Introduction
Hepatocellular carcinoma (HCC) is a highly prevalent
and lethal disease, which poses a threat to human health. HCC is
the fifth most common malignancy worldwide and the 5-year survival
rate of patients with HCC is <20% (1). However, the genetic factors and
pathogenesis of HCC remain unclear, and liver resection is the only
available curative treatment (2).
Notably, surgical treatment only benefits patients diagnosed with
early-stage HCC, and liver resection is not effective in patients
with late-stage HCC (2,3).
Chemotherapy is an effective strategy to increase
the survival rate of patients with late-stage HCC (4). Notably, targeted therapies for HCC
have been approved for clinical use (4). Sorafenib and doxorubicin (Dox) are
widely used chemical drugs that represent standard therapies for
patients with advanced HCC (5).
However, drug resistance mechanisms may limit the effectiveness of
chemotherapy in patients with HCC (6). Therefore, the identification of novel
clinical strategies able to promote chemotherapeutic sensitivity is
required.
Dox is a type of anthracycline, which inhibits
protein translation by interacting with DNA and RNA (7). Dox is the most common chemotherapy
drug for the treatment of various types of cancer, including breast
cancer, gastric carcinoma, liver cancer, lung cancer and lymphoma
(8). However, the molecular
mechanism underlying Dox function remains unclear. Notably,
chemoresistance to Dox represents a major challenge for the
treatment of HCC. A previous study demonstrated that AMP-activated
protein kinase family member 5 (ARK5) is able to modulate the
resistance of HCC to Dox via epithelial-mesenchymal transition
(EMT) (9). A previous study
identified that semaphorins regulate cell migration, enhancing the
resistance of HCC to Dox (10).
Additionally, salinomycin, an ionophore antibiotic, reverses the
resistance of HCC to Dox by inhibiting the β-catenin/TCF complex
and activating forkhead box O3 (11). Collectively, these previous studies
suggested that chemoresistance in HCC may be a multifactorial
mechanism that requires further investigation.
A previous study demonstrated that low intensity
ultrasound (LIUS) enhances the anticancer effects of chemotherapy
(12). LIUS can treat solid tumors
via sonodynamic therapy, ultrasound-mediated chemotherapy,
ultrasound-mediated gene delivery and antivascular ultrasound
therapy (12). A previous study
demonstrated that LIUS, in combination with chemical compounds,
suppresses proliferation of tongue squamous carcinoma cells
(13). In addition, it has been
demonstrated that LIUS increases Dox uptake, and inhibits cancer
cell proliferation and migration (14). Although various studies have
observed an association between treatment with LIUS and tumor
suppression, the mechanism underlying the antitumor effects of LIUS
remains unclear. Therefore, understanding the molecular mechanism
underlying LIUS may facilitate the development of clinical
strategies combining chemotherapy with LIUS to treat cancer.
Reactive oxygen species (ROS) are involved in
numerous pathophysiological processes. A previous study
demonstrated that ROS, by modulating the expression levels of
certain microRNAs (miRNAs/miRs), may regulate gene expression in
tumor cells (15). Oxidative
stress has been reported to induce the expression of miRNAs
belonging to the miR-200 family, and the crosstalk between ROS
signaling and miR-200 increases oxidative stress-mediated liver
cell death (16). Notably, the
ROS-MYC proto-oncogene, bHLH transcription factor-miR-27 pathway
increases HCC cell proliferation and liver cancer progression
(17). These findings indicate
that LIUS may affect the expression of miRNAs via the production of
ROS. The present study hypothesized that dysregulated miRNA
expression induced by ROS accumulation may represent the mechanism
underlying enhanced Dox sensitivity following treatment with
LIUS.
In the present study, a novel regulatory pathway
consisting of LIUS, ROS and miRNAs was identified in HCC cells. The
present results suggested that LIUS was able to significantly
increase sensitivity to Dox by activating the ROS pathway.
Furthermore, ROS decreased the expression levels of miR-21,
resulting in increased expression levels of PTEN and HCC cell
apoptosis. Therefore, the present results suggested that LIUS
together with Dox may represent a novel strategy to decrease
chemoresistance in HCC, improving the effectiveness of chemotherapy
in clinical settings.
Materials and methods
Cells and ultrasound device
Huh7 cells were purchased from The Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in an incubator with a humidified
atmosphere and 5% CO2 at 37°C. The different
concentrations (0–1.5 µg/ml) of Dox (Wako Pure Chemical Industries,
Ltd; Osaka, Japan) was added into cells, and cells were treated for
24 h, as previously described (18). Huh7 cells (1×104 cells)
were cultured in 3.5-cm diameter dishes (Corning, Inc., Corning,
NY, USA) and placed on an ultrasonic transducer (Onda Corporation,
Sunnyvale, CA, USA). LIUS waves of varying intensities (diameter:
40 mm; center frequency: 1.1 MHz; duty factor: 20%; repetition
frequency: 100 Hz) were transmitted for 15 min through the bottom
of the cultured dishes via a 2.5-cm thick aluminum block in a
humidified 37°C incubator with 5% CO2. Untreated cells
served as controls. In certain experiments, the ROS scavenger
N-acetylcysteine (NAC; 10 mM, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to cells 1 h prior to the administration of Dox
at 37°C; after 24 h of incubation, cell suspensions were
immediately subjected to LIUS exposure. After the treatment, the
cells were collected for further analyses.
Cell viability assay
Cells were treated as aforementioned. Cells were
seeded at 5,000 cells/well in 96-well plates. After 24 h, the
medium was replaced with DMEM supplemented with 10% FBS and Dox (0,
0.1, 0.2, 0.4, 0.8 or 1.5 µg/ml). Cells were cultured in an
incubator for 24 h at 37°C with 5% CO2. Cell viability
was measured using a Cell Counting Kit-8 assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan), according to the
manufacturer's protocol. A microplate reader (MRX II; Dynex
Technologies, Inc., Chantilly, VA, USA) was used to measure the
optical density at 450 nm.
Malondialdehyde (MDA) measurement
The level of lipid peroxidation was assessed by
measuring MDA levels using the thiobarbituric acid reactive
substance (TBARS) according to the method of Zhang et al
(19). In brief, Huh7 cells were
treated with LIUS and/or Dox for 24 h, and then the cells were
homogenized on ice in lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology, Haimen, China) and then centrifuged at
13,000 × g for 10 min at 4°C to remove insoluble material.
Supernatant (200 µl) were placed into a micro-centrifuge tube and
600 µl of the TBARS solution then added. This mixture was incubated
at 95°C for 60 min and cooled to room temperature in an ice bath
for 10 min. Finally, 200 µl was pipetted into each well of a
96-well plate, and the absorbance at 532 nm was measured using a
spectrophotometer (UV-1800 UV-vis spectrophotometer, SHIMADZU
Corporation, Tokyo, Japan). A standard curve was prepared using
various concentrations of 1,1,3,3-tetraethoxypropane (1–10 nM).
TBARS levels were indicated in nM. TBA was procured from
Sigma-Aldrich (Merck KGaA). Other chemicals required, such as EDTA
and trichloroacetic acid were procured from Merck KGaA.
Cell apoptosis assay
Cell apoptosis was assessed by staining the cells
with the BD Pharmingen™ Annexin V-fluorescein isothiocyanate and
propidium iodide kit (BD Biosciences), according to the
manufacturer's protocol. The cells were analyzed with a FACSCalibur
flow cytometer (BD Biosciences) and then analyzed by FlowJo 8.7.1
software (FlowJo LLC). Staining cells simultaneously with Annexin
V-FITC (green fluorescence) and the non-vital dye PI (red
fluorescence) allowed the discrimination of viable cells
(FITC−PI−), early apoptotic
(FITC+PI−), and late apoptotic or necrotic
cells (FITC+PI+). Finally, the apoptotic rate
was calculated from the percentage of early + late apoptotic
cells.
ROS detection
The generation of ROS was assessed using 2′,7′-DCFH
diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA). Briefly, at the end
of treatment, the cell culture medium was discarded and the cells
were incubated with DCFH (20 µmol/l) for 30 min at 37°C, followed
by two washes with PBS. Then the DCFH-DA stain detecting ROS
production was observed using a fluorescence microscope
(magnification, ×200; Nikon Corporation). Fluorescence was read at
485 nm for excitation and 530 nm for emission with an Infinite M200
Microplate Reader (Tecan Group, Ltd.) and analyzed with BD FACSDiva
(version 6.2; BD Biosciences) software.
Microarray analysis
Total RNA was extracted from Huh7 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly, the
quantity of RNA samples was evaluated via NanoDrop™ ND-1000
spectrophotometry (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Total RNA (200 ng) was labeled with fluorescence dye hy3 or
hy5 using a miRCURY Hy3/Hy5 Power Labeling kit (cat. no. 208031-A)
and hybridized on the miRCURY™ LNA Array (v.18.0), both obtained
from Exiqon (Qiagen, Inc.) according to the manufacturer's
protocol. Data were analyzed using GeneSpring software version 7.3
(Agilent Technologies, Inc.). The miRNAs with intensities ≥50 were
used to calculate a normalization factor in all samples.
Normalization was performed using median normalization. The miRNA
expression profiles (heatmaps) were determined using MEV software
(version 4.6; http://mev.tm4.org/#/welcome).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
miRNA was prepared using the miRNeasy Mini kit
(Qiagen, Inc.) and total RNA was prepared using TRIzol reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. For miRNA reverse transcription, cDNA was synthesized
using TaqMan® miRNA reverse transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) at 42°C for 1 h. For
mRNA reverse transcription, cDNA was synthesized using the Oligo dT
primer (Takara Biotechnology Co., Ltd.) at 42°C for 1 h. qPCR was
performed using an SYBR Green PCR mix (Takara Biotechnology Co.,
Ltd.). qPCR was conducted as follows: 95°C for 15 min, followed by
40 cycles of 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec,
and a final extension step at 72°C for 5 min. The following primers
were used for RT-qPCR analysis: miR-21 forward (F),
5′-GCCCGCTAGCTTATCAGACTGATG-3′ and miR-21 reverse (R),
5′-CAGTGCAGGGTCCGAGGT-3′; U6 F, 5′-TGCGGGTGCTCGCTTCGCAGC-3′ and U6
R, 5′-CCAGTGCAGGGTCCGAGGT-3′; PTEN F, 5′-TTGGCGGTGTCATAATGTCT-3′
and PTEN R, 5′-GCAGAAAGACTTGAAGGCGTA-3′; GAPDH F,
5′-AGGTCGGTGTGAACGGATTTG-3′ and GAPDH R,
5′-TGTAGACCATGTAGTTGAGGTCA-3′ The RT-qPCR assays were performed in
triplicate and the relative expression levels were calculated based
on the 2−ΔΔCq method (20).
Transfection
When Huh7 cells in 6-well plates had grown to ~80%
confluence, miR-21 mimics (20 nM) or miR-21 inhibitor (20 nM) were
transfected into cells at 37°C for 48 h, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 4 h, the transfection medium was
discarded. Cells were washed with serum-free DMEM, then cultured in
DMEM supplemented with 10% FBS. miR-21 mimics, mimics negative
control (NC), miR-21 inhibitor and inhibitor NC were obtained from
Guangzhou RiBoBio Co., Ltd. The sequences were as follows: miR-21
inhibitor, 5′-AUCGAAUAGUCUGACUACAACU-3′; miR-21 mimics,
5′-UAGCUUAUCAGACUGAUGUUGA-3′; mimics NC,
5′-CCCCCCCCCCCCCCCCCCCC-3′; inhibitor NC,
5′-CAGUACUUUUGUAGUACAA-3′. Cells were harvested after 24 h for
further analyses.
Western blotting
Huh7 cells were lysed in lysis buffer (Tris 50 mM,
pH 7.4, NaCl 150 mM, 1% Triton X-100 and EDTA 1 mM, pH 8.0)
containing cOmplete™ Mini Protease Inhibitor (Roche Diagnostics)
for 20 min on ice, and cell debris was removed by centrifugation at
15,000 × g for 20 min at 4°C. The protein concentration was
determined using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). The proteins (30 µg/lane) were separated via 10%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore).
Membranes were blocked with 5% milk for 2 h at room temperature,
and then the membranes were incubated overnight at 4°C with primary
antibodies against superoxide dismutase 1 (1:1,000; SOD-1; cat. no.
sc-101523; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
glutathione peroxidase (1:1,000; GPx; cat. no. sc-133160; Santa
Cruz Biotechnology, Inc.), PTEN (1:1,000; cat. no. 9188; Cell
Signaling Technology, Inc., Danvers, MA, USA), phosphorylated
(p-)AKT (1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.),
AKT (1:1,000; cat. no. 4685; Cell Signaling Technology, Inc.),
p-mTOR (1:1,000; cat. no. 5536; Cell Signaling Technology, Inc.),
mTOR (1:1,000; cat. no. 2983; Cell Signaling Technology, Inc.) and
β-actin (1:1,000; cat. no. 3700; Cell Signaling Technology, Inc.),
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit or mouse IgG secondary antibodies (1:10,000; cat. nos.
ab205718 or ab6789; Abcam, Cambridge, UK) at room temperature for 2
h. The protein bands were detected using an enhanced
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.).
Semi-quantification was performed using ImageJ version 1.46
(National Institutes of Health, Bethesda, MD, USA).
Bioinformatics
Online miRNA prediction websites were used for
initial analyses, including TargetScan 7.0 (http://www.targetscan.org/) and miRanda (http://www.microrna.org/).
Luciferase assay
The predicted and mutated sequences targeting the
3′-untranslated region (UTR) of PTEN were amplified and cloned into
the pGL3 vector (Promega Corporation). pGL3-PTEN-3′-UTR wild-type
(WT) and pGL3-PTEN-3′-UTR mutated (Mut) were synthesized by
GenePharma. Huh7 cells (1–2×105 cells per well) were
co-transfected with 10 ng pGL3 luciferase vectors and 20 ng
Renilla vector (pRL-TK; Promega Corporation), together with
20 nM miR-21 inhibitor, 20 nM miR-21 mimics, 20 nM mimics NC or 20
nM inhibitor NC using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 24 h at 37°C. Luciferase
activity was detected using the Dual-Luciferase Reporter Assay
system (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity.
Statistical analysis
Data are presented as the means ± standard
deviation. Experiments were performed at least three times in
triplicate. Differences were analyzed with one-way analysis of
variance among multiple groups followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Dox combined with LIUS promotes
apoptosis of HCC cells
Huh7 is a HCC cell line that is sensitive to Dox. To
examine the effectiveness of Dox in suppressing tumor growth, Huh7
cells were treated with various doses of Dox and cell viability was
measured after 24 h. The present results demonstrated that
treatment with Dox decreased the survival rate of Huh7 cells in a
dose-dependent manner (Fig. 1A).
The half maximal inhibitory concentration of Dox was 0.57 µg/ml; in
contrast, only minor reductions in viability were observed
following treatment with 0.1 µg/ml Dox. Therefore, 0.1 µg/ml Dox
was selected as a working concentration to investigate the ability
of LIUS to promote sensitivity to Dox. As presented in Fig. 1B and C, compared with Dox treatment
alone, cell viability was significantly reduced and the rate of
apoptosis was significantly increased in the LIUS + Dox group,
consistent with a previous study (14). The present results suggested that
LIUS may enhance HCC cell apoptosis in combination with
chemotherapy. A previous study reported that treatment with LIUS
increases intracellular ROS accumulation (21). Subsequently, the association
between ROS and LIUS-induced apoptosis was investigated. ROS in
Huh7 cells were detected by MitoSOX staining. The present study
revealed that treatment with LIUS or Dox alone could increase the
intracellular levels of ROS. Notably, ROS accumulation was
significantly enhanced following combined treatment with LIUS and
Dox (Fig. 1D). MDA is a marker of
oxidative stress, and its intracellular levels were slightly
increased in response to LIUS or DOX treatment. However, following
combined treatment with LIUS and Dox, the concentration of MDA
exhibited a ~2-fold increase compared with single treatments
(Fig. 1E). GPx and SOD-1 are
ROS-scavenging enzymes that serve important roles in the
oxidant/antioxidant balance, and are thus able to prevent oxidative
stress. To further investigate the antioxidative response, the
protein expression levels of GPx and SOD-1 were assessed by western
blotting. The protein expression levels of GPx and SOD-1 were
significantly decreased following combined treatment with LIUS and
Dox (Fig. 1F). The present results
suggested that LIUS combined with Dox increased apoptosis and ROS
accumulation in Huh7 cells.
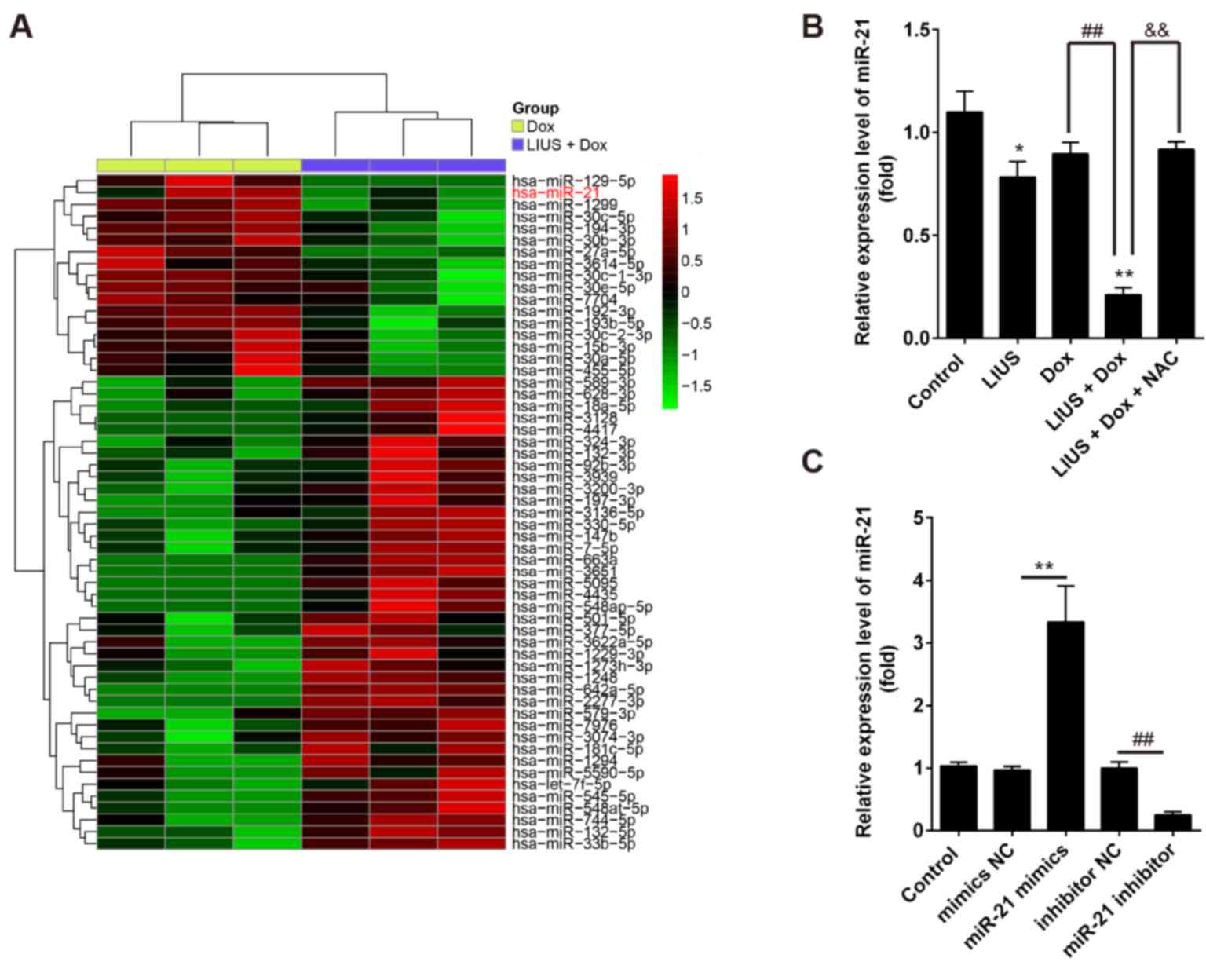
Dox combined with LIUS downregulates
the expression levels of miR-21 in HCC cells via ROS
To investigate the mechanisms underlying
LIUS-induced apoptosis, the miRNA expression profile of Huh7 cells
following treatment with Dox alone or in combination with LIUS was
investigated by microarray analysis. The microarray results
suggested that LIUS combined with Dox affected the expression
levels of certain miRNAs compared with Dox treatment alone
(Fig. 2A). Among the miRNAs
downregulated following combined treatment with LIUS and Dox, the
expression levels of miR-21 were markedly decreased. A previous
study demonstrated that miR-21 may serve as an oncogene with a role
in cancer pathogenesis, invasion and metastasis (22). Additionally, miR-21 has been
identified to mediate chemotherapy resistance in HCC cells
(23), and to increase HCC cell
growth and invasion (24). A
previous study suggested that miR-21 may be used as a biomarker
associated with poor prognosis in patients with HCC (25). Therefore, miR-21 was selected for
further experiments. RT-qPCR was performed to validate the
expression levels of miR-21 in Huh7 cells following single and
combined treatments. In line with the microarray results, the
expression levels of miR-21 were significantly decreased following
combined treatment with LIUS and Dox compared with single
treatments (Fig. 2B). Notably,
NAC, a ROS inhibitor, restored the expression levels of miR-21 in
Huh7 cells. The present results suggested that LIUS decreased the
expression levels of miR-21 in Dox-treated cells via activation of
the ROS pathway. In order to investigate the role of miR-21 in the
effect of Dox and LIUS on cell survival, miR-21 was overexpressed
or silenced using mimics or inhibitor, respectively.
Post-transfection with miR-21 mimics or miR-21 inhibitor, the
expression levels of miR-21 were significantly increased or
decreased, respectively (Fig.
2C).
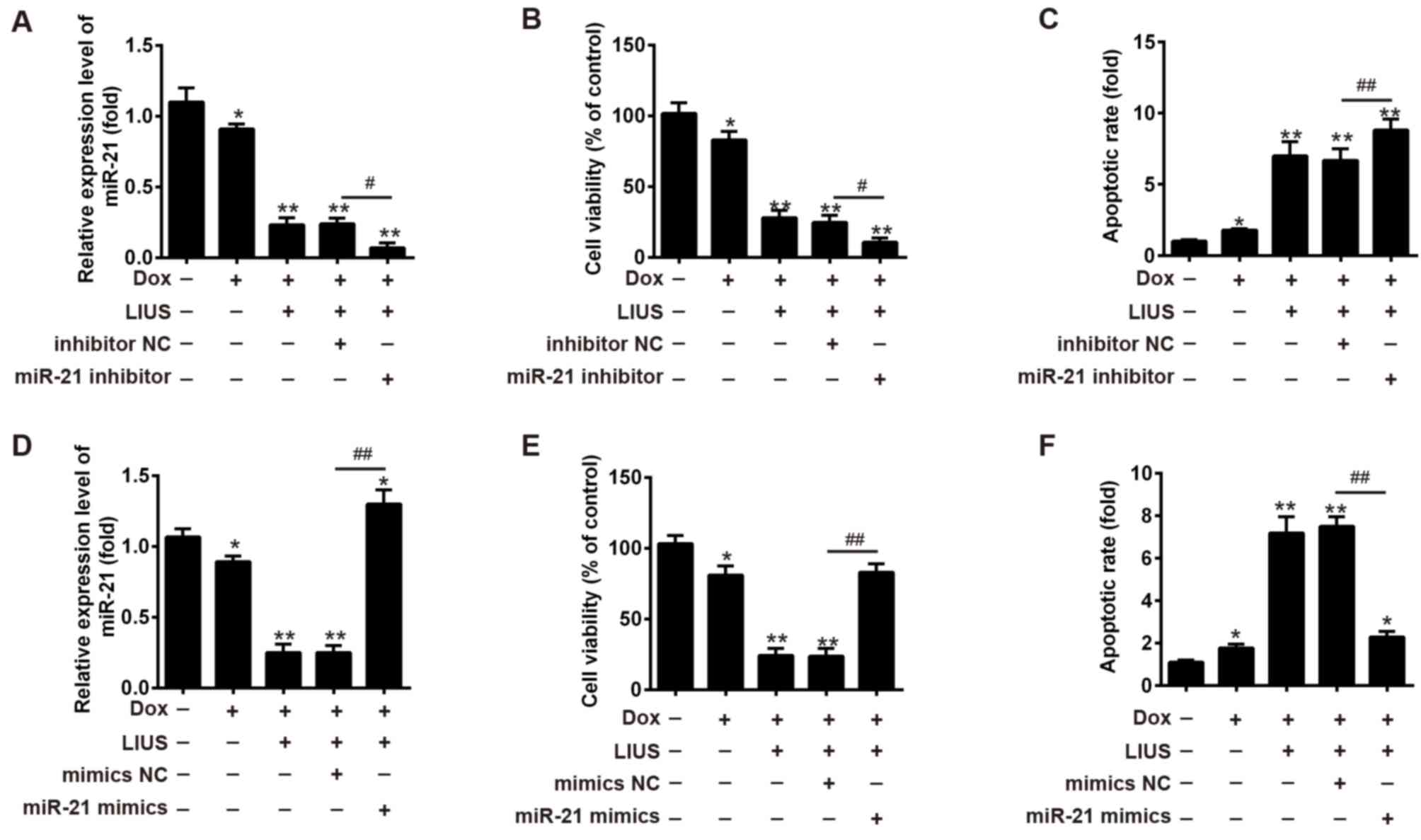
miR-21 regulates the effects of Dox
and LIUS on HCC cell apoptosis
The present study hypothesized that Dox combined
with LIUS may affect HCC cell survival via the ROS/miR-21 pathway.
To examine the function of miR-21 on cell viability and apoptosis
following treatment with Dox and/or LIUS, miR-21 inhibitor or
miR-21 mimics were transfected into Huh7 cells, and cell viability
and apoptosis were investigated (Fig.
3). In Huh7 cells cotreated with LIUS and Dox, the expression
levels of miR-21 were decreased and increased following
transfection with miR-21 inhibitor and mimics, respectively
(Fig. 3A and D). Transfection with
miR-21 inhibitor increased cell apoptosis and decreased cell
viability following combined treatment with Dox and LIUS (Fig. 3B and C), whereas miR-21 mimics
increased cell viability and decreased apoptosis (Fig. 3E and F, respectively). The present
results suggested that miR-21 regulated the effects of Dox and LIUS
on apoptosis of Huh7 cells.
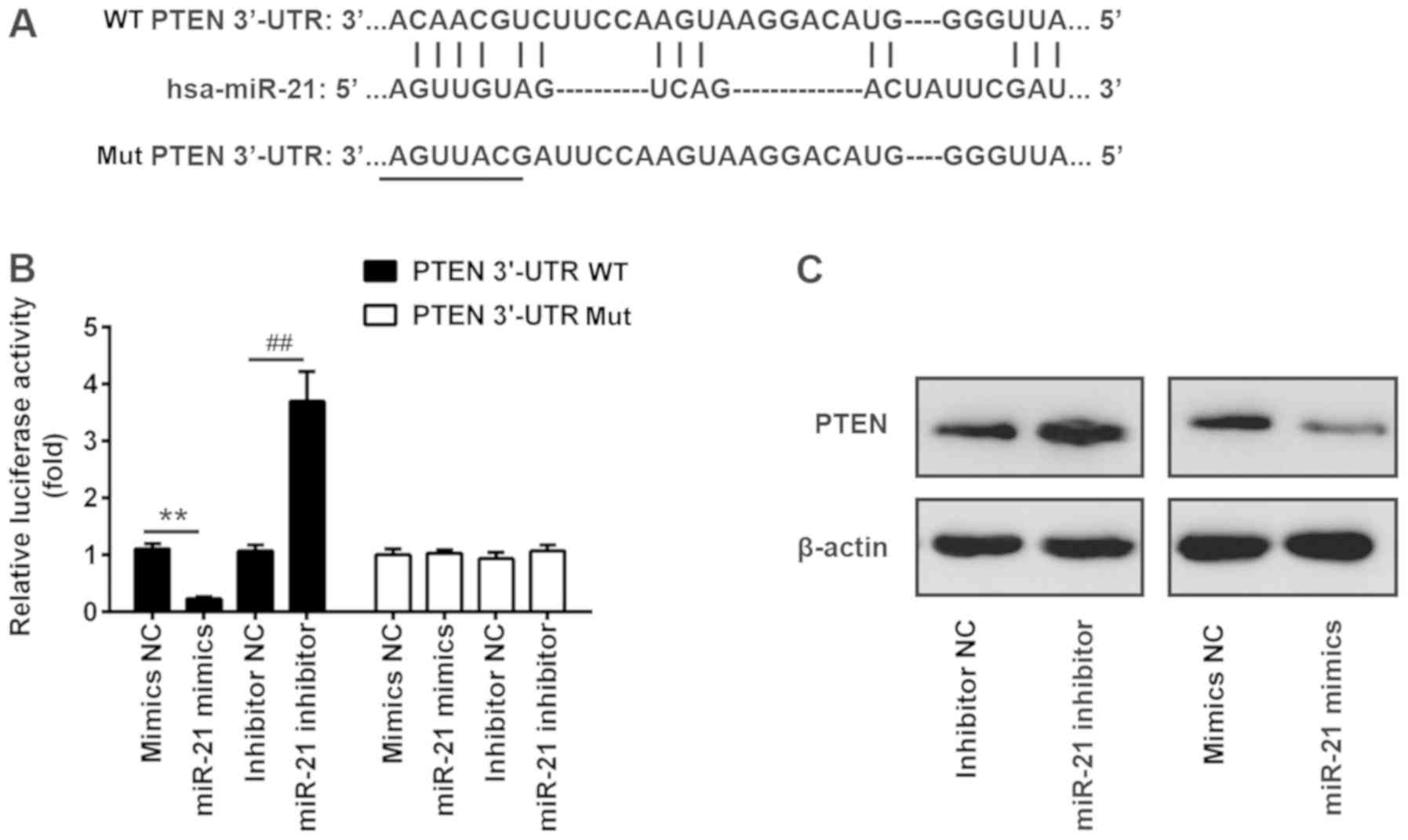
PTEN is a target of miR-21
Via bioinformatics prediction using TargetScan 7.0
and miRanda, a putative target site of miR-21 was identified in the
3′-UTR of PTEN mRNA, an important regulator of the AKT/mTOR pathway
(Fig. 4A) (26) To investigate the interaction
between miR-21 and the 3′-UTR of PTEN, a luciferase assay was
performed. The WT or Mut 3′-UTR sequences of PTEN were cloned
upstream of a luciferase gene and the constructed plasmids were
transfected into Huh7 cells together with miR-21 inhibitor or
mimics. The results of a dual-luciferase reporter assay suggested
that miR-21 mimics suppressed the luciferase activity by ~70%
compared with control mimics. Conversely, miR-21 inhibitor
increased the luciferase activity by ~3-fold (Fig. 4B). In contrast, transfection with
miR-21 mimics or inhibitor did not affect the luciferase activity
of a plasmid carrying the Mut 3′-UTR sequence of PTEN (Fig. 4B). In line with the luciferase
assay results, western blotting suggested that miR-21 inhibitor
enhanced the protein expression levels of PTEN, whereas miR-21
mimics decreased the protein expression levels of PTEN (Fig. 4C).
Treatment with LIUS increases
sensitivity of cells to Dox via the ROS/miR-21/PTEN axis
PTEN is a tumor suppressor gene, and tumor growth is
decreased following overexpression of PTEN (27,28).
To determine whether LIUS could enhance PTEN expression via the
ROS/miR-21 pathway in HCC cells, Huh7 cells were treated with LIUS
and/or Dox. After 48 h, western blot analysis was performed. Dox
combined with LIUS increased the protein expression levels of PTEN,
in line with the present results suggesting that LIUS suppressed
cell viability and survival (Fig.
5A). Notably, NAC, a ROS inhibitor, significantly decreased the
protein expression levels of PTEN following combined treatment with
Dox and LIUS (Fig. 5B). A previous
study reported that miR-21 regulated the expression of PTEN and
phosphorylation of its downstream kinase AKT, and that the
reduction of p-AKT was associated with enhanced chemosensitivity
(29). To investigate the effects
of Dox and LIUS cotreatment on activation of the AKT/mTOR pathway
in Huh7, western blot analysis was performed. The present results
suggested that the phosphorylation levels of AKT and mTOR were
significantly decreased following combined treatment with Dox and
LIUS compared with in the control group (Fig. 5C). However, treatment with NAC
reversed this effect, suggesting that activation of the
PTEN/AKT/mTOR pathway following treatment with Dox and LIUS was
dependent on the accumulation of ROS. Collectively, the present
results provided novel insights into the mechanism underlying the
combination of LIUS and chemotherapy. Notably, LIUS was identified
to promote chemotherapy sensitivity, inducing apoptosis of HCC
cells and increasing the antitumor effects of Dox via the
ROS/miR-21/PTEN pathway.
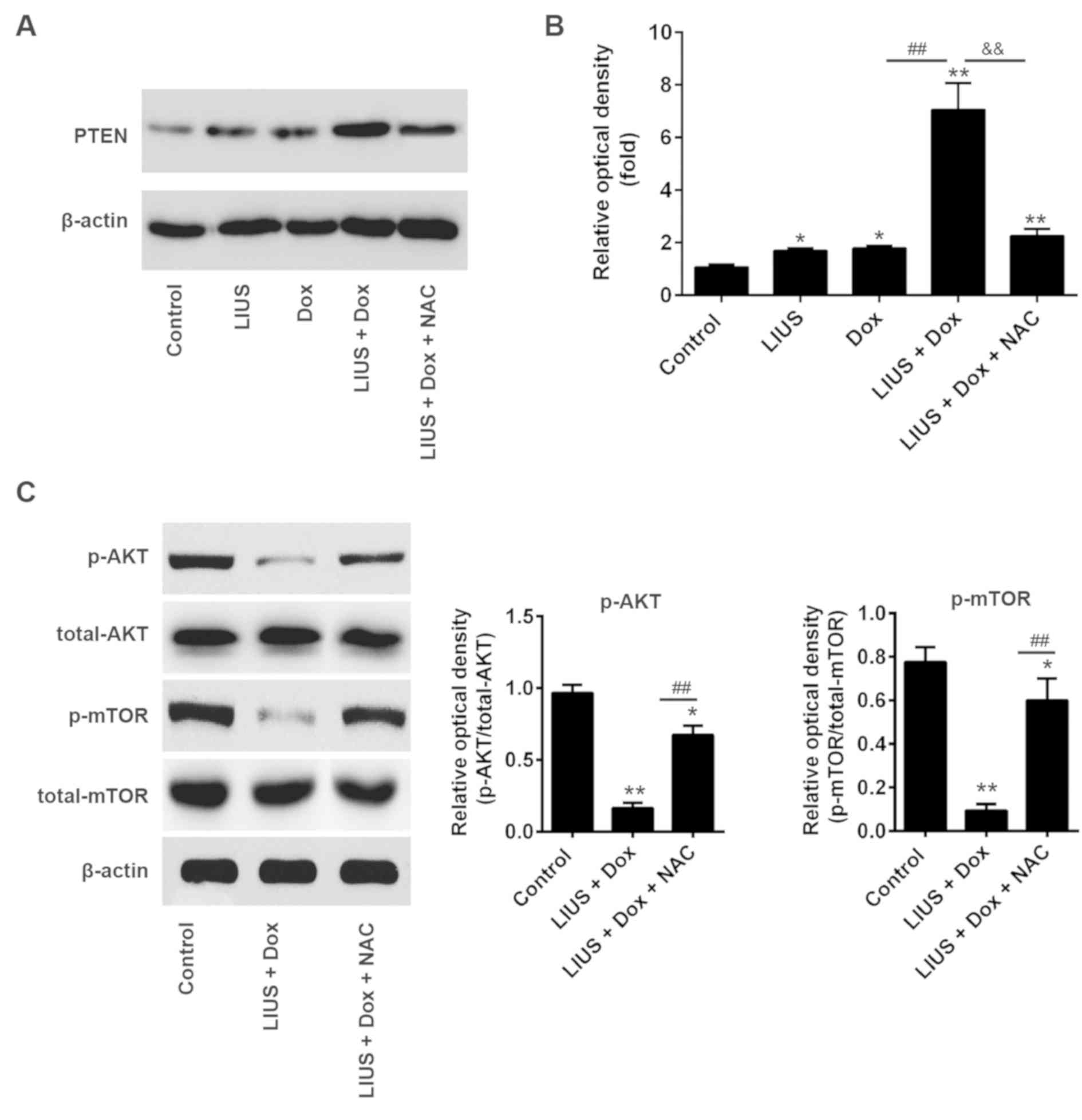 | Figure 5.Treatment with LIUS enhances
sensitivity to Dox via the reactive oxygen species/miR-21/PTEN
axis. (A) Huh7 cells were treated with various compounds and the
protein expression levels of PTEN were analyzed by western
blotting. (B) Semi-quantification of the protein expression levels
of PTEN normalized to β-actin. (C) Protein expression levels of
p-AKT, AKT, p-mTOR and mTOR were measured by western blotting, and
the protein expression levels were semi-quantified using ImageJ.
*P<0.05, **P<0.01 vs. control; ##P<0.01 vs. Dox
group; &&P<0.01 vs. LIUS + Dox group. AKT,
AKT serine/threonine kinase; Dox, doxorubicin; LIUS, low intensity
ultrasound; miR-21, microRNA-21; mTOR, mechanistic target of
rapamycin kinase; NAC, N-acetylcysteine; p-, phosphorylated; PTEN,
phosphatase and tensin homolog; t-, total. |
Discussion
In China, the incidence of HCC is increasing; in
total, ~466,000 patients are diagnosed with HCC every year and it
leads to ~422,000 cases of mortality (30). Surgery is an effective approach to
treat HCC; however, it is suitable only for patients with
early-stage HCC (31). In
contrast, for patients with late-stage HCC, the available
treatments are limited (32).
Transarterial chemoembolization represents a standard treatment for
patients with advanced HCC (33).
However, patients with HCC treated with Dox or sorafenib exhibit
resistance to chemotherapy (34).
Epigenetic alterations, cellular export of drugs and evasion of
apoptosis are frequently identified in resistant HCC cells, and
these processes markedly limit the effectiveness of chemotherapy
(35). Therefore, it is necessary
to develop novel strategies to improve the effect of chemotherapy
and prevent chemoresistance.
Ultrasound is a therapeutic approach that has been
used in recent decades, and the identification of the optimal
parameters is necessary for an effective treatment (36). Although LIUS has been demonstrated
to be an effective anticancer treatment (12), high intensity focused ultrasound
represents an additional non-invasive therapy to treat cancer
(37). LIUS is characterized by a
decreased intensity, and may alter the tumor environment and gene
expression (38). However, the
molecular mechanisms underlying ultrasound therapy remain unclear.
Previous studies have demonstrated that the biological effects
induced by ultrasound are primarily caused by thermal effects,
inertial cavitation and ROS accumulation (12,39).
Thermal effects and inertial cavitation may cause protein
denaturation and tissue damage (39,40).
The association between ultrasound treatment and ROS production has
attracted increasing attention (41). A previous study on HCC revealed
that LIUS increases ROS production, decreasing chemotherapy
resistance and increasing the cellular uptake of DNA-damaging drugs
(22). In line with these previous
studies, the present results suggested that treatment with LIUS
exhibited synergistic effects with Dox, and increased the
sensitivity of HCC cells to Dox, promoting apoptosis of Huh7
cells.
ROS has been reported to regulate miRNAs involved in
tumorigenesis; however, the association between ROS-induced miRNA
dysregulation and chemotherapy resistance remains unclear. In the
present study, ROS were identified to decrease the expression
levels of miR-21 and treatment with NAC reversed this effect.
miR-21 is an oncogene, and was identified to be upregulated in
various types of cancer (22,42).
miR-21 regulates cancer cell proliferation, migration and various
anti-apoptotic processes (22,43,44).
In the present study, the expression levels of miR-21 were
significantly decreased following treatment with LIUS, as
identified by microarray analysis. Furthermore, the present results
suggested that the expression levels of PTEN were increased
following miR-21 knockdown. PTEN is a tumor suppressor gene that
has attracted increasing attention in cancer therapy (45). Additionally the PTEN/AKT signaling
pathway has been identified to regulate cell growth and survival
(45). In line with these previous
studies, the present results suggested that treatment with LIUS
increased the expression levels of PTEN by suppressing miR-21
expression and increased the sensitivity of HCC cells to Dox.
To the best of our knowledge, the present study is
the first to suggest that LIUS combined with the chemotherapy drug
Dox may induce apoptosis of HCC cells, increase chemotherapy
sensitivity and exhibit potent antitumor effects. ROS production
increased following treatment with LIUS and this decreased the
expression levels of miR-21. The present results suggested that the
expression levels of PTEN were regulated by the ROS/miR-21 axis,
suggesting that LIUS affected tumor cell survival by regulating the
PTEN/AKT signaling pathway. Collectively, this study provided novel
insights into the molecular mechanism underlying the role of LIUS
in promoting the effects of chemotherapy. In particular, treatment
with LIUS increased chemotherapy sensitivity via the
ROS/miR-21/PTEN pathway. The present results suggested that the
combined treatment with LIUS and Dox may represent a novel strategy
to treat HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Nature Fund
Projects of The Inner Mongolia Autonomous Region (grant no.
2016MS08105).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CX, HZ and YZ performed the experiments, analyzed
the data and wrote the paper. HZ designed the present study and
provided experimental materials. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yii AC, Tan GL, Tan KL, Lapperre TS and
Koh MS: Fixed airways obstruction among patients with severe
asthma: Findings from the Singapore general hospital-severe asthma
phenotype study. BMC Pulm Med. 14:1912014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lurje G, Lesurtel M and Clavien PA:
Multimodal treatment strategies in patients undergoing surgery for
hepatocellular carcinoma. Dig Dis. 31:112–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lope CR, Tremosini S, Forner A, Reig M
and Bruix J: Management of HCC. J Hepatol. 56 Suppl 1:S75–S87.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giordano S and Columbano A: Met as a
therapeutic target in HCC: Facts and hopes. J Hepatol. 60:442–452.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X
and Han G: Combination therapy of sorafenib and TACE for
unresectable HCC: A systematic review and meta-analysis. PLoS One.
9:e911242014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishida N, Kitano M, Sakurai T and Kudo M:
Molecular mechanism and prediction of sorafenib chemoresistance in
human hepatocellular carcinoma. Dig Dis. 33:771–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Momparler RL, Karon M, Siegel SE and Avila
F: Effect of adriamycin on DNA, RNA, and protein synthesis in
cell-free systems and intact cells. Cancer Res. 36:2891–2895.
1976.PubMed/NCBI
|
|
8
|
Rivankar S: An overview of doxorubicin
formulations in cancer therapy. J Cancer Res Ther. 10:853–858.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan JX, Wang F and Ye LY:
Doxorubicin-induced epithelial-mesenchymal transition through SEMA
4A in hepatocellular carcinoma. Biochem Biophys Res Commun.
479:610–614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Liang C, Xue F, Chen W, Zhi X,
Feng X, Bai X and Liang T: Salinomycin decreases doxorubicin
resistance in hepatocellular carcinoma cells by inhibiting the
β-catenin/TCF complex association via FOXO3a activation.
Oncotarget. 6:10350–10365. 2015.PubMed/NCBI
|
|
12
|
Wood AK and Sehgal CM: A review of
low-intensity ultrasound for cancer therapy. Ultrasound Med Biol.
41:905–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv Y, Fang M, Zheng J, Yang B, Li H,
Xiuzigao Z, Song W, Chen Y and Cao W: Low-intensity ultrasound
combined with 5-aminolevulinic acid administration in the treatment
of human tongue squamous carcinoma. Cell Physiol Biochem.
30:321–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan H, Li H, Liu G, Cong W, Zhao H, Cao W
and Zheng J: Doxorubicin combined with low intensity ultrasound
suppresses the growth of oral squamous cell carcinoma in culture
and in xenografts. J Exp Clin Cancer Res. 36:1632017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan J, Huang Z, Han J, Shao J and Huang C:
Redox regulation of microRNAs in cancer. Cancer Lett. 418:250–259.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Y, Yan W, Lu L, Wang Y, Lu W, Cao Y
and Cai W: p38/p53/miR-200a-3p feedback loop promotes oxidative
stress-mediated liver cell death. Cell Cycle. 14:1548–1558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Li TW, Zhou Y, Peng H, Liu T,
Zandi E, Martínez-Chantar ML, Mato JM and Lu SC: Activation of a
novel c-Myc-miR27-prohibitin 1 circuitry in cholestatic liver
injury inhibits glutathione synthesis in mice. Antioxid Redox
Signal. 22:259–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang
C, Wang F, Zhang CY, Zen K and Li L: MiR-26 enhances
chemosensitivity and promotes apoptosis of hepatocellular carcinoma
cells through inhibiting autophagy. Cell Death Dis. 8:e25402017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Liu X, Qiang H, Li K, Wang J,
Chen D and Zhuang Y: Inhibitory effects of rosa roxburghii tratt
juice on in vitro oxidative modification of low density lipoprotein
and on the macrophage growth and cellular cholesteryl ester
accumulation induced by oxidized low density lipoprotein. Clin Chim
Acta. 313:37–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, Yang
B and Cao W: Enhancement of anti-tumor effects of 5-fluorouracil on
hepatocellular carcinoma by low-intensity ultrasound. J Exp Clin
Cancer Res. 35:712016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: MiR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZB, Li ZZ, Li L, Chu HT and Jia M:
MiR-21 and miR-183 can simultaneously target SOCS6 and modulate
growth and invasion of hepatocellular carcinoma (HCC) cells. Eur
Rev Med Pharmacol Sci. 19:3208–3217. 2015.PubMed/NCBI
|
|
25
|
Huang CS, Yu W, Cui H, Wang YJ, Zhang L,
Han F and Huang T: Increased expression of miR-21 predicts poor
prognosis in patients with hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:7234–7238. 2015.PubMed/NCBI
|
|
26
|
Lim HJ, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–689. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leslie NR and Downes CP: PTEN function:
How normal cells control it and tumour cells lose it. Biochem J.
382:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li
JH, Xu XM, Liu S, Chen J, Liu F, et al: Phosphoinositide
3-kinase/Akt pathway plays an important role in chemoresistance of
gastric cancer cells against etoposide and doxorubicin induced cell
death. Int J Cancer. 122:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirstein MM and Vogel A: The pathogenesis
of hepatocellular carcinoma. Dig Dis. 32:545–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rich NE, Yopp AC and Singal AG: Medical
management of hepatocellular carcinoma. J Oncol Pract. 13:356–364.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reig M, Darnell A, Forner A, Rimola J,
Ayuso C and Bruix J: Systemic therapy for hepatocellular carcinoma:
The issue of treatment stage migration and registration of
progression using the BCLC-refined RECIST. Semin Liver Dis.
34:444–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan ST, Li ZL, He ZX, Qiu JX and Zhou SF:
Molecular mechanisms for tumour resistance to chemotherapy. Clin
Exp Pharmacol Physiol. 43:723–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Draper DO: Facts and misfits in ultrasound
therapy: Steps to improve your treatment outcomes. Eur J Phys
Rehabil Med. 50:209–216. 2014.PubMed/NCBI
|
|
37
|
Wu F: High intensity focused ultrasound: A
noninvasive therapy for locally advanced pancreatic cancer. World J
Gastroenterol. 20:16480–16488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia B, Zou Y, Xu Z and Lv Y: Gene
expression profiling analysis of the effects of low-intensity
pulsed ultrasound on induced pluripotent stem cell-derived neural
crest stem cells. Biotechnol Appl Biochem. 64:927–937. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duco W, Grosso V, Zaccari D and Soltermann
AT: Generation of ROS mediated by mechanical waves (ultrasound) and
its possible applications. Methods. 109:141–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jang HJ, Lee JY, Lee DH, Kim WH and Hwang
JH: Current and future clinical applications of high-intensity
focused ultrasound (HIFU) for pancreatic cancer. Gut Liver. 4 Suppl
1:S57–S61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi J, Chen Z, Wang B, Wang L, Lu T and
Zhang Z: Reactive oxygen species-manipulated drug release from a
smart envelope-type mesoporous titanium nanovehicle for tumor
sonodynamic-chemotherapy. ACS Appl Mater Interfaces. 7:28554–28565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma X, Conklin DJ, Li F, Dai Z, Hua X, Li
Y, Xu-Monette ZY, Young KH, Xiong W, Wysoczynski M, et al: The
oncogenic microRNA miR-21 promotes regulated necrosis in mice. Nat
Commun. 6:71512015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melnik BC: MiR-21: An environmental driver
of malignant melanoma? J Transl Med. 13:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sekar D, Krishnan R, Thirugnanasambantham
K, Rajasekaran B, Islam VI and Sekar P: Significance of microRNA 21
in gastric cancer. Clin Res Hepatol Gastroenterol. 40:538–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Worby CA and Dixon JE: Pten. Annu Rev
Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI
|