A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere
CA, et al: Amyloid-beta protein dimers isolated directly from
Alzheimer's brains impair synaptic plasticity and memory. Nat Med.
14:837–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu S, Okamoto S, Lipton SA and Xu H:
Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.
Mol Neurodegener. 9:482014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hampel H, Mesulam MM, Cuello AC, Farlow
MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo
E, Snyder PJ and Khachaturian ZS: The cholinergic system in the
pathophysiology and treatment of Alzheimer's disease. Brain.
141:1917–1933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D,
Choi J, Callaway EM and Xu X: Cell-type-specific circuit
connectivity of hippocampal CA1 revealed through Cre-dependent
rabies tracing. Cell Rep. 7:269–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Pan C, Xuan A, Xu L, Bao G, Liu F,
Fang J and Long D: Treatment efficacy of NGF nanoparticles
combining neural stem cell transplantation on Alzheimer's disease
model rats. Med Sci Monit. 21:3608–3615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mufson EJ, Counts SE, Fahnestock M and
Ginsberg SD: Cholinotrophic molecular substrates of mild cognitive
impairment in the elderly. Curr Alzheimer Res. 4:340–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu P, Jones LL, Snyder EY and Tuszynski
MH: Neural stem cells constitutively secrete neurotrophic factors
and promote extensive host axonal growth after spinal cord injury.
Exp Neurol. 181:115–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Gao Y, Zhang W and Xu JR: Regulation
and effects of neurotrophic factors after neural stem cell
transplantation in a transgenic mouse model of Alzheimer disease. J
Neurosci Res. 96:828–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conner JM, Franks KM, Titterness AK,
Russell K, Merrill DA, Christie BR, Sejnowski TJ and Tuszynski MH:
NGF is essential for hippocampal plasticity and learning. J
Neurosci. 29:10883–10889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mufson EJ, Counts SE, Perez SE and
Ginsberg SD: Cholinergic system during the progression of
Alzheimer's disease: Therapeutic implications. Expert Rev
Neurother. 8:1703–1718. 2008. View Article : Google Scholar : PubMed/NCBI
|